Abstract
This article provides an overview of the diverse applications of hydrogels in nutrient recovery from water and wastewater. Due to their unique properties, such as high water-retention capacity, nutrient rerelease, and tunable porosity, hydrogels have emerged as promising materials for efficient nutrient capture and recycling. It has been suggested that hydrogels, depending on their composition, can be reused in agriculture, especially in drought-prone areas. Further research paths have been identified that could expand their application in these regions. However, the main focus of the article is to highlight the current gaps in understanding how hydrogels bind nitrogen and phosphorus compounds. The study underscores the need for research that specifically examines how different components of hydrogel matrices interact with each other and with recovered nutrients. Furthermore, it is essential to assess how various nutrient-recovery parameters, such as temperature, pH, and heavy metal content, interact with each other and with specific matrix compositions. This type of research is crucial for enhancing both the recovery efficiency and selectivity of these hydrogels, which are critical for advancing nutrient-recovery technologies and agricultural applications. A comprehensive research approach involves using structured research methodologies and optimization techniques to streamline studies and identify crucial relationships.
1. Introduction
Rapid population growth drives an escalating demand for food, with projections suggesting that, by 2050, 70% of the global population will reside in urban areas, pushing the total population to 10 billion individuals [1]. To meet the needs of this expanding populace, food production must surge by 70%, with agriculture, already the most significant water consumer at 70%, facing heightened pressure. These demographic shifts pose substantial challenges to the global agricultural system, exacerbating issues such as soil overexploitation and nutrient depletion due to intensified cultivation practices.
Fertilization is a common practice in intensive agriculture to provide plants with nutrients such as the macronutrients (N, P, and K) and micronutrients (Cu, Zn, Mn, and B) necessary for proper growth [2]. The most commonly used are fast-acting fertilizers, which are expensive to produce and require non-renewable resources, such as phosphorus deposits, which are being depleted [3]. Fertilization partially replenishes the deficiencies of these elements in the soil; however, the migration of the elements in the soil layers and their leaching or erosion cause a large part of them to be lost and enter the surface water or be emitted into the atmosphere. Nevertheless, the most serious negative effects are those associated with excessive levels of nutrients. Such excesses can lead to harmful algal blooms that disrupt the balance of aquatic ecosystems, threatening aquatic life and drinking water quality. In addition, excess nutrients can lead to water oxygen deficiency, which negatively affects aquatic organisms and leads to habitat degradation [4]. In the context of global environmental challenges, including environmental degradation, it is necessary to take measures to reduce the adverse effects of intensive agriculture. Developing sustainable agricultural practices that consider soil conservation and the sustainable use of natural resources is becoming necessary.
Nutrients can be recovered from renewable resources, such as waste, wastewater, and surface runoff. The use of technology to recover nitrogen and phosphorus from water and wastewater can contribute to sustainable nutrient management, minimizing the excess of these substances in the aquatic environment. In addition, recovery of these nutrients can provide an alternative source of fertilizer for agriculture, reducing the need for chemical fertilizers. In the long term, nutrient recycling can contribute to protecting the environment, increasing agricultural production efficiency and ensuring food security. It is, therefore, an essential strategy for achieving sustainable development and preserving a healthy environment for future generations.
The recovery of nutrients from waste, wastewater, and water is possible through various physical and chemical separation methods. The available techniques must enable the effective separation of phosphorus and nitrogen from water and wastewater with the possibility of reusing these components. Hydrogel materials offer such opportunities, which, thanks to their properties, are an interesting alternative to conventional methods. Recent review papers in the field of hydrogels for nutrient recovery have provided concise insights into the utilization of innovative materials and methodologies for environmental sustainability. Jayakumar et al. (2020) underscored the significance of integrating health and environmental research, emphasizing the challenges and innovative solutions in material development for human health and environmental monitoring [5]. Khan and colleagues (2016) highlighted the superior performance of hydrogels in pollutant adsorption and recovery, stressing the importance of controlled synthesis and surface chemistry for efficient pollutant removal and reuse [6]. Additionally, Wujcicki and Kluczka (2023) focused on the potential of hybrid chitosan sorbents for removing orthophosphate(V) from natural waters, stressing the importance of phosphorus recovery for sustainable resource management [7]. Furthermore, Hu et al. (2023) discussed the increasing interest in using hydrogel materials for phosphate removal and recovery, emphasizing the need for further research to ensure long-term sustainability and mitigate potential environmental impacts [8]. These reviews collectively provide valuable insights and directions for advancing nutrient-recovery technologies using hydrogel materials, contributing to developing environmentally friendly solutions for wastewater treatment and resource conservation.
Hydrogels have become promising materials for efficient nutrient capture and recycling due to their unique properties, including a high water-holding capacity and tunable porosity. This article discusses various methods using hydrogels to remove nutrients, such as nitrogen and phosphorus, from different sources, i.e., water and wastewater. In addition, recent developments in the development of new hydrogel-based systems for nutrient recovery are included, including their potential difficulties and future directions in the field.
The proposed review aims to explore the potential of biodegradable hydrogels in nutrient-recovery applications. The authors will mainly focus on the key issue of recovering nitrogen (N) and phosphorus (P) from aquatic environments using hydrogel materials, synthesizing the latest developments in the field. Overall, this review will highlight the important role of hydrogels in sustainable water management practices by promoting nutrient recycling and minimizing environmental pollution.
Our review will shed light on innovative strategies for nutrient reclamation, focusing on the sustainable utilization of hydrogel materials to mitigate the escalating pressures of population growth and agricultural intensification on water resources.
2. Materials and Methods
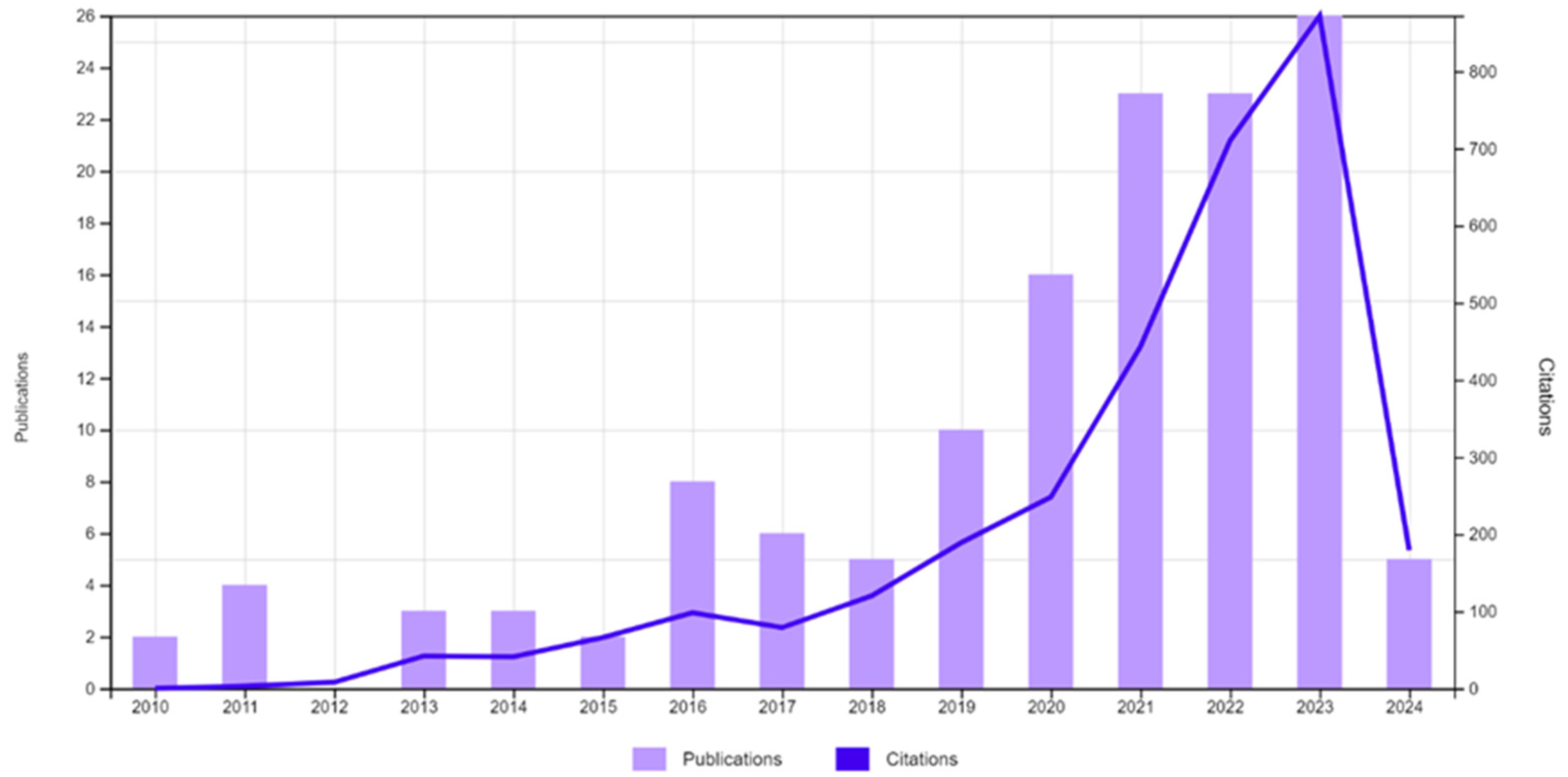
A bibliometric analysis on nitrogen and phosphorus removal/recovery from water bodies and wastewater was conducted concerning the relevant literature from the Web of Science database. The literature search encompassed a variety of keywords, such as recovery, remediation, nutrients, phosphorus, phosphate, nitrogen, nitrate, ammonia, hydrogel, water, and wastewater, in various combinations. The results suggest that the discussed topic is current and attracts considerable interest, as the chart illustrates the emergence of papers and citations (Figure 1). The timeframe was unrestricted due to the topic’s novelty and the recent surge in research activity. The literature predominantly consisted of articles, totaling 149 papers collected.
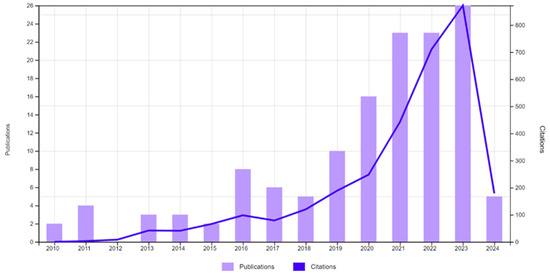
Figure 1.
Emergence of publications and citations indicating the topic’s current relevance and interest (access date 5 April 2024).
3. Results and Discussion
3.1. Nutrient Composition in Water and Wastewater—Implications for Resource Recovery
Phosphorus (P) and nitrogen (N) are among the most essential macronutrients, playing a significant role in plant and animal development. P affects cellular processes, such as photosynthesis and respiration, and participates in nucleic acid synthesis. Moreover, it is involved in regulating many enzymes [9]. This macronutrient plays a role in mitigating abiotic stresses, like salinity, heat, acid stresses, and heavy metals [10]. P is also crucial for plant growth and development by stimulating seeds, roots, stem growth, and flower formation and germination. Furthermore, its availability enhances the nitrogen-fixing ability of leguminous plants [11]. N is one of the elements of amino acids, nucleic acids, proteins, chlorophyll, and secondary metabolites [12,13]. Large amounts of N are contained in the atmosphere but not in the form usable for plants. Only leguminous plants have the unique ability to symbiose with rhizobial bacteria and convert N2 into plant-available forms [14]. Plants take up N by their roots in the forms of nitrate, ammonium, or organic nitrogen available in the soil. For optimal development and growth, all plants need a well-balanced N supply [12,13].
Despite the positives of N and P, we are dealing with an excess of these elements in the aquatic environment due to the significant increase in agricultural production in recent years. Their high levels in the NH4+-N and PO43− forms are present in industrial, as well as domestic and agricultural, wastewater, contributing to the eutrophication of water bodies [15,16]. Water’s eutrophication negatively impacts various sectors, including food security, ecosystem health, and the economy. It disrupts industries, such as tourism, fisheries, and the medical sector. Moreover, algal blooms create dead zones, leading to aquatic animals’ deaths and altering plant species diversity [17].
Due to significant N and P biological properties, which are essential for plant growth and development, and the negative impact of their excess on the environment, it is crucial to recover these elements from biowaste. Large amounts of these nutrients are found in wastewater sources [16].
It is estimated that around 380 billion m3 of wastewater are produced annually worldwide. It is expected to increase by 24% by 2030 and by 51% by 2050, which is over the current level, and it will reach about 574 billion m3. Among global regions, Asia is the largest producer of wastewater, with current annual estimates at 159 billion m3, accounting for 42% of the urban wastewater generated worldwide. By the end of 2030, Asia will produce approximately 44% of the world’s wastewater. Other regions with significant wastewater production include North America, generating 67 billion m3, and Europe, with 68 billion m3 annually. The average nitrogen concentration in urban wastewater is 43.7 mg/L, resulting in an estimated 16.6 teragrams (Tg) of N present in the wastewater produced globally each year. Similarly, with an average P concentration of 7.8 mg/L, the phosphorus content in wastewater is approximately 3.0 Tg annually. The current global demand for nitrogen in the agriculture sector is 115.5 Tg. Regarding phosphorus, the global demand for this nutrient is 43.8 Tg. Consequently, nitrogen in wastewater can cover 14.4% of the worldwide nitrogen-fertilizer demand, while phosphorus can contribute to 6.8% [18].
