A Review of the Benefits of the Sustainable Utilization of Shrimp Waste to Produce Novel Foods and the Impact on Human Health
Abstract
1. Introduction
2. Methodology
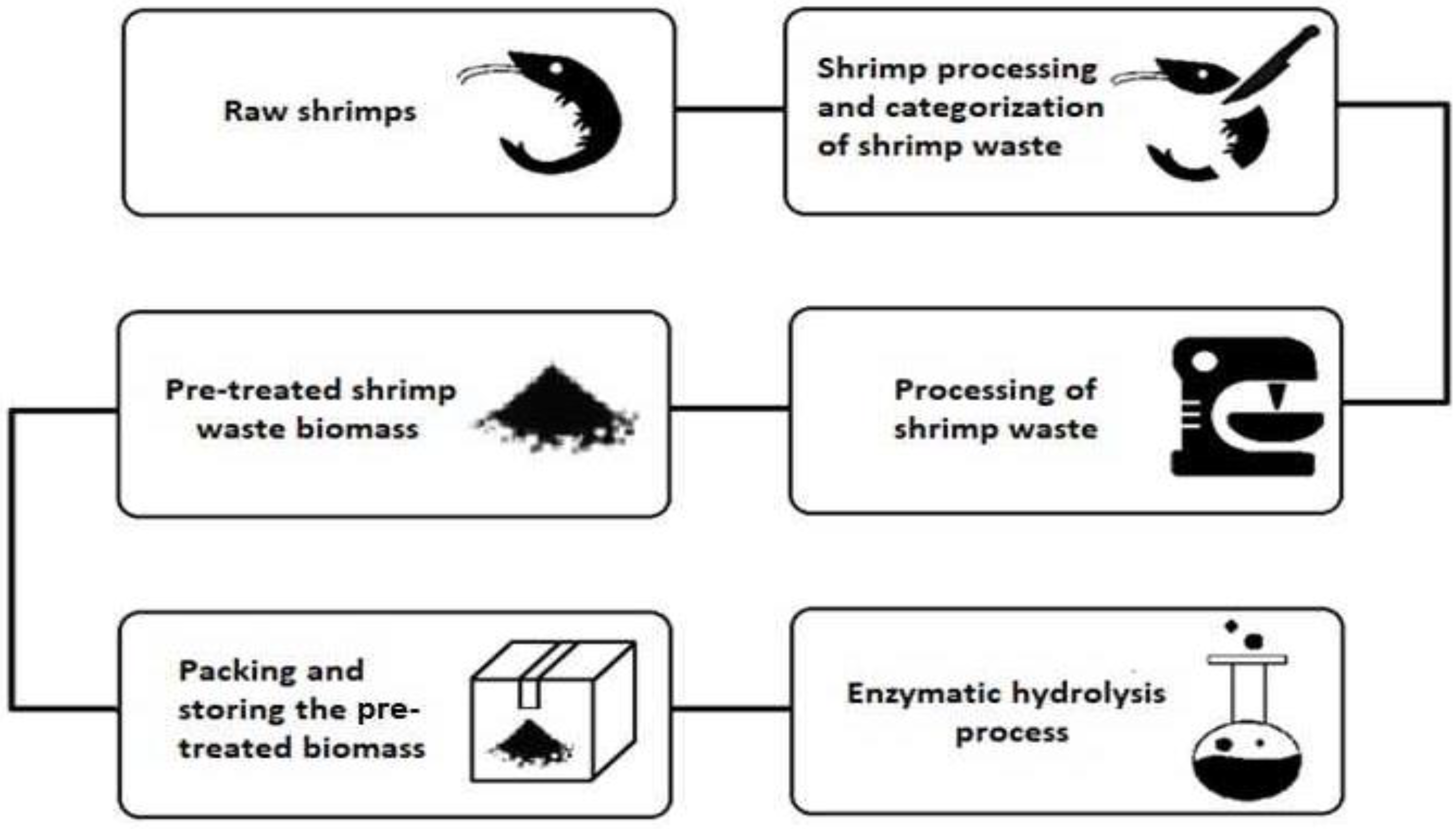
3. General Techniques Reported for Processing Shrimp Waste
3.1. Preparation of Raw Waste Shrimp Samples
3.2. Description of the Enzymatic Hydrolysis Process
3.3. Recovered Bioactive Substances from Shrimp Waste and Applications
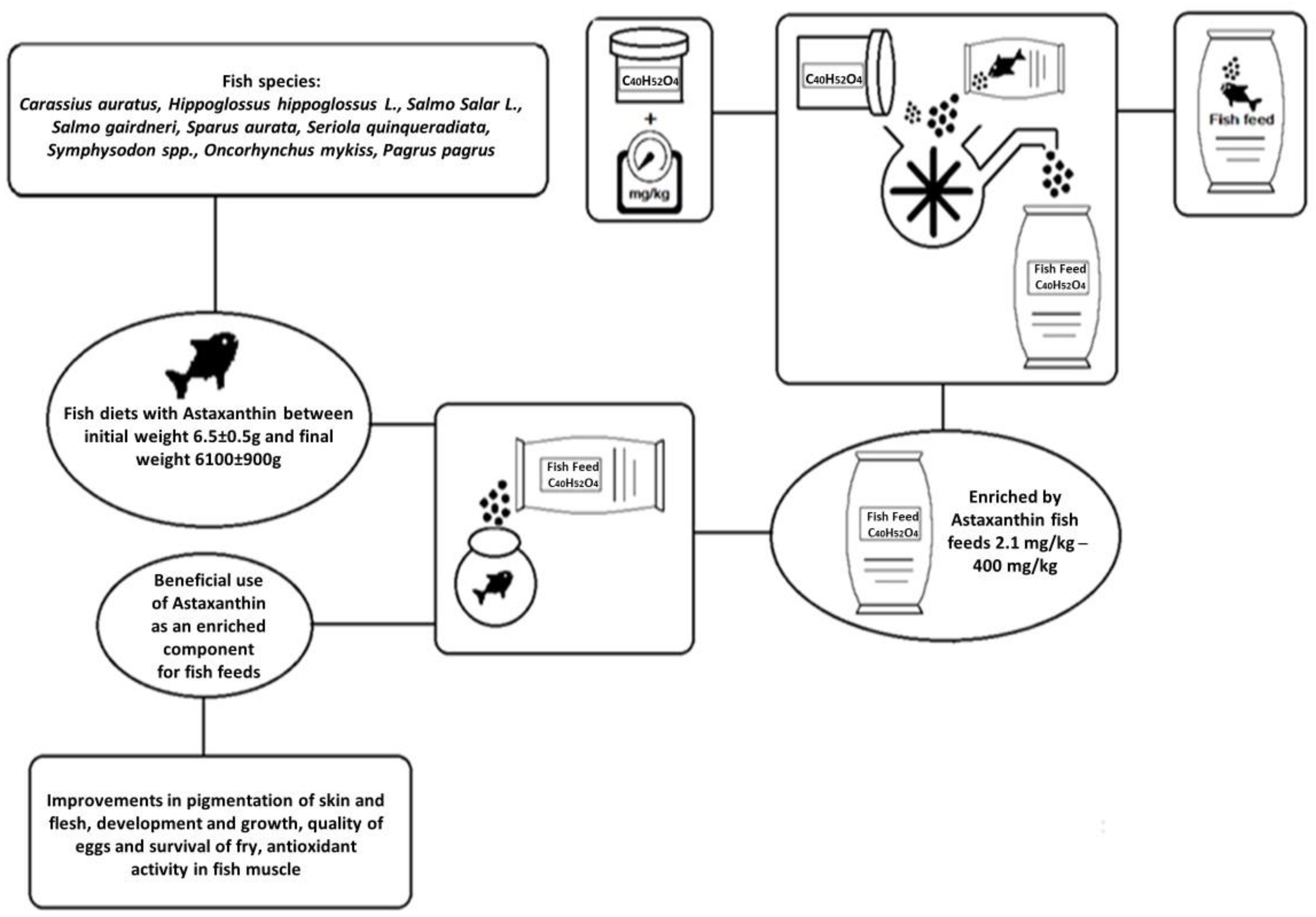
4. The Application of Astaxanthin as a Complementary Feed Colorant in Fish Diets
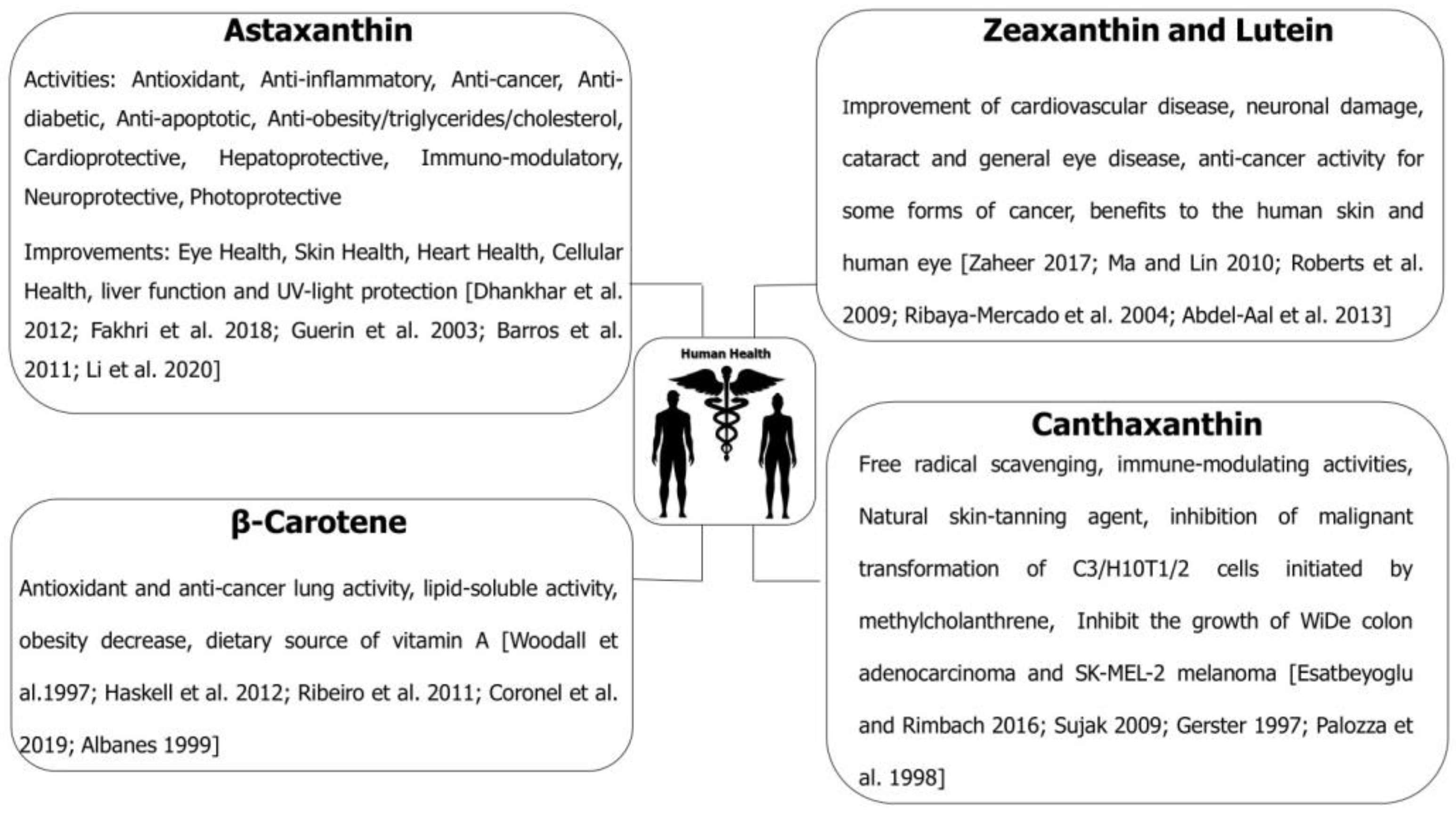
5. Carotenoids and Their Impact on Human Health
6. Trends and Future Challenges of Shrimp Waste Utilization
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Fricke, E.; Koch, M.; Dietz, H.; Slater, M.J.; Saborowski, R. Brown shrimp (Crangon crangon) processing remains as ingredient for Litopenaeus vannamei feeds: Biochemical characterisation and digestibility. Aquac. Rep. 2022, 25, 101225. [Google Scholar] [CrossRef]
- Bataille, M.P.; Bataille, P.F. Extraction of proteins from shrimp processing waste. J. Chem. Technol. Biotechnol. 1983, 33, 203–208. [Google Scholar] [CrossRef]
- Babu, C.M.; Chakrabarti, R.; Sambasivarao, K.R.S. Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT-Food Sci. Technol. 2008, 41, 227–235. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Gavlighi, H.A. Protein hydrolysates derived from aquaculture and marine byproducts through autolytic hydrolysis. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4872–4899. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. Food Saf. 2022, 22, 46–106. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of fish byproducts: Sources to end-product applications of bioactive protein hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Ho, T.C.; Chae, S.-J.; Cho, Y.-J.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Sato, N.; Daimon, H.; Fujie, K. Production of valuable materials by hydrothermal treatment of shrimp shells. Ind. Eng. Chem. Res. 2001, 40, 5885–5888. [Google Scholar] [CrossRef]
- Cretton, M.; Malanga, G.; Sobczuk, T.M.; Mazzuca, M. Lipid fraction from industrial crustacean waste and its potential as a supplement for the feed industry: A case study in Argentine Patagonia. Waste Biomass Valorization 2020, 12, 2311–2319. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Z.; Li, X.; Wang, S.; Pei, H.; Sun, H.; Zhang, Z. Green, simple, and effective process for the comprehensive utilization of shrimp shell waste. ACS Omega 2020, 5, 19227–19235. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Masci, P.; Gobe, G.; Osborne, S. Current and potential uses of bioactive molecules from marine processing waste. J. Sci. Food Agric. 2015, 96, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Abuzar, N.; Sharif, H.R.; Sharif, M.K.; Arshad, R.; Rehman, A.; Ashraf, W.; Karim, A.; Awan, K.A.; Raza, H.; Khalid, W.; et al. Potential industrial and nutritional applications of shrimp by-products: A review. Int. J. Food Prop. 2023, 26, 3407–3432. [Google Scholar] [CrossRef]
