Homing Pigeons as Biomonitors of Atmospheric Metal Exposure and Health Effects to Promote Environment Sustainability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Tissue Collection
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Lung Tissue Histology
2.3. Particulate Matters Collection and Processing
2.4. Contaminant Analyses and Quality Control
2.5. Data Analysis
3. Results
3.1. Metal Accumulation under Ingestion and Respiratory Routes of Exposure (Experiment 1)
3.1.1. Metal Accumulation Characteristics of Pigeon Tissues
3.1.2. Metal Concentrations in Pigeon Food
3.1.3. Metal Concentrations in Atmospheric Particulate Matter
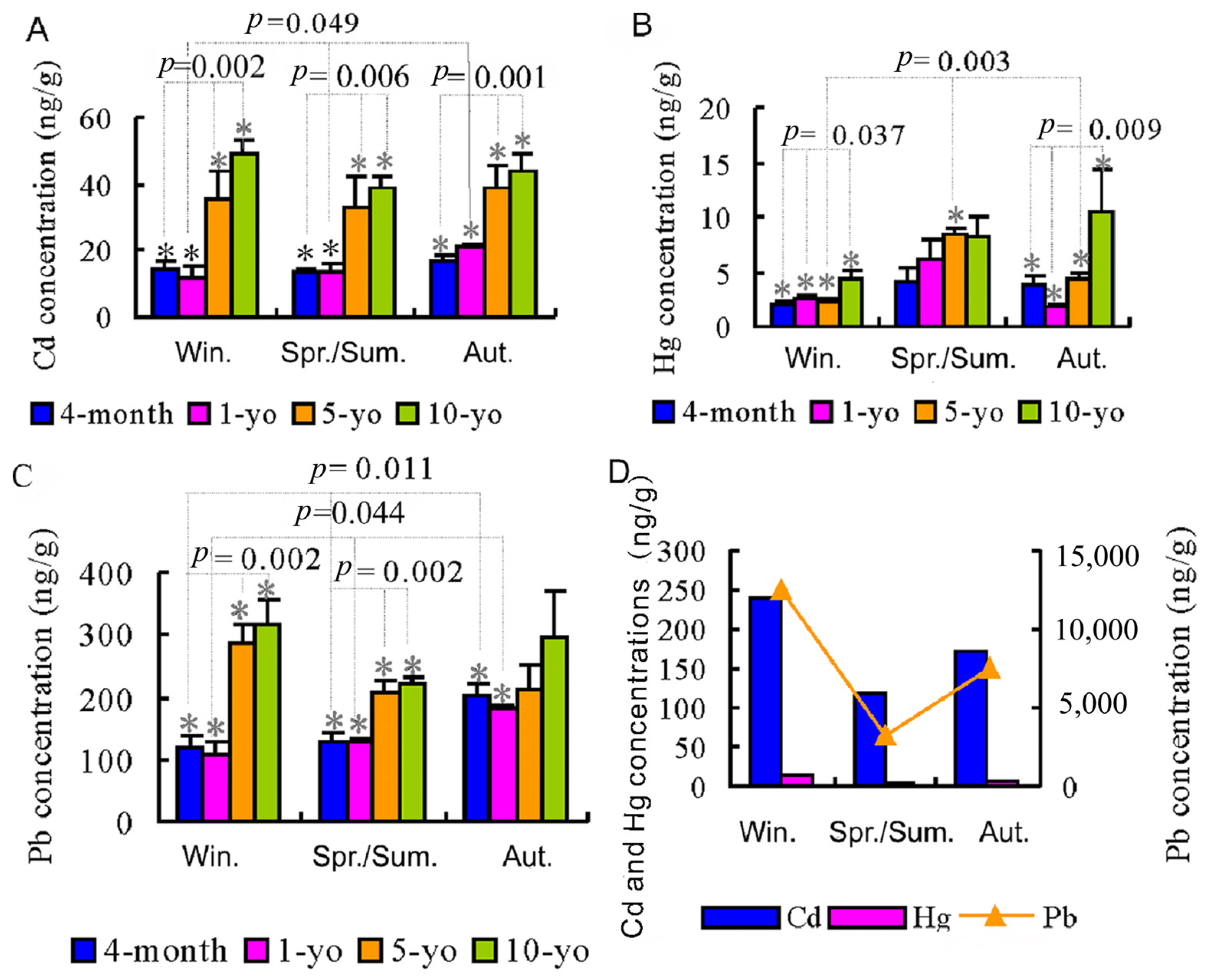
3.2. Seasonal Metal Concentrations Measured in Atmospheric Particulate Matter and Homing Pigeon Lung Tissue (Experiment 2)
3.3. Histology Analysis of Lung Tissue
3.3.1. Histology Analysis (Experiment 1)
3.3.2. Histology Analysis (Experiment 2)
4. Discussion
4.1. Ingestion and Respiratory Routes of Exposure
4.2. Tissue and Atmospheric Particulate Matter Metal Concentrations
4.3. Evaluation of the Toxic Effects of Atmospheric Pollution on Homing Pigeon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; et al. Managing the health effects of climate change. Lancet 2009, 373, 1693–1733. [Google Scholar] [CrossRef]
- Vohra, K.; Marais, E.A.; Bloss, W.J.; Schwartz, J.; Mickley, L.J.; Damme, M.V.; Clarisse, L.; Coheur, P.F. Rapid rise in premature mortality due to anthropogenic air pollution in fast-growing tropical cities from 2005 to 2018. Sci. Adv. 2022, 8, eabm4435. [Google Scholar] [CrossRef]
- Wolf, M.J.; Esty, D.C.; Kim, H.; Bell, M.L.; Brigham, S.; Nortonsmith, Q.; Zaharieva, S.; Wendling, Z.A.; de Sherbinin, A.; Emerson, J.W. New Insights for Tracking Global and Local Trends in Exposure to Air Pollutants. Environ. Sci. Technol. 2022, 56, 3984–3996. [Google Scholar] [CrossRef]
- Zuidema, C.; Paulsen, M.; Simpson, C.D.; Jovan, S.E. Evaluation of Orthotrichum lyellii moss as a biomonitor of diesel exhaust. Sci. Total Environ. 2024, 922, 171306. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. PM 2.5. Proc. Natl. Acad. Sci. USA 2013, 110, 8756. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Jung, C.R.; Lin, C.Y.; Hwang, B.F. Combined exposure to heavy metals in PM2.5 and pediatric asthma. J. Allergy Clin. Immunol. 2021, 147, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.W.; Wang, H.Y.; Du, C.L.; Yuan, T.H.; Chen, C.Y.; Yu, C.J.; Chan, C.C. Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: A population-based longitudinal cohort study. Sci. Total Environ. 2022, 810, 152186. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Chen, C.; Sun, Q.; Wang, Y.; Du, H.; Wang, J.; Zhong, Y.; Shi, W.; Li, T.; et al. Impact of heavy PM2.5 pollution events on mortality in 250 Chinese counties. Environ. Sci. Technol. 2022, 56, 8299–8307. [Google Scholar] [CrossRef]