Due to the differences between natural water bodies and wastewater, the hydrogel composition must be adjusted to suit the specific conditions of each environment. One key difference lies in environmental factors; the hydrogels used in lakes or rivers need to withstand UV radiation, temperature fluctuations, and the challenges of operating in large volumes of water with high flow rates [19]. They must also possess mechanical strength and biodegradability to facilitate their removal after the recovery. In contrast, wastewater presents challenges, such as numerous chemical and biological pollutants, which can lower hydrogel reusability, prevent the possibility of utilizing it as fertilizer applications, or accelerate their degradation. Therefore, it is crucial to ensure that hydrogels are selective, avoiding the accumulation of heavy metals and preventing microbial fouling. To address these challenges, staged purification processes using hydrogels are used, initially adsorbing harmful substances and, subsequently, selectively recovering nutrients. In natural water bodies, where nitrogen and phosphorus compounds are typically the primary concern due to eutrophication risks, the concentration of other pollutants is generally lower compared to wastewater. Furthermore, hydrogel compositions must be biocompatible in natural environments to minimize the disruption to aquatic ecosystems, whereas in wastewater treatment, the focus is on cost-effectiveness and practical scalability within controlled environments [20]. Therefore, determining the operational environment is crucial for adjusting hydrogel composition, efficacy, and selectivity accordingly. Economic aspects are crucial in the application of hydrogels for water purification and nutrient recovery. Recovering nutrients could lead to the production of a substitute for commercial fertilizer, potentially lowering overall costs. This approach also benefits by mitigating environmental remediation expenses in eutrophic water bodies and sewage treatment, thereby enhancing water quality and the ecosystem. Hydrogel properties can be adjusted easily through composition adjustments or crosslinking methods, offering flexibility during installation design. Equipment development allows for modifying hydrogel composition, affecting matrix efficiency and selectivity. While initial installation costs may be manageable, significant investments are required for future modifications like changing crosslinking methods to adjust mechanical properties. Therefore, it is important to design the initial installation to expand, modify, and adapt it to the production of hydrogels with a wide range of applications. The scope of applications of hydrogels is strongly dependent on the type of pollutants present in a given wastewater and the operating parameters (temperature, pH, biological contamination, and UV radiation). Most hydrogel-application research is conducted on a laboratory scale, limiting information on the production costs on a bigger scale. For this reason, it is necessary to research the scaling of this type of production to estimate the potential costs in commercial use. Due to their biodegradability, ongoing production is required to replenish filters, particularly in nonreusable hydrogels. The industrial application of hydrogels remains novel, necessitating further research to assess costs accurately and consider scenarios where hydrogels produce secondary fertilizers versus not. In conclusion, the economic viability of hydrogel technology in industrial settings requires comprehensive exploration, balancing potential cost savings with production scalability and application versatility.
Recovering nutrients, such as N and P, from water and wastewater is key to sustainable resource management. Through appropriate and innovative technologies, it is possible to reduce the environmental pollution of water bodies and utilize the recovered components to produce fertilizers, thereby decreasing the demand for primary resources. The available global data indicate that the recovery potential is huge, and its utilization can bring significant ecological and economic benefits.
3.2. Overview of Methods for Nutrient Recovery from Water and Wastewater
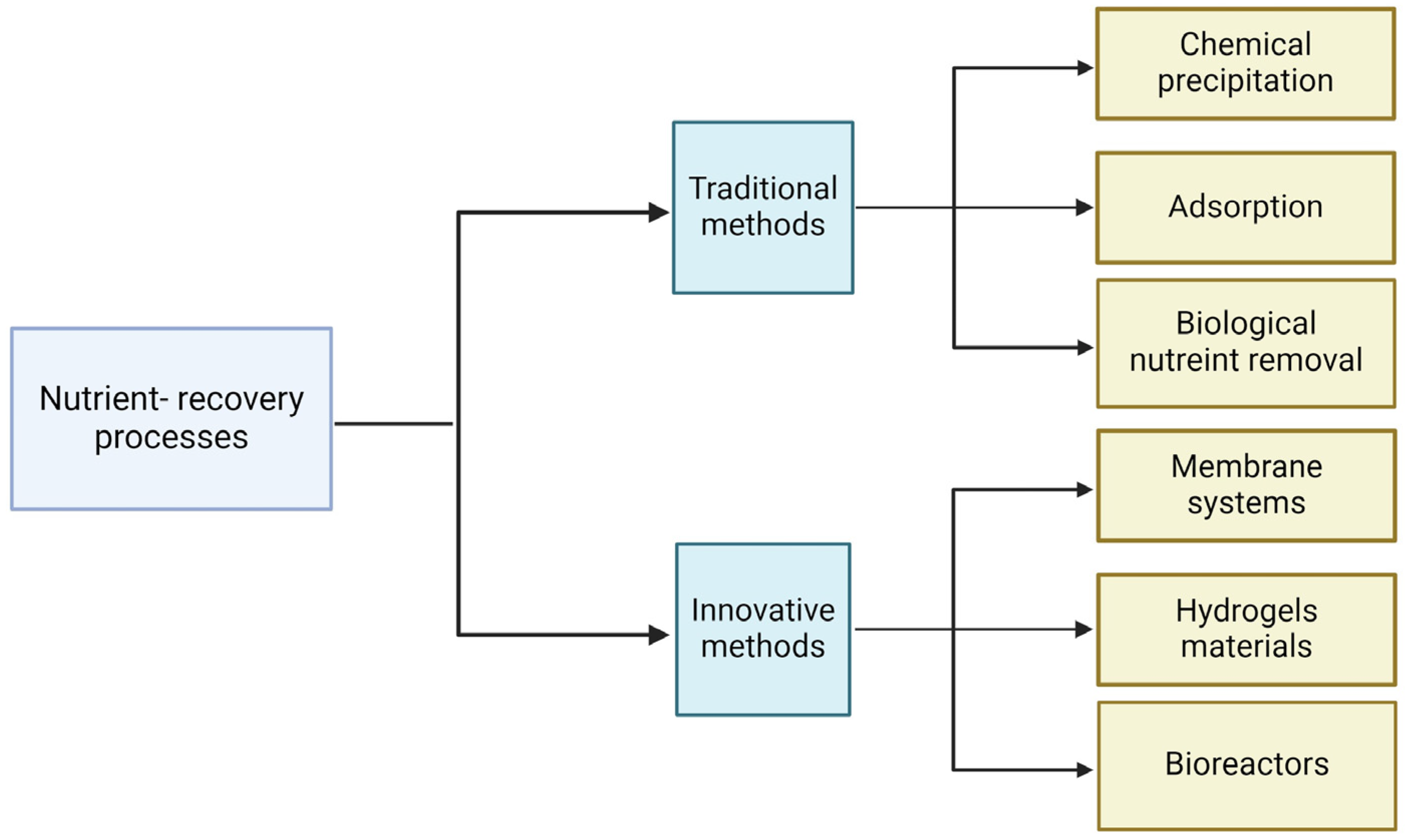
Currently, numerous technologies are designed to recover nutrients from wastewater and water. These can be divided into two groups: traditional and innovative methods. The first includes techniques such as chemical precipitation, adsorption, and biological nutrient removal with enhanced biological phosphorus removal. The innovative techniques include membrane systems, hydrogel materials, and bioreactors (Figure 2).
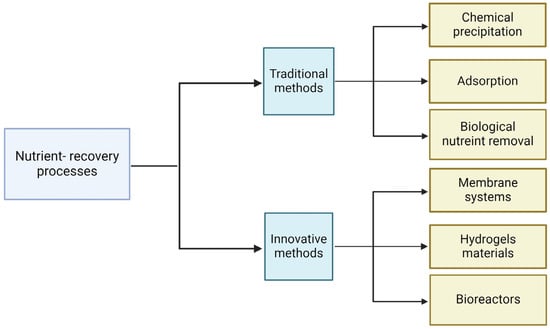
Figure 2.
Methods of nutrient recovery. Created with Biorender.com.
Chemical precipitation involves the recovery of ammonium and phosphates from wastewater as struvite (MgNH4PO4·6H2O), a white orthorhombic crystalline substance. Precipitation of struvite occurs when the combined concentrations of ammonium, magnesium, and phosphate exceed the solubility product in an alkaline environment [21]. The precipitation of struvite is shown in Equation (1) [22].
The recovery of phosphates requires fewer reagents than the recovery of ammonium, which makes it more sustainable. Precipitate is known for having only small amounts of impurities and can be used as a fertilizer in agriculture [21]. The main advantages of the chemical nutrient-recovery method are efficiency (effectively removes phosphorus and nitrogen from water), simplicity (easy to implement and control), and speed, as the process is relatively quick. However, it also has disadvantages, the most important of which are cost (it requires the use of chemical reagents, which can be expensive) and environmental impact (risk of secondary pollution after decomposition).
Adsorption can be an effective and selective removal method for nutrients combined with their recovery through desorption or directly using the adsorbent as a fertilizer. Synthesized metal oxides and hydroxides, layered double hydroxides, carbonate minerals, clay minerals, zeolites, porous silica, activated carbon, biochar, and polymers are the main adsorbents used for nutrient recovery. Bio-derived resources and industrial wastes, such as shells from eggs and clams, can serve as low-cost materials. These materials are advantageous due to their environmental friendliness, and they are often biodegradable, renewable, and have low toxicity [23]. Biochar is one of the better types of adsorbents due to the possibility of using them in recovery, recovering nitrogen and phosphorus from agricultural runoff, wastewater, and soil. Its porous structure efficiently adsorbs these nutrients, preventing their leaching and runoff into water bodies. This means that, after nutrient recovery, it can be reused in the form of soil amendments because they slowly release the adsorbed nutrients. In wastewater treatment, biochar effectively adsorbs pollutants, thereby improving water quality. The utilization of biochar for nutrient recovery can vary based on its production cycle and factors such as feedstock type and production, and parameters techniques can change its efficiency [24,25,26]. The main advantages of nutrient recovery by adsorption include specificity (adaptability to specific contaminants), regeneration (adsorbent materials can be regenerated and reused), and, of course, efficiency (high adsorption capacity for phosphorus and nitrogen). However, it also has drawbacks, including the high cost of producing advanced adsorbents, material consumption (limited durability of some adsorbents), and complexity (requires advanced technology to produce and regenerate materials).
Biological nutrient removal (BNR) relies mainly on the removal of N and P from water and wastewater. Enhanced biological phosphorus removal (EBPR) is a BNR option, where phosphates are incorporated into activated sludge with polyphosphorus-accumulating organisms (PAOs) playing a crucial role in process play. Under anaerobic conditions, with PAO assistance, cells release phosphate into the solution, leading to its accumulation in wastewater. Metal ions, like Mg2+ and K+, can also be concentrated in wastewater using the BNR method. Moreover, the produced biomass can be stored in surplus sludge form. However, this method is not perfect because of the presence of pathogens and heavy metals in sludge [16]. Nitrification and denitrification are the most commonly used methods for the biological removal of nitrogen. In nitrification, ammonium-oxidizing bacteria (AOB) convert ammonia to nitrite, and nitrite-oxidizing bacteria (NOB) convert nitrite to nitrate, while denitrifying bacteria carry out denitrification. Unfortunately, these processes require a high energy input [27]. The main advantages of biological nutrient recovery include naturalness (it uses natural microbial processes), sustainability (low need for chemicals), and multifunctionality (it can remove different pollutants at the same time). However, it also has disadvantages, including length of time (biological processes can be slower), sensitivity (requires careful control of environmental conditions), and operating costs (may require complex control and monitoring systems).
Membrane technologies, such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, are the most used. These methods produce fertilizers with a concentration of nutrients. Adding substances, such as surfactants or polymers soluble in water like chitosan, can increase membrane-separation efficiency. Problems with salt precipitation can be solved with pH regulations. Non-pressure-driven membrane processes include electrodialysis, and its advanced option is bipolar membrane electrodialysis, which enables the dissociation of water into hydrogen and hydroxide ions [28]. The main advantages of membrane as a nutrient-recovery method are efficiency (efficient separation and concentration of nutrients) and cleanliness (minimal secondary contamination). However, it also has disadvantages, including high operating costs due to electricity consumption, technical complexity (requires specialized equipment and maintenance), and limitations in large-scale applications.
A membrane photobioreactor (MPBR) combines the cultivation of microalgae and membrane technology to produce recycled water without pathogens and significant contamination. This method involves nutrient removal and recovery from pre-treated sewage from an anaerobic membrane bioreactor (AnMBR). BPR and AnMBR systems reduce the energy demand for wastewater treatment and the carbon footprint [29]. An anoxic–aerobic algal–bacterial photobioreactor (AA-ABPh) is another innovative method of removing nutrients from wastewater and can be connected with upgrading biogas because of nitrification and denitrification processes. AA-ABPh enables the recovery of not only P and N but also carbon. The experiment concluded with the enrichment of a unialgal culture, achieved through continuous biomass settling and recycling [30]. Membrane photobioreactors offer many advantages for nutrient recovery from water and wastewater, including high efficiency, production of valuable products, and sustainability. However, their implementation is associated with high costs, technological complexity, and some biological limitations and waste-management issues. However, the proper design and optimization of these systems can contribute to their effective and efficient use.
Electrochemical systems (BESs) are membrane-based bioreactors that utilize two electrodes—an anode and a cathode—and employ exoelectrogenic microorganisms to catalyze oxidation and reduction reactions. This technology allows nutrients and energy to carry recovery or production. Ion-exchange membranes are the most widely used to recover ammonium and phosphates, which can be reused as a fertilizer. BESs do not require the use of chemicals or an increase in the temperature of the process [31]. Electrochemical systems (ECS) for nutrient recovery from water and wastewater offer many advantages, including nitrogen- and phosphorus-recovery efficiencies, low energy consumption, and the potential to produce valuable products, such as bioelectricity and biogas. However, their implementation presents technological challenges, high capital and operating costs, and the need to address biological and engineering issues, such as membrane fouling. Despite these drawbacks, BESs have the potential to make a significant contribution to balanced water and wastewater management practices, especially with proper optimization and system management.
These methods have the potential to solve problems associated with water pollution. Proposing green, cheap, and effective methods is crucial for nutrient recovery. Sustainable water and wastewater management, technologies, and regulations are the main elements of the circular nutrient system. This system includes the technologies and practices that lead to nutrient recovery from wastewater streams and the reuse of them as fertilizers and energy resources. Recycling nutrients brings resource, societal, and environmental benefits. To enhance markets for these technologies, it is essential to align legislation with existing regulations [32]. By adopting innovative technologies, these systems can improve resource efficiency, protect the environment, and support sustainable agriculture. Integrating nutrient recovery into broader waste management and recycling strategies is essential for developing a sustainable and resilient water and wastewater management system.
