Hydrometallurgical Extraction of Valuable Metals by Mixed Acid Leaching System for Used Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Collection of Samples
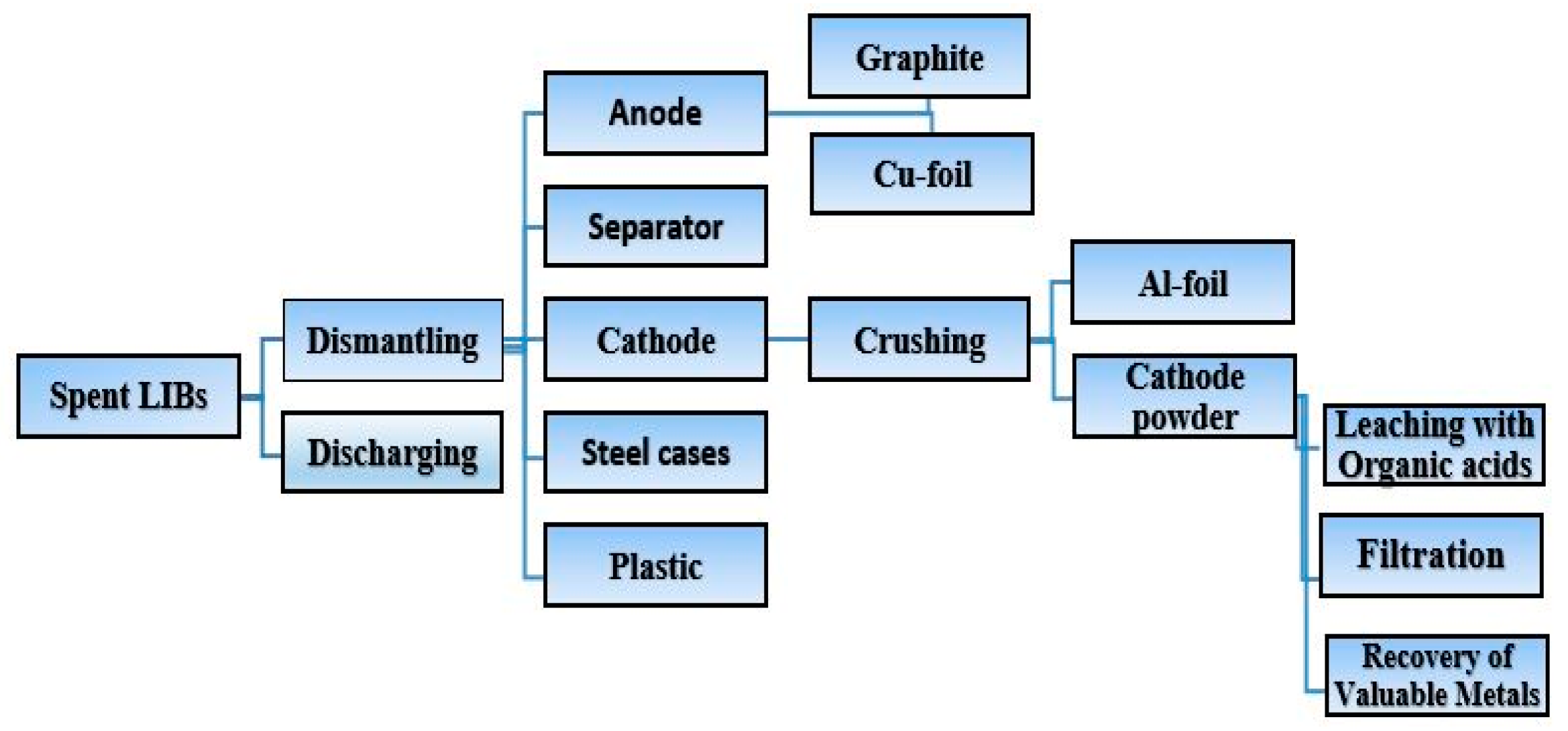
2.3. Sample Pre-Treatment
2.3.1. Discharging
2.3.2. Dismantling
2.3.3. Brine Dissolution Procedure
2.4. Leaching Experiment
2.4.1. Leaching of Cathode Powder
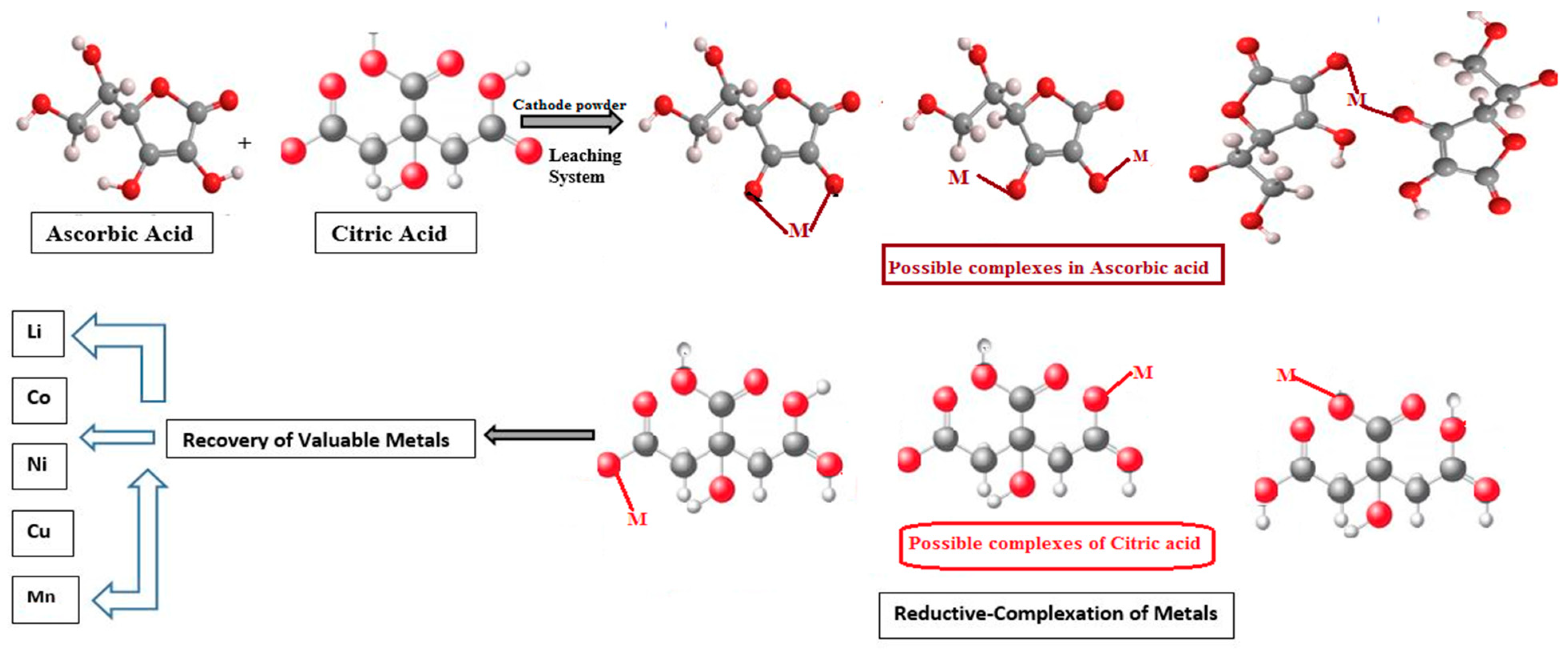
2.4.2. Leaching of Cathode Powder with Mixed Organic Acid
2.4.3. Reductive Leaching
2.5. Recovery of Valuable Metals
3. Analytical Methods
4. Results and Discussion
4.1. Optimization of Leaching Conditions
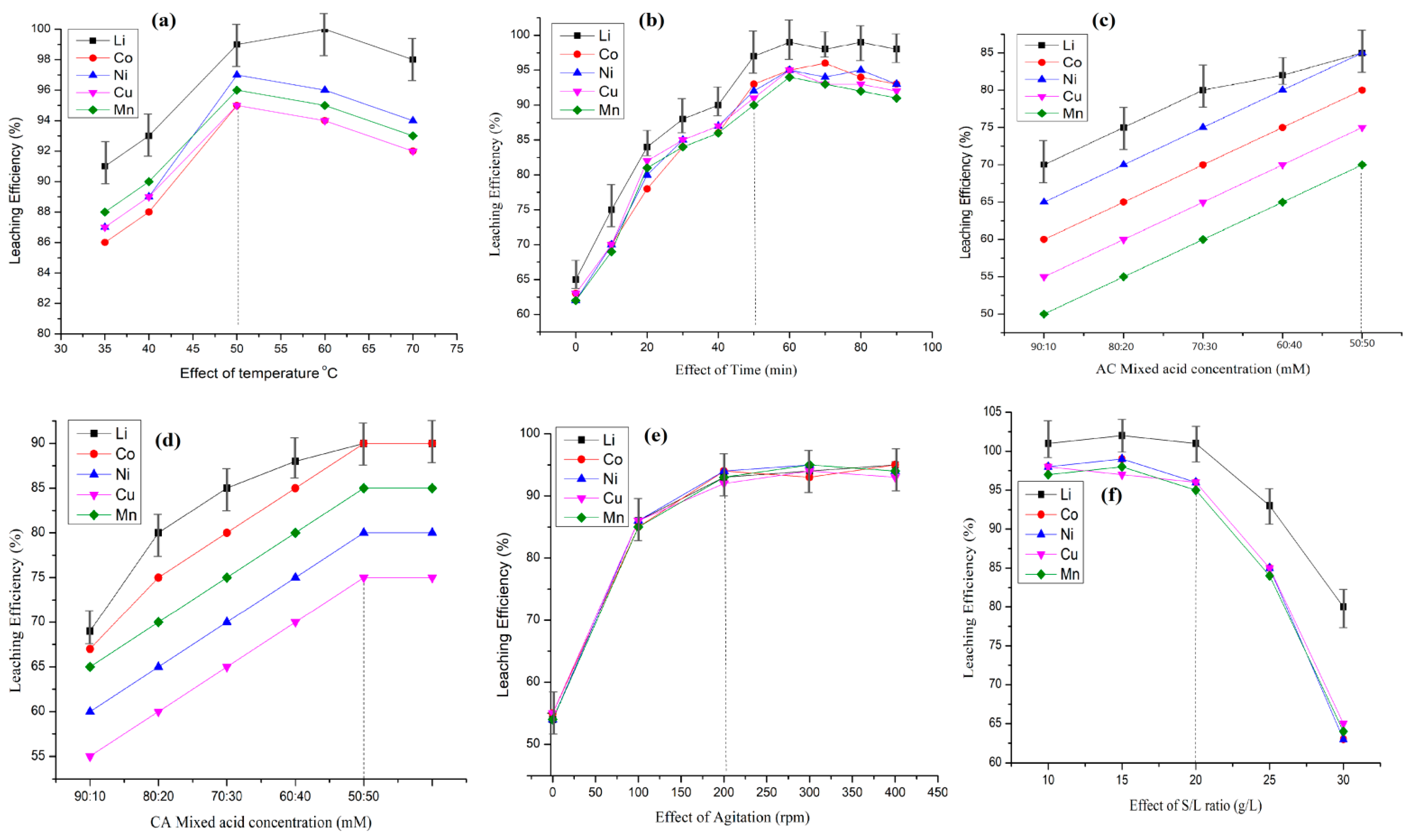
4.1.1. Effect of Temperature
4.1.2. Effect of Retention Time
4.1.3. Effect of Concentration
4.1.4. Effect of Stirring Speed
4.1.5. Effect of Solid–Liquid Ratio
4.1.6. Leaching Kinetics
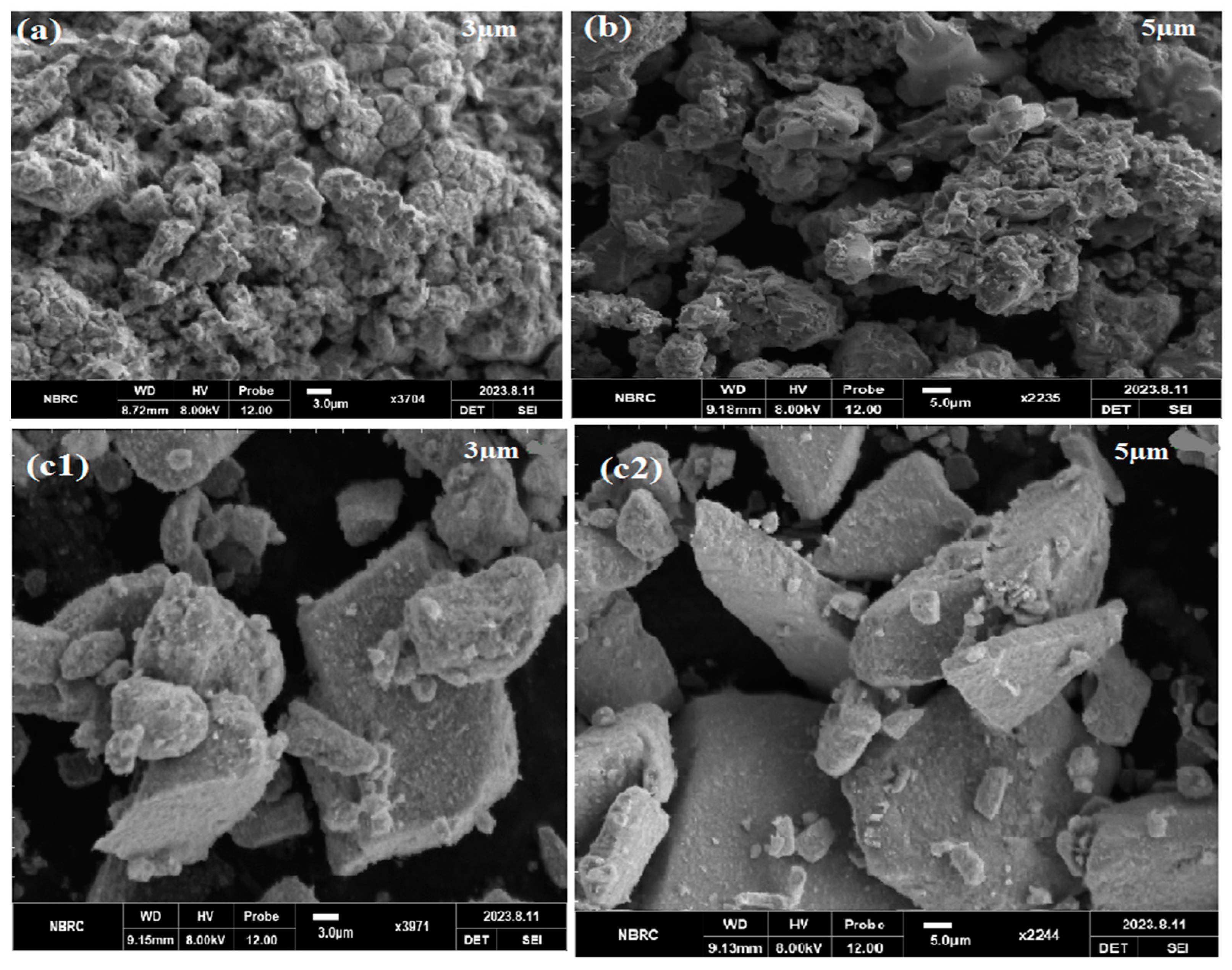
4.2. SEM Analysis
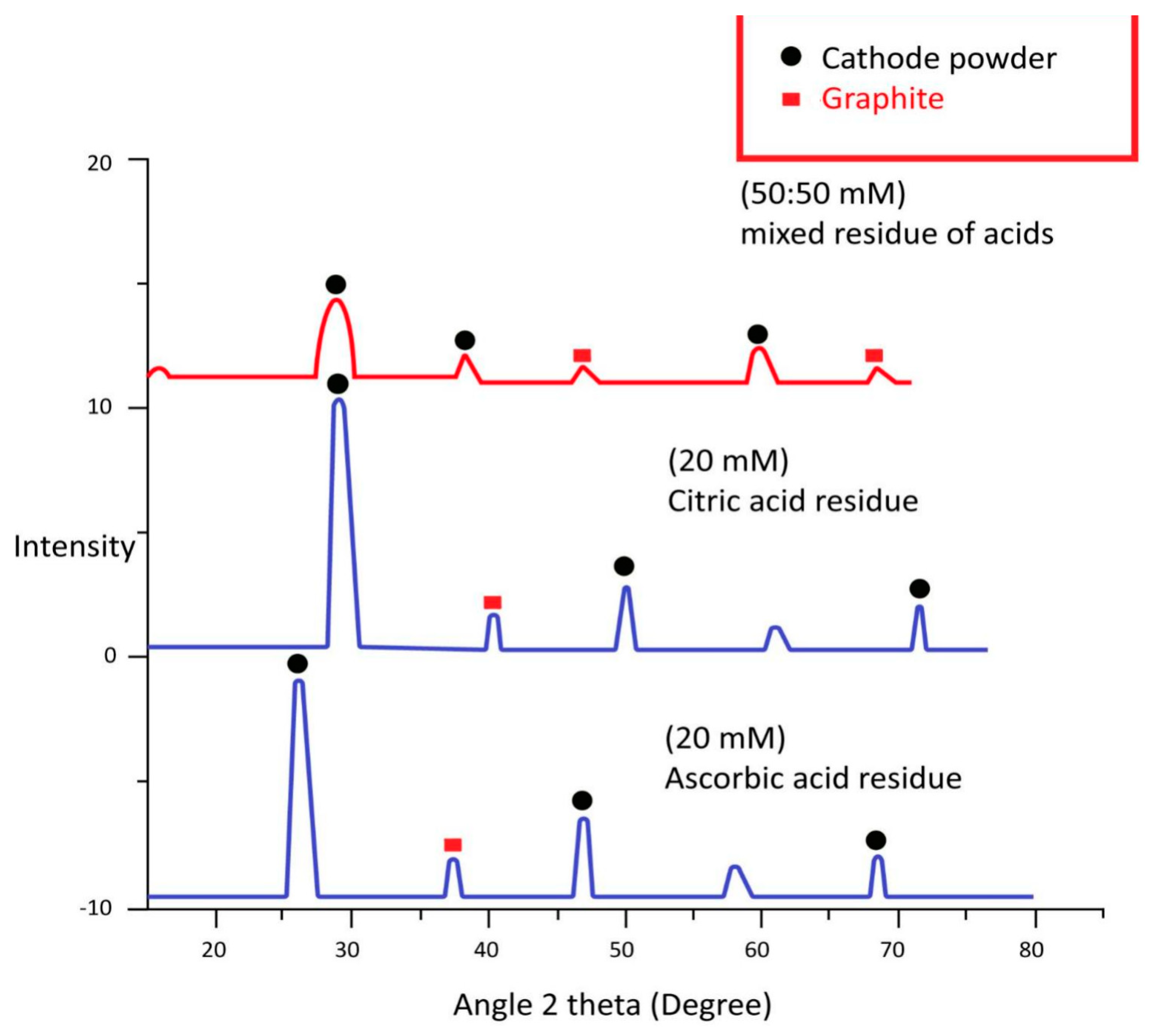
4.3. XRD Analysis
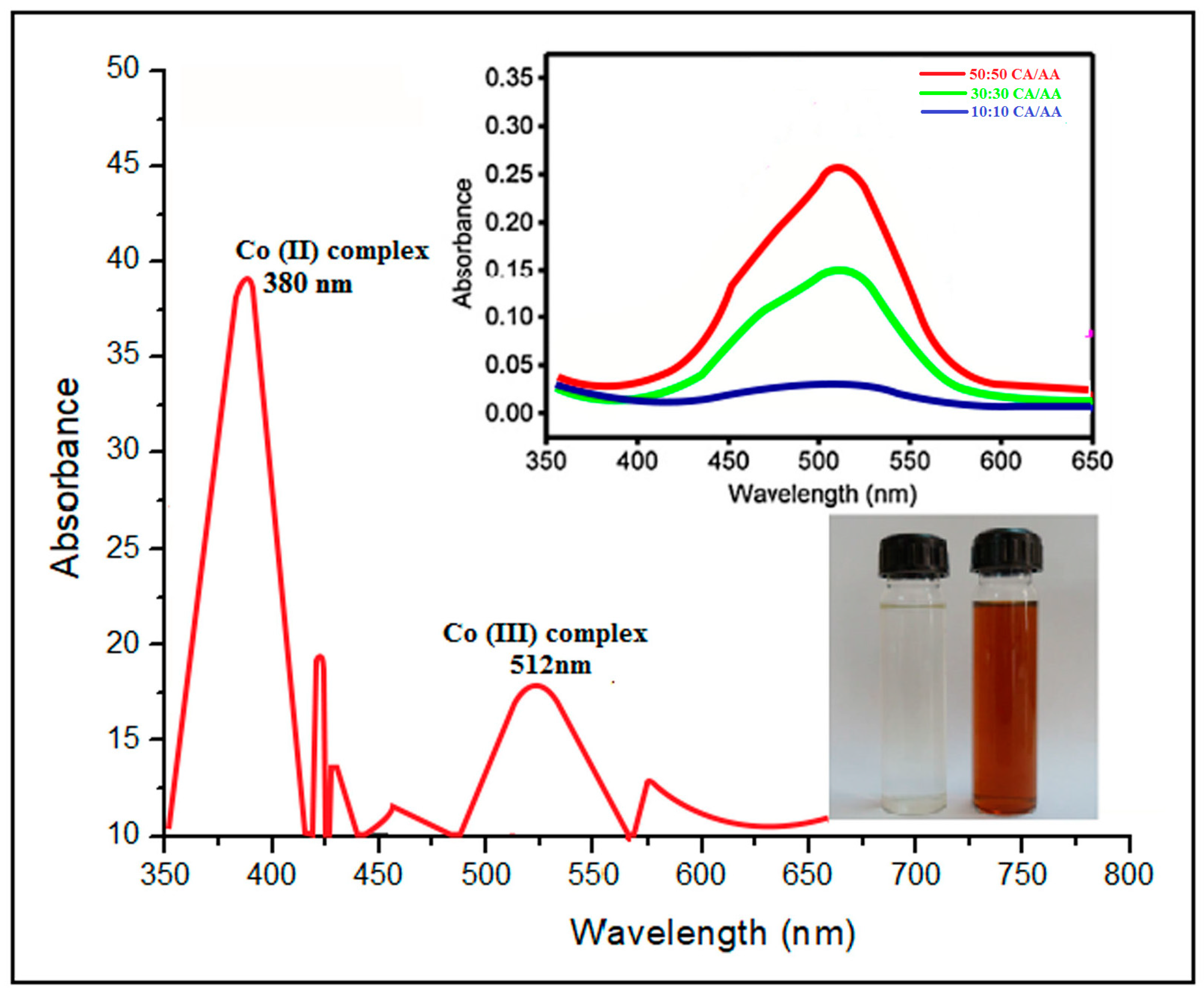
4.4. UV–Visible Analysis
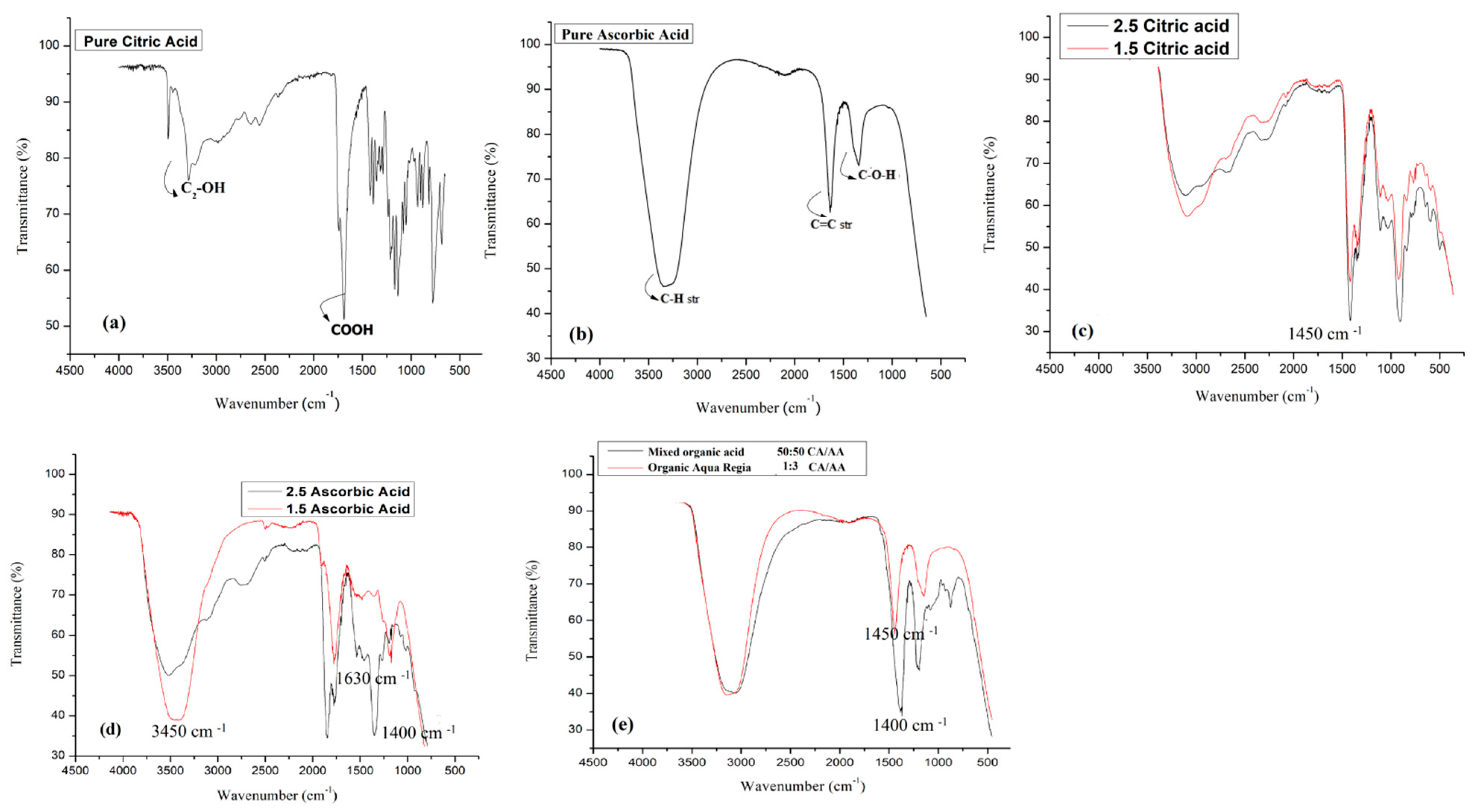