- Rahman, M.; Khosravi, S.; Chang, K.H.; Lee, S.-M. Effects of dietary inclusion of astaxanthin on growth, muscle pigmentation and antioxidant capacity of juvenile rainbow trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 2016, 21, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, A.; Shaikh, S.A.; Ramesh, M.; Sikarwar, M.S. Chapter 6—The structure–activity relationship of marine products for neuroinflammatory disorders. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, F.R.S., Ed.; Dennis S.: Amsterdam, The Netherlands, 2021; Volume 70, pp. 151–194. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Canthaxanthin (accessed on 27 March 2005).
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Dhankhar, J.; Kadian, S.S.; Sharma, A. Astaxanthin: A potential carotenoid. Int. J. Pharm. Sci. Rev. Res. 2012, 3, 1246–1259. [Google Scholar]
- Zimmer, T.B.R.; Mendonça, C.R.B.; Zambiazi, R.C. Methods of protection and application of carotenoids in foods—A bibliographic review. Food Biosci. 2022, 48, 101829. [Google Scholar] [CrossRef]
- Stringheta, P.; Nachtigall, A.; Oliveira, T.T.; Junqueira-Goncalves, M.P. Luteína: Propriedades antioxidantes e benefícios à saúde. Aliment. E Nutr. Araraquara 2009, 17, 229–238. [Google Scholar]
- Bakan, E.; Akbulut, Z.T.; İnanç, A.L. Carotenoids in foods and their effects on hu-man health. Akad. Gıda 2014, 12, 61–68. [Google Scholar]
- Giuffrida, D.; Salvo, F.; Salvo, A.; La Pera, L.; Dugo, G. Pigments composition in monovarietal virgin olive oils from various sicilian olive varieties. Food Chem. 2007, 101, 833–837. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Lycopene: A Biologically Important Carotenoid for Humans? Arch. Biochem. Biophys. 1996, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Roh, S.K.; Park, K.H.; Yoon, K.-R. Effective extraction of astaxanthin pigment from shrimp using proteolytic enzymes. Biotechnol. Bioprocess Eng. 1999, 4, 199–204. [Google Scholar] [CrossRef]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Agric. Food Chem. 2007, 55, 10345–10350. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tian, M.; Zhou, J.; Row, K.H. Task-specific ionic liquid-assisted extraction and separation of astaxanthin from shrimp waste. J. Chromatogr. B 2010, 878, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian red spotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, B.; Silva, L.; Rodrigues, A. Drying and extraction of astaxanthin from pink shrimp waste (Farfantepenaeus subtilis): The applicability of spouted beds. Food Sci. Technol. 2018, 38, 454–461. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar] [CrossRef]
- Chakrabarti, R. Carotenoprotein from tropical brown shrimp shell waste by enzymatic process. Food Biotechnol. 2002, 16, 81–90. [Google Scholar] [CrossRef]
- Gildberg, A.; Stenberg, E. A new process for advanced utilisation of shrimp waste. Process Biochem. 2001, 36, 809–812. [Google Scholar] [CrossRef]
- Sila, A.; Nasri, M.; Bougatef, A. Isolation and characterisation of carotenoproteins from deep-water pink shrimp processing waste. Int. J. Biol. Macromol. 2012, 51, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Guerard, F.; Sumaya-Martinez, M.T.; Laroque, D.; Chabeaud, A.; Dufossé, L. Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem. 2007, 42, 1486–1491. [Google Scholar] [CrossRef]
