Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Calculations
- Change in percentage crystallinity with respect to sulfur
- Melting enthalpy of composite materials (PBJS90)
- Cold crystallization enthalpy of composite materials
- Melting enthalpy of sulfur
- Cold crystallization enthalpy of sulfur
2.2. Preparation of Jar for Thiocracking
2.3. Synthesis
2.3.1. Preparation of PBJS90
2.3.2. Heating of PBJ in the Absence of Sulfur to Give hPBJ
2.3.3. Depolymerization of PBJS90
2.4. Determination of Dark Sulfur Content
2.5. Mechanical Strength Analysis
3. Results and Discussion
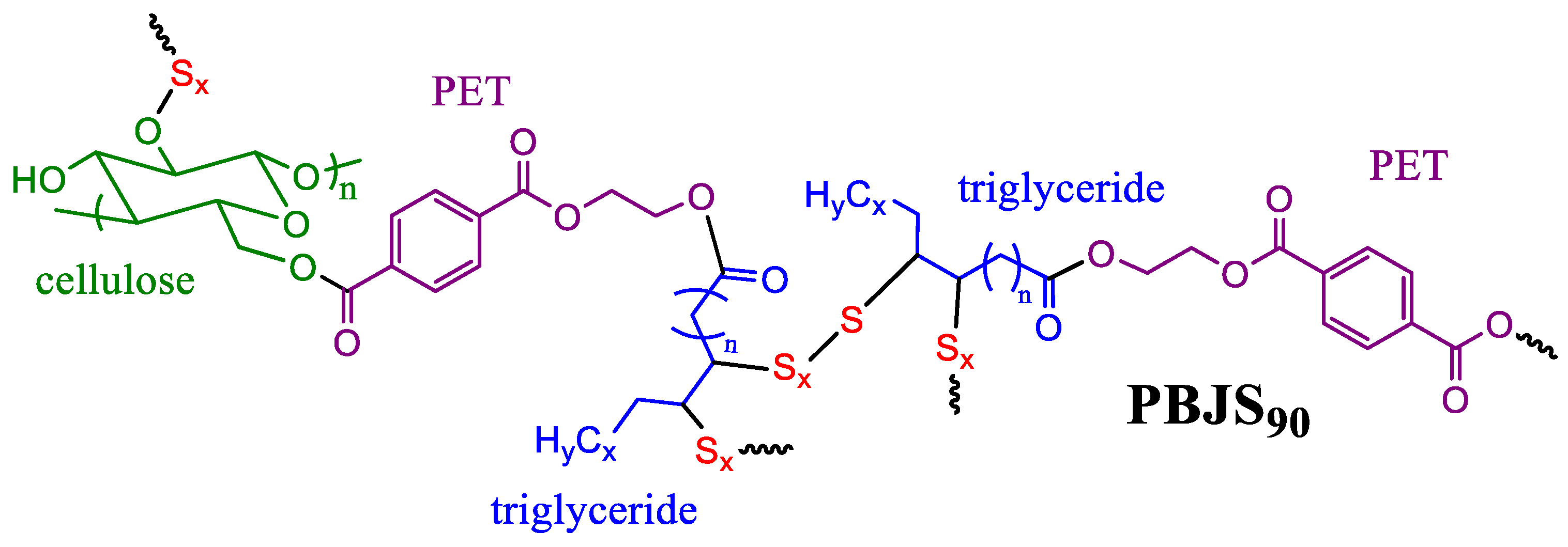
3.1. Design and Preliminary Analysis of Multi-Material
3.2. Reactivity of Individual Components with Elemental Sulfur
3.3. Thiocracking and Chemical Analysis of Composite PBJS90
3.4. Thermal and Mechanical Properties
3.5. Environmental and Sustainability Impact Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Poly. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Krausmann, F.; Schaffartzik, A.; Mayer, A.; Gingrich, S.; Eisenmenger, N. Global trends and patterns in material use. MRS Online Proc. Libr. 2013, 1545, mrss13-1545. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Fagnani, D.E.; Tami, J.L.; Copley, G.; Clemons, M.N.; Getzler, Y.D.Y.L.; McNeil, A.J. 100th Anniversary of Macromolecular Science Viewpoint: Redefining Sustainable Polymers. ACS Macro Lett. 2021, 10, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Martinho, G. Waste hierarchy index for circular economy in waste management. Waste Manag. 2019, 95, 298–305. [Google Scholar] [CrossRef]
- Oberoi, I.S.; Rajkumar, P.; Das, S. Disposal and recycling of plastics. Mater. Today Proc. 2021, 46, 7875–7880. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 14, e202300142. [Google Scholar] [CrossRef]
- Clark, J.H.; Matharu, A.S. Waste to wealth using green chemistry. Issues Environ. Sci. Technol. 2013, 37, 66–82. [Google Scholar]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Barria, C.; Martins, M.A.; Franco-Martinez, L.; Barreto, A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. Hazard. Mater. 2021, 403, 123590. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in marine environment review of methods for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Lahtela, V.; Hyvarinen, M.; Karki, T. Composition of plastic fractions in waste streams: Toward more efficient recycling and utilization. Polymers 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A. Microalgae and microplastic removal. Purasuchikkusu 2022, 73, 41–45. [Google Scholar]
- Schwarzbauer, J.; Heim, S.; Brinker, S.; Littke, R. Occurrence and alteration of organic contaminants in seepage and leakage water from a waste deposit landfill. Water Res. 2002, 36, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Venancio, C.; Melnic, I.; Tamayo-Belda, M.; Oliveira, M.; Martins, M.A.; Lopes, I. Polymethylmethacrylate nanoplastics can cause developmental malformations in early life stages of Xenopus laevis. Sci. Total Environ. 2022, 806, 150491. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, L.; Han, X.; Wang, X.; Zhang, Y.; Ma, X.; Lin, K.; Wang, R.; Ni, Z.; Wang, J.; et al. Microplastic-induced apoptosis and metabolism responses in marine Dinoflagellate, Karenia mikimotoi. Sci. Total Environ. 2022, 804, 150252. [Google Scholar] [CrossRef]
- Zhou, X.-X.; He, S.; Gao, Y.; Chi, H.-Y.; Wang, D.-J.; Li, Z.-C.; Yan, B. Quantitative Analysis of Polystyrene and Poly(methyl methacrylate) Nanoplastics in Tissues of Aquatic Animals. Environ. Sci. Technol. 2021, 55, 3032–3040. [Google Scholar] [CrossRef]
- Thomas, D.; Schuetze, B.; Heinze, W.M.; Steinmetz, Z. Sample preparation techniques for the analysis of microplastics in soil-a review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; M’Hamdi Alaoui, F.E.; El Ouinani, A.; Sahibed-Dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Effect of materials mixture on the higher heating value: Case of biomass, biochar and municipal solid waste. Waste Manag. 2017, 61, 78–86. [Google Scholar] [CrossRef]
