Seasonal Variation in the Element Composition of Dried, Powdered Green Sea Urchin (Strongylocentrotus droebachiensis) from Northern Norway
Abstract
1. Introduction
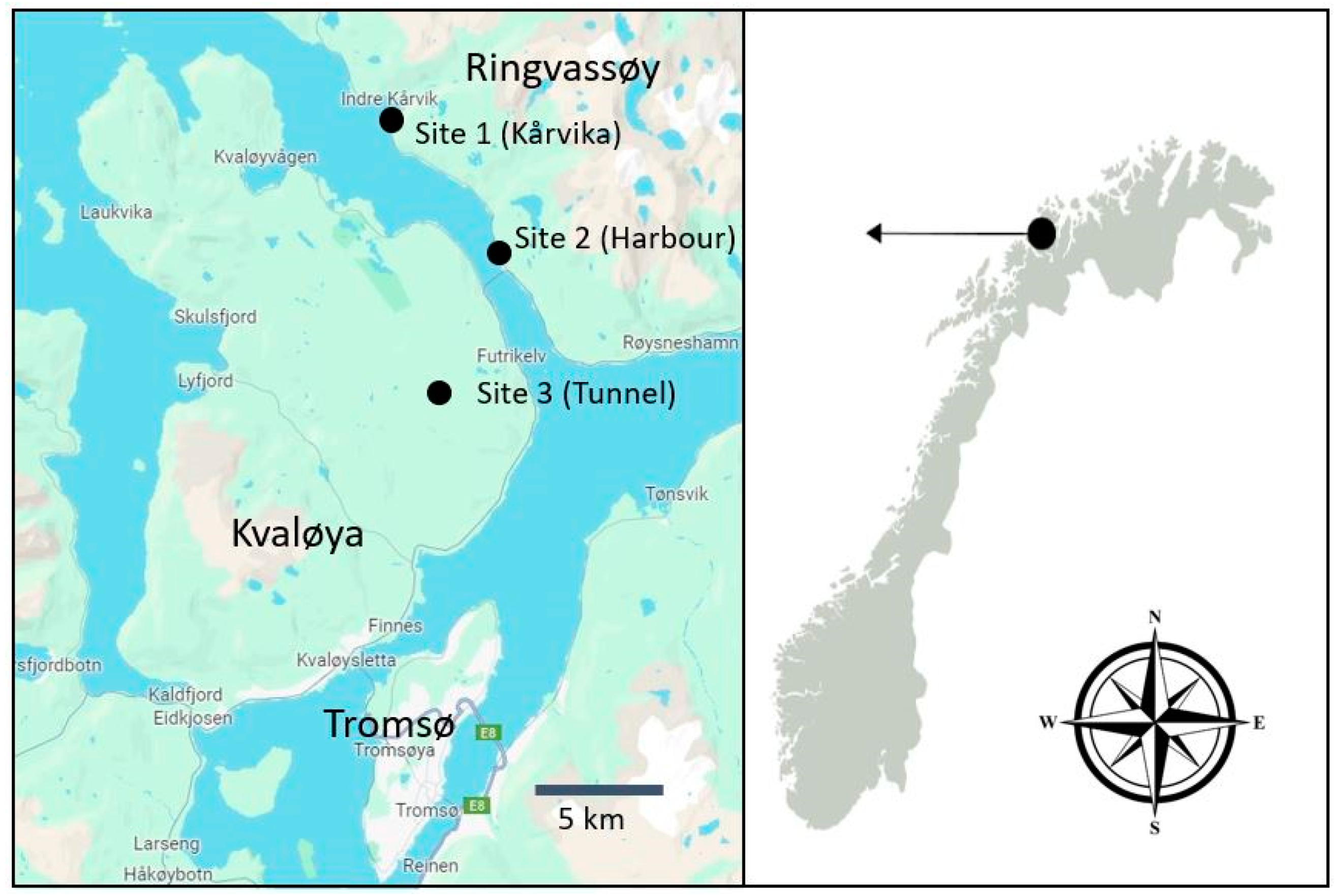
2. Materials and Methods
3. Results
3.1. Sea Urchin Gonad Index
3.2. Primary Nutrients
3.3. Secondary Nutrients
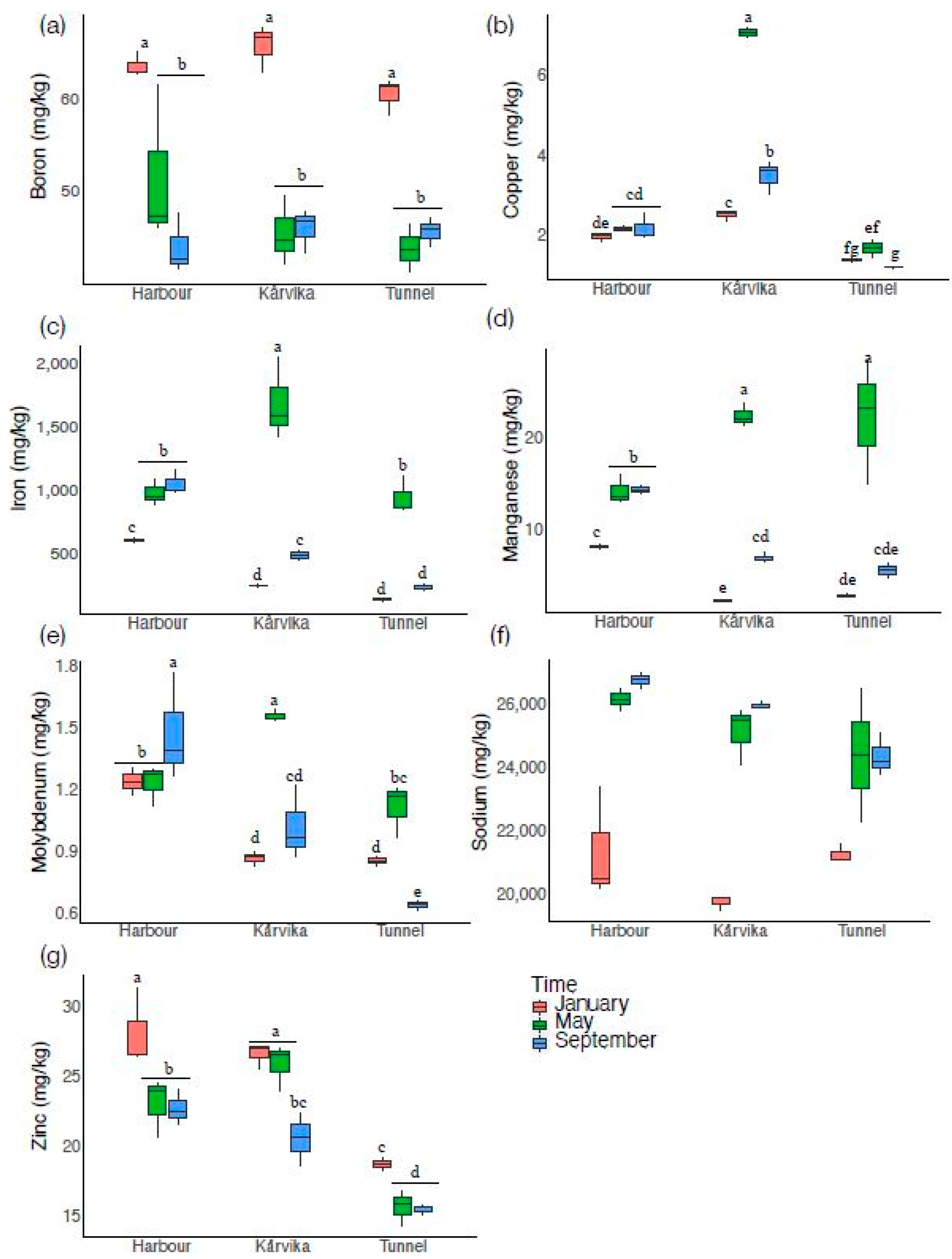
3.4. Micronutrients
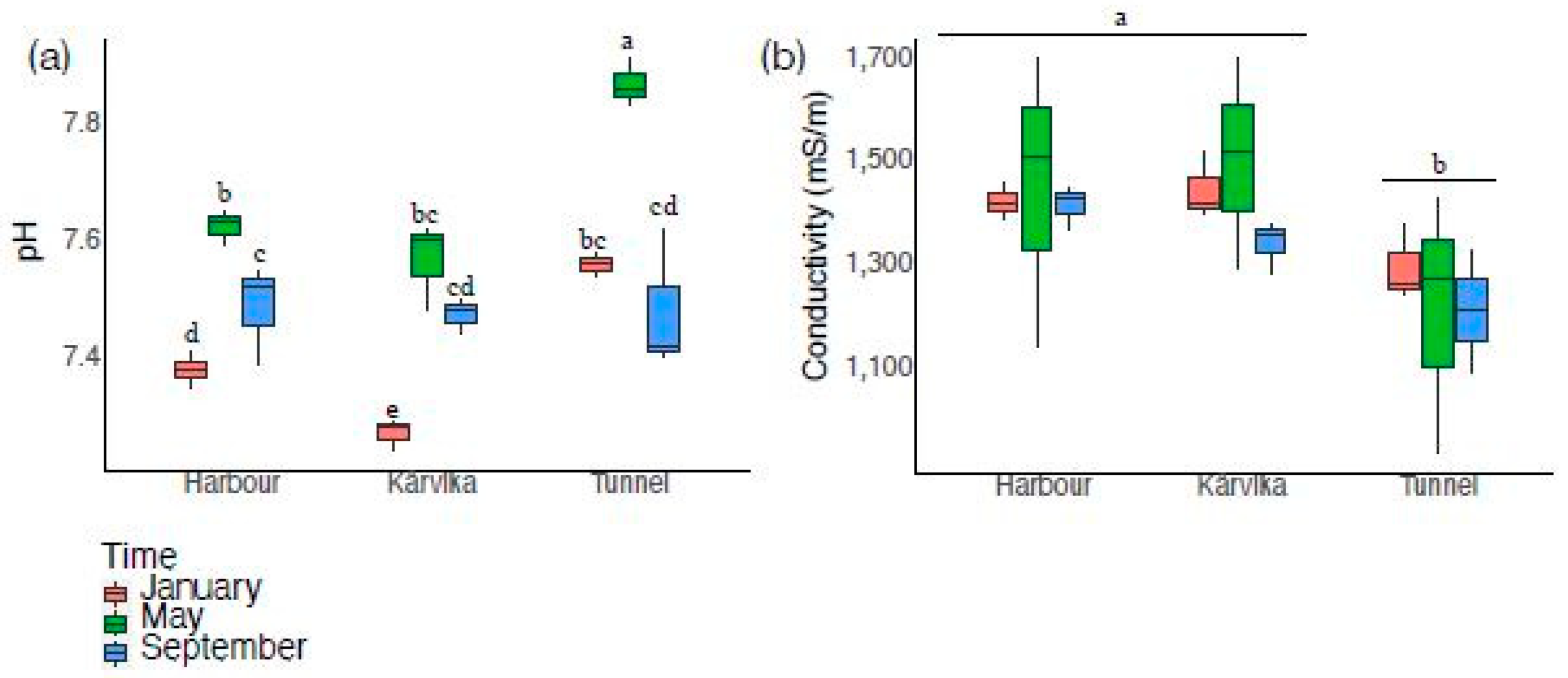
3.5. Other Elemental Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamilton, S.L.; Gleason, M.G.; Godoy, N.; Eddy, N.; Grorud-Colvert, K. Ecosystem-based Management for Kelp Forest Ecosystems. Mar. Policy 2022, 136, 104919. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience, and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Stewart, N.L.; Konar, B. Kelp Forests versus Urchin Barrens: Alternate Stable States and Their Effect on Sea Otter Prey Quality in the Aleutian Islands. J. Mar. Biol. 2012, 2012, 492308. [Google Scholar] [CrossRef]
- Steneck, R.S. Sea urchins as drivers of shallow benthic marine community structure. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 195–212. [Google Scholar] [CrossRef]