- Trung, T.; Phuong, P. Bioactive compounds from by-products of shrimp processing industry in Vietnam. J. Food Drug Anal. 2012, 20, 194–197. [Google Scholar] [CrossRef]
- Cheung, I.W.; Li-Chan, E.C. Angiotensin-I-converting enzyme inhibitory activity and bitterness of enzymatically-produced hydrolysates of shrimp (Pandalopsis dispar) processing byproducts investigated by Taguchi design. Food Chem. 2010, 122, 1003–1012. [Google Scholar] [CrossRef]
- Dey, S.S.; Dora, K.C. Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J. Food Sci. Technol. 2011, 51, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Peña, A.U.; Espinoza-Perez, J.D.; Sandoval-Fabian, G.C.; Balagurusamy, N.; Hernandez-Rivera, A.; De-La-Garza-Rodriguez, I.M.; Contreras-Esquivel, J.C. Screening of industrial enzymes for deproteinization of shrimp head for chitin recovery. Food Sci. Biotechnol. 2010, 19, 553–557. [Google Scholar] [CrossRef]
- Sowmya, R.; Ravikumar, T.M.; Vivek, R.; Rathinaraj, K.; Sachindra, N.M. Optimization of enzymatic hydrolysis of shrimp waste for recovery of antioxidant activity rich protein isolate. J. Food Sci. Technol. 2012, 51, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ren, L.; Jiang, J. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur. Food Res. Technol. 2010, 232, 281–287. [Google Scholar] [CrossRef]
- De Holanda, H.D.; Netto, F.M. Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J. Food Sci. 2006, 71, 298–303. [Google Scholar] [CrossRef]
- Limam, Z.; Sadok, S.; El Abed, A. Enzymatic Hydrolysis of Shrimp Head Waste: Functional and Biochemical Properties. Food Biotechnol. 2008, 22, 352–362. [Google Scholar] [CrossRef]
- Randriamahatody, Z.; Sylla, K.S.; Nguyen, H.T.; Donnay-Moreno, C.; Razanamparany, L.; Bourgougnon, N.; Bergé, J.P. Proteolysis of shrimp by-products (Peaneus monodon) from Madagascar. CyTA-J. Food 2011, 9, 220–228. [Google Scholar] [CrossRef][Green Version]
- Messina, C.; Renda, G.; Randazzo, M.; Laudicella, A.; Gharbi, S.; Pizzo, F.; Morghese, M.; Santulli, A. Extraction of bioactive compounds from shrimp waste. Bull. Inst. Natn. Scien. Tech. Mer Salammbô 2015, 42, 27–29. [Google Scholar]
- Armenta, R.E.; Guerrero-Legarreta, I. Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 2009, 112, 310–315. [Google Scholar] [CrossRef]
- Mizani, M.; Aminlari, M.; Khodabandeh, M. An Effective method for producing a nutritive protein extract powder from shrimp-head waste. Food Sci. Technol. Int. 2005, 11, 49–54. [Google Scholar] [CrossRef]
- Huang, G.; Ren, Z.; Jiang, J. Separation of Iron-Binding Peptides from Shrimp Processing By-products Hydrolysates. Food Bioprocess Technol. 2010, 4, 1527–1532. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.; Chen, B.; Jing, L.; Zhu, Z.; Kazemi, K. Modeling and optimization of newfoundland shrimp waste hydrolysis for microbial growth using response surface methodology and artificial neural networks. Mar. Pollut. Bull. 2016, 109, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.W.Y.; Li-Chan, E.C.Y. Application of taste sensing system for characterisation of enzymatic hydrolysates from shrimp processing by-products. Food Chem. 2014, 145, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Armenta-López, R.; Guerrero, I.L.; Huerta, S. Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. J. Food Sci. 2002, 67, 1002–1006. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.O.; de Souza, E.O.; Bora, P.S. Utilization of shrimp industry waste in the formulation of tilapia (Oreochromis niloticus Linnaeus) feed. Bioresour. Technol. 2007, 98, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Ayed-Ajmi, Y.; Sayari, N.; Nasri, M.; Martinez-Alvarez, O.; Bougatef, A. Antioxidant and Anti-proliferative Activities of Astaxanthin Extracted from the Shell Waste of Deep-water Pink Shrimp (Parapenaeus longirostris). Nat. Prod. J. 2013, 3, 82–89. [Google Scholar] [CrossRef][Green Version]
- Goldsmith, P.; Amankwah, F.; Gunjal, K.; Smith, J. Financial Feasibility of Producing Value-Added Seafood from Shrimp Waste in Quebec. J. Aquat. Food Prod. Technol. 2003, 12, 39–61. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Páez-Osuna, F.; Guerrero-Galván, S.R.; Ruiz-Fernández, A.C. The environmental impact of shrimp aquaculture and the coastal pollution in Mexico. Mar. Pollut. Bull. 1998, 36, 65–75. [Google Scholar] [CrossRef]
- Razafindrainibe, H. Baseline Study of the Shrimp Trawl Fishery in Madagascar and Strategies for Bycatch Management; Project TCP/MAG/3201-REBYC2; United Nations Food and Agriculture Organization: Rome, Italy, 2010. [Google Scholar]
- Kumar, A.; Kumar, D.; George, N.; Sharma, P.; Gupta, N. A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. Int. J. Biol. Macromol. 2018, 109, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.A.; Routroy, S.; Thakur, M. Understanding fish waste management using bibliometric analysis: A supply chain perspective. Waste Manag. Res. 2022, 41, 531–553. [Google Scholar] [CrossRef] [PubMed]
- Ravanipour, M.; Bagherzadeh, R.; Mahvi, A.H. Fish and shrimp waste management at household and market in Bushehr, Iran. J. Mater. Cycles Waste Manag. 2021, 23, 1394–1403. [Google Scholar] [CrossRef]
- Pfeiffer, N. Disposal and Re-Utilisation of Fish and Fish Processing Waste (Including Aquaculture Wastes). In NDP Marine RTDI Desk Study Series; Marine Institute: Galway, Ireland, 2003. [Google Scholar]
- Anh, P.T.; Dieu, T.T.M.; Mol, A.P.; Kroeze, C.; Bush, S.R. Towards eco-agro industrial clusters in aquatic production: The case of shrimp processing industry in Vietnam. J. Clean. Prod. 2011, 19, 2107–2118. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research progress on applications of calcium derived from marine organisms. Sci. Rep. 2020, 10, 18425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Song, L.; Farag, M.A. Maximizing crustaceans (shrimp, crab, and lobster) by-products value for optimum valorization practices: A comparative review of their active ingredients, extraction, bioprocesses and applications. J. Adv. Res. 2024, 57, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Salama, M.F.; El-Banna, H.A. Shrimp’s waste: Chemical composition, nutritional value and utilization. Nahrung 1999, 43, 418–423. [Google Scholar] [CrossRef]
- Dayal, J.S.; Ponniah, A.G.; Ambasankar, K. Shrimp as Health Food-Advisory Fact Sheet. e-publication Series No.15, 2012. Available online: http://14.139.181.163/Books/ciba0595.pdf (accessed on 1 August 2024).