- Bora, R.R.; Wang, R.; You, F. Waste Polypropylene Plastic Recycling toward Climate Change Mitigation and Circular Economy: Energy, Environmental, and Technoeconomic Perspectives. ACS Sust. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Cepeliogullar, O.; Putun, A.E. A pyrolysis study for the thermal and kinetic characteristics of an agricultural waste with two different plastic wastes. Waste Manag. Res. 2014, 32, 971–979. [Google Scholar] [CrossRef]
- Ephraim, A.; Minh, D.P.; Lebonnois, D.; Peregrina, C.; Sharrock, P.; Nzihou, A. Co-pyrolysis of wood and plastics: Influence of plastic type and content on product yield, gas composition and quality. Fuel 2018, 231, 110–117. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Khalili, S.; Vahidifar, A.; Naderi, G.; Dubois, C. Waste Polymethyl Methacrylate (PMMA): Recycling and High-Yield Monomer Recovery. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–33. [Google Scholar]
- Godiya, C.B.; Gabrielli, S.; Materazzi, S.; Pianesi, M.S.; Stefanini, N.; Marcantoni, E. Depolymerization of waste poly(methyl methacrylate) scraps and purification of depolymerized products. J. Environ. Manag. 2019, 231, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I. Recent advancements in thermolysis of plastic solid wastes to liquid fuel. J. Therm. Anal. Calorim. 2022, 147, 3495–3508. [Google Scholar] [CrossRef]
- Khatwa, M.A.; Salem, H.G.; Haggar, S.M. Building material from waste. Can. Metall. Q. 2005, 44, 339–350. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.-B. Chemical recycling to monomers: Industrial Bisphenol-A-Polycarbonates to novel aliphatic polycarbonate materials. J. Polym. Sci. 2022, 60, 3256–3268. [Google Scholar] [CrossRef]
- Luo, X.; Zhan, J.; Mei, Q.; Zhang, S. Selective oxidative upgrade of waste polystyrene plastics by nitric acid to produce benzoic acid. Green Chem. 2023, 25, 6717–6727. [Google Scholar] [CrossRef]
- Munyaneza, N.E.; Posada, C.; Xu, Z.; De Altin Popiolek, V.; Paddock, G.; McKee, C.; Liu, G. A Generic Platform for Upcycling Polystyrene to Aryl Ketones and Organosulfur Compounds. Angew. Chem. Int. Ed. 2023, 62, e202307042. [Google Scholar] [CrossRef] [PubMed]
- Ozsin, G.; Putun, A.E. A comparative study on co-pyrolysis of lignocellulosic biomass with polyethylene terephthalate, polystyrene, and polyvinyl chloride: Synergistic effects and product characteristics. J. Clean. Prod. 2018, 205, 1127–1138. [Google Scholar] [CrossRef]
- Salvilla, J.N.V.; Ofrasio, B.I.G.; Rollon, A.P.; Manegdeg, F.G.; Abarca, R.R.M.; de Luna, M.D.G. Synergistic co-pyrolysis of polyolefin plastics with wood and agricultural wastes for biofuel production. Appl. Energy 2020, 279, 115668. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Q.; Sun, K.; Chi, Y.; Yan, J. Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Bioresour. Technol. 2018, 249, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.C.; Dove, A.P. 100th Anniversary of Macromolecular Science Viewpoint: Toward Catalytic Chemical Recycling of Waste (and Future) Plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass with plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kugelmass, L.H.; Tagnon, C.; Stache, E.E. Photothermal Mediated Chemical Recycling to Monomers via Carbon Quantum Dots. J. Am. Chem. Soc. 2023, 145, 16090–16097. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.; Teo, J.Y.Q.; Feng, Z.; Tan, T.T.Y.; Lim, J.Y.C. Organocatalytic Aerobic Oxidative Degradation of Polystyrene to Aromatic Acids. ACS Sust. Chem. Eng. 2023, 11, 12514–12522. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Soares, C.T.d.M.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- Huang, P.; Pitcher, J.; Mushing, A.; Lourenço, F.; Shaver, M.P. Chemical recycling of multi-materials from glycol-modified poly(ethylene terephthalate). Resour. Conserv. Recycl. 2023, 190, 106854. [Google Scholar] [CrossRef]