- Norderhaug, K.M.; Christie, H. Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Mar. Biol. Res. 2009, 5, 515–528. [Google Scholar] [CrossRef]
- James, P.; Siikavuopio, S.I.; Mortensen, A. Sea Urchin Aquaculture in Norway. In Echinoderm Aquaculture (s. 147–173); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Verbeek, J.; Louro, I.; Christie, H.; Carlsson, P.M.; Matsson, S.; Renaud, P.E. Restoring Norway’s Underwater Forests, a Strategy to Recover Kelp Ecosystems from Urchin Barrens; SeaForester, NIVA, Akvaplan-niva: Oslo, Norway, 2012. [Google Scholar]
- Zilia, F.; Bacenetti, J.; Sugni, M.; Matarazzo, A.; Orsi, L. From waste to product: Circular economy applications from Sea Urchin. Sustainability 2021, 13, 5427. [Google Scholar] [CrossRef]
- Zilia, F.; Orsi, L.; Costantini, M.; Tedesco, D.E.A.; Sugni, M. Case study of Life Cycle Assessment and sustainable business model for sea urchin waste. Clean. Environ. Syst. 2023, 8, 100108. [Google Scholar] [CrossRef]
- Solstad, R.G.; Philip, J. Observations from the Hydrolysis of the Green Sea Urchin (Strongylocentrotus droebachiensis). Glob. Chall. 2022, 7, 2200078. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S. Cultivation of Saccharina latissima (Sugar kelp) Using Sea Urchins Crush as a Nutrient Source. Master’s Thesis, UiT The Arctic University of Norway, Tromsø, Norway, 2021. Available online: https://hdl.handle.net/10037/26548 (accessed on 21 January 2024).