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive fatty acids in mantis shrimp, crab and caramote prawn: Their content and distribution among the main lipid classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Ghorbel-Bellaaj, O.; Jellouli, K.; Maalej, H. Shrimp processing by-products protein hydrolysates: Evaluation of antioxidant activity and application in biomass and proteases production. Biocatal. Biotransformation 2017, 35, 287–297. [Google Scholar] [CrossRef]
- Sookying, D.; Davis, D.; da Silva, F.S.D. A review of the development and application of soybean-based diets for Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 441–448. [Google Scholar] [CrossRef]
- Raju, N.; Benjakul, S. Application of Saponin for Cholesterol Removal from Pacific White Shrimp (Litopenaeus vannamei) Lipid. Eur. J. Lipid Sci. Technol. 2020, 122, 1–9. [Google Scholar] [CrossRef]
- Bassig, R.; Obinque, A.; Nebres, V.; Santos, V.D.; Peralta, D.; Madrid, A.J. Utilization of Shrimp Head Wastes into Powder Form as Raw Material for Value-Added Products. Philipp. J. Fish. 2021, 28, 181–190. [Google Scholar] [CrossRef]
- Subasinghe, R.; Bueno, P.; Phillips, M.; Hough, C.; McGladdery, S.; Arthur, J. Aquaculture in the Third Millennium. Technical. In Report of the Conference on Aquaculture in the Third Millennium, Proceedings of the Conference on Aquaculture in the Third Millennium, Bangkok, Thailand, 20–25 February 2000; NACA and FAO: Italy, Rome, 2001. [Google Scholar]
- Grigorakis, K. Fillet proximate composition, lipid quality, yields, and organoleptic quality of Mediterranean-farmed marine fish: A review with emphasis on the new species. Crit. Rev. Food Sci. Nutr. 2017, 57, 2956–2969. [Google Scholar] [CrossRef]
- Rao, R.N.; Alvi, S.N.; Rao, B.N. Preparative isolation and characterization of some minor impurities of astaxanthin by high-performance liquid chromatography. J. Chromatogr. A 2005, 1076, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, C.T.; Robaina, L.; Fernández-Palacios, H.; Schuchardt, D.; Izquierdo, M. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture 2005, 244, 223–231. [Google Scholar] [CrossRef]
- Das, A.P.; Biswas, S.P. Carotenoids and Pigmentation in Ornamental Fish. J. Aquac. Mar. Biol. 2016, 4, 146–148. [Google Scholar] [CrossRef]
- Christiansen, R.; Torrissen, O.J. Growth and survival of Atlantic salmon, Salmo salar L., fed different dietary levels of astaxanthin juveniles. Aquac. Nutr. 1996, 2, 55–62. [Google Scholar] [CrossRef]
- Paripatananont, T.; Tangtrongpairoj, J.; Sailasuta, A.; Chansue, N. Effect of astaxanthin on the pigmentation of goldfish Carassius auratus. J. World Aquac. Soc. 1999, 30, 454–460. [Google Scholar] [CrossRef]
- Verakunpiriya, V.; Mushiake, K.; Kawano, K.; Watanabe, T. Supplemental Effect of astaxanthin in broodstock diets on the quality of Yellowtail eggs. Fish. Sci. 1997, 63, 816–823. [Google Scholar] [CrossRef]
- Nickell, D.; Bromage, N. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture 1998, 169, 233–246. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Li, X.; Chen, Z.; Liang, G.; Leng, X. Dietary astaxanthin improved the body pigmentation and antioxidant function, but not the growth of discus fish (Symphysodon spp.). Aquac. Res. 2016, 48, 1359–1367. [Google Scholar] [CrossRef]
- Torrissen, O.J. Pigmentation of salmonids: Interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 1989, 79, 363–374. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Naevdal, G. Pigmentation of salmonids—Variation in flesh carotenoids of Atlantic salmon. Aquaculture 1988, 68, 305–310. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Du, X.; Yao, J.; He, F.; Niu, X.; Wang, G.; Zhang, D. Effects of dietary astaxanthin on the growth, innate immunity and antioxidant defence system of Paramisgurnus dabryanus. Aquac. Nutr. 2020, 26, 1453–1462. [Google Scholar] [CrossRef]
- Christiansen, R.; Glette, J.; Lie, O.; Torrissen, O.J.; Waagbø, R. Antioxidant status and immunity in Atlantic salmon, Salmo salar L., fed semi-purified diets with and without astaxanthin supplementation. J. Fish Dis. 1995, 18, 317–328. [Google Scholar] [CrossRef]
- Sawanboonchun, J.; Roy, W.J.; Robertson, D.A.; Bell, J.G. The impact of dietary supplementation with astaxanthin on egg quality in Atlantic cod broodstock (Gadus morhua, L.). Aquaculture 2008, 283, 97–101. [Google Scholar] [CrossRef]
- Palma, J.; Andrade, J.; Bureau, D. The impact of dietary supplementation with astaxanthin on egg quality and growth of long snout seahorse (Hippocampus guttulatus) juveniles. Aquac. Nutr. 2016, 23, 304–312. [Google Scholar] [CrossRef]
- Jensen, C.; Birk, E.; Jokumsen, A.; Skibsted, L.H.; Bertelsen, G. Effect of dietary levels of fat, α-tocopherol and astaxanthin on colour and lipid oxidation during storage of frozen rainbow trout (Oncorhynchus mykiss) and during chill storage of smoked trout. Z. Lebensm. Unters. Forsch. A 1998, 207, 189–196. [Google Scholar] [CrossRef]
- Kiessling, A.; Dosanjh, B.; Higgs, D.; Deacon, G.; Rowshandeli, N. Dorsal aorta cannulation: A method to monitor changes in blood levels of astaxanthin in voluntarily feeding Atlantic salmon, Salmo salar L. Aquac. Nutr. 1995, 1, 43–50. [Google Scholar] [CrossRef]
- Storebakken, T.; Choubert, G. Flesh pigmentation of rainbow trout fed astaxanthin or canthaxanthin at different feeding rates in freshwater and saltwater. Aquaculture 1991, 95, 289–295. [Google Scholar] [CrossRef]
- Wathne, E.; Bjerkeng, B.; Storebakken, T.; Vassvik, V.; Odland, A.B. Pigmentation of Atlantic salmon (Salmo salar) fed astaxanthin in all meals or in alternating meals. Aquaculture 1998, 159, 217–231. [Google Scholar] [CrossRef]