- Tito, E.; dos Passos, J.S.; Bensaid, S.; Pirone, R.; Biller, P. Multilayer plastic film chemical recycling via sequential hydrothermal liquefaction. Resour. Conserv. Recycl. 2023, 197, 107067. [Google Scholar] [CrossRef]
- Samorì, C.; Pitacco, W.; Vagnoni, M.; Catelli, E.; Colloricchio, T.; Gualandi, C.; Mantovani, L.; Mezzi, A.; Sciutto, G.; Galletti, P. Recycling of multilayer packaging waste with sustainable solvents. Resour. Conserv. Recycl. 2023, 190, 106832. [Google Scholar] [CrossRef]
- Vagnoni, M.; Pitacco, W.; Arpaia, V.; Catelli, E.; Gualandi, C.; Mastroddi, R.; Mezzi, A.; Samorì, C.; Sciutto, G.; Tagliavini, E.; et al. Recycling of multilayer packaging waste with switchable anionic surfactants. Resour. Conserv. Recycl. 2023, 198, 107141. [Google Scholar] [CrossRef]
- Belyamani, I.; Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Toward recycling “unsortable” post-consumer WEEE stream: Characterization and impact of electron beam irradiation on mechanical properties. J. Clean. Prod. 2021, 294, 126300. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Detoxification of bisphenol A via sulfur-mediated carbon–carbon σ-bond scission. RSC Sustain. 2023, 1, 535–542. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Tennyson, A.G.; Smith, R.C. Single-stage chemical recycling of plastic waste to yield durable composites via a tandem transesterification-thiocracking process. J. Polym. Sci. 2023, 61, 787–793. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Derr, K.M.; Maladeniya, C.P.; Sauceda-Oloño, P.Y.; Tennyson, A.G.; Smith, R.C. Upcycling waste PMMA to durable composites via a transesterification-inverse vulcanization process. J. Polym. Sci. 2023, 62, 554–563. [Google Scholar] [CrossRef]
- Derr, K.M.; Smith, R.C. One-Pot Method for Upcycling Polycarbonate Waste to Yield High-Strength, BPA-Free Composites. J. Polym. Sci. 2023, 62, 1115–1122. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-Selective Catalytic Oxidation: Catalysts and Processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Demirbas, A.; Alidrisi, H.; Balubaid, M.A. API Gravity, Sulfur Content, and Desulfurization of Crude Oil. Pet. Sci. Technol. 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Wolfs, J.; Ribca, I.; Meier, M.A.R.; Johansson, M. Polythionourethane Thermoset Synthesis via Activation of Elemental Sulfur in an Efficient Multicomponent Reaction Approach. ACS Sust. Chem. Eng. 2023, 11, 3952–3962. [Google Scholar] [CrossRef]
- Conen, P.; Nickisch, R.; Meier, M.A.R. Synthesis of highly substituted alkenes by sulfur-mediated olefination of N-tosylhydrazones. Commun. Chem. 2023, 6, 255. [Google Scholar] [CrossRef]
- Nickisch, R.; Conen, P.; Gabrielsen, S.M.; Meier, M.A.R. A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv. 2021, 11, 3134–3142. [Google Scholar] [CrossRef]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and Applications of Polymers Made by Inverse Vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Sulfur-based polymers by inverse vulcanization: A novel path to foster green chemistry. Green Mater. 2020, 8, 172–180. [Google Scholar]
- Wagenfeld, J.-G.; Al-Ali, K.; Almheiri, S.; Slavens, A.F.; Calvet, N. Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Manag. 2019, 95, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Copolymerization of vegetable oils and bio-based monomers with elemental sulfur: A new promising route for bio-based polymers. Sust. Chem. Pharm. 2019, 13, 100158. [Google Scholar] [CrossRef]
