Abstract
The objective of this study was to assess the toxicity of leaf and bark extracts of Strycns pseudoquina and their fractions, as well as flavonoids isolated from its bark, in a population of Spodoptera frugiperda. Crude bark and leaf extracts, as well as fractions of these extracts, were obtained using a rotary evaporator and different solvents; the ethyl acetate fraction was obtained, and flavonoid compounds were isolated, consisting mostly of 3-O-methylquercetin and minimally of strychnobiflavone. The crude extracts, extract fractions, and isolated flavonoids were applied separately to maize leaves, which were offered to S. frugiperda larvae; then, biological parameters of this insect population were evaluated. Toxic effects of topical applications of the isolated flavonoids on the larvae were also evaluated. The results highlighted the larvicidal effects of the crude bark extract and its fractions and the significant toxicity of this crude extract (LC50 = 0.048%), which was six times more toxic to larvae than its ethyl acetate fraction (LC50 = 0.288%). The investigated flavonoids showed no significant larvicidal effect. The pronounced larvicidal effect of S. pseudoquina bark crude extract and its fractions on S. frugiperda indicates that this plant presents an insecticidal potential to be explored in integrated pest management programs.
1. Introduction
Spodoptera frugiperda (Lepidoptera: Noctuidae) is a polyphagous pest known as the fall armyworm that, during its larval stage, can attack more than 353 plant species of 76 families, including economically important crops such as maize, soybean, cotton, sorghum, and wheat [1]. Despite its being predominantly found in Latin American countries [2], studies have shown occurrences in African and Asian countries and, more recently, in Australia [1,3].
Maize (Zea mays) is the fall armyworm’s preferred plant host; the pest attacks during the entire phenological cycle of this crop, mainly at the vegetative stage and during the reproductive stage, when they attack parts of ears, such as the style–stigmas and even developing grains [4,5]. These attacks during the plant’s development can decrease yields by 21% to 73%, depending on the affected stage [6]. In addition to the pest’s polyphagous activity, its characteristics include a short life cycle, a high reproduction rate, and migratory capacity, which result in its occurrence during the entire year and in different places, making its control difficult [7].
The control of this pest is usually carried out with organosynthetic insecticides that, despite being efficient, can cause damage to the environment and human health when applied in excess or incorrectly [8,9]. The use of genetically modified crops, such as Bt-transgenic maize, is another strategy to control this pest, as they contain proteins from the bacterium Bacillus thuringiensis which have insecticidal action [10]. However, these proteins can increase plant resistance to S. frugiperda. Michelotto et a. [11] pointed out that this strategy is more efficient in decreasing damage, even when combined with the application of insecticides, as the selection pressure of continuous planting of Bt maize may have been one of the causes of the evolution of S. frugiperda that resulted in its resistance to these proteins [10]. Bioactive compounds extracted from plants have often been shown to be promising alternatives to synthetic compounds to control insects, as they contain substances that are less harmful to non-target organisms, such as natural enemies, pollinators, and humans [8,12,13]. Therefore, these compounds have been used as models for obtaining synthetic insecticides, as well as extracts from specific plant parts, which have been used as low-cost insecticidal alternatives [14,15]. Despite the insecticidal potential of several plant species, according to Vieira et al. [16], the toxic mechanisms of action of their bioactive compounds on organisms need to be better understood. These secondary metabolites exert significant larvicidal effects on insects by ingestion or contact [16,17].
In Brazil, the Cerrado biome alone has more than 12,000 records of seed plant species, 35% of which are endemic [18]. Biotic and abiotic stresses caused by dry winters and rainy summers, combined with the high incidence of solar radiation, affect the production of secondary metabolites of plants in the Cerrado biome, making them abundant in natural compounds, such as flavonoids, terpenes, alkaloids, and tannins [16,19]. This situation on its own would explain the need for more studies on potential insecticides from plants in this environment.