3.3. Nutrient-Recovery Strategies Using Hydrogels
3.3.1. Hydrogel Types
Hydrogels are three-dimensional (3D) networks capable of absorbing and swelling large amounts of water due to the presence of hydrophilic groups, such as -NH2, -OH, -COOH, -CONH, -CONH2, and -SO3H, in their polymer networks and osmotic pressure [6,7,8,33,34,35]. These networks typically consist of crosslinked polymer chains [34,35,36,37,38,39,40]. Physical or chemical crosslinking of natural or synthetic polymer chains is used to design hydrogels [39,40,41,42]. Due to crosslinking, hydrogels do not dissolve in water and can maintain an unchanged 3D structure during swelling. The water the material absorbs is stored in the interstitial spaces between the crosslinked hydrogel polymer chains. When the swelling capacity of hydrogels is very high (e.g., above 100%), they are called superabsorbent (SH) hydrogels. Physical crosslinking involves temporary bonding through hydrogen bonding or hydrophobic or electrostatic interactions between polar groups. Chemical crosslinking, on the other hand, is a permanent bond formed by covalent bonds, enhanced by relatively stronger ionic interactions between the different functional groups of the introduced crosslinking agents [41]. Hydrogel properties, such as swelling rate, degradability, stiffness, and size, can be modified by changing the hydrophilic–hydrophobic ratio, initiator or polymer concentration, and reaction conditions (temperature, time, etc.) [35,41,42].
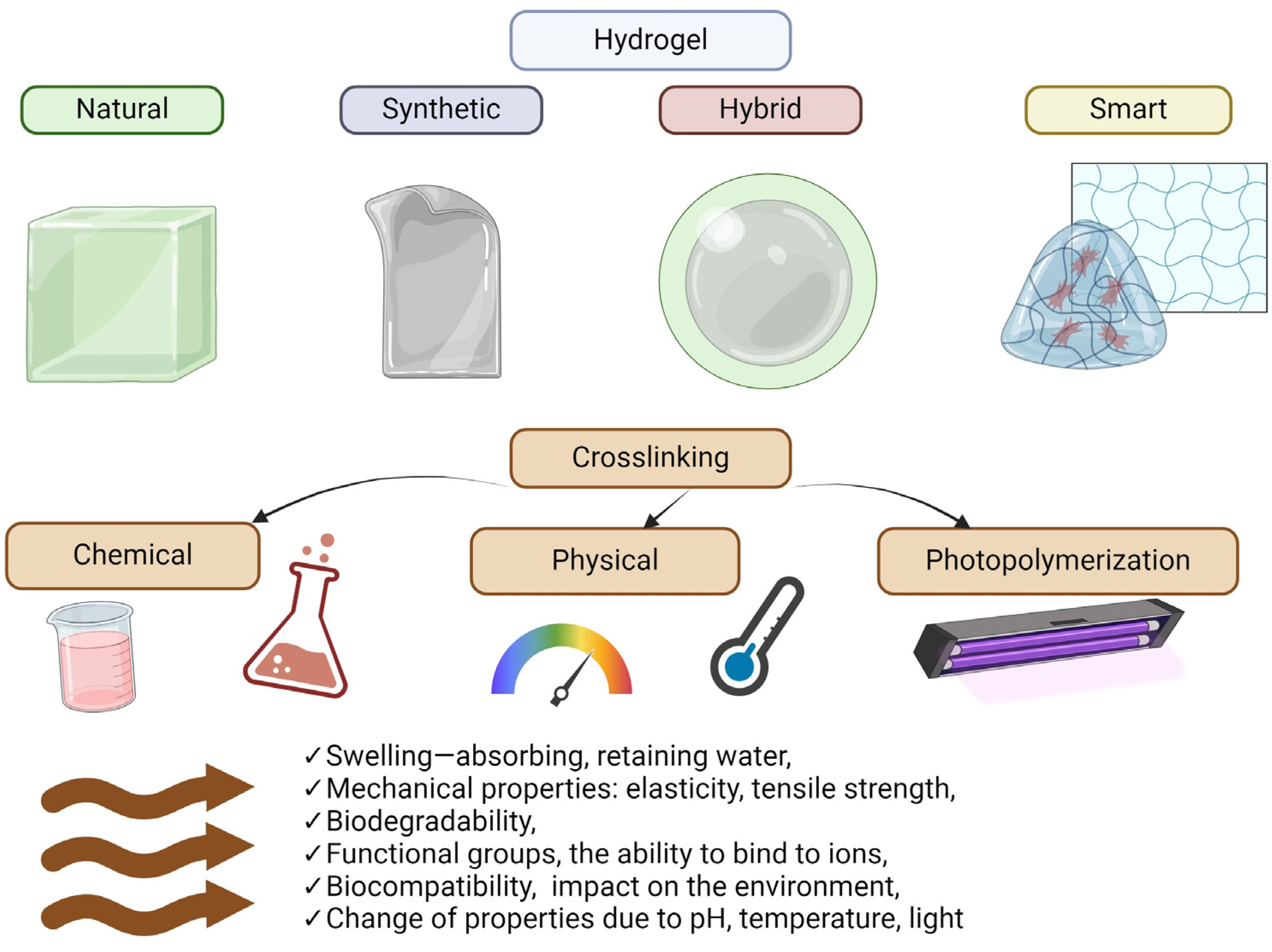
Hydrogels can be classified according to several criteria [38], such as material origin, polymer composition, configurations, crosslinking types, physical appearance, and ionic charges (Figure 3). Hydrogel sources can be classified as synthetic or biological materials [39]. Conversely, depending on the polymer source, these materials can be classified as natural, synthetic, or semi-synthetic. Synthetic polymers are derived from petroleum products [6,43], whereas biological hydrogels have natural sources, such as cellulose, starch, lignin, kenaf fiber, collagen, chitosan, agarose, alginate, hyaluronic acid, gelatine, and fibrin [8,33]. Natural hydrogels have many advantages, such as biocompatibility and biodegradability, making them attractive for various biomedical applications. However, their low stability and mechanical strength can be challenging, especially for applications requiring greater material durability. In addition, the fact that some of these materials can be allergenic in rare cases highlights the need to consider individual body reactions when using them. Synthetic hydrogels offer several advantages over natural hydrogels, including greater stability and mechanical strength. They are mainly composed of synthetic polymers manufactured by polymerizing suitable monomers. The polymers listed, such as PVA, PEG, PEO, PHEMA, PNIPAM, PAA, and PAAM, form the basis of synthetic hydrogels and offer varying degrees of biocompatibility. Some, such as PAAM, are known to be biocompatible, meaning they are less likely to cause immune reactions or other adverse effects when in the body. Due to their synthetic origin, synthetic hydrogels can be carefully designed to meet specific application requirements, allowing for control over their physical and chemical properties [6,33]. This design flexibility makes them attractive candidates for various biomedical applications, from passive drug carriers to implantable materials. Semisynthetic polymers are an exciting class of materials that combine the properties of both natural and synthetic polymers. They are obtained by chemical modification of natural polymers or by combining natural polymers with synthetic polymers, resulting in materials with unique properties. Examples of semi-synthetic polymers are GelMA (methacrylate-modified gelatine) and AcHyA (acrylate-modified hyaluronic acid). These chemical modifications make it possible to tailor the properties of natural polymers to specific application needs and to control their behaviors under different environmental conditions. In addition, combining natural polymers with synthetic polymers, such as fibrinogen conjugated with PEG or gelatine with albumin, opens the way to hydrogels with diverse physical and chemical properties. These materials retain the bioactivity of natural hydrogels and allow their properties to be regulated by manipulating different chemical parameters [7,39,40].
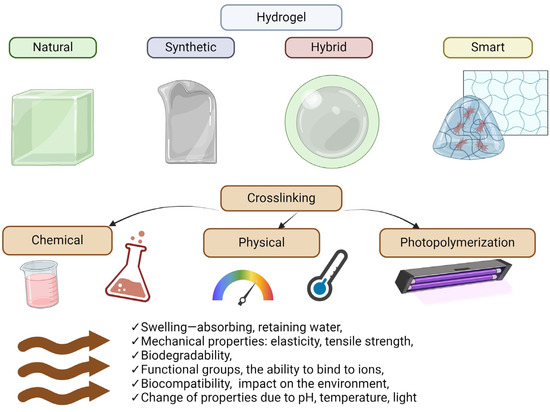
Figure 3.
Main types of hydrogels and their properties. Created with Biorender.com.
Recently, hydrogels have attracted attention in the fields of environmental engineering [44,45], wastewater treatment [7,46], and soft robotics [47]. With technological advances in hydrogel production, their application in an even wider range of fields can be expected. Studies in recent years [33,40,48] indicate that hydrogels are used in various fields, such as agriculture, biomaterials, the food industry, drug delivery, tissue engineering, and regenerative medicine. Significant research interest in hydrogels can be found in the literature [41,42] due to their potential in water and wastewater treatment. Hydrogels exhibit excellent performance in the adsorptive removal of a wide range of aqueous contaminants, including heavy metals, nutrients, and toxic dyes [8].
Hydrogels can be designed to have a large contact surface area and appropriate functional groups to enhance their ability to adsorb various components. Due to their structure, they can effectively retain and remove unwanted substances from water, making them a promising solution for environmental protection [40]. Their application in water treatment can range from industrial processes to domestic filtration systems, helping to improve drinking-water quality and protect water resources. Research into new hydrogels focuses on improving their efficiency, durability, and regenerability, which can increase their practical value and application on a broader scale [6,33,40] Specific functional groups, such as -OH, -NH2, -COOH, and -CONH2, in hydrogel networks are critical for enabling the adsorptive recovery of aqueous nutrients [49]. These groups are actively involved in chemical interactions with various contaminants, increasing the efficiency of the adsorption process. Contaminants can be adsorbed on the surface of the hydrogel and within its swollen three-dimensional network. Adsorption mechanisms can include hydrogen bonding, electrostatic interactions, and complexation with metal ions [6]. The high efficiency of hydrogels in capturing nutrients from water is due to their large contact surface area and ability to tailor their chemical structure to specific contaminants. The properties of the hydrogel can be fine-tuned by controlling the synthesis parameters, which include the type of reactor, reaction time, reaction temperature, type, and amount of components [41]. Specific hydrogels can be modified as required by copolymerizing more than one type of monomer or by post-modifying the polymerized products. High swelling capacity, low production cost, non-toxic nature, photostability, high adsorption coefficient, and swelling capacity are the most important characteristics of a material for removing contaminants from water [46]. Depending on the type of contaminant to be removed, specially functionalized hydrogels can be prepared. For example, the functional groups of polyacrylamide (PAAM) can be converted into different groups by appropriate chemical reactions, including sulfonate groups by a sulfomethylation reaction, amine groups by a Hofmann reaction, carboxylate groups by a hydrolysis reaction, and hydroxyl groups by a reaction with formaldehyde [42]. These modifications allow the properties of the hydrogel to be adapted to specific tasks, such as the adsorption of particular pollutants [8]. Some researchers [50] have also shown that hydrogels can be reused by the desorption of contaminants and recovering the adsorption capacity in subsequent treatment cycles. Hydrogels offer many advantages, including ease of separation and handling, large-scale applicability, and regeneration and reuse [46]. At present, attempts to use hydrogels for water treatment are mainly at the laboratory stage, and further research is needed to move towards large-scale industrial applications.
The recovery of nutrients from water and wastewater using hydrogels is a complex process that is influenced by many process factors. These include the properties of the hydrogel, its type (biodegradable and synthetic hydrogels have different adsorption properties), porosity (higher porosity increases the surface area that is available for nutrient adsorption), particle size and shape (affects the availability of adsorption sites), and hydrophilicity/hydrophobicity (affects the ability to bind to water and dissolved compounds) [6,8,33]. Also important is the environment from which the nutrients are recovered, e.g., what is the nutrient concentration (higher concentrations of nitrogen and phosphorus may increase the adsorption efficiency), the presence of other contaminants (may compete for adsorption sites on the hydrogel), and pH (affects the surface charge of the hydrogel and the degree of nutrient ionization) [6,7]. Operating conditions affect the kinetics of nutrient adsorption and desorption. These conditions include temperature, contact time (longer contact time can increase adsorption efficiency but can also lead to hydrogel saturation), and water/wastewater flow rate (flow rate can affect contact time and nutrient availability to the hydrogel). Environmental and technical aspects of the application of a particular hydrogel are also important. These include the availability and cost of materials, the environmental friendliness, biodegradability and environmental impact of the hydrogels used, the technical feasibility of implementation, and the scalability and ability to integrate with existing water and wastewater treatment systems.
Each of these factors can affect the efficiency and practicality of using hydrogels to recover nutrients, such as nitrogen and phosphorus, from water and wastewater. In practice, optimizing these factors is key to achieving the best recovery results.
The mechanism of nutrient recovery from water and wastewater using hydrogels is complex and depends on many factors. However, properly designed and optimized hydrogels can be a very effective tool for water management and the recovery of valuable nutrients. The recovery of nutrients from water and wastewater using hydrogels is primarily based on their ability to adsorb, retain, and release nutrients. This mechanism can be broken down into several key steps. The ability to adsorb nutrients (e.g., nitrogen and phosphorus) from water or wastewater is primarily based on attraction and binding to the hydrogel surface. There are three methods of adsorption, i.e., physical, chemical, and complexation [39]. Physical adsorption is, first, the weak interactions between nutrient molecules and the hydrogel surface through van der Waals forces. Second, however, are electrostatic interactions. When the hydrogel and the nutrients have opposite charges, they attract each other. Chemical adsorption is mainly related to the formation of chemical bonds between functional groups on the hydrogel surface and the nutrients [41,42]. For example, amino groups on a hydrogel can form bonds with phosphate ions.
Another type of mechanism for recovering nutrients from water and wastewater using hydrogels is diffusion [33,48]. After adsorption to the surface of a hydrogel, nutrients can diffuse into the hydrogel, increasing its adsorption capacity. On the other hand, the porosity and internal structure of the hydrogel affect the rate and efficiency of nutrient diffusion.