- Sigurgisladottir, S.; Parrish, C.; Lall, S.; Ackman, R. Effects of feeding natural tocopherols and astaxanthin on Atlantic salmon (Salmo salar L.) fillet quality. Food Res. Int. 1994, 27, 23–32. [Google Scholar] [CrossRef]
- Christiansen, R.; Lie, O.; Torrissen, O.J. Growth and survival of Atlantic salmon, Salmo salar L., fed different dietary levels of astaxanthin. First-feeding fry. Aquac. Nutr. 1995, 1, 189–198. [Google Scholar] [CrossRef]
- Gomes, E.; Dias, J.; Silva, P.; Valente, L.; Empis, J.; Gouveia, L.; Bowen, J.; Young, A. Utilization of natural and synthetic sources of carotenoids in the skin pigmentation of gilthead seabream (Sparus aurata). Eur. Food Res. Technol. 2002, 214, 287–293. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Christiansen, R.; Struksnæs, G.; Estermann, R. Astaxanthin deposition in the flesh of Atlantic salmon, Salmo salar L., in relation to dietary astaxanthin concentration and feeding period. Aquac. Nutr. 1995, 1, 77–84. [Google Scholar] [CrossRef]
- Baker, R.; Pfeiffer, A.-M.; Schöner, F.-J.; Smith-Lemmon, L. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar. Anim. Feed Sci. Technol. 2002, 99, 97–106. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Struksnæs, G.; Rørvik, K.-A.; Koppe, W.; Bjerkeng, B. Astaxanthin digestibility as affected by ration levels for Atlantic salmon, Salmo salar. Aquaculture 2006, 261, 215–224. [Google Scholar] [CrossRef]
- Řehulka, J. Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture 2000, 190, 27–47. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Hatlen, B.; Wathne, E. Deposition of astaxanthin in fillets of Atlantic salmon (Salmo salar L.) fed diets with herring, capelin, sandeel, or Peruvian high PUFA oils. Aquaculture 1999, 180, 307–319. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Berge, G. Apparent digestibility coefficients and accumulation of astaxanthin E/Z isomers in Atlantic salmon (Salmo salar L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. B Biochem. 2000, 127, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Choubert, G.; Storebakken, T. Dose response to astaxanthin and canthaxanthin pigmentation of rainbow trout fed various dietary carotenoid concentrations. Aquaculture 1989, 81, 69–77. [Google Scholar] [CrossRef]
- Blumberg, M.J.; Halpner, D.A. Physiological Stage and anti-oxidant status and Health. In Antioxidant Status, Diet, Nutrition and Health, 1st ed.; Papas, M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 251–276. [Google Scholar]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta-Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.J. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion—Evidence in humans. Am. J. Clin. Nutr. 2012, 96, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological Aspects of β-Carotene Production. Food Bioprocess Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Coronel, J.; Pinos, I.; Amengual, J. β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Machate, D.J.; De Cássia Freitas, K.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; De Cássia Avellaneda Guimarães, R. β-Carotene: Preventive role for Type 2 diabetes mellitus and obesity: A review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D. β-Carotene and lung cancer: A case study. Am. J. Clin. Nutr. 1999, 69, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From molecule to function. Mol. Nutr. Food Res. 2016, 61, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A. Interactions between canthaxanthin and lipid membranes—Possible mechanisms of canthaxanthin toxicity. Cell. Mol. Biol. Lett. 2009, 14, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. The potential role of lycopene for human health. J. Am. Coll. Nutr. 1997, 16, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Maggiano, N.; Calviello, G.; Lanza, P.; Piccioni, E.; Ranelletti, O.F.; Bartoli, M.G. Canthaxanthin induces apoptosis in human cancer cell lines. Carcinogenesis 1998, 19, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K. Hen egg carotenoids (lutein and zeaxanthin) and nutritional impacts on human health: A review. CyTA-J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.-M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 2004, 23, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of Lutein and Zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Poppe, S.C.; Souza-Junior, T.P. Putative benefits of microalgal astaxanthin on exercise and human health. Rev. Bras. Farmacogn. 2011, 21, 283–289. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Huang, S.-C.; Chang, W.-T.; Chen, S.-C.; Hsu, C.-L. Effect of astaxanthin on the inhibition of lipid accumulation in 3T3-L1 adipocytes via modulation of lipogenesis and fatty acid transport pathways. Molecules 2020, 25, 3598. [Google Scholar] [CrossRef] [PubMed]
- Suganya, V.; Anuradha, V.; Ali, M.S.; Sangeetha, P.; Bhuvana, P. In vitro anti-diabetic activity of microencapsulated and non-encapsulated astaxanthin. Int. J. Curr. Pharm. Res. 2017, 9, 90–96. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wu, J. Astaxanthin in Liver Health and Disease: A Potential Therapeutic Agent. Drug Des. Dev. Ther. 2020, 14, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T. Canthaxanthin in aquafeed applications: Is there any risk? Trends Food Sci. Technol. 2001, 12, 240–243. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Rutella, G.S.; Saa, D.L.T.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Dmytryk, A.; Śmieszek, A.; Marycz, K. Chemical Characterization of Enteromorpha prolifera Extract Obtained by Enzyme-Assisted Extraction and Its Influence on the Metabolic Activity of Caco-2. Int. J. Mol. Sci. 2017, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2018, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]

| Scientific Names of Species/by-Products Used | Country/ State/ Company | Raw Waste Shrimp | General Description of Waste Preparation before Hydrolysis | References |