Species of the genus Strychnos L. have been highlighted in folk medicine for years due to the presence of important secondary metabolites in their chemical composition [20]. This genus belongs to the Loganeaceae family, which comprises approximately 200 species distributed over the Americas, Africa, Australia, and Asia [20,21]. The target plant in the present study was Strychnos pseudoquina, popularly known as quina-do-campo or falsa-quina, an arboreous species that is native to South America and commonly found in the Cerrado biome in Brazil [22].
Phytochemical characteristics and pharmacological properties of S. pseudoquina have been relatively well studied [23,24,25,26,27,28,29], although the use of these plants is still primarily based on folk medicine, and, to date, there are no published studies reporting the insecticidal potential of Strychnos species.
Importantly, anthropic pressure caused by urbanization, farming (mainly), and tourism has heavily affected the Brazilian Cerrado. Nowadays, every plant that is born in the natural areas of the Brazilian Cerrado, even in those protected by law, is threatened with disappearance [30]. Also, as reported by Leite et al. [31], for purposes such as our work, it is important to emphasize that the herbal market represents an additional threat, due to the deleterious effect of intensive bark extraction and the consequent felling of S. pseudoquina. Therefore, the purpose of our study was to discover other properties of the plant and to warn about the importance of its preservation.
In this context, the objective of this study was to assess the toxicity of leaf and bark extracts of S. pseudoquina, extract fractions, and metabolite isolates (flavonoids) extracted from the bark in an S. frugiperda population, under laboratory conditions.
2. Materials and Methods
2.1. Plant Material Collection and Preparation of Extracts
Leaves and barks of S. pseudoquina were collected from adult plants in Niquelândia, GO, Brazil (14°23′33.5″ S, 47°55′37.8″ W). Exsiccates of the collected plants were registered with SisGen (number A36DDE3) and placed in the herbarium of the State University of Goiás, Brazil (record HUEG 14494).
Fresh leaves and barks of S. pseudoquina were separately dried at room temperature and crushed in a knife mill. The product of this process was dipped in ethanol (96%) in an Erlenmeyer flask and left to rest for seven days. The material was then filtered through a separatory funnel using cotton; the solvent used was removed in a rotary evaporator, obtaining the final crude ethanolic extract (CEE). The CEE was used in a fractionation with the following solvents: ethyl acetate and hexane (for leaves) and ethyl acetate and methanol (for barks). The CEE was dissolved in the solvents for seven days and then vacuum-filtered with the incorporation of the adjuvant microcrystalline cellulose. The following leaf and bark extracts and fractions of these extracts were obtained at the end of this process, after removing the solvents in the rotary evaporator: crude bark extract; crude leaf extract; ethyl acetate fraction of bark extract; ethyl acetate fraction of leaf extract; methanol fraction of bark extract; and hexane fraction of leaf extract. Figure 1 shows the sequence of actions for obtaining the fractions of S. pseudoquina extracts used in the bioassays.
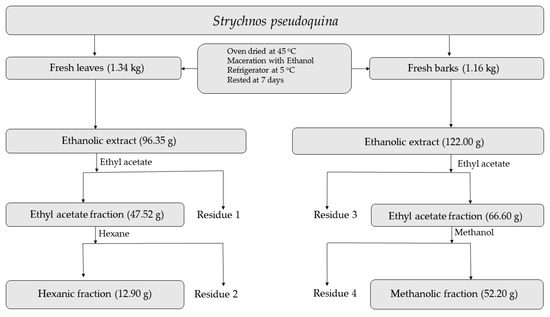
Figure 1.
Procedures adopted for producing bark and leaf extracts of Strycnos pseudoquina, as well as fractions of these extracts, used in the toxicity bioassays.
All fractions of leaf and bark extracts obtained were placed in glass bottles with screw caps, covered with aluminum foil, and maintained in a refrigerator until the biological tests.
2.2. Rearing of S. frugiperda
The S. frugiperda larvae used in the experiment were obtained by artificial rearing with a common bean-based diet, according to the methodology described by Kasten Júnior et al. [32]. The entomology laboratory in which the biological tests were conducted was maintained at an air temperature of 25 ± 2 °C and a relative air humidity of 67 ± 2% with a 12 h photoperiod, according to the conditions adopted by Bezerra et al. [30].
