Advances in Geochemical Monitoring Technologies for CO2 Geological Storage
Abstract
1. Introduction
2. Overview of Geochemical Monitoring Techniques
2.1. Gas Monitoring
- Enhancing the accuracy of CO2 concentration monitoring through sensor upgrades or data calibration. Sensors are critical components when collecting environmental parameters. In recent years, CO2 concentration monitoring equipment such as Fourier Transform Infrared Spectrometer (FT-IR) and Photoacoustic Spectroscopy (PAS) sensors have developed rapidly. FT-IR, combined with a long-path gas absorption cell, can improve detection sensitivity to the ppb level, meeting monitoring needs in complex environments such as high-temperature and high-humidity environments [25,26,27]. PAS can monitor multi-component mixed gasses with high sensitivity, making it ideal for online CO2 monitoring, although the stability of PAS still needs to be improved [28,29]. Since the precision components of sensors are susceptible to environmental influences (Figure 1a) [30,31,32], designs typically include temperature, humidity, and pressure compensation mechanisms or algorithms to correct the initial measurements and enhance the credibility of the data [30,31]. By integrating measurements from different sensors, reliable data under specific environmental conditions can be output based on algorithms, collectively representing the CO2 concentration changes in the region.
- The coordinated monitoring of multiple gasses to indirectly reflect gas leakages. During biological photosynthesis and respiration, changes in O2 and CO2 concentrations have a good linear relationship, so the ratio of O2 to CO2 concentration changes can be used to determine whether CO2 leakage has occurred. If CO2 leakage occurs, there will be a significant abrupt leakage signal (Figure 1b) [33]. However, factors such as water–rock–CO2 interactions, methane oxidation, rock weathering, and groundwater flow can generate or consume CO2 or O2 [34,35]. Therefore, monitoring changes in the composition and concentration of multiple gasses, such as CO2, O2, N2, CH4, Ar, and He, is needed to assist in analyses of the CO2 source and corresponding geochemical processes (Figure 1c) [36]; this can reduce the impact of environmental background changes and achieve effective monitoring [37].
2.2. Water Monitoring
2.3. Tracer Monitoring
2.4. Isotope Monitoring
3. Research Developments
3.1. Development of Monitoring Strategies
3.2. Storage State and Leakage Assessment
4. Outlook
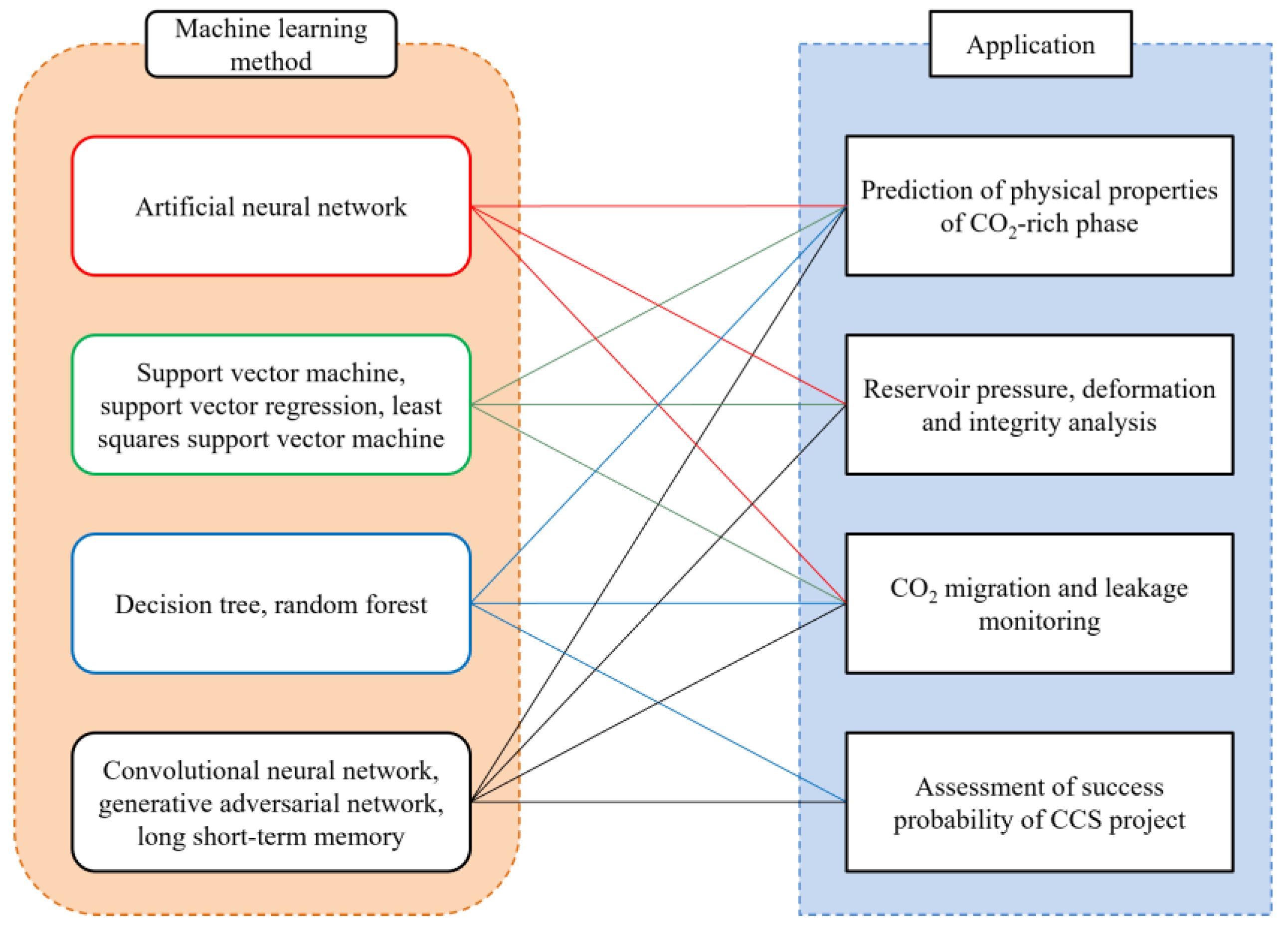
4.1. Application of Artificial Intelligence and Machine Learning
4.2. Baseline Survey and Internet of Things Monitoring
5. Conclusions
- Implement a comprehensive multi-method monitoring approach to improve accuracy and coverage.
- Strengthen baseline surveys to establish reliable environmental reference standards.
- Utilize Internet of Things (IoT) technology for real-time data collection.
- Integrate artificial intelligence (AI) and machine learning (ML) to enhance data processing and achieve more accurate anomaly detection.