- Campus, P.; Swarts, N.D.; Mundy, C.; Keane, J.P.; Gardner, C. Assessing Processing Waste from the Sea Urchin (Centrostephanus rodgersii) Fishery as an Organic Fertilizer. Agronomy 2022, 12, 2919. [Google Scholar] [CrossRef]
- Bergquist, T. Assessing dried, powdered green sea urchin (Strongylocentrotus droebachiensis) as a biostimulant. Bachelor Thesis, number BIO-2203-H04. UiT. The Arctic University of Norway, Tromsø, Norway, 2023; p. 34. [Google Scholar]
- Ling, S.D.; Keane, J.P. Resurvey of the Longspined Sea Urchin (Centrostephanus rodgersii) and Associated Barren Reef in Tasmania. University of Tasmania: Hobart, Australia, 2018; Report. Available online: https://www.imas.utas.edu.au/__data/assets/pdf_file/0005/1176026/129569-Resurvey-of-the-Longspined-Sea-Urchin-Centrostephanus-rodgersii-and-associated-barren-reef-in-Tasmania.pdf (accessed on 4 February 2024).
- Zandvakili, O.R.; Barker, A.V.; Hashemi, M.; Etemadi, F.; Autio, W.R. Comparisons of commercial organic and chemical fertilizer solutions on growth and composition of lettuce. J. Plant Nutr. 2019, 42, 990–1000. [Google Scholar] [CrossRef]