4.5. FT-IR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kotkar, A.; Dash, S.; Bhanja, P.; Sahu, S.; Verma, A.; Mukherjee, A.; Mohapatra, M.; Basu, S. Microwave assisted recycling of spent Li-ion battery electrode material into efficient oxygen evolution reaction catalyst. Electrochimica Acta 2023, 442, 141842. [Google Scholar] [CrossRef]
- Du, K.-D.; Ang, E.H.; Wu, X.-L.; Liu, Y. Progresses in sustainable recycling technology of spent lithium-ion batteries. Energy Environ. Mater. 2022, 5, 1012–1036. [Google Scholar] [CrossRef]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries E-waste: A circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef]
- Vuppaladadiyam, S.S.V.; Thomas, B.S.; Kundu, C.; Vuppaladadiyam, A.K.; Duan, H.; Bhattacharya, S. Can e-waste recycling provide a solution to the scarcity of rare earth metals? An overview of e-waste recycling methods. Sci. Total. Environ. 2024, 924, 171453. [Google Scholar] [CrossRef]
- Akcil, A.; Ibrahim, Y.A.; Meshram, P.; Panda, S. Abhilash Hydrometallurgical recycling strategies for recovery of rare earth elements from consumer electronic scraps: A review. J. Chem. Technol. Biotechnol. 2021, 96, 1785–1797. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Yetim, D.; Svecova, L.; Leprêtre, J.C. Lithium-Ion Battery Cathode Recycling through a Closed-Loop Process Using a Choline Chloride-Ethylene Glycol-Based Deep-Eutectic Solvent in the Presence of Acid. ChemistryOpen 2024, 13, e202300061. [Google Scholar] [CrossRef]
- Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals 2023, 13, 1330. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Ren, L.; Liu, B.; Bao, S.; Ding, W.; Zhang, Y.; Hou, X.; Lin, C.; Chen, B. Recovery of Li, Ni, Co and Mn from spent lithium-ion batteries assisted by organic acids: Process optimization and leaching mechanism. Int. J. Miner. Met. Mater. 2024, 31, 518–530. [Google Scholar] [CrossRef]
- Sidiq, A.L.; Floweri, O.; Karunawan, J.; Abdillah, O.B.; Santosa, S.P.; Iskandar, F. NCM cathode active materials reproduced from end-of-life Li-ion batteries using a simple and green hydrometallurgical recycling process. Mater. Res. Bull. 2022, 153, 111901. [Google Scholar] [CrossRef]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Sumboja, A.; Santosa, S.P.; Ogi, T.; Iskandar, F. Regeneration of LiNi1/3Co1/3Mn1/3O2 cathode active materials from end-of-life lithium-ion batteries through ascorbic acid leaching and oxalic acid coprecipitation processes. ACS Sustain. Chem. Eng. 2020, 8, 16104–16114. [Google Scholar] [CrossRef]