The hydrogel structure can retain nutrient molecules mechanically and by creating stable chemical and physical interactions [39]. The desorption and release of nutrients can be achieved by changing environmental conditions (e.g., pH, temperature) or by using specific chemical reactants. The advantage is that the rate and amount of nutrients released from the hydrogel can be controlled, which can be used in agriculture for fertilization [48]. The main factors influencing the effectiveness of the mechanism for recovering nutrients from water and wastewater using hydrogels include the chemical composition of the hydrogel (type and number of functional groups on the hydrogel surface), operating conditions (pH, temperature, contact time, and nutrient concentration), water and wastewater characteristics (presence of other contaminants that may compete for adsorption sites), and hydrogel regeneration (effectiveness and durability of hydrogel regeneration processes) [33,48]. Specific examples of hydrogel applications for nutrient recovery, such as nitrogen and phosphorus, are described in the following sections of the article.
3.3.2. Nitrogen Recovery with Hydrogels
Nitrogen is a fundamental macronutrient essential for plant development and is typically supplemented through synthetic fertilizers. The intensification of agriculture has led to an increased use of these fertilizers. This consequently results in an enlargement in the degree of environmental pollution. Ammonium (NH4+) and nitrate (NO3−) groups penetrate surface waters, causing eutrophication. It causes the development of organic matter in water reservoirs and the depletion of oxygen, which translates into the destruction of the ecosystem, and the production of toxins. Biological methods are used to purify water from nitrogen forms, and they involve bacteria to bind individual nitrogen forms. This is often an expensive process with a complex application and control system that is difficult to apply on a larger scale. Next are physicochemical methods involving changes in pH and temperature and the addition of precipitating chemical compounds, which translates into high energy costs and the emission of additional waste that requires further management. Reverse osmosis is used on a large scale and with high efficiency. Despite its high efficiency and degree of nitrogen removal, it requires a continuous cleaning of the membranes and high operating and investment costs. A much cheaper method with a wide range of applications is the adsorption process, e.g., activated carbon, which involves the adsorption of individual nitrogen groups on the material surface. Despite its high efficiency and simplicity of application, the method has drawbacks related to the need to regenerate the adsorbent used or replace it, and activated carbon is not very effective for the adsorption of inorganic nitrogen compounds.
Due to the growing problem with agricultural, industrial, and municipal waste, research began to intensify on the use of environmentally friendly methods with a broader range of modifiability to adapt to a given type of sewage. The study proposes the application of biodegradable hydrogels, which are polymer networks that can absorb water and, thus, absorb various forms of nitrogen. Table 1 summarizes the latest works examining the possibilities of using hydrogels to recover ammonium and nitrate groups. Most hydrogels can absorb more than one pollutant, and most often, the ability to bind nitrogen groups is associated with the absorption of phosphorus groups and heavy metals. Most of the work focused on conducting research in an artificial environment with a given value of a given form of nitrogen to examine the obtained hydrogel composite’s adsorption properties precisely. This approach allows for a thorough examination of the impact of individual components of the produced hydrogel on adsorption.
Most often, hydrogels with acrylic acid were applied (seven articles), based on publications [51] showing excellent properties for binding NH4+ groups. A low pH, <4, causes the -COO− groups to protonate to the -COOH group, reducing the possibility of combining positive NH4+ groups and the adsorption degree. An increase in pH causes the predominance of -COO− bonds and is associated with electrostatic interactions with the ammonium group. Other studies have indicated that pH levels are too high, which reduces the adsorption degree around pH 8–9 [51,52] The mentioned bonds with the -COO− group are weak, making the regeneration of the hydrogel and the removal of adsorbed ammonium groups possible. This property allows for its use in agriculture [53]. The binding of ammonium groups is also affected by the considerable quantity of hydrogen bonds, which exchange positive hydrogen ions with ammonium ions, increasing the adsorption capacity and making hydrogel matrices an ideal medium for binding ammonia. This tendency is facilitated by the presence of the following functional groups in the hydrogel structure: NH2, -COOH, CONH2, and OH [51]. It is worth noting that acrylic acid forms appear in each of the tested hydrogels for NH4+ adsorption, except for the hydrogel in work [54], where the acquired hydrogel-bound ammonium and nitrate groups simultaneously, with a high recovery rate of 63.83% and 86.93%, respectively. The 3.5% w/v sodium alginate crosslinked with a CaCl2 solution (4% w/v) with the addition of an axenic culture (Nitzschia palea) was used and compared with the control group using only hydrogel and only bacteria. It has been shown that encapsulation in a biopolymer matrix promotes the development of binding microorganisms and causes an increase in the removal of nitrogen groups compared to the control groups. Due to the use of microorganisms and the high tightness of the obtained structures, hydrogels cannot be reused. However, they are highly biodegradable and can be used in fertilizer applications.
The basis for ammonia removal is the forms of acrylic acid, where in publication [55], it was decided to apply the addition of natural rubber to produce a superabsorbent with high mechanical properties to simultaneously remove ammonia and increase its water absorption and retention properties for soil applications. The acquired component demonstrated sufficient ammonia absorption at 13 mmol/g. After 30 min, the equilibrium state was achieved. The composition used mainly improved the mechanical properties of the composite and water absorption and retention, making it an ideal water reservoir for application in areas at risk of drought. Additionally, it is a biodegradable material due to the appropriate composition, which degrades by 16% only after 21 days. Research indicates the potential for modifying the structure with natural materials to produce more comprehensive solutions for environmental remediation. The addition of chitosan and biochar implanted in a hydrogel based on the acrylic acid variety was also tested in application as a soil conditioner. The principal assumption of the research was to investigate whether the hydrogel would reduce ammonia losses during flow. The efficiency recovery of 90% was proven with a capacity of 149.25 mg/g. The retained ammonia can be quickly released depending on demand. This is possible due to the weak bonds between ammonium groups and the -COO− group [53].
Some studies examined the behavior of the acquired hydrogels in uncontrolled ammonia-recovery environments, where publication [19] revealed that the presence of organic compounds may contribute to a slight reduction in the recovery rate. The research was carried out by comparing the usage of hydrogels in synthetic and domestic wastewater with similar content of ammonium groups and using different amounts of hydrogels. However, in work [20], the differences in nitrogen recovery were significant and reached approximately 50%, even though the same amount of hydrogel was applied, and the ammonia content in the compared waste was comparable. What caused differences in recovery depending on a given waste medium has not been established. Yet, in this publication, a detailed determination of the influence of pH, temperature, and ammonia concentration on the recovery rate was conducted, confirming the conclusions from previous works. In this study, nanomagnetic particles of Fe3O4 were utilized, which, according to the research results, indicate an increase in hydrogels’ water adsorption, retention, and life-expectancy properties. According to the research, after six recovery cycles after regeneration, it still has 43% of its starting properties.
There are fewer articles on NO3− recovery, although other hydrogel compositions have been applied to their recovery. It is related to a different mechanism of binding of nitrate groups, where the charge of the ion is negative, thus binding to positive groups, such as amino and hydroxyl groups, and ammonium ions to negative groups, such as carboxyl and sulfonic groups. For this reason, obtaining structures with the recovery properties of both groups is quite difficult, but as previously shown in the publication [54], it is not impossible. This requires the use of microorganisms, thus providing comprehensive solutions that combine several approaches to waste disposal. In another work, it is suggested to utilize a combination of chitosan and ethylene glycol, where it was presumed that the presence of -OH and -NH groups in the hydrogel structure will allow for effective adsorption. According to the research, electrostatic interactions between the specified functional groups were observed, resulting in a high adsorption rate. It was noted that a low pH of around three led to a more significant adsorption of protons, which increased the formation of electrostatic bonds. Due to the nature of the exchange, it was decided to investigate the use of polyamine polymetric hydrogel [56], which, due to the high density of amino groups that are easily modified, makes them ideal for binding NO3− groups. The recovery of nitrate groups was 70%, along with the recovery of 99% PO43− and 95% SO42−. Due to the opposite binding mechanisms of the ammonium and nitrate groups, research into creating hydrogels with a dual bond containing both carboxyl and amine groups, which will allow for the simultaneous recovery of NH4+ and NO3− groups, could be the solution.

Table 1.
Hydrogel application in nitrogen recovery.
Table 1.
Hydrogel application in nitrogen recovery.
| Nutrient | Source (Water, Wastewater, Industrial, etc.) [mg/L] | Hydrogel Type | Process Parameters (T, pH, t) | Sorption Models | Sorption Efficiency [mg/L] | Reusability | References |
|---|---|---|---|---|---|---|---|
| NH4+ | model solution 18–1800 | Superabsorbent with natural rubber-graft–poly (acrylic acid-co-acrylamide) network and linear poly (diallyldimethyl ammonium chloride) | t—30 min | Adsorption kinetic and isotherms described by the pseudo-second-order kinetic and Langmuir isotherm model | 234 | Biodegradable for soil applications, disintegration approximately 16% after 21 days | [55] |
| NH4+ | Prepared solution 0–500 | Poly(acrylic acid)-grafted chitosan and biochar composite | T—25 °C, t–20 min | Langmuir model, pseudosecond-order model. | 149.25 (90%) | Fertilizer application due to the possibility of redistribution of adsorbed nutrients | [53] |
| NH4+ | Domestic and synthetic wastewater | Tailored poly(acrylic acid)-based hydrogels | Hydrogel loadings 2.5, 5.0, 7.5 g/L, t—10 min | - | 8.3–10.1 (53–77%) | - | [19] |
| NH4+ | Drainage water, main drainage water, fish pool water 6.70, 5.39, 6.56, respectively (sugarcane field waste) | Acrylic acid polymer hydrogel, nano Fe3O4 | pH 2–9.86, T 15–95 °C | - | Based on the medium type: 89.16%, 32.50%, 31.11% (40 mg of hydrogel applied) | The recovery after the 6th hydrogel recovery cycle using NaCl is still 43% of the starting value. | [20] |
| NO3−, NH4+ | Aquaculture water | Ca-alginate beads (gelation method) with Nitzschia palea (bacteria) | - | - | Nitrate removal: 86.93%, ammonia 63.83% | Biodegradable with microorganisms for single use | [54] |
| NH4+ | Prepared solution 900 | Sodium alginate-grafted poly(acrylic acid)/graphene oxide | t—1 h, T—25 °C, | Adsorption kinetic and isotherm described by the pseudo-second-order and Freundlich model | 118.8 | After adsorption, the hydrogel can be used as a fertilizer with a slow release of nutrients effect | [57] |
| NO3− | - | Chitosan–ethylene glycol hydrogel | pH 3–11, T 20–40 °C | Isotherms of nitrate followed the Langmuir model | 49.04 | - | [58] |
| NH4+ | Prepared solution 1–2000 | Poly (acrylic acid) hydrogel | t—30 min, T—10–50 °C, flow 0.5 mL/min | All models have been utilized to describe adsorption: Langmuir, Freundlich, Redlich–Peterson | 110.6–120.8 | After use in the bioreactor, still 81.1% of adsorption properties, regeneration per 10 adsorption | [59] |
| NH4+ | Prepared solution 10–100 | Hydrogel composite with chitosan, acrylic acid, acrylamide, | t—30 min (equilibrium state), t—4 h, t—5–120 min (adsorption kinetic), T—25 °C, pH 3–10 | Langmuir isotherm model, the adsorption kinetics described by the pseudo-second-order model | 40.2 | After adsorption, the hydrogel can be used as a fertilizer with a slow release of nutrients effect | [51] |
| NO3− | Industrial wastewater | Magnetic hydrogel | t—5 min | - | 81.7% | Capacity 188 mg/g, use for 60 cycles adsorption–desorption | [50] |
| NH4+ | Prepared solution 100 | Feather protein-grafted poly(potassium acrylate)/polyvinyl alcohol semi-interpenetrating polymer networks | pH 2–12, T—20–40 °C, t—30 min–4 h | Kinetic analysis the pseudo-second-order model, adsorption isotherms of hydrogel described by the Freundlich model | 287.68 | After adsorption, the hydrogel can be used as a fertilizer with a slow release of nutrients effect | [52] |
3.3.3. Phosphorus Recovery with Hydrogels
Phosphorus is an essential element. It plays a key role in human health and development [60]. However, its widespread use in agriculture and industry, often with low productivity, results in excess phosphorus entering the environment through wastewater discharges [8]. Therefore, the effective recovery of phosphates from water and wastewater and their reuse as a nutrient appears to be crucial for sustainable development [60,61].