2.3. Maize Production for Feeding Larvae
Maize plants were grown in a greenhouse at the State University of Goiás, in Ipameri, GO, Brazil, in eight-liter pots with a substrate composed of a mixture of soil, sand, and cattle manure (2:1:1), according to the methodology adopted by [32]. Six seeds of the non-transgenic maize cultivar AG-1051 were sown in each pot. Irrigation was carried out daily after planting, and no insecticide or other natural or synthetic agrochemical was applied. Leaves from young plants (approximately 30 days of age) were harvested daily and taken to the laboratory to be fed to S. frugiperda larvae.
2.4. Preparation and Offering of Different Fractions of Plant Extracts to Larvae
The dilution of all tested bark and leaf extracts was carried out using acetone and water (1:1). This solvent was applied to the maize plants and caused no noticeable repellent effects on S. frugiperda larvae. This solvent was used for diluting the plant extracts applied on the larvae in a similar experiment conducted by [31].
The plant extract concentrations (w/v) used were as follows: 0.01%, 0.05%, 0.1%, 0.50%, 1.0%, 2.5%, and 5.0%. These extracts were applied to freshly harvested leaves of young maize plants with the aid of a brush, covering their entire surface; the leaves were then placed on filter paper sheets for natural drying on the laboratory benches, which lasted approximately 25 min, and then offered to S. frugiperda larvae.
The highest concentration (5%) was established considering that those above 8%, tested preliminarily, resulted in a significant repellent effect, i.e., the larvae did not feed on the treated maize leaves. This methodology was adapted from that used by Barbosa et al. and Pires Júnior et al. [30,33], who also assessed adverse effects of tree plant extracts on S. frugiperda.
2.5. Antixenosis Effect of S. pseudoquina Extracts and Their Fractions on S. frugiperda
Second-instar S. frugiperda larvae were removed from the artificial diet and placed in 1 L plastic cups with lids to evaluate the attractiveness or repellency of the crude bark and leaf extracts of S. pseudoquina and fractions of these extracts at different concentrations. These larvae were kept in this environment, without any food, for 1.5 h. They were then transferred to trays containing maize leaves treated with the S. pseudoquina extracts, using a small brush.
The experimental unit of this bioassay consisted of a 30 cm diameter plastic tray with filter paper on the bottom and leaf fragments placed equidistantly at the edges. These leaf fragments (approximately 3.0 × 3.0 cm) from the conventional maize hybrid were treated with bark and leaf extracts of S. pseudoquina at concentrations of 0.5 and 5% (w/v) (Figure 2). Fourteen larvae were released in the center of the tray, where they moved freely to choose the different maize leaf fragments treated with the extracts. These trays were sealed with transparent lids to facilitate the visualization of the movement and location of the larvae. The times established for counting the larvae on the maize leaf fragments after the release of larvae in the center of the tray were 15, 30, 60, 120, 240, 720, and 1440 min. A completely randomized experimental design with six replications was used, consisting of six treatments and a control (with only the solvent used for diluting the extracts, applied similarly to those containing plant extracts).

Figure 2.
(a) Container with artificial diet used to rear neonatal larvae of Spodoptera frugiperda. (b–e) The sampling units used for the antixenosis test, with a chance to choose. Trays containing maize leaves treated with bark and leaf extracts of Strychnos pseudoquina. (c) Fourteen larvae of S. frugiperda (second instar) were released in the center of the tray. (d,e) Recordings at different times of where larvae were quantified on treated maize leaves.
2.6. Effects of Antibiosis of S. pseudoquina Extracts on S. frugiperda
The sampling unit in this experiment consisted of a 250 mL plastic cup with a lid, with a filter paper slightly moistened with deionized water on the bottom, on which a leaf fragment treated with extract and an S. frugiperda larva at the beginning of the second instar were placed; the larva was removed from the artificial diet and placed to feed on this leaf fragment. The evaluated treatments were bark extracts (crude extract and its ethyl acetate and methanol fractions) and leaf extracts (crude extract and its ethyl acetate and hexane fractions) at concentrations of 0.01%, 0.05%, 0.1%, 0.5%, 1.0%, 2.5%, and 5% (w/v). A completely randomized design with thirty replications was used.