- Milian, Y.E.; Jamett, N.; Cruz, C.; Herrera-León, S.; Chacana-Olivares, J. A comprehensive review of emerging technologies for recycling spent lithium-ion batteries. Sci. Total. Environ. 2023, 910, 168543. [Google Scholar] [CrossRef]
- Chen, H.; Gu, S.; Guo, Y.; Dai, X.; Zeng, L.; Wang, K.; He, C.; Dodbiba, G.; Wei, Y.; Fujita, T. Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3. Hydrometallurgy 2021, 205, 105746. [Google Scholar] [CrossRef]
- Cai, L.; Lin, J.; Fan, E.; Wu, F.; Chen, R.; Li, L. Eco-friendly organic acid-assisted mechanochemical process for metal extraction from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 10649–10657. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Nayaka, G.; Pai, K.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Ruan, Z.; Xie, M.; Chen, J.; Ren, X. Recycling of lithium batteries—A review. Energies 2022, 15, 1611. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Morcali, M.H. Recycling of Silver and Zinc from Silver Oxide Battery Waste. ChemistrySelect 2019, 4, 9011–9017. [Google Scholar] [CrossRef]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Chaudhary, V.; Lakhera, P.; Kim, K.-H.; Deep, A.; Kumar, P. Insights into the Eco-Friendly Recovery Process for Valuable Metals from Waste Lithium-ion Batteries by Organic Acids Leaching. Sep. Purif. Rev. 2024, 53, 82–99. [Google Scholar] [CrossRef]
- Kozak, T.; Kars, H.; Gül, M.; Şahin, G.; Atagün, C.; Aydin, C.; Hamarat, İ.; Halvacı, E.; Bayat, R.; Akın, M. Recycling of valuable elements contained in waste lithium ion batteries. J. Sci. Rep.-C 2024, 006, 19–36. [Google Scholar]
- Yan, S.; Sun, C.; Zhou, T.; Gao, R.; Xie, H. Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep. Purif. Technol. 2021, 257, 117930. [Google Scholar] [CrossRef]
- Almeida, J.R.; Moura, M.N.; Barrada, R.V.; Barbieri, E.M.S.; Carneiro, M.T.W.D.; Ferreira, S.A.D.; Lelis, M.d.F.F.; de Freitas, M.B.J.G.; Brandão, G.P. Composition analysis of the cathode active material of spent Li-ion batteries leached in citric acid solution: A study to monitor and assist recycling processes. Sci. Total. Environ. 2019, 685, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundström, M. Leaching of metals from spent lithium-ion batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef]