- Drozdov, A.L.; Sharmankina, V.V.; Zemnukhova, L.A.; Polyakova, N.V. Chemical composition of spines and tests of sea urchins. Biol. Bull. 2016, 43, 521–531. [Google Scholar] [CrossRef]
- Amarowicz, R.; Synowiecki, J.; Shahidi, F. Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem. 2012, 133, 822–826. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Deiana, S.; Campus, P.; Mazza, A.; Deiana, P.; Pais, A. Assessment of the use potential of edible sea urchins (Paracentrotus lividus) processing waste within the agricultural system: Influence on soil chemical and biological properties and bean (Phaseolus vulgaris) and wheat (Triticum vulgare) growth in an amended acidic soil. J. Environ. Manag. 2012, 109, 12–18. [Google Scholar] [CrossRef]
- James, P.; Evensen, T.; Johansson, G. Effect of Season and Increased Temperature on Survival, Roe Enhancement, and Reproductive Cycle of the Green Sea Urchin (Strongylocentrotus droebachiensis) Collected from Four Relatively Close Sites in Northern Norway. Aquac. Res. 2023, 2023, 6360865. [Google Scholar] [CrossRef]
- SS-EN 13805:2014; Foodstuffs—Determination of Trace Elements—Pressure Digestion. CEN: Brussels, Belgium, 2014.