- Lee, T.; Dirlam, P.T.; Njardarson, J.T.; Glass, R.S.; Pyun, J. Polymerizations with Elemental Sulfur: From Petroleum Refining to Polymeric Materials. J. Am. Chem. Soc. 2022, 144, 5–22. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Lim, J.; Pyun, J.; Char, K. Recent Approaches for the Direct Use of Elemental Sulfur in the Synthesis and Processing of Advanced Materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Gupta, A.; Worthington, M.J.H.; Patel, H.D.; Johnston, M.R.; Puri, M.; Chalker, J.M. Reaction of Sulfur and Sustainable Algae Oil for Polymer Synthesis and Enrichment of Saturated Triglycerides. ACS Sustain. Chem. Eng. 2022, 10, 9022–9028. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Bu Najmah, I.; Lundquist, N.A.; Stanfield, M.K.; Stojcevski, F.; Campbell, J.A.; Esdaile, L.J.; Gibson, C.T.; Lewis, D.A.; Henderson, L.C.; Hasell, T.; et al. Insulating Composites Made from Sulfur, Canola Oil, and Wool**. ChemSusChem 2021, 14, 2352–2359. [Google Scholar] [CrossRef]
- Eder, M.L.; Call, C.B.; Jenkins, C.L. Utilizing Reclaimed Petroleum Waste to Synthesize Water-Soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Appl. Polym. Mater. 2022, 4, 1110–1116. [Google Scholar] [CrossRef]
- Gomez, I.; Mecerreyes, D.; Blazquez, J.A.; Leonet, O.; Ben Youcef, H.; Li, C.; Gómez-Cámer, J.L.; Bondarchuk, O.; Rodriguez-Martinez, L. Inverse vulcanization of sulfur with divinylbenzene: Stable and easy processable cathode material for lithium-sulfur batteries. J. Power Sources 2016, 329, 72–78. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Inverse Vulcanization of Sulfur using Natural Dienes as Sustainable Materials for Lithium–Sulfur Batteries. ChemSusChem 2016, 9, 3419–3425. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem. A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Müller, F.G.; Lisboa, L.S.; Chalker, J.M. Inverse Vulcanized Polymers for Sustainable Metal Remediation. Adv. Sustain. Syst. 2023, 7, 2300010. [Google Scholar] [CrossRef]
- Chalker, J.M.; Mann, M.; Worthington, M.J.H.; Esdaile, L.J. Polymers Made by Inverse Vulcanization for Use as Mercury Sorbents. Org. Mater. 2021, 3, 362–373. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Smith, J.A.; Mulhall, R.; Goodman, S.; Fleming, G.; Allison, H.; Raval, R.; Hasell, T. Investigating the Antibacterial Properties of Inverse Vulcanized Sulfur Polymers. ACS Omega 2020, 5, 5229–5234. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Westerman, C.R.; Walker, P.M.; Jenkins, C.L.; Westerman, C.R.; Walker, P.M. Synthesis of Terpolymers at Mild Temperatures Using Dynamic Sulfur Bonds in Poly(S-Divinylbenzene). JoVE 2019, e59620. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Sayer, K.B.; Miller, V.L.; Merrill, Z.; Davis, A.E.; Jenkins, C.L. Allyl sulfides in garlic oil initiate the formation of renewable adhesives. Polym. Chem. 2023, 14, 3091–3098. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef]
- Diez, S.; Hoefling, A.; Theato, P.; Pauer, W. Mechanical and Electrical Properties of Sulfur-Containing Polymeric Materials Prepared via Inverse Vulcanization. Polymers 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Ceper, E.B.; Li, X.; Yang, J.; Dong, W.; Ozmen, M.M.; Theato, P. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, 1800650. [Google Scholar] [CrossRef]
- Gomez, I.; De Anastro, A.F.; Leonet, O.; Blazquez, J.A.; Grande, H.-J.; Pyun, J.; Mecerreyes, D. Sulfur Polymers Meet Poly(ionic liquid)s: Bringing New Properties to Both Polymer Families. Macromol. Rapid Commun. 2018, 39, 1800529. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Alberto Blazquez, J.; Grande, H.-J.; Mecerreyes, D. Poly(anthraquinonyl sulfides): High Capacity Redox Polymers for Energy Storage. ACS Macro Lett. 2018, 7, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A role for terpenoid cyclization in the atom economical polymerization of terpenoids with sulfur to yield durable composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Smith, A.D.; Tennyson, A.G.; Smith, R.C. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Bear, J.C.; Peveler, W.J.; McNaughter, P.D.; Parkin, I.P.; O’Brien, P.; Dunnill, C.W. Nanoparticle-sulphur “inverse vulcanization” polymer composites. Chem. Commun. 2015, 51, 10467–10470. [Google Scholar] [CrossRef]