- Nayaka, G.; Manjanna, J.; Pai, K.; Vadavi, R.; Keny, S.; Tripathi, V. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, L.; Fu, B.; Ahn, J.W.; Wang, X. Recycling of mixed lithium-ion battery cathode materials with spent lead-acid battery electrolyte with the assistance of thermodynamic simulations. J. Clean. Prod. 2020, 266, 121827. [Google Scholar] [CrossRef]
- Munir, H.; Srivastava, R.R.; Kim, H.; Ilyas, S.; Khosa, M.K.; Yameen, B. Leaching of exhausted LNCM cathode batteries in ascorbic acid lixiviant: A green recycling approach, reaction kinetics and process mechanism. J. Chem. Technol. Biotechnol. 2020, 95, 2286–2294. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2018, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Fan, E.; Yang, J.; Huang, Y.; Lin, J.; Arshad, F.; Wu, F.; Li, L.; Chen, R. Leaching mechanisms of recycling valuable metals from spent lithium-ion batteries by a malonic acid-based leaching system. ACS Appl. Energy Mater. 2020, 3, 8532–8542. [Google Scholar] [CrossRef]
- Santana, I.; Moreira, T.; Lelis, M.; Freitas, M. Photocatalytic properties of Co3O4/LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent. Mater. Chem. Phys. 2017, 190, 38–44. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Lin, L.; Lu, Z.; Zhang, W. Recovery of lithium and cobalt from spent Lithium- Ion batteries using organic aqua regia (OAR): Assessment of leaching kinetics and global warming potentials. Resour. Conserv. Recycl. 2021, 167, 105416. [Google Scholar] [CrossRef]
- Gao, R.; Sun, C.; Zhou, T.; Zhuang, L.; Xie, H. Recycling of LiNi0. 5Co0. 2Mn0. 3O2 Material from Spent Lithium-ion Batteries Using Mixed Organic Acid Leaching and Sol-gel Method. ChemistrySelect 2020, 5, 6482–6490. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]

| Time °C | Model 1 | Model 2 | Model 3 | Model 4 | Fitting Result | ||||
|---|---|---|---|---|---|---|---|---|---|
| InK | R2 | InK | R2 | InK | R2 | InK | R2 | n | |
| 50 °C | −3.1806 | 0.97 | −3.1806 | 0.99 | −3.1806 | 0.99 | −3.1806 | 0.99 | 0.5 |
| 45 °C | −3.3287 | 0.91 | −3.1806 | 0.97 | −3.1806 | 0.99 | −3.1806 | 0.98 | 0.7 |
| 40 °C | −3.4527 | 0.89 | −3.4527 | 0.91 | −3.4527 | 0.99 | −3.4527 | 0.81 | 0 |
| 35 °C | −3.5575 | 0.85 | −3.5575 | 0.86 | −3.5575 | 0.98 | −3.5575 | 0.86 | 0 |