- SS-EN ISO 17294-2:2016; Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2016.
- Method 200.8:1994; Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma—Mass Spectrometry. US EPA: Washington, DC, USA, 1994.
- DIN EN 27888:1993-11; Water Quality; Determination of Electrical Conductivity. DIN: Berlin, Germany, 1993.
- Anas, M.; Lioa, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S. Effects of Nitrogen and Phosphorus Fertilization on Plant Growth and Nitrate Accumulation in Vegetables. J. Plant Nutr. 2004, 27, 539–556. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of Phosphate Solubilizing Microorganisms in Sustainable Agriculture—A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Prajapati, K.; Modi, H.A. The Importance of Patassium in Plant Growth—A Review. Indian J. Plant Sci. 2012, 1, 177–186. Available online: http://www.cibtech.org/jps.htm (accessed on 14 March 2024).
- Jaiswal, D.K.; Verma, J.P.; Prakash, S.; Meena, V.S.; Meena, R.S. Potassium as an Important Plant Nutrient in Sustainable Agriculture: A State of the Art. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Jilani, S.J.; Waseem, K.; Khaliq, G. Effect of different Nkp levels on the growth and yield of three onion (Allium cepa L.) varieties. Asian J. Plant Sci. 2003, 2, 342–346. [Google Scholar]
- Römheld, V.; Marschner, H. Function of micronutrients in plants. Micronutr. Agric. 1991, 4, 297–328. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Mishra, S.; Chauhan, D.K.; Dubey, N.K. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plant. 2015, 37, 139. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, B.T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Part 3 Chemical Methods, 5.3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996. First published: 1 January 1996. [Google Scholar] [CrossRef]
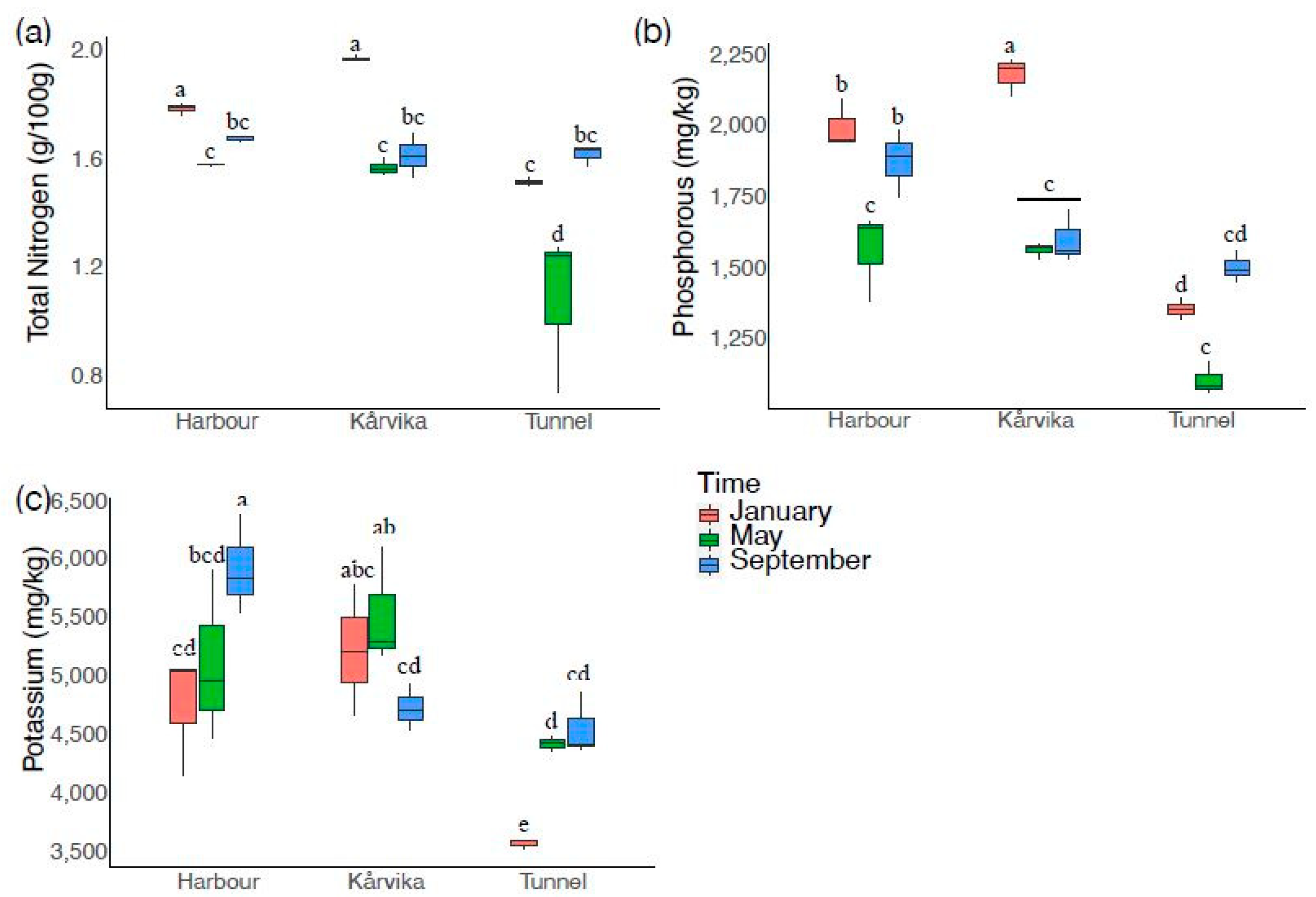

| Total Nitrogen | ||||
| Source of Variation | Df | Mean Squares | F-Value | p-Value |
| Site | 2 | 0.26018 | 23.805 | 8.80 × 10−6 |
| Time | 2 | 0.27634 | 25.283 | 5.92 × 10−6 |
| Site × Time | 4 | 0.06909 | 6.322 | 0.00233 |
| Residual | 18 | 0.01093 | ||