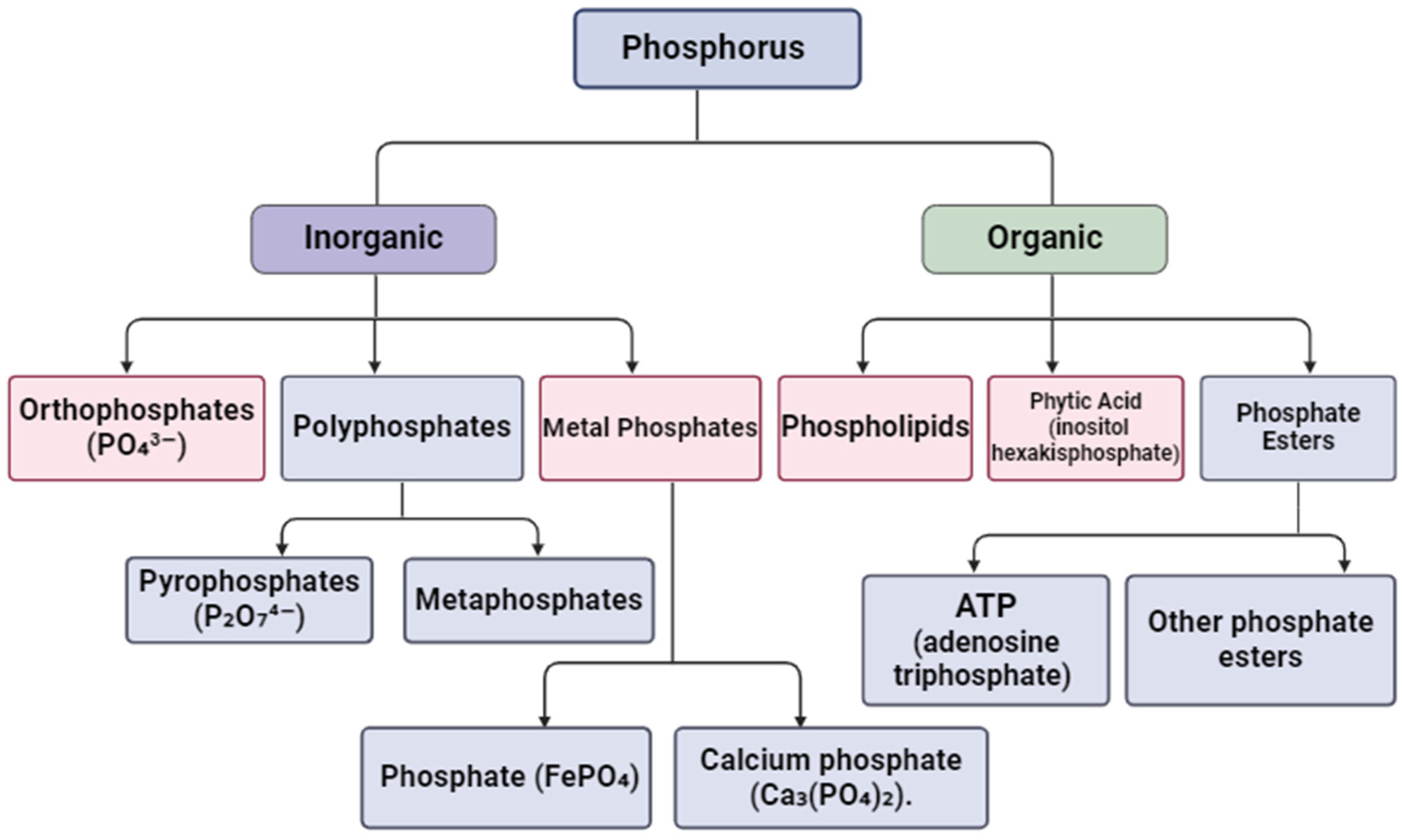
In the aquatic environment, forms of phosphorus exist in different chemical states and compounds. Figure 4 shows the main types of phosphorus that can be trapped and removed.
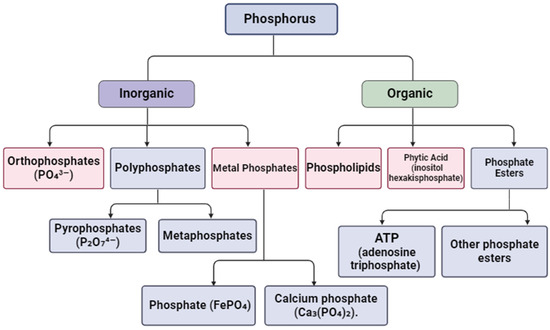
Figure 4.
Main types of phosphorus that hydrogels can bind. Created with Biorender.com.
The most common form of phosphorus in water and wastewater is inorganic phosphorus, i.e., ortho-phosphate (PO43−). Orthophosphates are commonly found in fertilizers and industrial waste [62]. Then, there are polyphosphates, which contain more than one phosphate group, such as pyrophosphates (P2O₇4−) and metaphosphates. These can be used in various industries, such as detergents and fertilizers. There are also metal phosphates. These are metal complex phosphates, such as ferric phosphate (FePO4) and calcium phosphate (Ca3(PO4)2). These can be present in surface water and wastewater due to chemical reactions. Another equally common form is organic phosphorus, which can include phospholipids, which are components of the cell membranes of living organisms that can break down and release organic phosphorus into water. This category also contains the phytic acid (inositol hexakisphosphate) found in plants, and it can be released into the aquatic environment [63,64]. Organic phosphate esters, i.e., compounds such as ATP (adenosine triphosphate) and other phosphate esters, may also be present in biological wastewater.
Due to the high interest in phosphorus removal from aquatic environments, several methods have been developed to remove phosphorus from water and wastewater. Hydrogels can effectively capture various types of inorganic and organic phosphorus from water and wastewater [62]. Applying chemical, physical, and biological modifications and optimizing environmental conditions can significantly improve the efficiency of phosphorus removal. This is key to preventing ecological problems, such as eutrophication, and ensuring the sustainable use of water resources.
The main mechanisms of phosphorus capture include physical adsorption, where phosphates can be adsorbed on the hydrogel surface by van der Waals forces and other weak interactions [64]. Another method is chemical adsorption, in which chemical reactions between phosphates and functional groups in hydrogels can lead to stronger phosphate binding [63,64]. Complexation with metals is where metal-modified hydrogels (e.g., Fe3⁺, Al3⁺) can form complexes with phosphates to enhance their uptake [65,66]. Also, there is ionic binding, where hydrogels containing amine, carboxyl, or hydroxyl groups can bind phosphates ionically, depending on pH and other environmental conditions.
These technologies are expensive. As a result, research using physicochemical methods has increased dramatically in recent years [7,67]. Cheaper natural adsorbents based on biopolymers, such as cellulose [68,69], alginate [70,71], starch [72,73] chitosan [74,75] etc., have attracted considerable research interest. Chitosan exhibits strong phosphate-adsorption capability through its amino groups, which is particularly effective under acidic pH conditions. Calcium alginate, on the other hand, utilizes calcium ions (Ca2⁺) for phosphate adsorption via complexation. Introducing metal oxide nanoparticles like Fe2O3 and Al2O3 as hydrogel additives enhances the overall adsorption efficiency in these systems.
Based on the latest literature [7,74,76,77,78,79,80] it has been shown that various forms of chitosan are among the most commonly used hydrogels for the removal of phosphate(V) (PO43−) from water and wastewater (Table 2). Its availability is high, as it is mainly produced by the chemical deacetylation of chitin using parts of mussels. The adsorption capacity and phosphorus removal rate are the parameters by which the applicability of modified chitosan sorbents should be compared. Among other biopolymers, chitosan is characterized by its ease in forming different morphological structures such as membranes, fibers, hydrogels, nanoparticles, and microspheres. Other valuable properties of chitosan are its biodegradability and bioavailability. Chitosan is a polycationic polymer. The presence of a positively charged amine group in its structure (pKa = 6.5) increases its solubility in acidic and neutral solutions. This is mainly due to the structure of the polymer [74,81,82], more specifically, its molecular weight and degree of acetylation, pH, temperature, polymer crystallinity or form (powder, granule, flake, membrane, etc.), and the presence of functional hydroxyl and amino groups, which can be easily modified by grafting and crosslinking. The most common forms for introducing chitin hydrogels into water or wastewater include dry or wet hydrogel beads, i.e., uncrosslinked and crosslinked, modified with metal ions or metal oxides, filled with carbon or biocarbon, zeolite and mineral composite beads, and magnetic beads [7]. One method of producing chitosan beads is the coagulation process.

Table 2.
Application of chitosan hydrogels in phosphorus recovery.
In addition to the bead form mentioned above, it can also be used as nanofibres, granules, flakes, nanoparticles, sponges, and bioflocculants [99,104,107,108,109]. Unmodified chitosan in the form of hydrogel granules (CSH) has been described by Jóźwiak and co-authors [52,75,101] in an equimolar mixture of P-PO4, N-NO2, and N-NO3 for the removal of anions from aqueous solutions. The adsorption capacity of orthophosphate by CSH was 15.72 mg/g at pH 4 after 60 min. Incorporating metal ions into chitosan hydrogels enhances their phosphate-adsorption capacity. The metals significantly increasing phosphate adsorption are multivalent metals such as La3+, Al3+, Zr4+, and Ce3+. Modifying these metals increases phosphate’s adsorption capacity above 100 mg/g [85,86]. Karthikeyan and co-workers [79] studied the removal of phosphate from aqueous solutions using hybrid chitosan and alginate beads containing metal (Fe3+) (Fe-CS-Alg). Modifications of chitosan hydrogel beads use less valuable metals, i.e., Zn2+, Cu2+, or Ca2+. However, sorbents formed from these cations generally do not have high adsorption capacities compared to sorbents modified with metals with higher positive charges.
Starch hydrogels are widely used as sorbents for removing phosphate (PO43−) from water and wastewater due to their biodegradability, availability, and ability to be chemically modified to improve adsorption properties [110,111]. Starch hydrogels, especially chemically modified ones, are highly capable of adsorbing phosphate from water and wastewater [43,112,113,114] Modifications with compounds such as chitosan, metal oxides, magnetic nanoparticles, or functional groups can significantly improve their adsorption properties.
Starch hydrogels modified with chitosan are an example of a starch hydrogel. Chitosan contains amino groups that can form phosphate complexes by chemical adsorption. Researchers [115,116,117] have shown that starch–chitosan composite hydrogels have a higher phosphate-adsorption capacity than pure starch or chitosan. Under optimal pH and temperature conditions, these hydrogels can effectively remove phosphates from aqueous solutions. Another example is starch hydrogels modified with alumina (Al2O3), which can form complexes with phosphates due to the presence of active hydroxyl groups on its surface. Alumina-modified starch increases the number of available adsorption sites and the chemical stability of the hydrogel. Composite starch-Al2O3 hydrogels show a significantly higher phosphate-adsorption capacity than unmodified ones. They are effective over a wide pH range, making them versatile sorbents for various applications. Starch hydrogels modified with magnetic nanoparticles (Fe3O4) are also popular [116]. Magnetic nanoparticles can be functionalized to increase the adsorption capacity of phosphates. Starch modified with Fe3O4 nanoparticles forms a composite that can be easily recovered from a solution using a magnetic field. Starch-Fe3O4 hydrogels show good phosphate-adsorption capacity and can be easily recovered from solution [116,118]. They are effective even at low phosphate concentrations, making them suitable for water treatment. Another group of starch hydrogels are those modified with carboxyl groups. Carboxyl groups can bind phosphates by ion exchange and hydrogen bonding. The modification of starch with carboxyl groups increases its ability to bind phosphate ions [116,119,120]. Starch–carboxyl hydrogels exhibit high adsorption capacity and fast phosphate-binding kinetics. They are effective over a wide range of phosphate concentrations and can be used in applications ranging from wastewater treatment to surface water treatment. Cerium oxide (CeO2)-modified hydrogels can adsorb phosphate due to active surface sites [116]. Modification of starch with cerium oxide produces a composite with high stability and adsorption capacity. Starch-CeO2 hydrogels exhibit high phosphate-adsorption capacity and chemical stability. They are effective over a wide pH range and can be used to treat industrial water and wastewater.
Alginate hydrogels are widely used in phosphorus-adsorption processes from water due to their properties such as biocompatibility, biodegradability, and ability to form stable complexes with various metal ions [121,122,123]. Alginate hydrogels, especially chemically modified ones, are effective sorbents for phosphate removal from water. Various modifications, such as the addition of metal ions, metal oxides, magnetic nanoparticles, or chitosan, can significantly improve the adsorption properties of these hydrogels. Several examples of using alginate hydrogels for phosphorus adsorption are discussed below, with a description of the mechanisms of action and experimental results. One example is an alginate hydrogel modified with calcium ions. Calcium ions can form ionic bridges with the carboxyl groups of the alginate, resulting in crosslinking that increases the stability of the hydrogel. In addition, calcium ions can react with phosphates to form insoluble calcium phosphates. Researchers [123] have shown that alginate hydrogels modified with calcium ions have a high phosphate-adsorption capacity. The efficiency of phosphate adsorption by these hydrogels is high, especially at neutral and slightly acidic pH. Another example is an alginate hydrogel modified with calcium ions [122,123]. Calcium ions can form ionic bridges with the carboxyl groups of the alginate, creating crosslinks that increase the stability of the hydrogel. Calcium ions can also react with phosphates to form insoluble calcium phosphates. Scientific studies [123] have shown that alginate hydrogels modified with calcium ions have a high phosphate-adsorption capacity. The efficiency of phosphate adsorption by these hydrogels is high, especially at neutral and slightly acidic pH. Hydrogels modified with magnetic nanoparticles and iron oxide have also been used [124].
The binding mechanisms of nutrients such as orthophosphates (PO43−), which are the most abundant in water and wastewater, are crucial to understanding adsorption processes and optimizing systems for phosphate removal from water and wastewater [41,46,64]. The kinetics of phosphate fixation depend on many chemical, physical, and biological factors. The kinetics of phosphate binding by hydrogel sorbents depends on a complex interaction of many factors, including pH, temperature, phosphate concentration, sorbent surface properties, and the presence of competing ions [46,63]. Understanding these mechanisms and factors is critical to optimizing adsorption processes and developing more effective sorbents for phosphate removal from water and wastewater. The ionization of functional groups on the sorbent surface and the speciation of phosphates in a solution are affected by pH [60]. At an alkaline pH, phosphates are more ionized, which can increase adsorption on positively charged surfaces. An increase in temperature usually increases the rate of chemical reactions and adsorption but can also lead to desorption at higher temperatures. Higher phosphate concentrations in a solution increase the drive for adsorption, which can lead to faster adsorption equilibrium. The specific surface area of the sorbent influences the kinetics of phosphate binding. A larger specific surface area increases the availability of adsorption sites, resulting in faster adsorption. Other anions (e.g., NO3, SO42−) can compete with phosphates for adsorption sites, reducing phosphate-adsorption efficiency [7,125,126]. The sorbent’s porous structure and nanostructured features can increase the availability of adsorption sites and the diffusion of phosphates into the pores.