- Adjust monitoring plans dynamically based on the results obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcculloch, M.T.; Winter, A.; Sherman, C.E.; Trotter, J.A. 300 years of sclerosponge thermometry shows global warming has exceeded 1.5 °C. Nat. Clim. Chang. 2024, 14, 171–177. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Lu, X. China Carbon Dioxide Capture, Utilization and Storage (CCUS) Annual Report (2023); The Administrative Center for China’s Agenda 21, Global CCS Institute, Tsinghua University: Beijing, China, 2023. [Google Scholar]
- Global CCS Institute. Global Status of CCS 2023; Global CCS Institute: Melbourne, Australia, 2023. [Google Scholar]
- Ringrose, P.; Mathieson, A.; Wright, I.; Selama, F.; Hansen, O.; Bissell, R.; Saoula, N.; Midgley, J. The in Salah CO2 Storage Project: Lessons Learned and Knowledge Transfer. Energy Procedia 2013, 37, 6226–6236. [Google Scholar] [CrossRef]
- Roberts, J.J.; Stalker, L. What have we learned about CO2 leakage from CO2 release field experiments, and what are the gaps for the future? Earth Sci. Rev. 2020, 209, 102939. [Google Scholar] [CrossRef]
- Ringrose, P.S.; Roberts, D.M.; Gibson-Poole, C.M.; Bond, C.; Wightman, R.; Taylor, M.; Raikes, S.; Iding, M.; Østmo, S. Characterization of the Krechba CO2 storage site: Critical elements controlling injection performance. Energy Procedia 2011, 4, 4672–4679. [Google Scholar] [CrossRef]
- Liu, E.; Zhu, L.; Raj, A.G.; McClellan, J.H.; Al-Shuhail, A.; Kaka, S.I.; Iqbal, N. Microseismic events enhancement and detection in sensor arrays using autocorrelation-based filtering. Geophys. Prospect. 2017, 65, 1496–1509. [Google Scholar] [CrossRef]
- Vermeul, V.R.; Amonette, J.E.; Strickland, C.E.; Williams, M.D.; Bonneville, A. An overview of the monitoring program design for the FutureGen 2.0 CO2 storage site. Int. J. Greenh. Gas Control 2016, 51, 193–206. [Google Scholar] [CrossRef]
- Myers, M.B.; Roberts, J.J.; White, C.; Stalker, L. An experimental investigation into quantifying CO2 leakage in aqueous environments using chemical tracers. Chem. Geol. 2019, 511, 91–99. [Google Scholar] [CrossRef]
- Gassara, O.; Estublier, A.; Garcia, B.; Noirez, S.; Cerepi, A.; Loisy, C.; Le Roux, O.; Petit, A.; Rossi, L.; Kennedy, S.; et al. The Aquifer-CO2Leak project: Numerical modeling for the design of a CO2 injection experiment in the saturated zone of the Saint-Emilion (France) site. Int. J. Greenh. Gas Control 2021, 104, 103196. [Google Scholar] [CrossRef]
- Cahill, A.G.; Jakobsen, R. Geochemical modeling of a sustained shallow aquifer CO2 leakage field study and implications for leakage and site monitoring. Int. J. Greenh. Gas Control 2015, 37, 127–141. [Google Scholar] [CrossRef]
- Cuoco, E.; Sacchi, E.; De Francesco, S.; Paolucci, V.; Maletic, E.L.; Darrah, T.H.; Sirna, M.; Tedesco, D. Groundwater mixing in a heterogeneous multilayer aquifer driven by geogenic CO2 fluxes: Evidence from chemical and isotopic composition of Ferrarelle waters (Riardo Plain, southern Italy). Appl. Geochem. 2020, 116, 104564. [Google Scholar] [CrossRef]
- Roberts, J.J.; Gilfillan, S.M.V.; Stalker, L.; Naylor, M. Geochemical tracers for monitoring offshore CO2 stores. Int. J. Greenh. Gas Control 2017, 65, 218–234. [Google Scholar] [CrossRef]
- Györe, D.; Gilfillan, S.M.V.; Stuart, F.M. Tracking the interaction between injected CO2 and reservoir fluids using noble gas isotopes in an analogue of large-scale carbon capture and storage. Appl. Geochem. 2017, 78, 116–128. [Google Scholar] [CrossRef]
- Stalker, L.; Boreham, C.; Underschultz, J.; Freifeld, B.; Perkins, E.; Schacht, U.; Sharma, S. Application of tracers to measure, monitor and verify breakthrough of sequestered CO2 at the CO2CRC Otway Project, Victoria, Australia. Chem. Geol. 2015, 399, 2–19. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 Sequestration in Deep Sedimentary Formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Gilfillan, S.; Haszedline, S.; Stuart, F.; Gyore, D.; Kilgallon, R.; Wilkinson, M. The application of noble gases and carbon stable isotopes in tracing the fate, migration and storage of CO2. Energy Procedia 2014, 63, 4123–4133. [Google Scholar] [CrossRef]
- Oldenburg, C.M.; Lewicki, J.L.; Hepple, R.P. Near-Surface Monitoring Strategies for Geologic Carbon Dioxide Storage Verification; University of California: Los Angeles, CA, USA, 2003. [Google Scholar]
- Oskarsson, N.; Pálsson, K.; Ólafsson, H.; Ferreira, T. Experimental monitoring of carbon dioxide by low power IR-sensors: Soil degassing in the Furnas Volcanic Centre, Azores. J. Volcanol. Geotherm. Res. 1999, 92, 181–193. [Google Scholar] [CrossRef]
- Lewicki, J.L.; Hilley, G.E. Eddy covariance mapping and quantification of surface CO2 leakage fluxes. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Lewicki, J.L.; Fischer, M.L.; Hilley, G.E. Six-week time series of eddy covariance CO2 flux at Mammoth Mountain, California: Performance evaluation and role of meteorological forcing. J. Volcanol. Geotherm. Res. 2008, 171, 178–190. [Google Scholar] [CrossRef]
- Radziemski, L.J.; Solarz, R.W.; Paisner, J.A. Laser Spectroscopy and its Applications; Routledge: London, UK, 1987. [Google Scholar]
- Yang, H.; Qin, Y.; Feng, G.; Ci, H. Online Monitoring of Geological CO2 Storage and Leakage Based on Wireless Sensor Networks. IEEE Sens. J. 2013, 13, 556–562. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration ESRL. Trends in Atmospheric Carbon Dioxide. 2023. Available online: https://gml.noaa.gov/ccgg/trends/gl_trend.html (accessed on 1 June 2024).