| Phosphorous | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 677,470 | 92.10 | 3.51 × 10−10 |
| Time | 2 | 425,615 | 57.86 | 1.45 × 10−8 |
| Site × Time | 4 | 102,126 | 13.88 | 2.46 × 10−5 |
| Residual | 18 | 7356 | ||
| Potassium | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 3,200,381 | 17.416 | 6.19 × 10−5 |
| Time | 2 | 849,604 | 4.623 | 0.0240 |
| Site × Time | 4 | 787,970 | 4.288 | 0.0131 |
| Residual | 18 | 183,763 | ||
| Magnesium | ||||
| Source of Variation | Df | Mean Squares | F-Value | p-Value |
| Site | 2 | 1,213,333 | 2.701 | 0.0942 |
| Time | 2 | 614,444 | 1.368 | 0.2799 |
| Site × Time | 4 | 574,444 | 1.279 | 0.3150 |
| Residual | 18 | 449,259 | ||
| Calcium | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 2.823 × 109 | 1.131 | 0.3447 |
| Time | 2 | 1.445 × 1010 | 5.786 | 0.0115 |
| Site × Time | 4 | 2.816 × 109 | 1.128 | 0.3746 |
| Residual | 18 | 2.497 × 109 | ||
| Sulphur | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 2,004,581 | 14.220 | 0.000197 |
| Time | 2 | 2,179,393 | 15.460 | 0.000124 |
| Site × Time | 4 | 839,259 | 5.953 | 0.003110 |
| Residual | 18 | 140,970 | ||
| Boron | ||||
| Source of Variation | Df | Mean Squares | F-Value | p-Value |
| Site | 2 | 24.4 | 1.713 | 0.208 |
| Time | 2 | 919.5 | 64.561 | 6.14 × 10−9 |
| Site × Time | 4 | 26.5 | 1.864 | 0.161 |
| Residual | 18 | 14.2 | ||
| Copper | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 21.133 | 477.4 | 2.54 × 10−16 |
| Time | 2 | 7.117 | 160.8 | 3.31 × 10−12 |
| Site × Time | 4 | 5.145 | 116.2 | 1.34 × 10−12 |
| Residual | 18 | 0.044 | ||
| Iron | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 506,271 | 30.72 | 1.58 × 10−6 |
| Time | 2 | 1,826,597 | 110.82 | 7.61 × 10−11 |
| Site × Time | 4 | 370,494 | 22.48 | 8.49 × 10−7 |
| Residual | 18 | 16,482 | ||
| Manganese | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 10.3 | 1.842 | 0.187 |
| Time | 2 | 532.6 | 94.827 | 2.76 × 10−10 |
| Site × Time | 4 | 74.8 | 13.325 | 3.22 × 10−5 |
| Residual | 18 | 5.6 | ||
| Molybdenum | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 0.4627 | 30.94 | 1.50 × 10−6 |