- Sanderfoot, O.V.; Holloway, T. Air pollution impacts on avian species via inhalation exposure and associated outcomes. Environ. Res. Lett. 2017, 12, 083002. [Google Scholar] [CrossRef]
- Wang, F.; Outridge, P.; Feng, X.; Meng, B.; Heimburger-Boavida, L.; Mason, R.P. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere?—Implications for evaluating the effectiveness of the Minamata Convention. Sci. Total Environ. 2019, 674, 58–70. [Google Scholar] [CrossRef]
- Saunders, L.J.; Wania, F. Cross-Species Evaluation of Bioaccumulation Thresholds for Air-Breathing Animals. Environ. Sci. Technol. 2023, 57, 10491–10500. [Google Scholar] [CrossRef]
- Groffen, T.; Lasters, R.; Bervoets, L.; Prinsen, E.; Eens, M. Are Feathers of a Songbird Model Species (The Great Tit, Parus major) Suitable for Monitoring Perfluoroalkyl Acids (PFAAs) in Blood Plasma? Environ. Sci. Technol. 2020, 54, 9334–9344. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Durmus, A.; Adizel, O.; Uyar, H.N. A bibliometric analysis: What do we know about metals (loids) accumulation in wild birds? Environ. Sci. Pollut. Res. 2021, 28, 10302–10334. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Vejsnæs, F.; Brodschneider, R.; Hatjina, F.; Van der Steen, J.J.M.; Oller-Serrano, J.L.; Fernández-Alba, A.R. Enhancing the environmental monitoring of pesticide residues through Apis mellifera colonies: Honey bees versus passive sampling. Sci. Total Environ. 2023, 884, 163847. [Google Scholar] [CrossRef]
- Frantz, A.; Pottier, M.A.; Karimi, B.; Corbel, H.; Aubry, E.; Haussy, C.; Gasparini, J.; Castrec-Rouelle, M. Contrasting levels of heavy metals in the feathers of urban pigeons from close habitats suggest limited movements at a restricted scale. Environ. Pollut. 2012, 168, 23–28. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, X.; Simal-Gándara, J.; Alvarez, L.E.F.; López-Beceiro, A.M.; Pérez-López, M.; Martínez-Carballo, E. Non-invasive biomonitoring of organic pollutants using feather samples in feral pigeons (Columba livia domestica). Environ. Pollut. 2020, 267, 115672. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, J.; Haastert, B.; Rehkämper, G. Asymmetry of different brain structures in homing pigeons with and without navigational experience. J. Exp. Biol. 2010, 213, 2219–2224. [Google Scholar] [CrossRef]
- da Silva, A.P.; Stoute, S.; Hauck, R.; Shivaprasad, H.L.; Jerry, C. A case report of avian malaria (Plasmodium spp.) in pen-reared pigeons (Columba livia). Avian Dis. 2021, 65, 213–218. [Google Scholar] [CrossRef]
- Tong, Y.; Zhao, X.; Li, H.; Pei, Y.; Ma, P.; You, J. Using homing pigeons to monitor atmospheric organic pollutants in a city heavily involving in coal mining industry. Chemosphere 2022, 307, 135679. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Rodewald, A.D. In a state of flux: The energetic pathways that move contaminants from aquatic to terrestrial environments. Environ. Toxicol. Chem. 2012, 31, 1175–1183. [Google Scholar] [CrossRef]