- Geng, Y.; Zhang, W.; Yan, X.; Zhang, F.; Xie, Y.; Zhou, C.; Yin, B.; Ge, H. Comparison and Analysis of Carbon Dioxide Online Monitoring System for Stationary Pollution Sources in Jinan City. Environ. Sci. Technol. 2023, 36, 58–63. [Google Scholar] [CrossRef]
- Jie, G.; Han, Z.; Yu, Z.; Qian, W.; Han, X. On-line Monitoring of Waste Incineration Flue Gas Based on Fourier Transform Infrared Spectroscopy. China Instrum. 2018, 63–66. [Google Scholar] [CrossRef]
- Lin, Z.; Shao, S.; Liu, Y.; Dong, X.; Doing, X. Progress of research on infrared spectrometry in gas quantitative analysis. Anal. Instrum. 2009, 6–9. [Google Scholar] [CrossRef]
- Shi, J. Study on Detection System of Carbon Dioxide on Photoacoustic Spectrum; Zhejiang University of Technology: Hangzhou, China, 2017. [Google Scholar]
- Liu, K.; Guo, X.; Yi, H.; Chen, W.; Zhang, W.; Gao, X. Off-beam quartz-enhanced photoacoustic spectroscopy. Opt. Lett. 2009, 34, 1594–1596. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Zhu, X.; Liu, Y.; Zhenyu, W. Temperature Compensation for Infrared Detection of Carbon Dioxide Concentration. Infrared Technol. 2023, 45, 671–677. [Google Scholar]
- Jun, L. High-temperature Compensation Method for Non-dispersiveInfrared Carbon Dioxide Based on Numerical Iteration. Instrum. Tech. Sens. 2017, 104–106. [Google Scholar] [CrossRef]
- Shuqin, L. Detection and Analysis System for CO2 Gas Based on TDLAS. Doctoral Dissertation, Harbin Institute of Technology, Harbin, China, 2013; p. 67. [Google Scholar]
- Ma, D.; Tan, W.; Zhang, Z.; Wang, X.; Xia, F.; Hu, J. Recognition of leak CO2 with wavelet analysis based on correlation monitoring between CO2 and O2 in atmosphere. Process Saf. Environ. Prot. 2018, 114, 64–78. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Berner, E.K.; Berner, R.A. Global Environment: Water, Air, and Geochemical Cycles, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2012; Available online: http://www.jstor.org/stable/j.ctv30pnvjd (accessed on 1 June 2024).
- Romanak, K.; Dobeck, L.; Dixon, T.; Spangler, L. Potential for a process-based monitoring method above geologic carbon storage sites using dissolved gases in freshwater aquifers. Procedia Earth Planet. Sci. 2013, 7, 746–749. [Google Scholar] [CrossRef]
- Risk, D.; Lavoie, M.; Nickerson, N. Using the Kerr investigations at Weyburn to screen geochemical tracers for near-surface detection and attribution of leakage at CCSJEOR sites. Int. J. Greenh. Gas. Control. 2015, 35, 13–17. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, S.; Guan, Y.; Yang, H. Open-path Halon 1301 NDIR sensor with temperature compensation. Infrared Phys. Technol. 2019, 97, 129–134. [Google Scholar] [CrossRef]
- Do, H.-K.; Yu, S.; Ryuh, Y.-G.; Ju, Y.; Kang, H.-J.; Ha, S.-W.; Yun, S.-T. Tracing CO2 leakage and migration using the hydrogeochemical tracers during a controlled CO2 release field test. Appl. Geochem. 2022, 143, 105390. [Google Scholar] [CrossRef]
- Rillard, J.; Pourret, O.; Censi, P.; Inguaggiato, C.; Zuddas, P.; Toulhoat, P.; Gombert, P.; Brusca, L. Behavior of rare earth elements in an aquifer perturbed by CO2 injection: Environmental implications. Sci. Total Environ. 2019, 687, 978–990. [Google Scholar] [CrossRef]
- Abbaspour, A.; Refahi, M.; Khalafi-Nezhad, A.; Soltani Rad, N.; Behrouz, S. Carbon composite–PVC based membrane coated platinum electrode for chromium determination. J. Hazard. Mater. 2010, 184, 20–25. [Google Scholar] [CrossRef]
- Lacroix, E.; de Donato, P.; Lafortune, S.; Caumon, M.-C.; Barres, O.; Liu, X.; Derrien, M.; Piedevache, M. In situ continuous monitoring of dissolved gases (N2, O2, CO2, H2) prior to H2 injection in an aquifer (Catenoy, France) by on-site Raman and infrared spectroscopies: Instrumental assessment and geochemical baseline establishment. Anal. Methods 2021, 13, 3806–3820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.-J.; Liu, P.-F.; Fu, L.; Laso-Pérez, R.; Yang, L.; Bai, L.-P.; Li, J.; Yang, M.; Lin, J.-Z.; et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef]
- Tyne, R.L.; Barry, P.H.; Lawson, M.; Byrne, D.J.; Warr, O.; Xie, H.; Hillegonds, D.J.; Formolo, M.; Summers, Z.M.; Skinner, B.; et al. Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs. Nature 2021, 600, 670–674. [Google Scholar] [CrossRef]
- Derakhshan-Nejad, Z.; Sun, J.; Yun, S.; Lee, G. Potential CO2 intrusion in near-surface environments: A review of current research approaches to geochemical processes. Environ. Geochem. Health 2019, 41, 2339–2364. [Google Scholar] [CrossRef] [PubMed]
- Mcling, T.; Smith, W.; Smith, R. Utilizing Rare Earth Elements as Tracers in High TDS Reservoir Brines in CCS Applications. Energy Procedia 2014, 63, 3963–3974. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.H.; Yun, S.; Jeen, S. Shallow groundwater system monitoring on controlled CO2 release sites: A review on field experimental methods and efforts for CO2 leakage detection. Geosci. J. 2016, 20, 569–583. [Google Scholar] [CrossRef]