The maize leaf fragments treated with the extracts were weighed before being offered to the larvae and after 24 h. Fresh maize leaves were then offered daily to the larvae (ad libitum) until pre-pupation.
The maize leaves were exchanged, and feces and exuviae were removed daily. Thus, the insects’ development was monitored through their entire life cycle. The evaluated variables were the following: duration of larval and pupal stages; larval weight after seven days of consumption of leaves treated with the extracts and their fractions; duration of larval and pupal stages; larval and pupal mortality; duration of the adult stage; sex ratio; and visible abnormalities in insects.
The procedure for the application of S. pseudoquina crude leaf and bark extracts and their fractions on fragments of maize leaves was the same as that described for the antixenosis test.
2.7. Flavonoids Isolated from S. pseudoquina Bark Extract and Their Larvicidal Activity against S. frugiperda
The flavonoids isolated and tested in this bioassay were obtained from the ethyl acetate fraction of the S. pseudoquina bark extract. This fraction was chosen because it presented more significant larvicidal effects on S. frugiperda compared to the other evaluated extract fractions. The procedures for the isolation and identification of flavonoids were carried out using 30.0 g of an ethyl acetate fraction of S. pseudoquina bark extract. This extract was subjected to chromatography by adsorption in a glass column, and the separated compounds were identified by nuclear magnetic resonance and high-resolution mass spectrometry analyses. A yellowish solid was produced (80.0 mg) by the end of this separation process, containing mostly the flavonoid 3-O-methylquercetin (C16H12O7) and, minimally, the biflavonoid strychnobiflavone (C32H22O14), which were separated. These isolated compounds had already been previously identified in this same plant [23,34]; thus, only results referring to the larvicidal effects of these compounds were presented. The methodology for the isolation and quantification of these compounds was described by Santos [34]. The isolated and identified flavonoids were placed in glass jars with screw caps, covered with aluminum foil, and kept in a refrigerator until biological testing was conducted.
The two flavonoids (yellowish solids) were completely dissolved for the biological tests using water + acetone (1:1) as the solvent. The toxicity by ingestion of flavonoids by neonate larvae was evaluated in a first test, and the topical toxicity of these flavonoids on larvae of the same age was evaluated in a second test. The procedure for the application of the flavonoids isolated from S. pseudoquina on fragments of maize leaves, which were offered to larvae for ingestion, was the same as that adopted for the tests with the bark and leaf extracts and their fractions at a concentration of 10.000 ppm. The topical toxicity was evaluated after the application of flavonoids (1.0 μL larva−1) at concentrations of 2.500, 5.000, and 10.000 ppm. The biological compounds were applied to the notal region of neonate larvae (n = 12) with the aid of a 10.0 μL microsyringe. After being treated with these compounds, the larvae were placed in 250 mL cups containing fresh leaves of non-transgenic maize, provided ad libitum as food. The larvae were monitored daily until de formation of pupae, and deaths caused by the topical application of the product were recorded. The control treatment was carried out similarly, but with only the solvent water + acetone (1:1).
2.8. Statistical Analyses
The antixenosis effect of the bark and leaf extracts of S. pseudoquina on S. frugiperda was evaluated by ANOVA (p < 0.05) in choice tests. This effect was evaluated by the non-parametric Mann–Whitney test (p < 0.05) in no-choice tests through the consumption of leaves treated with crude extracts and their fractions by the larvae. This test was also used to assess the effect of antibiosis on larval and pupal weights, the duration of different insect development stages, and the sex ratios of the insects under the different treatments. Pupal mortality in the different treatments was compared by the Kruskal–Wallis test (p < 0.05). The extract concentrations and larval mortality were correlated through logistic regression analysis (p < 0.05). The larvicidal effect of the extracts over time was determined by the non-parametric Kaplan–Meier method (p < 0.05). To obtain dose–response curves for each treatment, five to seven doses were used. All analyses were performed using the R 4.2.2 program [35].