- Glass, R.S.; Char, K.; Pyun, J. From waste to valuable plastics-Discovery of new paradigms from well-studied systems with elemental sulfur. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 157–161. [Google Scholar] [CrossRef]
- Gwon, S.; Jeong, Y.; Oh, J.E.; Shin, M. Sustainable sulfur composites with enhanced strength and lightweightness using waste rubber and fly ash. Constr. Build. Mater. 2017, 135, 650–664. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Cengiz, E.C.; Demir-Cakan, R.; Yagci, Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for lithium sulfur batteries. Eur. Polym. J. 2016, 80, 70–77. [Google Scholar] [CrossRef]
- Boyd, D.A. Sulfur and Its Role in Modern Materials Science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Hwang, J.; Lee, S.Y.; Park, J.; Han, N.; Lee, C.H.; Kang, S.-W.; Urbas, A.; Kim, J.O.; Ku, Z.; et al. Highly Sensitive and Cost-Effective Polymeric-Sulfur-Based Mid-Wavelength Infrared Linear Polarizers with Tailored Fabry-Perot Resonance. Adv. Mater. 2023, 35, 2209377. [Google Scholar] [CrossRef]
- Ghosh, A.; Shukla, S.; Khosla, G.S.; Lochab, B.; Mitra, S. Sustainable Sulfur-rich Copolymer/Graphene Composite as Lithium-Sulfur Battery Cathode with Excellent Electrochemical Performance. Sci. Rep. 2016, 6, 25207. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilizers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; El Gamal, M. Hydro-mechanical behavior of a newly developed sulfur polymer concrete. Cem. Concr. Compos. 2009, 31, 186–194. [Google Scholar] [CrossRef]
- Rappold, T.A.; Lackner, K.S. Large scale disposal of waste sulfur: From sulfide fuels to sulfate sequestration. Energy 2010, 35, 1368–1380. [Google Scholar] [CrossRef]
- Stojcevski, F.; Stanfield, M.K.; Hayne, D.J.; Mann, M.; Lundquist, N.A.; Chalker, J.M.; Henderson, L.C. Inverse Vulcanisation of canola oil as a route to recyclable chopped carbon fibre composites. Sustain. Mater. Technol. 2022, 32, e00400. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, C.; Fu, Y.; Guo, T.; Yan, G.; Hu, J. Sulfur-containing adsorbent made by inverse vulcanization of sulfur/oleylamine/potato starch for efficient removal of Hg(II) ions. J. Environ. Chem. Eng. 2023, 11, 109806. [Google Scholar] [CrossRef]
- Wongsirathat, C.; Chavalparit, O. Utilization of sulfur waste from petroleum refinery for sulfur concrete. Adv. Mater. Res. 2014, 856, 113–117. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Dop, R.A.; Yan, P.; Zhao, W.; Wang, H.; Dodd, L.J.; McDonald, T.O.; Hasell, T. Inverse vulcanised sulfur polymer nanoparticles prepared by antisolvent precipitation. J. Mater. Chem. A 2022, 10, 13704–13710. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Direksilp, C.; Falkenstein, P.; Welle, A.; Koenig, M.; Heissler, S.; Matysik, J.; Levkin, P.A.; Theato, P. Inverse Vulcanization of Styrylethyltrimethoxysilane-Coated Surfaces, Particles, and Crosslinked Materials. Angew. Chem. Int. Ed. 2020, 59, 18639–18645. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Astashkin, A.V.; Glass, R.S.; MacKay, M.E.; Char, K.; Pyun, J. Preparation of Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur. ACS Macro Lett. 2014, 3, 1258–1261. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Kalderis, D.; Arslan, M.; Yagci, Y.; Kiskan, B. Poly(benzoxazine-co-sulfur): An efficient sorbent for mercury removal from aqueous solution. J. Appl. Polym. Sci. 2017, 134, 45306. [Google Scholar] [CrossRef]
- Ali Ghazi, Z.; Zhu, L.; Wang, H.; Naeem, A.; Khattak, A.M.; Liang, B.; Ali Khan, N.; Wei, Z.; Li, L.; Tang, Z. Efficient Polysulfide Chemisorption in Covalent Organic Frameworks for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 6, 1601250. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Recycling and Self-Healing of Polybenzoxazines with Dynamic Sulfide Linkages. Sci. Rep. 2017, 7, 5207. [Google Scholar] [CrossRef]