- Wei, Y. Effects of Pure and Impure Carbon Dioxide (CO2) on Soil Chemistry. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Kasina, M.; Bock, S.; Würdemann, H.; Pudlo, D.; Picard, A.; Lichtschlag, A.; März, C.; Wagenknecht, L.; Wehrmann, L.M.; Vogt, C.; et al. Mineralogical and geochemical analysis of Fe-phases in drill-cores from the Triassic Stuttgart Formation at Ketzin CO2 storage site before CO2 arrival. Environ. Earth Sci. 2017, 76, 161. [Google Scholar] [CrossRef]
- Lions, J.; Devau, N.; de Lary, L.; Dupraz, S.; Parmentier, M.; Gombert, P.; Dictor, M.-C. Potential impacts of leakage from CO2 geological storage on geochemical processes controlling fresh groundwater quality: A review. Int. J. Greenh. Gas Control 2014, 22, 165–175. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Mayer, B.; Gunter, W.; Perkins, E. Monitoring of fluid–rock interaction and CO2 storage through produced fluid sampling at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Appl. Geochem. 2005, 20, 1131–1157. [Google Scholar] [CrossRef]
- Jeong, J.; Jeen, S.; Hwang, H.; Lee, K. Changes in Geochemical Composition of Groundwater Due to CO2Leakage in Various Geological Media. Water 2020, 12, 2597. [Google Scholar] [CrossRef]
- Zielinski, J.P.T.; Melo, C.L.; Iglesias, R.S.; Reginato, P.R. CO2-Shallow groundwater interaction and related hydrogeochemical mechanisms: A review on reduced-scale CO2 release field experiments. Green Gases 2023, 13, 829–859. [Google Scholar] [CrossRef]
- Bergmann, P.; Schmidt-Hattenberger, C.; Labitzke, T.; Wagner, F.; Just, A.; Flechsig, C.; Rippe, D. Five Years of CO2 Injection Monitoring at Ketzin, Germany, Using Electrical Resistivity Tomography. In Proceedings of the 78th EAGE Conference and Exhibition 2016, Online, 30 May–2 June 2016. [Google Scholar]
- Collins, G.F.; Bartlett, F.E.; Turk, A.; Edmonds, S.M.; Mark, H.L. A preliminary evaluation of gas air tracers. J. Air Pollut. Control. Assoc. 1965, 15, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.J.; Alei, M.; Cappis, J.H.; Guthals, P.R.; Mason, A.S.; Rokop, D.J. Detection of multiply deuterated methane in the atmosphere. Geophys. Res. Lett. 1989, 16, 677–678. [Google Scholar] [CrossRef]
- Stalker, L.; Boreham, C.; Underschultz, J.; Freifeld, B.; Perkins, E.; Schacht, U.; Sharma, S. Geochemical monitoring at the CO2CRC Otway Project: Tracer injection and reservoir fluid acquisition. Energy Procedia 2009, 1, 2119–2125. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Q.; Wang, B.-D.; Zhu, C.-X.; Li, Y.-L. Preliminary study on environmental monitoring assessment system for CO2 storage projects. Environ. Eng. 2018, 36, 15–20. [Google Scholar] [CrossRef]
- Flohr, A.; Matter, J.M.; James, R.H.; Saw, K.; Brown, R.; Gros, J.; Flude, S.; Day, C.; Peel, K.; Connelly, D.; et al. Utility of natural and artificial geochemical tracers for leakage monitoring and quantification during an offshore controlled CO2 release experiment. Int. J. Greenh. Gas Control 2021, 111, 103421. [Google Scholar] [CrossRef]
- Mathieson, A.; Midgely, J.; Wright, I.; Saoula, N.; Ringrose, P. In Salah CO2 Storage JIP: CO2 sequestration monitoring and verification technologies applied at Krechba, Algeria. Energy Procedia 2011, 4, 3596–3603. [Google Scholar] [CrossRef]
- Rock, L.; Villegas, E.I.; Becker, V.; Dalkhaa, C.; Humez, P.; Nightingale, M.; Shevalier, M.; Mayer, B.; Zhang, G. Investigation of Natural Tracers for MMV at the Quest Carbon Capture and Storage Project, Alberta, Canada. Energy Procedia 2014, 63, 4191–4198. [Google Scholar] [CrossRef]
- Mcling, T.L.; Neupane, G.; Armstrong, L.T.; Smith, R.W. The use of environmental tracers to characterize a leaky CO2 CCS natural analogue site, Soda Springs, Idaho, USA. Greenh. Gases Sci. Technol. 2020, 10, 50–74. [Google Scholar] [CrossRef]
- Joun, W.-T.; Lee, K.-K.; Ha, S.-W.; Lee, S.-S.; Kim, Y.; Do, H.-K.; Jun, S.-C.; Kim, Y.; Ju, Y. A modified and rapid method for the single-well push-pull (SWPP) test using SF6, Kr, and uranine tracers. Water Res. 2023, 236, 119955. [Google Scholar] [CrossRef]
- Ju, Y.; Gilfillan, S.M.; Lee, S.-S.; Kaown, D.; Hahm, D.; Lee, S.; Park, I.-W.; Ha, S.-W.; Park, K.; Do, H.-K.; et al. Application of noble gas tracers to identify the retention mechanisms of CO2 migrated from a deep reservoir into shallow groundwater. Int. J. Greenh. Gas Control 2020, 97, 103041. [Google Scholar] [CrossRef]
- Karolytė, R. Migration and Retention of CO2 and Methane in the Otway Basin and South-East Australia: An Integrated Geochemical and Structural Analysis. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2019. [Google Scholar]
- Ju, Y.; Beaubien, S.E.; Lee, S.-S.; Kaown, D.; Hahm, D.; Lee, S.; Park, I.-W.; Park, K.; Yun, S.-T.; Lee, K.-K. Application of natural and artificial tracers to constrain CO2 leakage and degassing in the K-COSEM site, South Korea. Int. J. Greenh. Gas Control 2019, 86, 211–225. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, P.; Wu, S.; Zhou, W. Atmospheric 14 CO2 observation: A novel method to evaluate carbon emissions. Bull. Chin. Acad. Sci. 2023, 38, 1866–1873. [Google Scholar]