3. Results
3.1. Antixenosis Effects of S. pseudoquina Extracts on S. frugiperda
In the choice test, second-instar S. frugiperda larvae did not exhibit repellence (lesser number of visits) to treatments with crude leaf or bark extract of S. pseudoquina when compared to the control treatment in any of the evaluations (ANOVA, at p < 0.01) (Table 1).

Table 1.
Attractiveness (mean number of visitors ± standard deviation) of maize leaves treated with bark and leaf extracts of Strychnos pseudoquina to Spodoptera frugiperda larvae.
In the no-choice test, there was a significant decrease in consumption of maize leaves treated with S. pseudoquina crude bark extract by larvae compared to the control treatment, which was not found for the crude leaf extract (Figure 2A,D). Maize leaves treated with the ethyl acetate leaf fraction (2.5% and 5%) were significantly less consumed by larvae (Mann–Whitney test, at p < 0.05) (Figure 3D).
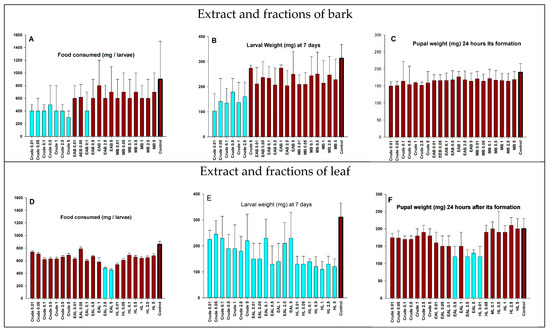
Figure 3.
(A–F) Biological variables of the Spodoptera frugiperda population after ingestion of maize leaves treated with crude extracts of Strychnos pseudoquina and extract fractions. Crude = crude plant extract; EAB = ethyl acetate fraction of bark extract; MB = methanol fraction of bark extract; EAL = ethyl acetate fraction of leaf extract; HL = hexane fraction of leaf extract; Control = only the solvent used for diluting the extracts and applied similarly to those containing plant extracts. Means represented by bars with the same color are not significantly different from each other by the Mann–Whitney test, at p < 0.05.
3.2. Effects of Antibiosis of S. pseudoquina Extracts on S. frugiperda
The larval weight of S. frugiperda seven days after ingestion of the crude leaf extract, as well as its fractions, at different concentrations, was significantly lower than that found for the control treatment (Figure 3E). Larval weight was significantly lower when the larvae ingested maize leaves treated with the crude bark extract compared to the other fractions of the bark extract and the control (Figure 3B). The ingestion of maize leaves treated with fractions of the bark extract at different concentrations did not cause a significant decrease in pupal weight (Figure 2C); the pupal weight of maize leaves treated with the ethyl acetate fraction (0.5%, 2.5%, and 5.0%) and the hexane fraction (0.01%) of the leaf extract was significantly lower compared to the other treatments and the control (Mann–Whitney test, at p < 0.05) (Figure 3F).
The larval mortality caused by ingestion of maize leaves treated with bark extracts (crude extract and its ethyl acetate and methanol fractions) at the different concentrations was significantly different (Kruskal–Wallis test, at p < 0.05) from that found for the control treatment (n = 30), which showed only one case of larval death. The larval mortality estimated through logistic regression was approximately 86% for the highest tested concentration of the crude bark extract, with a lethal concentration for 50% of the individuals (LC50) of 0.048% (Figure 4A); approximately 65% for the highest concentration of the ethyl acetate fraction of the bark extract (EABE), with an LC50 of 0.288% (Figure 4B); and approximately 60% for the highest concentrations of the ethyl acetate fraction of the leaf extract, with an LC50 of 0.043% (Figure 4E).
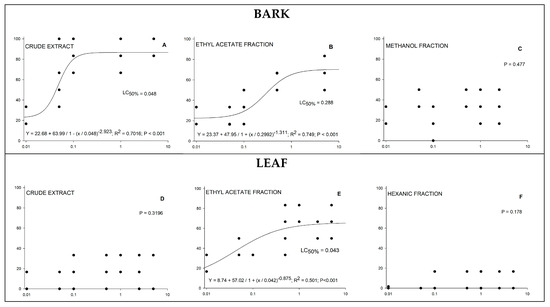
Figure 4.