- Berk, H.; Kaya, M.; Topcuoglu, M.; Turkten, N.; Karatas, Y.; Cihaner, A. Synthesis, characterization and application of high sulfur content polymeric materials from fatty acids. React. Funct. Polym. 2023, 187, 105581. [Google Scholar] [CrossRef]
- Bhargav, A.; Chang, C.-H.; Fu, Y.; Manthiram, A. Rationally Designed High-Sulfur-Content Polymeric Cathode Material for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.A.; Nguyen, V.Q.; McClain, C.C.; Kung, F.H.; Baker, C.C.; Myers, J.D.; Hunt, M.P.; Kim, W.; Sanghera, J.S. Optical Properties of a Sulfur-Rich Organically Modified Chalcogenide Polymer Synthesized via Inverse Vulcanization and Containing an Organometallic Comonomer. ACS Macro Lett. 2019, 8, 113–116. [Google Scholar] [CrossRef]
- Ding, N.; Lum, Y.; Chen, S.; Chien, S.W.; Hor, T.S.A.; Liu, Z.; Zong, Y. Sulfur-carbon yolk-shell particle based 3D interconnected nanostructures as cathodes for rechargeable lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 1853–1857. [Google Scholar] [CrossRef]
- Dop, R.A.; Neill, D.R.; Hasell, T. Sulfur-Polymer Nanoparticles: Preparation and Antibacterial Activity. ACS Appl. Mater. Interfaces 2023, 15, 20822–20832. [Google Scholar] [CrossRef]
- Franz, B.; Lichtenberg, H.; Hormes, J.; Modrow, H.; Dahl, C.; Prange, A. Utilization of solid elemental sulfur by the phototrophic purple sulfur bacterium Allochromatium vinosum: A sulfur K-edge X-ray absorption spectroscopy study. Microbiology 2007, 153, 1268–1274. [Google Scholar] [CrossRef]
- Gwon, S.; Ahn, E.; Shin, M. Self-healing of modified sulfur composites with calcium sulfoaluminate cement and superabsorbent polymer. Compos. Part B Eng. 2019, 162, 469–483. [Google Scholar] [CrossRef]
- Key, J.; Feng, Y.; Shen, J.; Wang, P.; Wang, H.; Liang, H.; Wang, R.; Ji, S. A highly crosslinked and conductive sulfur-rich copolymer with grafted polyaniline for stable cycling lithium-sulfur batteries. J. Electrochem. Soc. 2020, 167, 20530. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Yin, Y.; Mann, M.; Tonkin, S.J.; Slattery, A.D.; Andersson, G.G.; Gibson, C.T.; Chalker, J.M. Magnetic responsive composites made from a sulfur-rich polymer. Polym. Chem. 2022, 13, 5659–5665. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, R.C. Chemical Recycling of Poly(ethylene terephthalate) via Sequential Glycolysis, Oleoyl Chloride Esterification and Vulcanization to yield Durable Composites. Mater. Adv. 2023, 4, 2785–2793. [Google Scholar] [CrossRef]
- Derr, K.M.; Lopez, C.V.; Maladeniya, C.P.; Tennyson, A.G.; Smith, R.C. Transesterification-vulcanization route to durable composites from post-consumer poly(ethylene terephthalate), terpenoids, and industrial waste sulfur. J. Polym. Sci. 2023, 61, 3075–3086. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, R.C. Composites produced from Waste Plastic with Agricultural and Energy Sector by-Products. J. Appl. Polym. Sci. 2023, 141, e54828. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable Composites by Vulcanization of Oleyl-Esterified Lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Lauer, M.K.; Estrada-Mendoza, T.A.; McMillen, C.D.; Chumanov, G.; Tennyson, A.G.; Smith, R.C. Durable Cellulose-Sulfur Composites Derived from Agricultural and Petrochemical Waste. Adv. Sustain. Syst. 2019, 3, 1900062. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Thermomorphological and mechanical properties of vulcanized octenyl succinate/terpenoid-derivatized corn starch composites. Mater. Adv. 2022, 3, 4186–4193. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer-sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, sustainable, and stronger than portland cement: A composite from unseparated biomass and fossil fuel waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass-sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Insights, S.M. Peanut Butter—Worldwide. Available online: https://www-statista-com.libproxy.clemson.edu/outlook/cmo/food/spreads-sweeteners/spreads/peanut-butter/worldwide (accessed on 4 December 2023).