- Mayer, B.; Shevalier, M.; Nightingale, M.; Kwon, J.-S.; Johnson, G.; Raistrick, M.; Hutcheon, I.; Perkins, E. Tracing the movement and the fate of injected CO2 at the IEA GHG Weyburn-Midale CO2 Monitoring and Storage project (Saskatchewan, Canada) using carbon isotope ratios. Int. J. Greenh. Gas Control 2013, 16, S177–S184. [Google Scholar] [CrossRef]
- Mayer, B.; Humez, P.; Becker, V.; Dalkhaa, C.; Rock, L.; Myrttinen, A.; Barth, J. Assessing the usefulness of the isotopic composition of CO2 for leakage monitoring at CO2 storage sites: A review. Int. J. Greenh. Gas Control 2015, 37, 46–60. [Google Scholar] [CrossRef]
- Trumbore, S. Age of soil organic matter and soil respiration: Radiocarbon constraints on belowground C dynamics. Ecol. Appl. 2000, 10, 399–411. [Google Scholar] [CrossRef]
- Raistrick, M. Using Chemical and Isotopic Data to Monitor Geological Carbon Dioxide Storage at the International Energy Agency Weyburn Carbon Dioxide Monitoring and Storage Project, Saskatchewan, Canada. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2008. [Google Scholar]
- Gumm, L.P.; Bense, V.F.; Dennis, P.F.; Hiscock, K.M.; Cremer, N.; Simon, S. Dissolved noble gases and stable isotopes as tracers of preferential fluid flow along faults in the Lower Rhine Embayment, Germany. Hydrogeol. J. 2016, 24, 99–108. [Google Scholar] [CrossRef]
- Marty, B.; Jambon, A. C3He in volatile fluxes from the solid Earth: Implications for carbon geodynamics. Earth Planet Sci. Lett. 1987, 83, 16–26. [Google Scholar] [CrossRef]
- Jeandel, E.; Battani, A.; Sarda, P. Lessons learned from natural and industrial analogues for storage of carbon dioxide. Int. J. Greenh. Gas Control 2010, 4, 890–909. [Google Scholar] [CrossRef]
- Stalker, L.; Noble, R.; Pejcic, B.; Leybourne, M.; Hortle, A.; Michael, K.; Dixon, T.; Basava-Reddi, L. Feasibility of monitoring techniques for substances mobilised by CO2 storage in geological formations. Energy Procedia 2012, 23, 439–448. [Google Scholar] [CrossRef]
- Vermeul, V.R.; Strickland, C.E.; Thorne, P.D.; Bjornstad, B.N.; Mackley, R.D.; Kelly, M.E.; Sullivan, C.; Williams, M.D.; Amonette, J.E.; Downs, J.L.; et al. Future Gen 2.0 Monitoring Program: An Overview of the Monitoring Approach and Technologies Selected for Implementation. Energy Procedia 2014, 63, 4062–4070. [Google Scholar] [CrossRef]
- Györe, D. Noble Gases as Tracers of Injected CO2 in the Cranfield Enhanced Oil Recovery Field. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2015. [Google Scholar]
- Li, Q.; Liu, G. Risk Assessment of the Geological Storage of CO2: A Review. In Geologic Carbon Sequestration: Understanding Reservoir Behavior; Vishal, V., Singh, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 249–284. [Google Scholar] [CrossRef]
- Searchinger, T.D.; Wirsenius, S.; Beringer, T.; Dumas, P. Assessing the efficiency of changes in land use for mitigating climate change. Nature 2018, 564, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Guizhen, L.; Jian, Z.; Li, J. Status and suggestion of environmental monitoring for CO2 geological storage. Adv. Earth Sci. 2013, 28, 718–727. [Google Scholar]
- Wang, X. Optimization and Application of Soil Samples for the County’s Cultivated Land Quality Evaluation; Nanjing University: Nanjing, China, 2015. [Google Scholar]
- Ortega Romero, M.; Rincones Salinas, M.Á.; Elio Medina, J.D.; Gutiérrez del Olmo, J.; Nisi, B.; Mazadiego Martínez, L.F.; Iglesias, L.; Vaselli, O.; Grandia, F.; García, R.; et al. Gas monitoring methodology and application to ccs projects as defined by atmospheric and remote sensing survey in the natural analogue of campo de calatrava. Glob. Nest J. 2014, 16, 269–279. [Google Scholar]
- Johnson, G.; Dalkhaa, C.; Shevalier, M.; Nightingale, M.; Mayer, B.; Haszeldine, S. Pre-, Syn- and Post-CO2 Injection Geochemical and Isotopic Monitoring at the Pembina Cardium CO2 Monitoring Pilot, Alberta, Canada. Energy Procedia 2014, 63, 4150–4154. [Google Scholar] [CrossRef]
- Serno, S.; Johnson, G.; LaForce, T.C.; Ennis-King, J.; Haese, R.R.; Boreham, C.J.; Paterson, L.; Freifeld, B.M.; Cook, P.J.; Kirste, D.; et al. Using oxygen isotopes to quantitatively assess residual CO2 saturation during the CO2CRC Otway Stage 2B Extension residual saturation test. Int. J. Greenh. Gas Control 2016, 52, 73–83. [Google Scholar] [CrossRef]
- Johnson, G.; Mayer, B.; Shevalier, M.; Nightingale, M.; Hutcheon, I. Quantifying CO2 pore-space saturation at the Pembina Cardium CO2 Monitoring Pilot (Alberta, Canada) using oxygen isotopes of reservoir fluids and gases. Energy Procedia 2011, 4, 3942–3948. [Google Scholar] [CrossRef]
- Györe, D.; Stuart, F.M.; Gilfillan, S.M.V.; Waldron, S. Tracing injected CO2 in the Cranfield enhanced oil recovery field (MS, USA) using He, Ne and Ar isotopes. Int. J. Greenh. Gas Control 2015, 42, 554–561. [Google Scholar] [CrossRef]