Various innovative approaches can be used to improve phosphorus-uptake efficiency, such as the chemical modification of hydrogels [127,128]. Functionalizing the surface by adding functional groups, such as amine, carboxyl, thiol, or hydroxyl groups to increase the phosphate-binding capacity [33,46,63,64,92], can effectively improve phosphorus uptake. Another example is metal complexation [49,74,98,113,118,120,121] which involves the introduction of metal ions (e.g., Fe3⁺, Al3⁺, Ca2⁺) that can form stable complexes with phosphates. Physical modification of hydrogels to increase porosity can also contribute to more efficient phosphorus uptake, as confirmed in their study [129]. Electrospinning or cryogenic drying [130,131] can refine the hydrogel surface by creating nanopores or micropores. Incorporating nanoparticles, such as carbon nanotubes, graphene oxide, or metal nanoparticles [67,132], can improve adsorption sites and chemical interactions, known as nanostructural refinement. The biological modification of hydrogels primarily entails incorporating enzymes that enhance the availability of organic phosphorus compounds for adsorption [133,134]. For example, phosphatases can be immobilized in hydrogels to catalyze the degradation of complex phosphates into simpler forms. One method involves microbial immobilization [133,135], where microorganisms are incorporated to sequester phosphorus and convert it into biopolymers that hydrogels can more effectively bind. The optimization of adsorption and release conditions can also be used to achieve a more efficient uptake of phosphorus from the aquatic environment [136,137,138]. One method involves pH control, which optimizes conditions for phosphate adsorption. Chitosan, for instance, exhibits enhanced phosphate-adsorption efficiency under slightly acidic or neutral pH, where its amine groups are protonated. This approach also manages competing ions like chlorides and sulfates that may vie with phosphates for adsorption sites. Another strategy is employing dynamic release systems, where hydrogels are designed to release captured phosphates gradually. Such systems find application in slow-release fertilizers. Additionally, reactive systems can be developed, utilizing hydrogels that respond to environmental stimuli, such as pH, temperature, or light. These systems enable controlled adsorption and release processes tailored to changing environmental conditions.
3.4. Sustainable Hydrogels for Nutrient Recovery
To achieve sustainable nutrient management, it is essential to recover nutrients from sorption materials for reuse as raw materials in other processes. Creating an appropriate environment facilitates the release of ingredients from the matrix into the environment. Hydrogels can be reused to sorb ingredients several times (Table 3). It is important to allow the maximum concentration of ingredients to be recovered. The study demonstrated the feasibility of concentrating NH4+-N from wastewater using a small volume of recirculated eluent in a PAA hydrogel-filled column, offering valuable insights for future NH4+-N recovery. Varying the HCl concentration from 0.1 to 1 mol/L significantly enhanced NH4+-N desorption, reducing the required bed volume by 57%. Optimal desorption was achieved with 1 mol/L HCl, allowing effective reuse through multiple cycles and reaching a cumulative enrichment factor of ~10 [59]

Table 3.
Desorption of nutrients from loaded hydrogels.
Nutrient-loaded hydrogels are also excellent for agricultural applications. With controlled release, hydrogels minimize the loss of fertilizers through leaching and volatilization, increasing their efficiency in this sector. Hydrogels improve soil structure by increasing porosity and airiness, promoting the root system’s development. In particular, hydrogels based on biodegradable polymers provide an interesting solution, promoting precise nutrient-delivery and water-retention capabilities [148]. Their gradual biodegradation offers a slower release rate and enriches the soil with carbon compounds from the decomposition of biopolymers. Desorption and release of P and N allows them to be recovered and reused for plant growth but carries the risk of re-release with changing concentrations (e.g., due to varying rainfall strength). Some researchers recommend collecting sorbents after storms to reduce the excessive release of nutrients into the soil [149].
Hydrogels composed of an extracellular polymer of sodium alginate and humic acids, crosslinked with iron (III) ions, were designed, showing high efficiency in sorbing phosphate ions from model wastewater and actual piggery wastewater. In addition, the sorbents were quite large and porous, which improved the separation process after the sorption process. The authors calculated that the cost of treating a ton of wastewater with the help of this sorbent is only USD 25–80, and the obtained enriched sorbent can be used as a fertilizer in the sustainable management of P [149] The chitosan-montmorillonite-Fe nanocomposite adsorbent effectively adsorbed phosphate from solutions at different pHs and maintained its adsorption capacity in the presence of various anions, proving its applicability in recovering phosphate from wastewater. It released bioavailable phosphorus with controlled swelling, providing a potential slow-release fertilizer [141] A new hydrogel material derived from waste chicken feather protein (feather protein-grafted poly(potassium acrylate)/polyvinyl alcohol (FP-g-PKA/PVA) semi-IPN) effectively removed ammonium nitrogen and phosphate from agricultural water over a wide pH range, with the highest efficiencies obtained for both ions at pH 6–8. The release of nutrients from the enriched hydrogel and the ability to regenerate the sorbent were investigated, confirming the effectiveness of the release of nutrients [52].
Most of the work envisages potential agricultural applications of enriched hydrogels based on the potential to release N and P from matrices [150]. There are few studies on plants on the effectiveness of hydrogel fertilizers containing nutrients. Alginate matrices crosslinked with Fe(III) ions provided a sorbent for phosphate ions from sedimentary water and then tested their effect on the growth of Italian ryegrass (Lolium perenne) in pot tests. The elemental composition of the soil and plants after fertilization was also similar. Results comparable to the commercial fertilizer were obtained, confirming the potential for the agricultural application of enriched hydrogels [151]. A study by Skrzypczak et al. (2022) designed a multilayer hydrogel capsule for the controlled release of nutrients (including N and P) as an innovative fertilizer. The capsule consisted of a layer of sodium alginate with immobilized micronutrients (Cu, Mn, Zn) on eggshells, crosslinked with NPK solution, and an additional layer of biopolymers (0.79% alginate, 0.24% carboxymethylcellulose, and 8.07% starch). Studies have shown that the hydrogel capsules effectively release nutrients and increase the length of cucumber roots by 20% compared to commercial fertilizer [152].
3.5. Environmental Implications and Future Directions
Hydrogels are an innovative and environmentally friendly solution to growing amounts of wastewater with high and low levels of nitrogen and phosphorus. Recent studies have revealed that, depending on the composition, the properties of the hydrogel related to its mechanical strength, biodegradability, and tendency to bind individual waste compounds may change. This makes clear recommendations for a water treatment sector that is quite complex and requires further research, primarily related to the optimization process and the impact of other inorganic and organic compounds in the treated water. Most scientific research focuses on examining the influence of parameters such as pH, temperature, and ion concentration in the form of uncorrelated relationships with each other, causing problems with understanding the entire process. The obtained tests without correlation allow for the indication of the range of parameters in which hydrogels work satisfactorily, but without the correlation of individual parameters, using, e.g., the response surface method, it is impossible to determine whether more advanced dependencies occur. This is especially visible when looking at changes in the efficiency of hydrogels and strength depending on the hydrogel composition. The research was not conducted in a way that would allow for a precise determination of the differences between the hydrogel matrix and the hydrogel matrix with additives, which makes it difficult to estimate the nature of the occurring bonds, the processes, and the impact of the additive on improving the use of a given hydrogel. It is crucial for future research because it allows for efficiently excluding a given compound from the proposed hydrogel composition depending on the needs. It is suggested that research be conducted with a broader range of control groups compared to reconstituted hydrogels from the literature or control groups with the main matrix component. It will allow for a better understanding of their impact on the recovery and hydrogel parameters.
The tests showed a high hydrogel biodegradability, indicating that they can be utilized practically without any worries in an environment where the influence of the composition has a significant impact on this parameter. This state of affairs allows for the creation of composites with a reduced rate of biodegradability, which means that, after adsorption, they can be used as a soil amendment or fertilizer. This is the adequate application of hydrogels because it allows them to utilize their properties to store and release water, increasing the scope of application in areas at drought risk. However, this solution requires more detailed research on the adsorption of harmful organic compounds and heavy metals and their selectivity by the obtained hydrogels. The selectivity of structures is strongly dependent on the hydrogel composition. Understanding the mechanism of binding individual matrices and their additives with waste and nutrients is crucial when hydrogels are utilized for further fertilizer applications.
4. Conclusions
Hydrogels offer a promising and environmentally friendly solution for managing wastewater and promoting sustainable agriculture. Their composition influences their effectiveness in binding nitrogen and phosphorus compounds, which affects their mechanical strength, biodegradability, and selectivity. Despite extensive research, a comprehensive understanding of hydrogel performance is hindered by non-complex studies. Future research should focus on optimizing hydrogel compositions and investigate the impact of operating parameters and their interactions to enhance recovery efficiency. Additionally, distinguishing the effects of hydrogel matrices with and without additives is crucial for improving performance and understanding binding mechanisms.
High biodegradability suggests hydrogels can be safely used as soil amendments or fertilizers, particularly in drought-prone areas, due to their water storage and rerelease properties. Nonetheless, further investigations are required to refine their selectivity due to the adsorption of harmful compounds. Hydrogels also demonstrate the potential for sustainable nutrient management in agriculture by adsorbing, concentrating, and gradually releasing nutrients like phosphorus and nitrogen, with some studies indicating a performance comparable or superior to commercial fertilizers. Extensive field tests on various plant species are necessary to validate hydrogel efficacy compared to traditional fertilizers. Also, developing cost-effective and scalable production methods will be crucial for widespread adoption in sustainable agriculture.
Author Contributions
Conceptualization, A.W.-K. and D.S.; investigation, D.S., P.W., B.A. and A.W.-K.; writing—original draft preparation, D.S., P.W., B.A. and A.W.-K.; writing—review and editing, D.S., P.W., B.A. and A.W.-K.; supervision, A.W.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pathmudi, V.R.; Khatri, N.; Kumar, S.; Abdul-Qawy, A.S.H.; Vyas, A.K. A Systematic Review of IoT Technologies and Their Constituents for Smart and Sustainable Agriculture Applications. Sci. Afr. 2023, 19, e01577. [Google Scholar] [CrossRef]
- Quddus, M.A.; Anwar, M.B.; Alam, M.K.; Ahmed, R.; Sarker, K.K.; Islam, M.A.; Islam, M.T.; Kobeasy, M.I.; Gaber, A.; Ahmed, S. Modification of Nutrient Requirements for a Four Crop-Based Cropping System to Increase System Productivity, Maintain Soil Fertility, and Achieve Sustainable Intensification. Sustainability 2022, 14, 7194. [Google Scholar] [CrossRef]
- Ruffatto, K.; Emaminejad, S.A.; Juneja, A.; Kurambhatti, C.; Margenot, A.; Singh, V.; Cusick, R.D. Mapping the National Phosphorus Recovery Potential from Centralized Wastewater and Corn Ethanol Infrastructure. Environ. Sci. Technol. 2022, 56, 8691–8701. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; Taifouris, M.; Martín, M. Addressing the Contribution of Agricultural Systems to the Phosphorus Pollution Challenge: A Multi-Dimensional Perspective. Front. Chem. Eng. 2022, 4, 970707. [Google Scholar] [CrossRef]
- Jayakumar, A.; Jose, V.K.; Lee, J.M. Hydrogels for Medical and Environmental Applications. Small Methods 2020, 4, 1900735. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M.C. A Holistic Review of Hydrogel Applications in the Adsorptive Removal of Aqueous Pollutants: Recent Progress, Challenges, and Perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Wujcicki, Ł.; Kluczka, J. Recovery of Phosphate(V) Ions from Water and Wastewater Using Chitosan-Based Sorbents Modified—A Literature Review. Int. J. Mol. Sci. 2023, 24, 12060. [Google Scholar] [CrossRef]
- Hu, H.; Tong, Y.W.; He, Y. Current Insight into Enhanced Strategies and Interaction Mechanisms of Hydrogel Materials for Phosphate Removal and Recovery from Wastewater. Sci. Total Environ. 2023, 892, 164514. [Google Scholar] [CrossRef]