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Dale, J.J.; Stanley, J.; Dop, R.A.; Chronowska-Bojczuk, G.; Fielding, A.J.; Neill, D.R.; Hasell, T. Exploring Inverse Vulcanisation Mechanisms from the Perspective of Dark Sulfur. Eur. Polym. J. 2023, 195, 112198. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O.N., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef]
- Kang, K.-S.; Iyer, K.A.; Pyun, J. On the Fundamental Polymer Chemistry of Inverse Vulcanization for Statistical and Segmented Copolymers from Elemental Sulfur. Chem. A Eur. J. 2022, 28, e202200115. [Google Scholar] [CrossRef]
- Smith, J.A.; Wu, X.; Berry, N.G.; Hasell, T. High sulfur content polymers: The effect of crosslinker structure on inverse vulcanization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1777–1781. [Google Scholar] [CrossRef]
- Parker, D.J.; Chong, S.T.; Hasell, T. Sustainable inverse-vulcanised sulfur polymers. RSC Adv. 2018, 8, 27892–27899. [Google Scholar] [CrossRef]
- Dunn, J.; Jenkins, C.L. Making light of inverse vulcanization. Nat. Synth. 2022, 1, 835–836. [Google Scholar] [CrossRef]
- Lopez, C.V.; Karunarathna, M.S.; Lauer, M.K.; Maladeniya, C.P.; Thiounn, T.; Ackley, E.D.; Smith, R.C. High Strength, Acid-Resistant Composites from Canola, Sunflower, or Linseed Oils: Influence of Triglyceride Unsaturation on Material Properties. J. Poly. Sci. 2020, 58, 2259–2266. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef]
- Guinati, B.G.S.; Sauceda Oloño, P.Y.; Kapuge Dona, N.L.; Derr, K.M.; Wijeyatunga, S.K.; Tennyson, A.G.; Smith, R.C. Upcycling mixed-material waste with elemental sulfur: Applications to plant oil, unseparated biomass, and raw post-consumer food waste. RSC Sustain. 2024, 2, 1819–1827. [Google Scholar] [CrossRef]
- Dale, J.J.; Petcher, S.; Hasell, T. Dark Sulfur: Quantifying Unpolymerized Sulfur in Inverse Vulcanized Polymers. ACS Appl. Polym. Mater. 2022, 4, 3169–3173. [Google Scholar] [CrossRef]
- Kulkarni, P.; Ravekar, V.; Rama Rao, P.; Waigokar, S.; Hingankar, S. Recycling of waste HDPE and PP plastic in preparation of plastic brick and its mechanical properties. Clean. Mater. 2022, 5, 100113. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Mohammed, I.I.; Mohammed, S.A. Some properties of concrete with plastic aggregate derived from shredded PVC sheets. Constr. Build. Mater. 2019, 201, 232–245. [Google Scholar] [CrossRef]
- Attanasio, A.; Largo, A.; Alvarez, I.L.; Sonzogni, F.; Balaceanu, L. Sustainable aggregates from secondary materials for innovative lightweight concrete products. Heron 2015, 60, 5–26. [Google Scholar]
- Wu, H.; Liu, Z.; Sun, B.; Yin, J. Experimental investigation on freeze–thaw durability of Portland cement pervious concrete (PCPC). Constr. Build. Mater. 2016, 117, 63–71. [Google Scholar] [CrossRef]
- Phan, T.V.T.; Gallardo, C.; Mane, J. GREEN MOTION: A new and easy to use green chemistry metric from laboratories to industry. Green Chem. 2015, 17, 2846–2852. [Google Scholar] [CrossRef]
- Dormer, A.; Finn, D.P.; Ward, P.; Cullen, J. Carbon footprint analysis in plastics manufacturing. J. Clean. Prod. 2013, 51, 133–141. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Allwood, J.M.; Cullen, J.M.; Milford, R.L. Options for Achieving a 50% Cut in Industrial Carbon Emissions by 2050. Environ. Sci. Technol. 2010, 44, 1888–1894. [Google Scholar] [CrossRef]
- Brogaard, L.K.; Damgaard, A.; Jensen, M.B.; Barlaz, M.; Christensen, T.H. Evaluation of life cycle inventory data for recycling systems. Resour. Conserv. Recycl. 2014, 87, 30–45. [Google Scholar] [CrossRef]
- Mokhtar, A.; Nasooti, M. A decision support tool for cement industry to select energy efficiency measures. Energy Strategy Rev. 2020, 28, 100458. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Korniejenko, K.; Figiela, B.; Furtos, G. Review of Geopolymer Nanocomposites: Novel Materials for Sustainable Development. Materials 2023, 16, 3478. [Google Scholar] [CrossRef]
- Furtos, G.; Molnar, L.; Silaghi-Dumitrescu, L.; Pascuta, P.; Korniejenko, K. Mechanical and thermal properties of wood fiber reinforced geopolymer composites. J. Nat. Fibers 2022, 19, 6676–6691. [Google Scholar] [CrossRef]