|---|---|---|---|---|
| Penaeus monodon, Penaeus indicus, Metapenaeus monocerous, Penaeus Monodon | India Company | head | Raw shrimps were maintained on ice and beheaded. Shrimp waste from different species was stored separately, packed in a polyethylene bag, and stored at −20 °C. | [5] |
| Metapenaeus monoceros | India, Visakhapatnam, factories | cephalothorax, shell, tail, appendages | Shrimp shell waste was uniformly ground to a smooth paste. | [34] |
| Pandalus Borealis | Factory BioHenk AS | head, scale | The shrimp waste was packed in plastic bags and stored at −20 °C before use. | [35] |
| Parapenaeus Longirostris | Tunisia, Sfax, processing plant | head, cephalothorax, shell, appendix | The shrimp waste was washed with distilled water, ground, and stored at −20 °C before use. | [36] |
| Penaeus braziliensis and Penaeus subtilis | France, Saint Malo, Company comapeche | processing discards | The shrimp waste was ground, packed in polyethylene vacuum bags, and kept frozen at −20 °C. | [37] |
| Penaeus Vannamei | Vietnam, Khanh Hoa, seafood processing companies | head | Shrimp waste was transported on ice, washed, and ground into pieces of 0.3 to 0.5 cm. The shrimp waste was packed into plastic bags and frozen at −20 °C until use. | [38] |
| Pandalopsis Dispar | Canada, Vancouver, Albion Fisheries Ltd. | shell, tail, head | Cooked shrimps were hand-peeled, thawed overnight at 4 °C, and allocated to packages for storage at −25 °C until use. | [39] |
| Penaeus Monodon | - | head, shell | The shrimp waste was washed with water, milled, and dried. The shrimp waste was packed in a low-density polyethylene bag and frozen at −20 °C until use. | [40] |
| Litopenaeus vannamei | Mexico, Huatabampo City, Sonora State, Company El Camaron Dorado | head | Frozen shrimp waste was defrosted in a microwave at 55 °C. The dried shrimp waste was ground and stored under vacuum at −0.2 bar at room temperature. | [41] |
| Penaeus Indicus | Local market | head, carapace | Chilled shrimp waste was transported to the laboratory, homogenized with an equal volume of distilled water, and stored at −20 °C. | [42] |
| Shrimps | China, Zhejiang, Marine Fishery Co., Ltd. | by-products | Chilled shrimp waste was transported to the laboratory, homogenized with an equal volume of distilled water, and stored at −20 °C. | [43] |
| Xiphopenaeus Kroyeri | Brazil, Guaruja, Alpha Pescados | cephalothorax, shell, tail | The shrimp waste was washed with water, ground, packed in plastic bags, and frozen at −20 °C. | [44] |
| Penaeus Kerathurus | Processing factory | head | Shrimp waste was packed in plastic bags and stored at −40 °C until use. | [45] |
| Penaeus Monodon | Madagascar, UnIMA processing factory | head | Shrimp waste was packed in plastic bags and stored at −20 °C until use. | [46] |
| Parapenaeus Longirostris | - | exoskeleton, cephalothorax | The shrimp waste was thawed, minced, and dried at 45 °C for 40 h. | [47] |
| Litopenaeus vannamei | Mexico | discards | The shrimp discards were stabilized by lactic acid fermentation using Pediococcus pentosaceus | [48] |
| Penaeus Semisulcatus | Iran, Bushehr, fisheries from Bushehr | head, carapace | Fresh shrimp were cut by hand, separated into heads and carapaces, and ground into a paste. The paste was stored in a polyethylene bag and kept at −20 °C. | [49] |
| Shrimps | China. Wenzhou City, Zhejiang, aquatic product market | by-products | Shrimp by-products were stored at −18 °C until their use. | [50] |
| Shrimps | Canada, Newfoundland, local fish market | head, shell, tail | The shrimp waste was ground and packed in plastic bags. The ground shrimp materials were stored in plastic bags and kept frozen at −18 °C until their use. | [51] |
| Pandalopsis Dispar | Canada Vancouver, Albion Fisheries Ltd. | shell, head, tail | Cooked shrimps were hand-peeled in frozen form. The shrimp waste was kept overnight at 4 °C, distributed into packages, and stored at −25 °C until use. | [52] |
| Shrimp by-Product | Proteolytic Enzymes | Incubation (pH) | Incubation Temperature | Incubation Time | Enzyme Inhibition Temp./Time | Recovered Extracts | References |
|---|---|---|---|---|---|---|---|
| head | Single pH | Single temp. | Single time | Caroteinoids, astaxanthin | [5] | ||
| Trypsin | 7.6 | 45 °C | 2 h | 100 °C for 10 min | |||
| Papain | 6.2 | 55 °C | 2 h | 100 °C for 10 min | |||
| Pepsin | 4.0 | 45 °C | 2 h | 100 °C for 10 min | |||
| cephalothorax, shell, tail, appendages | Single pH | Single temp. | Single time. | Caroteinoids, proteins, carotenoproteins | [34] | ||
| Pepsin | 4.6 | 28 ± 2 °C | 3–4 h | ||||
| Papain | 6.2 | 28 ± 2 °C | 3–4 h | Boiled for 10 min | |||
| Trypsin | 7.6 | 28 ± 2 °C | 3–4 h | ||||
| head, scale | - | Single temp. | Single time | Protein hydrolysate, astaxanthin, chitosan | [35] | ||
| Alcalase | |||||||
| 2.4 l FG | 40 °C | 2 h | 90 °C for 20 min | ||||
| head, cephalothorax, shell, appendix | Trypsin | Single pH | Single temp. | Different periods: | Addition of acetic acid | Carotenoproteins | [36] |
| 10 | 25 °C | 1 h, 3 h, 5 h, 7 h | |||||
| processing discards | Alcalase | Single pH | Single temp. | - | Boiling water for 20 min | - | [37] |
| 2.4 L | 6.0–10.0 | 50 °C–70 °C | |||||
| head | Alcalase | - | Single temp. | Different periods: | - | Proteins, minerals, chitin, carotenoproteins, lipids | [38] |
| 55 °C | 2 h, 4 h, 6 h, 8 h | ||||||
| shell, tail, head | - | Single temp. | Different periods | Boiling water for 10 min | Protein hydrolysate | [39] | |
| Alcalase | 50 °C | 1 h, | |||||
| Bromelain | 50 °C | 4 h, | |||||
| Flavourzyme | 50 °C | 8 h, | |||||
| Protamex | 50 °C | 24 h | |||||
| head, shell | Single pH | Single temp. | Different periods: | 90 °C for 5 min | Protein hydrolysate | [40] | |