- Lambers, H. Annual Review of Plant Biology Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An Overview of Important Enzymes Involved in Nitrogen Assimilation of Plants. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; Volume 2057, pp. 1–13. [Google Scholar]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- diCenzo, G.C.; Tesi, M.; Pfau, T.; Mengoni, A.; Fondi, M. Genome-Scale Metabolic Reconstruction of the Symbiosis between a Leguminous Plant and a Nitrogen-Fixing Bacterium. Nat. Commun. 2020, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pal, P. Assessing the Feasibility of N and P Recovery by Struvite Precipitation from Nutrient-Rich Wastewater: A Review. Environ. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, J.; Liang, S. Nutrient Recovery from Wastewater: From Technology to Economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Ngatia, L.; Grace, J.M., III; Moriasi, D.; Taylor, R. Nitrogen and Phosphorus Eutrophication in Marine Ecosystems. In Monitoring of Marine Pollution; IntechOpen: London, UK, 2019. [Google Scholar]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Cruz, H.; Laycock, B.; Strounina, E.; Seviour, T.; Oehmen, A.; Pikaar, I. Modified Poly(Acrylic Acid)-Based Hydrogels for Enhanced Mainstream Removal of Ammonium from Domestic Wastewater. Environ. Sci. Technol. 2020, 54, 9573–9583. [Google Scholar] [CrossRef] [PubMed]
- Fazilati, A.; Mokhtarian, N.; Latifi, A.M.; Fazilati, M. Synthesis of Acrylic Acid Polymer Hydrogel Nano Fe3O4 to Remove Ammonia from Sugarcane Field Waste. Adv. Mater. Sci. Eng. 2020, 2020, 9204523. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Hasan, M.N.; Altaf, M.M.; Khan, N.A.; Khan, A.H.; Khan, A.A.; Ahmed, S.; Kumar, P.S.; Naushad, M.; Rajapaksha, A.U.; Iqbal, J.; et al. Recent Technologies for Nutrient Removal and Recovery from Wastewaters: A Review. Chemosphere 2021, 277, 130328. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and Prospects of Different Adsorbents for Phosphorus Uptake and Recovery from Water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Li, X.; Nan, H.; Jiang, H.; Wang, H.; Wang, C. Research Trends on Phosphorus Removal from Wastewater: A Review and Bibliometric Analysis from 2000 to 2022. J. Water Process Eng. 2023, 55, 104201. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus Adsorption by Functionalized Biochar: A Review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Zhang, X.; Show, P.L. Research Advances on Production and Application of Algal Biochar in Environmental Remediation. Environ. Pollut. 2024, 348, 123860. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.; Straka, L. New Directions in Biological Nitrogen Removal and Recovery from Wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring Technological Alternatives of Nutrient Recovery from Digestate as a Secondary Resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Viruela, A.; Robles, Á.; Durán, F.; Ruano, M.V.; Barat, R.; Ferrer, J.; Seco, A. Performance of an Outdoor Membrane Photobioreactor for Resource Recovery from Anaerobically Treated Sewage. J. Clean. Prod. 2018, 178, 665–674. [Google Scholar] [CrossRef]
- García, D.; Alcántara, C.; Blanco, S.; Pérez, R.; Bolado, S.; Muñoz, R. Enhanced Carbon, Nitrogen and Phosphorus Removal from Domestic Wastewater in a Novel Anoxic-Aerobic Photobioreactor Coupled with Biogas Upgrading. Chem. Eng. J. 2017, 313, 424–434. [Google Scholar] [CrossRef]
- Cerrillo, M.; Riau, V.; Bonmatí, A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes 2023, 13, 186. [Google Scholar] [CrossRef]
- Rosemarin, A.; Macura, B.; Carolus, J.; Barquet, K.; Ek, F.; Järnberg, L.; Lorick, D.; Johannesdottir, S.; Pedersen, S.M.; Koskiaho, J.; et al. Circular Nutrient Solutions for Agriculture and Wastewater—A Review of Technologies and Practices. Curr. Opin. Environ. Sustain. 2020, 45, 78–91. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Okamoto, M. Recent Progress on the Design and Applications of Polysaccharide-Based Graft Copolymer Hydrogels as Adsorbents for Wastewater Purification. Macromol. Mater. Eng. 2016, 301, 496–522. [Google Scholar] [CrossRef]
- Chen, J.; Park, K. Synthesis and Characterization of Superporous Hydrogel Composites. J. Control. Release 2000, 65, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R.; et al. Superabsorbent Hydrogels Based to Polyacrylamide/Cashew Tree Gum for the Controlled Release of Water and Plant Nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yap, J.X.; Leo, C.P.; Chang, C.K.; Show, P.-L. Polysaccharide Hydrogels for Controlling the Nutrient Release. Sep. Purif. Rev. 2023, 53, 276–288. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a Historical Perspective: From Simple Networks to Smart Materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally Friendly Hydrogel: A Review of Classification, Preparation and Application in Agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M.; Frazar, E.M.; Klaus, M.V.X.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment. Adv. Healthc. Mater. 2022, 11, e2101820. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Hydrogel Adsorbent in Industrial Wastewater Treatment and Ecological Environment Protection. Environ. Technol. Innov. 2020, 20, 101107. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.-S.; Bundschuh, J. Hydrogels: Novel Materials for Contaminant Removal in Water—A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1970–2014. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Piccoli, I.; Camarotto, C.; Squartini, A.; Longo, M.; Gross, S.; Maggini, M.; Cabrera, M.L.; Morari, F. Hydrogels for Agronomical Application: From Soil Characteristics to Crop Growth: A Review. Agron. Sustain. Dev. 2024, 44, 22. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of Toxic Metal Ions with Magnetic Hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yan, D.Y.S.; Khan, M.; Zhang, Z.; Lo, I.M.C. Application of Magnetic Hydrogel for Anionic Pollutants Removal from Wastewater with Adsorbent Regeneration and Reuse. J. Hazard. Toxic. Radioact. Waste 2017, 21, 04016008. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Mu, B.; Zong, L.; Wang, X.; Wang, A. One-Step Green Construction of Granular Composite Hydrogels for Ammonia Nitrogen Recovery from Wastewater for Crop Growth Promotion. Environ. Technol. Innov. 2024, 33, 103465. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Yue, Q.; Li, Q.; Gao, B. Adsorption of Ammonium and Phosphate by Feather Protein Based Semi-Interpenetrating Polymer Networks Hydrogel as a Controlled-Release Fertilizer. Environ. Technol. 2014, 35, 446–455. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Guan, Y. Excellent Adsorption-Desorption of Ammonium by a Poly(Acrylic Acid)-Grafted Chitosan and Biochar Composite for Sustainable Agricultural Development. ACS Sustain. Chem. Eng. 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, B.; Tiwari, A. Development of Diatom Entrapped Alginate Beads and Application of Immobilized Cells in Aquaculture. Environ. Technol. Innov. 2021, 23, 101736. [Google Scholar] [CrossRef]
- Cui, Y.; Xiang, Y.; Deng, Z.; Zhang, Z.; Li, L.; Wei, J.; Gui, W.; Xu, Y. Preparation of Natural Rubber Based Semi-IPNs Superabsorbent and Its Adsorption Behavior for Ammonium. Int. J. Biol. Macromol. 2021, 166, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kioussis, D.R.; Kofinas, P. Characterization of Anion Diffusion in Polymer Hydrogels Used for Wastewater Remediation. Polymer 2005, 46, 9342–9347. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Li, C.; Cheng, C.; Zheng, Y. Adsorption of Ammonium by Graphene Oxide-Based Composites Prepared by UV Irradiation and Using as Slow-Release Fertilizer. J. Polym. Environ. 2018, 26, 4311–4320. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Y.; Long, L.; Chen, K.; Hu, X.; Xue, Y. Biodegradable Chitosan-Ethylene Glycol Hydrogel Effectively Adsorbs Nitrate in Water. Environ. Sci. Pollut. Res. 2020, 27, 32762–32769. [Google Scholar] [CrossRef]
- He, M.; Ng, T.C.A.; Huang, S.; Xu, B.; Ng, H.Y. Ammonium Removal and Recovery from Effluent of AnMBR Treating Real Domestic Wastewater Using Polymeric Hydrogel. Sep. Purif. Technol. 2022, 296, 121376. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Kowalkowska, A.; Filipkowska, U.; Struk-Sokołowska, J.; Bolozan, L.; Gache, L.; Ilie, M. Recovery of Phosphorus as Soluble Phosphates from Aqueous Solutions Using Chitosan Hydrogel Sorbents. Sci. Rep. 2021, 11, 16766. [Google Scholar] [CrossRef]
- Tan, A.X.; Michalski, E.; Ilavsky, J.; Jun, Y.-S. Engineering Calcium-Bearing Mineral/Hydrogel Composites for Effective Phosphate Recovery. ACS ES T Eng. 2021, 1, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Sirajo, L.; Zaini, M.A.A. Existing and Emerging Technologies for the Removal of Orthophosphate from Wastewater by Agricultural Waste Adsorbents: A Review. Biomass Convers. Biorefinery 2023, 13, 12349–12365. [Google Scholar] [CrossRef]
- Ilia, G. Phosphorus Containing Hydrogels. Polym. Adv. Technol. 2009, 20, 707–722. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kong, H.; Wang, J.; Zhang, G.; Shen, F.; Liu, F.; Huang, Z. Lanthanum-calcium Bimetallic-Modified Attapulgite- Chitosan Hydrogel Beads for Efficient Phosphate Removal from Water: Performance Evaluation, Mechanistic and Life Cycle Assessment. Carbohydr. Polym. 2024, 338, 122183. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, Y.; Li, Z.; Wang, X.; Duan, C.; Zhao, W.; Xiong, C.; Nie, S.; Xu, Y.; Ni, Y. A Multifunctional Self-Crosslinked Chitosan/Cationic Guar Gum Composite Hydrogel and Its Versatile Uses in Phosphate-Containing Water Treatment and Energy Storage. Carbohydr. Polym. 2020, 244, 116472. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Gelatin Hydrogel Nanocomposites for Water Purification and Beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef]
- Hasan, M.N.; Shenashen, M.A.; Hasan, M.M.; Znad, H.; Awual, M.R. Assessing of Cesium Removal from Wastewater Using Functionalized Wood Cellulosic Adsorbent. Chemosphere 2021, 270, 128668. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Deng, L.; Li, K.; Lu, H.; Wang, R.; Li, W. Adsorption of Malachite Green in Aqueous Solution Using Sugarcane Bagasse-Barium Carbonate Composite. Colloid. Interface Sci. Commun. 2021, 44, 100485. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Jiang, W.; Yang, P. Study on the Efficacy of Sodium Alginate Gel Particles Immobilized Microorganism SBBR for Wastewater Treatment. J. Environ. Chem. Eng. 2022, 10, 107134. [Google Scholar] [CrossRef]
- Kube, M.; Fan, L.; Roddick, F. Alginate-Immobilised Algal Wastewater Treatment Enhanced by Species Selection. Algal Res. 2021, 54, 102219. [Google Scholar] [CrossRef]
- Munjur, H.M.; Hasan, M.N.; Awual, M.R.; Islam, M.M.; Shenashen, M.A.; Iqbal, J. Biodegradable Natural Carbohydrate Polymeric Sustainable Adsorbents for Efficient Toxic Dye Removal from Wastewater. J. Mol. Liq. 2020, 319, 114356. [Google Scholar] [CrossRef]
- Grover, A.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Vikrant, K.; Kim, K.-H.; Brown, R.J.C. Magnesium/Aluminum Layered Double Hydroxides Intercalated with Starch for Effective Adsorptive Removal of Anionic Dyes. J. Hazard. Mater. 2022, 424, 127454. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into Recent Advances of Chitosan-Based Adsorbents for Sustainable Removal of Heavy Metals and Anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for Wastewater Treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Thagira Banu, H.; Karthikeyan, P.; Meenakshi, S. Lanthanum (III) Encapsulated Chitosan-Montmorillonite Composite for the Adsorptive Removal of Phosphate Ions from Aqueous Solution. Int. J. Biol. Macromol. 2018, 112, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.; Meenakshi, S. In-Situ Fabrication of Cerium Incorporated Chitosan-β-Cyclodextrin Microspheres as an Effective Adsorbent for Toxic Anions Removal. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100272. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, H.; Rong, H. Development of Polyaminated Chitosan-Zirconium(IV) Complex Bead Adsorbent for Highly Efficient Removal and Recovery of Phosphorus in Aqueous Solutions. Int. J. Biol. Macromol. 2020, 164, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.; Banu, H.A.T.; Meenakshi, S. Synthesis and Characterization of Metal Loaded Chitosan-Alginate Biopolymeric Hybrid Beads for the Efficient Removal of Phosphate and Nitrate Ions from Aqueous Solution. Int. J. Biol. Macromol. 2019, 130, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Nthumbi, R.M.; Catherine Ngila, J.; Moodley, B.; Kindness, A.; Petrik, L. Application of Chitosan/Polyacrylamide Nanofibres for Removal of Chromate and Phosphate in Water. Phys. Chem. Earth Parts A/B/C 2012, 50–52, 243–251. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can Fungi Compete with Marine Sources for Chitosan Production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Liu, Y.; Zhang, T. Review on Preparation and Adsorption Properties of Chitosan and Chitosan Composites. Polym. Bull. 2022, 79, 2633–2665. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.; Han, Q.; Lou, S.; Zhang, L.; Zhang, W. Fast and Efficient Phosphate Removal on Lanthanum-Chitosan Composite Synthesized by Controlling the Amount of Cross-Linking Agent. Int. J. Biol. Macromol. 2020, 157, 247–258. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Mielcarek, A. Sorption of Nutrients (Orthophosphate, Nitrate III and V) in an Equimolar Mixture of P–PO4, N–NO2 and N–NO3 Using Chitosan. Arab. J. Chem. 2019, 12, 4104–4117. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Ding, J.; Zhang, Z.; Gao, C.; Halimi, M.; Demey, H.; Yang, Z.; Yang, W. High Phosphate Removal Using La(OH)3 Loaded Chitosan Based Composites and Mechanistic Study. J. Environ. Sci. 2021, 106, 105–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Shen, W.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Function Integrated Chitosan-Based Beads with throughout Sorption Sites and Inherent Diffusion Network for Efficient Phosphate Removal. Carbohydr. Polym. 2020, 230, 115639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zhang, Z.; Chen, Z. Removal of Phosphate from Aqueous Solution by Chitosan Coated and Lanthanum Loaded Biochar Derived from Urban Dewatered Sewage Sludge: Adsorption Mechanism and Application to Lab-Scale Columns. Water Sci. Technol. 2021, 84, 3891–3906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L. Removal of Phosphate Anions Using the Modified Chitosan Beads: Adsorption Kinetic, Isotherm and Mechanism Studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J. Facile Synthesis of Zr(IV)-Crosslinked Carboxymethyl Cellulose/Carboxymethyl Chitosan Hydrogel Using PEG as Pore-Forming Agent for Enhanced Phosphate Removal. Int. J. Biol. Macromol. 2021, 176, 558–566. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Q.; Wang, J.; Huang, R. Phosphate Removal by Ce(III)-impregnated Crosslinked Chitosan Complex from Aqueous Solutions. Polym. Eng. Sci. 2017, 57, 44–51. [Google Scholar] [CrossRef]