- Brudi, K.; Dahmen, N.; Schmieder, H. Partition coefficients of organic substances in two-phase mixtures of water and carbon dioxide at pressures of 8 to 30 MPa and temperatures of 313 to 333 K. J. Supercrit. Fluids 1996, 9, 146–151. [Google Scholar] [CrossRef]
- Magnier, C.; Rouchon, V.; Bandeira, C.; Goncalves, R.; Miller, D.; Dino, R. Surface and Subsurface Geochemical Monitoring of an EOR-CO2 Field: Buracica, Brazil. Oil Gas Sci. Technol. 2012, 67, 355–372. [Google Scholar] [CrossRef]
- Flude, S.; Györe, D.; Stuart, F.M.; Zurakowska, M.; Boyce, A.J.; Haszeldine, R.S.; Chalaturnyk, R.; Gilfillan, S.M.V. The inherent tracer fingerprint of captured CO2. Int. J. Greenh. Gas Control 2017, 65, 40–54. [Google Scholar] [CrossRef]
- Karolyte, R.; Johnson, G.; Serno, S.; Gilfillan, S.M.V. The influence of water-rock reactions and O isotope exchange with CO2 on water stable isotope composition of CO2 springs in SE Australia. stable isotope composition of CO2 springs in SE Australia. Energy Procedia 2017, 114, 3832–3839. [Google Scholar] [CrossRef]
- Johnson, G. Stable Isotope Approaches to Monitoring and Verification of Injected Carbon Dioxide at the Pembina Cardium Carbon Dioxide Monitoring Pilot, Alberta, Canada. Doctoral Thesis, The University of Strathclyde, Glasgow, UK, 2011. [Google Scholar]
- Humez, P.; Lions, J.; Negrel, P.; Lagneau, V. CO2 intrusion in freshwater aquifers: Review of geochemical tracers and monitoring tools, classical uses and innovative approaches. Appl. Geochem. 2014, 46, 95–108. [Google Scholar] [CrossRef]
- Johnson, G.; Raistrick, M.; Mayer, B.; Shevalier, M.; Taylor, S.; Nightingale, M.; Hutcheon, I. The use of stable isotope measurements for monitoring and verification of CO2 storage. Energy Procedia 2009, 1, 2315–2322. [Google Scholar] [CrossRef]
- Donders, T.H.; Decuyper, M.; Beaubien, S.E.; van Hoof, T.B.; Cherubini, P.; Sass-Klaassen, U. Tree rings as biosensor to detect leakage of subsurface fossil CO2. Int. J. Greenh. Gas Control 2013, 19, 387–395. [Google Scholar] [CrossRef]
- Blackford, J.; Bull, J.M.; Cevatoglu, M.; Connelly, D.; Hauton, C.; James, R.H.; Lichtschlag, A.; Stahl, H.; Widdicombe, S.; Wright, I.C. Marine baseline and monitoring strategies for carbon dioxide capture and storage (CCS). Int. J. Greenh. Gas Control 2015, 38, 221–229. [Google Scholar] [CrossRef]
- Gal, F.; Lions, J.; Pokryszka, Z.; Gombert, P.; Grellier, S.; Prevot, F.; Yacine, D.; Patrice, S. CO2 leakage in a shallow aquifer—Observed changes in case of small release. Energy Procedia 2014, 63, 4112–4122. [Google Scholar]
- Park, K.; Kim, C.Y.; Kirk, M.F.; Chae, G.; Kwon, M.J. Effects of natural non-volcanic CO2 leakage on soil microbial community composition and diversity. Sci. Total Environ. 2023, 862, 160754. [Google Scholar] [CrossRef] [PubMed]
- Beaubien, S.E.; Graziani, S.; Annunziatellis, A.; Bigi, S.; Ruggiero, L.; Tartarello, M.C.; Lombardi, S. Spatial-temporal water column monitoring using multiple, low-cost GasPro-pCO2 sensors: Implications for monitoring, modelling, and potential impact. Energy Procedia 2014, 63, 3840–3847. [Google Scholar] [CrossRef]
- Gal, F.; Michel, B.; Gilles, B.; Frederic, J.; Karine, M. CO2 escapes in the Laacher See region, East Eifel, Germany: Application of natural analogue onshore and offshore geochemical monitoring. Int. J. Greenh. Gas Control 2011, 5, 1099–1118. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.; Bai, Y. Numerical Simulation of CO2-ECBM Based on Multi-Physical Field Coupling Model. Sustainability 2022, 14, 11789. [Google Scholar] [CrossRef]
- Kang, Y.; Hong, Y.; Shen, Z.; Liu, Y.; Liang, Q.; Yang, Q.; Wang, H.; Yao, Z.; Wang, K.; Ma, Z. Numerical simulation of atmospheric diffusion of CO2 and optimization of monitoring points in Yanchang Oil Field. China Mining Magazine 2024, 33, 201–207. [Google Scholar]
- Zhou, Y.; Zuo, R.; Liu, G.; Yuan, F.; Mao, X.; Guo, Y. The Great-leap-forward Development of Mathematical Geoscience during 2010–2019: Big Data and Artificial Intelligence Algorithm are Changing Mathematical Geoscience. Bull. Mineral. Petrol. Geochem. 2021, 40, 556–573. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liu, S.; Xu, J.; Fan, C. Prediction method of CO2 injectivity in saline aquifer based on BP neural network. J. Cent. South Univ. 2022, 53, 4678–4686. [Google Scholar] [CrossRef]
- Lu, D.; Painter, S.L.; Azzolina, N.A.; Burton-Kelly, M.; Jiang, T.; Williamson, C. Accurate and Rapid Forecasts for Geologic Carbon Storage via Learning-Based Inversion-Free Prediction. Front. Energy Res. 2022, 9, 752185. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Davoodi, S.; Vo Thanh, H.; Wood, D.A.; Mehrad, M.; Rukavishnikov, V.S.; Dai, Z. Machine-learning predictions of solubility and residual trapping indexes of carbon dioxide from global geological storage sites. Expert Syst. Appl. 2023, 222, 119796. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Thanh, H.V.; Dashtgoli, D.S.; Yasin, Q.; Radwan, A.E.; Ashraf, U.; Lee, K.-K. Application of robust intelligent schemes for accurate modelling interfacial tension of CO2 brine systems: Implications for structural CO2 trapping. Fuel 2022, 319, 123821. [Google Scholar] [CrossRef]