| Time | 2 | 0.2523 | 16.87 | 7.46 × 10−5 |
| Site × Time | 4 | 0.1861 | 12.45 | 5.00 × 10−5 |
| Residual | 18 | 0.0150 | ||
| Sodium | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 7,023,981 | 0.288 | 0.753 |
| Time | 2 | 55,996,204 | 2.300 | 0.129 |
| Site × Time | 4 | 24,518,148 | 1.007 | 0.430 |
| Residual | 18 | 24,348,796 | ||
| Zinc | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 186.12 | 73.16 | 2.27 × 10−9 |
| Time | 2 | 55.09 | 21.65 | 1.62 × 10−5 |
| Site × Time | 4 | 7.73 | 3.04 | 0.0445 |
| Residual | 18 | 2.54 | ||
| Conductivity | ||||
| Source of Variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 110,464 | 4.566 | 0.0249 |
| Time | 2 | 12,958 | 0.536 | 0.5943 |
| Site × Time | 4 | 7735 | 0.320 | 0.8610 |
| Residual | 18 | 24,192 | ||
| pH | ||||
| Source of variation | Df | Mean Squares | F-value | p-value |
| Site | 2 | 0.09367 | 25.342 | 5.83 × 10−6 |
| Time | 2 | 0.19167 | 51.855 | 3.38 × 10−8 |
| Site × Time | 4 | 0.02349 | 6.354 | 0.00227 |
| Residual | 18 | 0.00370 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, P.; Evensen, T.; Kinnby, A. Seasonal Variation in the Element Composition of Dried, Powdered Green Sea Urchin (Strongylocentrotus droebachiensis) from Northern Norway. Sustainability 2024, 16, 6727. https://doi.org/10.3390/su16166727
James P, Evensen T, Kinnby A. Seasonal Variation in the Element Composition of Dried, Powdered Green Sea Urchin (Strongylocentrotus droebachiensis) from Northern Norway. Sustainability. 2024; 16(16):6727. https://doi.org/10.3390/su16166727
Chicago/Turabian StyleJames, Philip, Tor Evensen, and Alexandra Kinnby. 2024. "Seasonal Variation in the Element Composition of Dried, Powdered Green Sea Urchin (Strongylocentrotus droebachiensis) from Northern Norway" Sustainability 16, no. 16: 6727. https://doi.org/10.3390/su16166727
APA StyleJames, P., Evensen, T., & Kinnby, A. (2024). Seasonal Variation in the Element Composition of Dried, Powdered Green Sea Urchin (Strongylocentrotus droebachiensis) from Northern Norway. Sustainability, 16(16), 6727. https://doi.org/10.3390/su16166727