| Alcalase | 7.0–8.0 | 56 °C–60 °C 47 °C–50 °C 50 °C–52 °C 50 °C–55 °C | 30 min, 45 min, 60 min, 75 min, 90 min | ||||
| Neutrase | 6.3–6.5 | ||||||
| Protamex | 7.2–8.0 | ||||||
| Flavourzyme | 5.5–7.5 | ||||||
| head | Alcalase Flavourzyme Lysozyme Inovapure 300 Papain Trypsin VI | - | - | - | - | Chitin | [41] |
| head, carapace | Alcalase | - | Different temp.: | Different periods: | - | Caroteinoids, proteins, carotenoproteins | [42] |
| 20 °C, | 60 min, | ||||||
| 35 °C, | 150 min, | ||||||
| 50 °C | 240 min | ||||||
| nonspecified by-products | Alcalase | Single pH | Single temp. | - | Boiling water for 10 min | Iron-binding peptides | [43] |
| 7.8 | 55 °C. | ||||||
| cephalothorax, shell, tail | Alcalase | Single pH | Single temp.: | - | Proteins, astaxanthin, chitin | [44] | |
| 8.5 | 60 °C | 90 °C for 5 min | |||||
| 8.5 | 60 °C | ||||||
| head | Trypsin | Single pH | Single temp.: | Single time | Proteins | [45] | |
| 7.9–8.0 | 50 °C | 1 h | 90 °C for 5 min | ||||
| head | Single pH | Single temp. | Single time | Addition of NaOH | Protein hydrolysate, lipids, chitin | [46] | |
| Pepsin | 2.0 | 40 °C | 24 h | ------ | |||
| Novozyme | 3.0 | 50 °C | 24 h | 85 °C for 25 min. | |||
| Protex 6L | 9.5 | 60 °C | 24 h | 85 °C for 20 min. | |||
| Delvolase | 10 | 60 °C | 24 h | 90 °C for 20 min. | |||
| exoskeleton, cephalothorax | Protamex Flavourzyme Alcalase | - | - | - | - | Astaxanthin | [47] |
| discards | pH | Single temp. | Single time | - | Carotenoproteins | [48] | |
| Savinase, | 8.0 | 30 °C | 24 h | ||||
| Lipase | 8.0 | 30 °C | 24 h | ||||
| head, carapace | Alcalase | Single pH | Single temp. | Single time | - | Proteins | [49] |
| 8.0 | 50–60 °C | 1 h | |||||
| nonspecified by-products | Single pH | Single temp. | Single time | 95 °C for 10 min | Proteins, calcium | [50] | |
| Flavourzyme | 7.0 | 50 °C | 6 h | ||||
| Protamex | 6.5 | 50 °C | 6 h | ||||
| Alcalase | 8.0 | 55 °C | 6 h | ||||
| Pepsin | 2.0 | 37 °C | 6 h | ||||
| Trypsin | 8.2 | 40 °C | 6 h | ||||
| head, shell, tail | Single | Single | Single | - | [51] | ||
| pH | temp. | time | 90 °C for 20 min | ||||
| Alcalase 2.4 L | 8.0 | 40 °C | 1 h | ||||
| shell, heads tail | Alcalase Bromelain Flavourzyme Protamex | - | - | - | - | - | [52] |
| Scientific Names of Species | Fish Diet A: Initial (g) Weight B: Final (g) Weight | Dose of Astaxanthin mg/kg | Beneficial Effects on Fishes | References |
|---|---|---|---|---|
| Oncorhynchus mykiss | A: 800 g–900 g B: Not mentioned (g) | 40–100 mg/kg | Pigmentation improvement | [93] |
| Salmo salar L. | A: 580 g B: Doubled weight (g) | 75 mg/kg | Pigmentation improvement of the flesh | [94] |
| Pagrus pagrus | A: 44 g B: Not mentioned (g) | 20–40 mg/kg | Pigmentation improvement of the skin and growth | [80] |
| Carassius auratus | A: 10 g B: Not mentioned (g) | 0–100 mg/kg | Pigmentation improvement of the skin | [83] |
| Yellowtail | A: 6100 ± 900 g B: Not mentioned (g) | 0–40 mg/kg | Improvement of egg quality | [84] |
| Oncorhynchus mykiss | A: 111 ± 6 g B: Not mentioned (g) | 0–50 mg/kg | Pigmentation improvement of the skin | [95] |
| Salmo gairdneri | A: 63 g B: 96 and 123 g | 200 mg/kg | Pigmentation improvement of the skin and growth | [87] |
| Salmo salar | A: 510 g B: Not mentioned (g) | 41.4 mg/kg | Pigmentation improvement | [96] |
| Salmo salar | A: 309 g B: Not mentioned (g) | 84.2 mg/kg | Pigmentation improvement of the muscle | [97] |
| Salmo salar L. | A: 23 g B: Not mentioned (g) | 100 mg/kg | Growth and survival improvement | [98] |
| Sparus aurata | A: 97 ± 2 g and 150 ± 5 g B: Not mentioned (g) | 40 mg/kg and 40 mg/kg | Pigmentation improvement of the skin | [99] |
| Oncorhynchus mykiss | A: 6.5 ± 0.5 g, 25 ± 2 g, and 120 ± 5 g B: 400 g | 5.5 ± 0.3 mg/kg | Pigmentation improvement | [85] |
| Salrno salar L. | A: 115 ± 30 g B: 3275 ± 837 g | 0–200 mg/kg | Pigmentation improvement | [100] |
| Salmo salar | A: 408 g B: 1200 g | 60 mg/kg | Pigmentation improvement of flesh | [101] |
| Salmo salar | A: 2000 g B: Not mentioned (g) | 47 mg/kg | Investigation of carotenoids as plasma bioavailability indicator | [102] |
| Oncorhynchus mykiss | A: 178 ± 23 g B: Not mentioned (g) | 49.8 mg/kg | Improvement of growth | [103] |
| Symphysodon spp. | A: 10.3 ± 0.8 g B: Not mentioned (g) | 0–400 mg/kg | Improvements in pigmentation of body and skin and antioxidant properties | [86] |
| Salmo salar | A: 569 g B: Not mentioned (g) | 40 mg/kg | - | [104] |
| Salmo salar L. & Hippoglossus hippoglossus L. | A: 144 ± 2 g and 445 ± 16 g B: Not mentioned (g) | 66 mg/kg | - | [105] |
| Salmo gairdneri Richardson | A: 135 ± 5 g B: Not mentioned (g) | 0–200 mg/kg | Pigmentation improvement | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotodimas, I.; Ioannou, Z.; Kanlis, G. A Review of the Benefits of the Sustainable Utilization of Shrimp Waste to Produce Novel Foods and the Impact on Human Health. Sustainability 2024, 16, 6909. https://doi.org/10.3390/su16166909
Fotodimas I, Ioannou Z, Kanlis G. A Review of the Benefits of the Sustainable Utilization of Shrimp Waste to Produce Novel Foods and the Impact on Human Health. Sustainability. 2024; 16(16):6909. https://doi.org/10.3390/su16166909
Chicago/Turabian StyleFotodimas, Ioannis, Zacharias Ioannou, and Grigorios Kanlis. 2024. "A Review of the Benefits of the Sustainable Utilization of Shrimp Waste to Produce Novel Foods and the Impact on Human Health" Sustainability 16, no. 16: 6909. https://doi.org/10.3390/su16166909
APA StyleFotodimas, I., Ioannou, Z., & Kanlis, G. (2024). A Review of the Benefits of the Sustainable Utilization of Shrimp Waste to Produce Novel Foods and the Impact on Human Health. Sustainability, 16(16), 6909. https://doi.org/10.3390/su16166909