- Amar, M.N.; Ghahfarokhi, A.J. Prediction of CO2 diffusivity in brine using white-box machine learning. J. Pet. Sci. Eng. 2020, 190, 107037. [Google Scholar] [CrossRef]
- Aviso, K.B.; Janairo, J.I.B.; Promentilla, M.A.B.; Tan, R.R. Prediction of CO2 storage site integrity with rough set-based machine learning. Clean Technol. Environ. Policy 2019, 21, 1655–1664. [Google Scholar] [CrossRef]
- Yao, P.; Yu, Z.; Zhang, Y.; Xu, T. Application of machine learning in carbon capture and storage: An in-depth insight from the perspective of geoscience. Fuel 2023, 333, 126296. [Google Scholar] [CrossRef]
- Najafi-Marghmaleki, A.; Barati-Harooni, A.; Mohammadi, A.H. Impact of gas impurities on CO2 mole fraction: Application in carbon capture and storage (CCS) processes. Int. J. Greenh. Gas Control 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Wu, H.; Lubbers, N.; Viswanathan, H.S.; Pollyea, R.M. A multi-dimensional parametric study of variability in multi-phase flow dynamics during geologic CO2 sequestration accelerated with machine learning. Appl. Energy 2021, 287, 116580. [Google Scholar] [CrossRef]
- Hubert, P.; Padovese, L. A machine learning approach for underwater gas leakage detection. arXiv 2019, arXiv:1904.05661. [Google Scholar]
- Cao, C.; Liao, J.; Hou, Z.; Wang, G.; Feng, W.; Fang, Y. Parametric uncertainty analysis for CO2 sequestration based on distance correlation and support vector regression. J. Nat. Gas Sci. Eng. 2020, 77, 103237. [Google Scholar] [CrossRef]
- You, J.; Ampomah, W.; Sun, Q.; Kutsienyo, E.J.; Balch, R.S.; Dai, Z.; Cather, M.; Zhang, X. Machine learning based co-optimization of carbon dioxide sequestration and oil recovery in CO2-EOR project. J. Clean. Prod. 2020, 260, 120866. [Google Scholar] [CrossRef]
- Deng, T.; Liu, F.; Jia, G. Prediction carbon dioxide solubility in ionic liquids based on deep learning. Mol. Phys. 2020, 118, e1652367. [Google Scholar] [CrossRef]
- Wen, G.; Tang, M.; Benson, S.M. Towards a predictor for CO2 plume migration using deep neural networks. Int. J. Greenh. Gas Control 2021, 105, 103223. [Google Scholar] [CrossRef]
- Zhong, Z.; Sun, A.Y.; Yang, Q.; Ouyang, Q. A deep learning approach to anomaly detection in geological carbon sequestration sites using pressure measurements. J. Hydrol. 2019, 573, 885–894. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Bai, X.; Cao, Y.; Wu, L. Spatiotemporal evolution of carbon sequestration of limestone weathering in China. Sci. China Earth Sci. 2019, 62, 974–991. [Google Scholar] [CrossRef]
- Gerstenberger, M.; Nicol, A.; Stenhouse, M.; Berryman, K.; Stirling, M.; Webb, T.; Smith, W. Modularised logic tree risk assessment method for carbon capture and storage projects. Energy Procedia 2009, 1, 2495–2502. [Google Scholar] [CrossRef]
- Graziani, S.; Beaubien, S.E.; Bigi, S.; Lombardi, S. Spatial and Temporal pCO2 Marine Monitoring Near Panarea Island (Italy) Using Multiple Low-Cost GasPro Sensors. Environ. Sci. Technol. 2014, 48, 12126–12133. [Google Scholar] [CrossRef] [PubMed]
- Hortle, A.; de Caritat, P.; Stalvies, C.; Jenkins, C. Groundwater monitoring at the Otway Project site, Australia. Energy Procedia 2011, 4, 5495–5503. [Google Scholar] [CrossRef]
- Brydie, J.; Jones, D.; Jones, J.P.; Perkins, E.; Rock, L.; Taylor, E. Assessment of Baseline Groundwater Physical and Geochemical Properties for the Quest Carbon Capture and Storage Project, Alberta, Canada. Energy Procedia 2014, 63, 4010–4018. [Google Scholar] [CrossRef]
- Romanak, K.; Dixon, T. CO2 storage guidelines and the science of monitoring: Achieving project success under the California Low Carbon Fuel Standard CCS Protocol and other global regulations. Int. J. Greenh. Gas Control 2022, 113. [Google Scholar] [CrossRef]
- de Donato, P.; Pironon, J.; Sterpenich, J.; Laurent, A.; Piedevache, M.; Pokryszka, Z.; Quisel, N.; Barrès, O.; Thomas, S.; Rampnoux, N. CO2 flow baseline: Key factors of the geochemical monitoring program of future CO2 storage at Claye-Souilly (Paris basin). Energy Procedia 2011, 4, 5438–5446. [Google Scholar] [CrossRef]
- Weimer, J.E.; Sinopoli, B.; Krogh, B.H. A Relaxation Approach to Dynamic Sensor Selection in Large-Scale Wireless Networks. In Proceedings of the 28th International Conference on Distributed Computing Systems Workshops, Beijing, China, 17–20 June 2008; IEEE: New York, NY, USA, 2008; pp. 501–506. [Google Scholar]
- Ma, J.; Liu, J.; Zhou, Y.; Zheng, Y.; Lu, K.; Lin, X.; Wang, H.; Zhang, C. Online monitoring of CO2 using loT for assessment of leakage risks associated with geological sequestration. Earth Sci. Front. 2024, 31, 139–146. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Xu, Y.; Wang, W.; Cao, W.; Liu, Y.; He, J.; Lu, K. Study on IOT monitoring of urban soil pollution and visualized system based on microservice architecture. Earth Sci. Front. 2024, 31, 1–11. [Google Scholar] [CrossRef]