- Liu, X.L.; Ouyang, W.Y.; Shu, Y.L.; Tian, Y.Z.; Feng, Y.C.; Zhang, T.; Chen, W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ. Pollut. 2019, 254, 113113. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jia, B.; Tian, Y.Z.; Feng, Y.C. Source-specific health risk assessment of PM2.5-bound heavy metals based on high time-resolved measurement in a Chinese megacity: Insights into seasonal and diurnal variations. Ecotoxicol. Environ. Saf. 2021, 216, 112167. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, X.N.; Xue, Q.Q.; Tian, Y.Z.; Feng, Y.C. Inhalation bioaccessibility and risk assessment for PM-bound organic components: Co-effects of component physicochemical properties, PM properties, and sources. J. Hazard. Mater. 2023, 459, 132291. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, B.; Halbrook, R.S.; Zang, S.Y. Age-dependent accumulation of heavy metals in liver, kidney and lung tissues of homing pigeons in Beijing, China. Ecotoxicology 2013, 22, 1490–1497. [Google Scholar] [CrossRef]
- Pei, Y.; Halbrook, R.S.; Li, H.; You, J. Homing pigeons as a biomonitor for atmospheric PAHs and PCBs in Guangzhou, a megacity in South China. Mar. Pollut. Bull. 2017, 124, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, K.; Eeden, S.V. Contribution of Lung Macrophages to the Inflammatory Responses Induced by Exposure to Air Pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef]
- Chau, T.T.; Wang, K.Y. An association between air pollution and daily most frequently visits of eighteen outpatient diseases in an industrial city. Sci. Rep. 2020, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, K.; Ma, L.; Tang, B.; Sun, S.; Meng, F.; Jiang, P.; Qi, H. Source-specific health risks induced by PM2.5-bound metallic species under different pollution scenarios in a cold megacity of Northeast China. Urban Clim. 2022, 44, 101205. [Google Scholar] [CrossRef]
- Xu, H.M.; He, K.L.; Feng, R.; Shen, Z.X.; Cao, J.J.; Liu, S.X.; Ho, K.F.; Huang, R.J.; Guinot, B.; Wang, Q.Y.; et al. Metallic elements and pb isotopes in pm2.5 in three chinese typical megacities: Spatial distribution and source apportionment. Environ. Sci. Process. Impacts 2020, 22, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, Y.; Huang, L.; Wang, K.; Shen, H.; Li, Z. Pollution characteristics and toxic effects of PM1.0 and PM2.5 in Harbin, China. Environ. Sci. Pollut. Res. 2021, 28, 13229–13242. [Google Scholar] [CrossRef]
- USEPA. National Ambient Air Quality Standards (NAAQS). U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards 2012. Available online: https://www.epa.gov/criteria-air-pollutants (accessed on 26 July 2024).