- Furtos, G.; Silaghi-Dumitrescu, L.; Pascuta, P.; Sarosi, C.; Korniejenko, K. Mechanical Properties of Wood Fiber Reinforced Geopolymer Composites with Sand Addition. J. Nat. Fibers 2021, 18, 285–296. [Google Scholar] [CrossRef]
- Arena, U.; Ardolino, F. Technical and environmental performances of alternative treatments for challenging plastics waste. Resour. Conserv. Recycl. 2022, 183, 106379. [Google Scholar] [CrossRef]
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Science of the Total Environment 2021, 769, 144483. [Google Scholar] [CrossRef]
- Lewis, G.N.; Randall, M. The Heat Content Of the Various Forms of Sulfur. J. Am. Chem. Soc. 1911, 33, 476–488. [Google Scholar] [CrossRef]
- McCarty, J.A.; Sandefur, H.N.; Matlock, M.; Thoma, G.; Kim, D. Life cycle assessment of greenhouse gas emissions associated with production and consumption of peanut butter in the US. Trans. ASABE 2014, 57, 1741–1750. [Google Scholar]
- Passarini, F.; Ciacci, L.; Santini, A.; Vassura, I.; Morselli, L. Auto shredder residue LCA: Implications of ASR composition evolution. J. Clean. Prod. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- Peukert, W. Material properties in fine grinding. Int. J. Miner. Process. 2004, 74, S3–S17. [Google Scholar] [CrossRef]
- Schubert, G.; Bernotat, S. Comminution of non-brittle materials. Int. J. Miner. Process. 2004, 74, S19–S30. [Google Scholar] [CrossRef]
- Tomberlin, K.E.; Venditti, R.; Yao, Y. Life cycle carbon footprint analysis of pulp and paper grades in the united states using production-line-based data and integration. BioResources 2020, 15, 3899–3914. [Google Scholar] [CrossRef]
- Wüstenberg, D.; Kasper, J. Required energy and structural breakdown at the process of dynamic cutting—Comminution of polypropylene and aluminium. Int. J. Miner. Process. 2004, 74, S417–S424. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]



| Component | Mass | % of Upcycled Mass | Primary Chemical Components |
|---|---|---|---|
| Jar Without Lid | 38.800 g | 100% | Muti-Material (breakdown below) |
| Induction Seal | 0.265 g | 0.68% | Aluminum, cellulose, wax, polymer(s) |
| Adhesive | 0.020 g | 0.05% | Acrylic/rubber polymer(s) |
| Label | 1.473 g | 3.79% | Cellulose |
| Residual Peanut Butter | 15.482 g | 39.9% | Triglycerides |
| Jar Body | 21.560 g | 55.6% | Poly(ethylene terephthalate) |
| Material | Td [a] °C | Tm [b] °C | Tg [c] °C | Cold Crystal. Peaks/°C | ΔHm J/g | ΔHcc J/g | Percent Crystallinity [d] | Dark Sulfur (%) [e] |
|---|---|---|---|---|---|---|---|---|
| PBJS90 | 218 | 117 | NA | 36 | 27 | –5 | 29 | 14 |
| S8 | 229 | 118 | NA | NA | 44.8 | NA | 100 | 0 |
| Sample | Compressive Strength (MPa) | After Acid (MPa) | Strength Retained (%) | Compressive Modulus | Flexural Strength (MPa) | Flexural Modulus |
|---|---|---|---|---|---|---|
| PBJS90 | 37.7 ± 2.9 | 35.4 ± 4.5 | 94% | 74 ± 5 | 5.64 ± 0.32 | 631 ± 20.8 |
| APS95 [a] | 35.7 | ND [h] | ND | ND | 4.8 | 690 |
| SPG [b] | 23.1 | ND | ND | ND | 4.7 | ND |
| mPES [c] | 26.9 | ND | ND | ND | 7.7 | ND |
| Brick 1 [d] | 11.2 | ND | ND | ND | ND | ND |
| Brick 2 [e] | 16.4 | ND | ND | ND | 2.75 | ND |
| Brick 3 [f] | 9.0 | ND | ND | ND | ND | ND |
| C62 Brick [g] | 8.6 | ND | ND | ND | ND | ND |
| OPC | 17 | ND | ND | ND | 3.7 | 580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derr, K.M.; Smith, R.C. Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars. Sustainability 2024, 16, 7023. https://doi.org/10.3390/su16167023
Derr KM, Smith RC. Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars. Sustainability. 2024; 16(16):7023. https://doi.org/10.3390/su16167023
Chicago/Turabian StyleDerr, Katelyn M., and Rhett C. Smith. 2024. "Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars" Sustainability 16, no. 16: 7023. https://doi.org/10.3390/su16167023
APA StyleDerr, K. M., & Smith, R. C. (2024). Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars. Sustainability, 16(16), 7023. https://doi.org/10.3390/su16167023