| 30 °C | −3.3287 | 0.86 | −3.1806 | 0.97 | −3.1806 | 0.99 | −3.1806 | 0.86 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.; Khosa, M.K.; Noor, A.; Qayyum, S.; El Oirdi, M. Hydrometallurgical Extraction of Valuable Metals by Mixed Acid Leaching System for Used Lithium-Ion Batteries. Sustainability 2024, 16, 6817. https://doi.org/10.3390/su16166817
Fatima S, Khosa MK, Noor A, Qayyum S, El Oirdi M. Hydrometallurgical Extraction of Valuable Metals by Mixed Acid Leaching System for Used Lithium-Ion Batteries. Sustainability. 2024; 16(16):6817. https://doi.org/10.3390/su16166817
Chicago/Turabian StyleFatima, Sadaf, Muhammad Kaleem Khosa, Awal Noor, Sadaf Qayyum, and Mohamed El Oirdi. 2024. "Hydrometallurgical Extraction of Valuable Metals by Mixed Acid Leaching System for Used Lithium-Ion Batteries" Sustainability 16, no. 16: 6817. https://doi.org/10.3390/su16166817
APA StyleFatima, S., Khosa, M. K., Noor, A., Qayyum, S., & El Oirdi, M. (2024). Hydrometallurgical Extraction of Valuable Metals by Mixed Acid Leaching System for Used Lithium-Ion Batteries. Sustainability, 16(16), 6817. https://doi.org/10.3390/su16166817