- Yang, H.; Fan, H.; Xu, X.; Zhang, Y.; Wang, W.; Yan, Z.; Wang, C.; Wang, J.; Liu, L.; Wang, R.; et al. Analysis of spatio-temporal variations and influencing factors of atmospheric CO2 concentrations in energy resources development areas. Earth Sci. Front. 2024, 31, 147–164. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Cao, W.; Liu, J. Research on a CO2 Internet of Things Online Monitoring System for Geotechnical Engineering Construction; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Quattrocchi, F.; Cantucci, B.; Cinti, D.; Galli, G.; Pizzino, L.; Sciarra, A.; Voltattorni, N. Continuous/discrete geochemical monitoring of CO2 Natural Analogues and of Diffuse Degassing Structures (DDS): Hints for CO2 storage sites geochemical monitoring protocol. Energy Procedia 2009, 1, 2135–2142. [Google Scholar] [CrossRef]

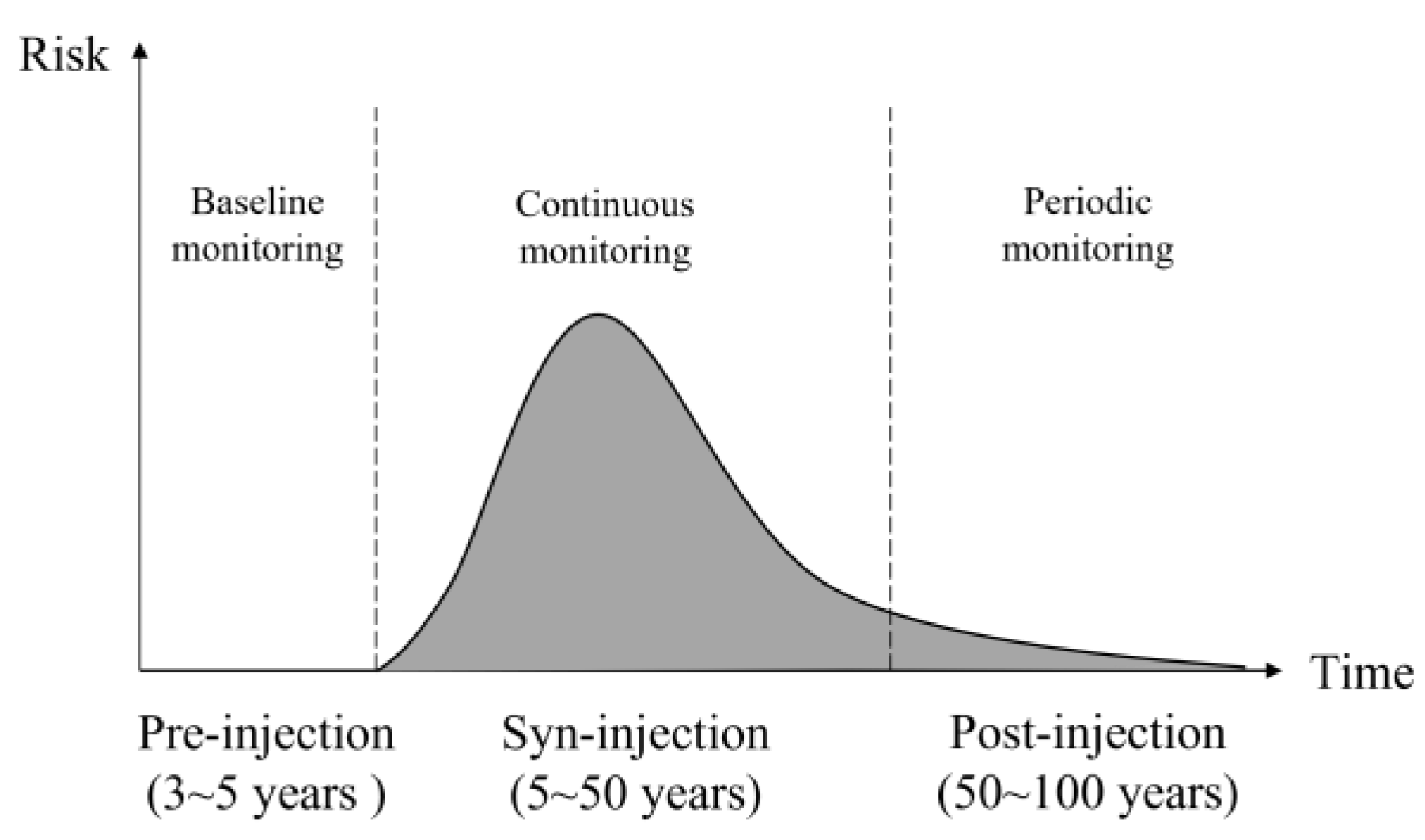






| Period | Pre-Injection | Syn-Injection | Post-Injection |
|---|---|---|---|
| pH |  | ||
| Type 1 |  | ||
| Type 2 |  | ||
| Type 3 |  | ||
| Tracers | Cost | Environment Impact | Type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost per Mt (CO2) | Logistics Cost | GWP (100 Years) | Biological Impact | Bio-Degradable | Use Restricted | In CO2 Stream? | In Storage Reservoir? | ||
| Artificial | SF6 | £1~100 | Acceptable | 22,850 | - | Uncertain | Yes | No | No |
| PFCs | £1~100 | Acceptable | 9540 | Possible | Yes | No | No | No | |
| CD4 | £1000~10,000 | Acceptable | >36 | Possible | Yes | No | No | No | |
| Natural | 14C (in CO2) | £10,000~100,000 | Acceptable | 1 | Possible | - | Yes | Yes | No |
| 14C/12C | £1~100 | Acceptable | 1 | Possible | - | Yes | Yes | No | |
| 13C/12C | £100,000~1,000,000 | Restrictive | 1 | No | - | No | Yes | Yes | |
| 18O | - | Restrictive | 1 | No | - | No | Yes | Yes | |
| CH4 | £1000~10,000 | Acceptable | 36 | Possible | Yes | No | No | Yes | |
| 3He/4He | £100~1000 | Acceptable | None | No | No | No | Yes | Yes | |
| 124,129Xe/130Xe | £1000~10,000 | Acceptable | None | No | No | No | Yes | Yes | |
| 80,83,86Kr/84Kr | £100,000~1,000,000 | Restrictive | None | No | No | No | Yes | Yes | |
| Method | Principle | Application | Main Mechanism Types | Leakage Scale | CO2 Sources | Leakage Path | Cycle | Accuracy | Case |
|---|---|---|---|---|---|---|---|---|---|
| gas | gas flux and composition | surface seepage or leakage spread | shallow/surface effects | direct | No | Yes | continuous/regular | normal | almost all |
| water | pH, ion concentration, and composition | cap integrity, plume migration | geochemical effects | indirect | No | Yes | continuous/regular | normal | InSalah, Outway, CO2SINK, Weyburn, Cranfield |
| noble gas | species and composition of noble gasses | plume migration, sequestration state | physical effects | indirect | Yes | Yes | regular | high | Outway, Weyburn, Cranfield |
| isotope | isotopic value | underground characteristics, storage state | geochemical effects | indirect | Yes | Yes | regular | high | Otway, Weyburn |
| tracer | the amount of tracer | plume migration, sequestration state | geochemical effects | indirect | Yes | Yes | regular | high | SECARB (SF6, (PFCs), InSalah (PFCs), Outway (CD4), Weyburn (PFCs), Shenhua (SF6) |
| Well | Measured CO2 | Loss of CO2 from CO2 Mix (%) | |||||
|---|---|---|---|---|---|---|---|
| 3He/4He | 40Ar/4He | C3F8 | SF6 | Kr | CH4 | ||
| 28F-2 2009 | 0.9% | 93 | 96 | / | / | / | / |
| 29F-1 2009 | 3.3% | 85 | 91 | / | / | / | / |
| 44-2 2009 | 8.4% | 77 | 86 | / | / | / | / |
| 29-5 2009 | 40.0% | 30 | 22 | / | / | / | / |
| 27-5 2009 | 46.9% | 28 | 42 | / | / | / | / |
| 370-1 | 86 kg | / | / | 27.9 | 34.6 | 40.0 | 42.1 |
| 370-2 | 86 kg | / | / | 34.7 | 41.0 | 47.9 | 43.4 |
| 372 | 86 kg | / | / | 64.3 | 70.6 | 70.8 | 51.6 |
| 373 | 86 kg | / | / | 46.1 | 52.1 | 55.2 | 44.3 |
| 376 | 143 kg | / | / | 36.6 | 52.5 | 53.0 | 40.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhou, Y.; Zheng, Y.; He, L.; Wang, H.; Niu, L.; Yu, X.; Cao, W. Advances in Geochemical Monitoring Technologies for CO2 Geological Storage. Sustainability 2024, 16, 6784. https://doi.org/10.3390/su16166784
Ma J, Zhou Y, Zheng Y, He L, Wang H, Niu L, Yu X, Cao W. Advances in Geochemical Monitoring Technologies for CO2 Geological Storage. Sustainability. 2024; 16(16):6784. https://doi.org/10.3390/su16166784
Chicago/Turabian StyleMa, Jianhua, Yongzhang Zhou, Yijun Zheng, Luhao He, Hanyu Wang, Lujia Niu, Xinhui Yu, and Wei Cao. 2024. "Advances in Geochemical Monitoring Technologies for CO2 Geological Storage" Sustainability 16, no. 16: 6784. https://doi.org/10.3390/su16166784
APA StyleMa, J., Zhou, Y., Zheng, Y., He, L., Wang, H., Niu, L., Yu, X., & Cao, W. (2024). Advances in Geochemical Monitoring Technologies for CO2 Geological Storage. Sustainability, 16(16), 6784. https://doi.org/10.3390/su16166784