- Ma, P.; Ding, W.; Yuan, J.; Yi, L.; Zhang, H. Total Recycle Strategy of Phosphorus Recovery from Wastewater Using Granule Chitosan Inlaid with γ-AlOOH. Environ. Res. 2020, 184, 109309. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, A.; Meenakshi, S. Effective Utilization of the Functional Groups in Chitosan by Loading Zn(II) for the Removal of Nitrate and Phosphate. Desalination Water Treat. 2014, 54, 1674–1683. [Google Scholar] [CrossRef]
- An, B.; Jung, K.-Y.; Lee, S.-H.; Lee, S.; Choi, J.-W. Effective Phosphate Removal from Synthesized Wastewater Using Copper–Chitosan Bead: Batch and Fixed-Bed Column Studies. Water Air Soil. Pollut. 2014, 225, 2050. [Google Scholar] [CrossRef]
- Kumar, I.A.; Viswanathan, N. Preparation and Testing of a Tetra-Amine Copper(II) Chitosan Bead System for Enhanced Phosphate Remediation. Carbohydr. Polym. 2018, 183, 173–182. [Google Scholar] [CrossRef]
- Deng, L.; Guan, Q.; Ning, P.; He, L.; Zhang, D. Synthesis of 3D Calcium-Modified Microspheres for Fast Purification of Simulated Phosphate Wastewater. J. Water Process Eng. 2021, 42, 102182. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Zhao, C.; Guo, B.; Xu, X.; Liu, T.; Zhao, L. A Novel Chitosan Modified Magnesium Impregnated Corn Straw Biochar for Ammonium and Phosphate Removal from Simulated Livestock Wastewater. Environ. Technol. Innov. 2022, 26, 102519. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Macazo, F.C.; Cai, R.; Minteer, S.D. A Sustainable Adsorbent for Phosphate Removal: Modifying Multi-Walled Carbon Nanotubes with Chitosan. J. Mater. Sci. 2018, 53, 12641–12649. [Google Scholar] [CrossRef]
- Kumar, I.A.; Viswanathan, N. Development of Multivalent Metal Ions Imprinted Chitosan Biocomposites for Phosphate Sorption. Int. J. Biol. Macromol. 2017, 104, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Hosseinifard, M. Highly Efficient Removal of Phosphate by Lanthanum Modified Nanochitosan-Hierarchical ZSM-5 Zeolite Nanocomposite: Characteristics and Mechanism. Cellulose 2020, 27, 4637–4664. [Google Scholar] [CrossRef]
- Aswin Kumar, I.; Viswanathan, N. Development and Reuse of Amine-Grafted Chitosan Hybrid Beads in the Retention of Nitrate and Phosphate. J. Chem. Eng. Data 2018, 63, 147–158. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, N.; Feng, C.; Zhang, Z. Adsorption for Phosphate by Crosslinked/Non-Crosslinked-Chitosan-Fe(III) Complex Sorbents: Characteristic and Mechanism. Chem. Eng. J. 2018, 353, 361–372. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Yao, Z.; Shen, Y.; He, Z.; Yang, X.; Ng, H.Y.; Wang, C.-H. Removal of Nitrate and Phosphate by Chitosan Composited Beads Derived from Crude Oil Refinery Waste: Sorption and Cost-Benefit Analysis. J. Clean. Prod. 2019, 207, 846–856. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Cross-linked Chitosan Beads for Phosphate Removal from Aqueous Solution. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Bozorgpour, F.; Ramandi, H.F.; Jafari, P.; Samadi, S.; Yazd, S.S.; Aliabadi, M. Removal of Nitrate and Phosphate Using Chitosan/Al2O3/Fe3O4 Composite Nanofibrous Adsorbent: Comparison with Chitosan/Al2O3/Fe3O4 Beads. Int. J. Biol. Macromol. 2016, 93, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, A.; Meenakshi, S. A Novel Quaternized Chitosan–Melamine–Glutaraldehyde Resin for the Removal of Nitrate and Phosphate Anions. Int. J. Biol. Macromol. 2014, 64, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, P.; Liu, J. Triethylene Tetramine Functionalized Magnetic Graphene Oxide Chitosan Composite with Superior Capacity for the Removal of Phosphate. J. Chem. Eng. Data 2017, 62, 3341–3352. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Z.; Cong, H.; Wang, Q.; Wang, X. Surface Chitosan-Coated Fe3O4 Immobilized Lignin for Adsorbed Phosphate Radicals in Solution. Biochem. Eng. J. 2022, 187, 108662. [Google Scholar] [CrossRef]
- Salehi, S.; Hosseinifard, M. Optimized Removal of Phosphate and Nitrate from Aqueous Media Using Zirconium Functionalized Nanochitosan-Graphene Oxide Composite. Cellulose 2020, 27, 8859–8883. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Zhang, Z.; Jin, P.; Zhang, Q.; Wang, X. The Removal of Phosphate from Aqueous Solution through Chemical Filtration Using a Sponge Filter. Chem. Lett. 2018, 47, 89–91. [Google Scholar] [CrossRef]
- Aguado, R.J.; Mazega, A.; Tarrés, Q.; Delgado-Aguilar, M. The Role of Electrostatic Interactions of Anionic and Cationic Cellulose Derivatives for Industrial Applications: A Critical Review. Ind. Crops Prod. 2023, 201, 116898. [Google Scholar] [CrossRef]
- Tagyan, A.I.; Yasser, M.M.; Mousa, A.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Marzouk, M.A. Potential Application of Innovative Aspergillus Terreus/ Sodium Alginate Composite Beads as Eco-Friendly and Sustainable Adsorbents for Alizarin Red S Dye: Isotherms and Kinetics Models. Microorganisms 2023, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Mehmood, S.; Haroon, M.; Kiran, M.; Waseem, K.; Aziz, T.; Farid, A. Role of Starch Based Materials as a Bio-Sorbents for the Removal of Dyes and Heavy Metals from Wastewater. J. Polym. Environ. 2022, 30, 1730–1748. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in Chemical Modifications of Starches and Their Applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Dong, Y.; Ghasemzadeh, M.; Khorsandi, Z.; Sheibani, R.; Nasrollahzadeh, M. Starch-Based Hydrogels for Environmental Applications: A Review. Int. J. Biol. Macromol. 2024, 269, 131956. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, H.; Liu, Y.; Li, S.; Wang, G.; Guo, T.; Han, H. An in Situ Reactive Spray-Drying Strategy for Facile Preparation of Starch-Chitosan Based Hydrogel Microspheres for Water Treatment Application. Chem. Eng. Process.-Process Intensif. 2021, 168, 108548. [Google Scholar] [CrossRef]
- Shrivastava, J.; Bajpai, A.K. Starch-Based Hydrogels. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–112. [Google Scholar]
- Wang, X.; Tarahomi, M.; Sheibani, R.; Xia, C.; Wang, W. Progresses in Lignin, Cellulose, Starch, Chitosan, Chitin, Alginate, and Gum/Carbon Nanotube (Nano)Composites for Environmental Applications: A Review. Int. J. Biol. Macromol. 2023, 241, 124472. [Google Scholar] [CrossRef]
- Saberi, A.; Alipour, E.; Sadeghi, M. Superabsorbent Magnetic Fe3O4-Based Starch-Poly (Acrylic Acid) Nanocomposite Hydrogel for Efficient Removal of Dyes and Heavy Metal Ions from Water. J. Polym. Res. 2019, 26, 271. [Google Scholar] [CrossRef]
- Atnafu, T.; Leta, S. Plasticized Magnetic Starch-Based Fe3O4 Clay Polymer Nanocomposites for Phosphate Adsorption from Aqueous Solution. Heliyon 2021, 7, e07973. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent Trends in the Application of Modified Starch in the Adsorption of Heavy Metals from Water: A Review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef]
- Chen, M.; Long, A.; Zhang, W.; Wang, Z.; Xiao, X.; Gao, Y.; Zhou, L.; Li, Y.; Wang, J.; Sun, S.; et al. Recent Advances in Alginate-Based Hydrogels for the Adsorption–Desorption of Heavy Metal Ions from Water: A Review. Sep. Purif. Technol. 2025, 353, 128265. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent Advances in Cellulose- and Alginate-Based Hydrogels for Water and Wastewater Treatment: A Review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; Elshishini, H.M.; El-Aqapa, H.G.; Hosny, M.; Abdelfatah, A.M.; Ahmed, M.S.; Hammad, E.N.; El-Subruiti, G.M.; Fawzy, M.; et al. Recent Developments in Alginate-Based Adsorbents for Removing Phosphate Ions from Wastewater: A Review. RSC Adv. 2022, 12, 8228–8248. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, H.; Yang, H. Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads. Minerals 2022, 12, 171. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Chen, H.; Gao, Y.; Zhou, L.; Wan, J.; Li, Y.; Tang, M.; Peng, Y.; Wang, B.; et al. Efficient Phosphate Removal from Water by Multi-Engineered PVA/SA Matrix Double Network Hydrogels: Influencing Factors and Removal Mechanism. Sep. Purif. Technol. 2024, 336, 126261. [Google Scholar] [CrossRef]
- Rahni, S.Y.; Mirghaffari, N.; Rezaei, B.; Ghaziaskar, H.S. Removal of Phosphate from Aqueous Solutions Using a New Modified Bentonite-Derived Hydrogel. Water Air Soil. Pollut. 2014, 225, 1916. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Dong, S.; Li, X.; Liu, C. Synchronously Construction of Hierarchical Porous Channels and Cationic Surface Charge on Lanthanum-Hydrogel for Rapid Phosphorus Removal. Environ. Res. 2023, 236, 116730. [Google Scholar] [CrossRef]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Zong, D.; Zhang, X.; Yin, X.; Wang, F.; Yu, J.; Zhang, S.; Ding, B. Electrospun Fibrous Sponges: Principle, Fabrication, and Applications. Adv. Fiber Mater. 2022, 4, 1434–1462. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Naushad, M.; Kumar, A.; Stadler, F.J.; Alfadul, S.M.; Mola, G.T. Applications of Nanocomposite Hydrogels for Biomedical Engineering and Environmental Protection. Environ. Chem. Lett. 2018, 16, 113–146. [Google Scholar] [CrossRef]
- Niazi, M.; Alizadeh, E.; Zarebkohan, A.; Seidi, K.; Ayoubi-Joshaghani, M.H.; Azizi, M.; Dadashi, H.; Mahmudi, H.; Javaheri, T.; Jaymand, M.; et al. Advanced Bioresponsive Multitasking Hydrogels in the New Era of Biomedicine. Adv. Funct. Mater. 2021, 31, 2104123. [Google Scholar] [CrossRef]
- Sharma, A.; Thatai, K.S.; Kuthiala, T.; Singh, G.; Arya, S.K. Employment of Polysaccharides in Enzyme Immobilization. React. Funct. Polym. 2021, 167, 105005. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent Advances in Nano-Engineered Approaches Used for Enzyme Immobilization with Enhanced Activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mauck, R.L. (Eds.) Biomaterials for Tissue Engineering Applications; Springer: Vienna, Austria, 2011; ISBN 978-3-7091-0384-5. [Google Scholar]
- Sharma, M.; Tellili, N.; Kacem, I.; Rouissi, T. Microbial Biopolymers: From Production to Environmental Applications—A Review. Appl. Sci. 2024, 14, 5081. [Google Scholar] [CrossRef]
- Kolodynska, D.; Skiba, A.; Gorecka, B.; Hubicki, Z. Hydrogels from Fundaments to Application. In Emerging Concepts in Analysis and Applications of Hydrogels; InTech: London, UK, 2016. [Google Scholar]
- Li, I.C.; Chen, Y.H.; Chen, Y.C. Sodium Alginate-g-Poly(Sodium Acrylate) Hydrogel for the Adsorption–Desorption of Ammonium Nitrogen from Aqueous Solution. J. Water Process Eng. 2022, 49, 102999. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Y.; Wang, A. Rapid and Wide PH-Independent Ammonium-Nitrogen Removal Using a Composite Hydrogel with Three-Dimensional Networks. Chem. Eng. J. 2012, 179, 90–98. [Google Scholar] [CrossRef]
- Dou, Z.; Xie, X. Chitosan-Montmorillonite-Fe Nanocomposite Hydrogel for Phosphate Recovery and Reuse. ACS ES T Eng. 2023, 3, 682–689. [Google Scholar] [CrossRef]
- Manna, A.; Lahiri, S.; Sen, K.; Banerjee, K. Fe(III) Cross-Linked Cellulose-Agar Hydrogel Beads for Efficient Phosphate Removal from Aqueous Solutions. Environ. Monit. Assess. 2024, 196, 1–23. [Google Scholar] [CrossRef]
- Wang, B.; Hu, X.; Zhou, D.; Zhang, H.; Chen, R.; Guo, W.; Wang, H.; Zhang, W.; Hong, Z.; Lyu, W. Highly Selective and Sustainable Clean-up of Phosphate from Aqueous Phase by Eco-Friendly Lanthanum Cross-Linked Polyvinyl Alcohol/Alginate/Palygorskite Composite Hydrogel Beads. J. Clean. Prod. 2021, 298, 126878. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly Effective Removal of Phosphate from Complex Water Environment with Porous Zr-Bentonite Alginate Hydrogel Beads: Facile Synthesis and Adsorption Behavior Study. Appl. Clay Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- Wang, B.; Hu, X.; Li, L.; Xie, Y.; Chen, R.; Guo, W.; Wang, H.; Wang, M.; Shi, J.; Chen, L.; et al. Enhanced Phosphate Removal by Filler Encapsulation and Surface Engineering Using SA/PVA Matrix: Fabrication Optimization, Adsorption Behaviors and Inner Removal Mechanism. Chem. Eng. J. 2023, 472, 145073. [Google Scholar] [CrossRef]
- Fu, J.; Leo, C.P.; Chang, C.K. Nutrient Recovery and Pollutant Removal Using Carboxymethyl Cellulose/Microfibrillated Cellulose Hydrogel: Templating Effects of CaCO3 Nanoparticles. J. Water Process Eng. 2024, 58, 104869. [Google Scholar] [CrossRef]
- Malicevic, S.; Garcia Pacheco, A.P.; Lamont, K.; Estepa, K.M.; Daguppati, P.; van de Vegte, J.; Marangoni, A.G.; Pensini, E. Phosphate Removal from Water Using Alginate/Carboxymethylcellulose/Aluminum Beads and Plaster of Paris. Water Environ. Res. 2020, 92, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Izydorczyk, G.; Mironiuk, M.; Szopa, D.; Chojnacka, K.; Witek-Krowiak, A. Selection of Matrix Material for Hydrogel Carriers of Plant Micronutrients. J. Mater. Sci. 2024, 59, 3031–3048. [Google Scholar] [CrossRef]
- Tan, X.; Yi, L.; Duan, Z.; Wu, X.; Ali, I.; Gao, L. Phosphorus Recovery from Wastewater Using Extracellular Polymeric Substances (EPS)-like Hydrogels. J. Water Process Eng. 2023, 52, 103512. [Google Scholar] [CrossRef]
- Huang, Y.X.; Liu, M.J.; Chen, S.; Jasmi, I.I.; Tang, Y.; Lin, S. Enhanced Adsorption and Slow Release of Phosphate by Dolomite–Alginate Composite Beads as Potential Fertilizer. Water Environ. Res. 2019, 91, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.; Siwek, H.; Kitczak, T.; Włodarczyk, M. Fertilization with Municipal Wastewater Phosphorus Adsorbed to Alginate Beads: Results from a Pot Experiment with Italian Ryegrass. Agronomy 2021, 11, 2142. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Gil, F.; Izydorczyk, G.; Mikula, K.; Gersz, A.; Hoppe, V.; Chojnacka, K.; Witek-Krowiak, A. Innovative Bio-Waste-Based Multilayer Hydrogel Fertilizers as a New Solution for Precision Agriculture. J. Environ. Manag. 2022, 321, 116002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).