- The State Council of the People’s Republic of China. Air Pollution Prevention and Control Action Plan; The State Council: Beijing, China, 2013.
- Cai, S.; Wang, Y.; Zhao, B.; Wang, S.; Chang, X.; Hao, J. The impact of the “Air Pollution Prevention and Control Action Plan” on PM2.5 concentrations in Jing-Jin-Ji region during 2012–2020. Sci. Total Environ. 2017, 580, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Chen, K.; Xiao, Z.; Tang, M.; Zheng, N.; Yang, N.; Gao, J.Y.; Li, Y.; Kong, J.; Xu, H. Health benefit assessment of China’s national action plan on air pollution in the Beijing-Tianjin-Hebei area. Aerosol Air Qual. Res. 2019, 19, 383–389. [Google Scholar] [CrossRef]
- Huang, L.K.; Wang, G.Z. Chemical characteristics and source apportionment of atmospheric particles during heating period in Harbin, China. J. Environ. Sci. 2014, 26, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.; Li, L.; Li, J.; Wei, L.; Chi, W.; Hong, L.; Zhao, Q.; Jiang, J. Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: Effect of the species distribution of heavy metals and heat supply. Sci. Rep. 2020, 10, 8160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Huang, C.L.; Xiao, T.; Yuan, Y.; Maob, Q.J.; Tan, H.P. Characterization of atmospheric aerosols and source apportionment analyses in urban Harbin, northeast China. Infrared Phys. Technol. 2019, 103, 103109. [Google Scholar] [CrossRef]
- Dauwe, T.; Bervoets, L.; Pinxten, R.; Blust, R.; Eens, M. Variation of heavy metals within and among feathers of birds of prey: Effects of molt and external contamination. Environ. Pollut. 2003, 124, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.; Cristol, D. Rapid depuration of mercury in songbirds accelerated by feather molt. Environ. Toxicol. Chem. 2017, 36, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Wang, X.X.; Zhang, R.X.; Chen, R.T.; Ma, L. A review on the potential risks and mechanisms of heavy metal exposure to Chronic Obstructive Pulmonary Disease. Biochem. Biophys. Res. Commun. 2023, 684, 149124. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Hong, Y.T.; Duan, X.H.; Qiming Zhou, Q.M.; Chen, J.; Liu, S.Y.; Su, J.Y.; Han, L.; Zhang, J.L.; Niu, B.F. Unveiling the metal mutation nexus: Exploring the genomic impacts of heavy metal exposure in lung adenocarcinoma and colorectal cancer. J. Hazard. Mater. 2024, 461, 132590. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Halbrook, R.S.; Zang, S.; Masdo, M.A.; Sun, L.; Han, S. Homing Pigeons as Biomonitors of Atmospheric Metal Exposure and Health Effects to Promote Environment Sustainability. Sustainability 2024, 16, 7014. https://doi.org/10.3390/su16167014
Cui J, Halbrook RS, Zang S, Masdo MA, Sun L, Han S. Homing Pigeons as Biomonitors of Atmospheric Metal Exposure and Health Effects to Promote Environment Sustainability. Sustainability. 2024; 16(16):7014. https://doi.org/10.3390/su16167014
Chicago/Turabian StyleCui, Jia, Richard S. Halbrook, Shuying Zang, Mary A. Masdo, Li Sun, and Shuang Han. 2024. "Homing Pigeons as Biomonitors of Atmospheric Metal Exposure and Health Effects to Promote Environment Sustainability" Sustainability 16, no. 16: 7014. https://doi.org/10.3390/su16167014
APA StyleCui, J., Halbrook, R. S., Zang, S., Masdo, M. A., Sun, L., & Han, S. (2024). Homing Pigeons as Biomonitors of Atmospheric Metal Exposure and Health Effects to Promote Environment Sustainability. Sustainability, 16(16), 7014. https://doi.org/10.3390/su16167014