(A–F) Toxicity of Strychnos pseudoquina extracts in Spodoptera frugiperda. Lethal concentration (LC) estimated based on concentration–mortality bioassay using logistic regression. The tested concentration is on the x-axis, and the observed mortality is on the y-axis.
The larval mortality found for the crude leaf extract and its hexane fraction did not fit a logistic regression model (Figure 4D,F), confirming the low larvicidal effect of these fractions, as the lowest (0.01%) and highest (5%) concentrations resulted in larval mortalities similar to the control (Kruskal–Wallis test, at p < 0.05). The larval mortality found for the bark extract methanol fraction did not fit a logistic regression model (Figure 4C); however, even the lowest tested concentration differed significantly from the control treatment.
Ingestion of the ethyl acetate fraction of the bark extract by larvae at the highest concentration (5%) resulted in faster death than the crude bark extract and its methanol fraction, according to the Kaplan–Meier survival estimation (p < 0.001). Larval mortality of 50% occurred at seven days after application of the crude bark extract and at three days after application of the ethyl acetate fraction of the bark extract. The bark extract methanol fraction did not reach a larval mortality above 50% (Figure 5).
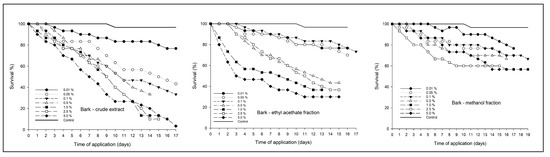
Figure 5.
Survival of Spodoptera frugiperda larvae over time after treatment with crude bark extract of Strychnos pseudoquina and extract fractions. Symbols represent the tested concentrations. Control = only the solvent used for diluting the extracts and applied similarly to those containing plant extracts. All treatments (crude extract and its fractions, with their respective concentrations) were significantly different from the control treatment by the Kaplan–Meier test, at p < 0.001.
The analysis of pupal mortality was carried out using larval survivors of treatments. For example, with the crude extract 5% treatment, two deaths of pupae out of the total of five formed (40%) were recorded. In the control treatment, of 29 pupae formed, 1 died naturally (3.44%). The mortality of S. frugiperda pupae found for the S. pseudoquina crude bark extract and its fractions at the different concentrations was not significantly different from that of the control treatment (Kruskal–Wallis test, at p < 0.05) (Table 2), even in treatments with the ethyl acetate fraction of the leaf extract (0.5%, 2.5%, and 5%), in which these extracts resulted in a significant decrease in pupal weight (Mann–Whitney test, at p < 0.05) (Figure 3F).

Table 2.
Variables used to measure the antifeedant effect of Spodoptera frugiperda individuals fed with maize leaves treated with crude bark extract of Strychnos pseudoquina and extract fractions.
The results on the effect of ingestion of the different S. pseudoquina bark extract fractions on the duration of the different development stages of the S. frugiperda population are shown in Table 2. The ingestion of the crude bark extract and its fractions did not significantly affect the life cycle duration of the S. frugiperda population; however, the pupal cycle duration presented a significant increase for all concentrations of the bark extract methanol fraction and some EABE concentrations (Mann–Whitney test, at p < 0.05).
Some visible abnormalities were found in the S. frugiperda population treated with the different extracts of S. pseudoquina but in insignificant quantities: two cases of abnormal darkening of larvae; one case of abnormal darkening of pupae; and two cases of wing deformation in adults, all found for the crude bark extract and ethyl acetate bark fractions. The insects in the other treatments, including the control, presented no visible abnormality.
3.3. Larvicidal Effect of Secondary Metabolites Isolated from S. pseudoquina Extract on S. frugiperda
The concentrations of flavonoids (3-O-methylquercetin and strychnobiflavone) evaluated in ingestion and contact tests did not result in significantly different larval mortality rates when compared to the control treatment (only the solvent used for diluting the extracts and applied similarly to those containing plant extracts) (Mann–Whitney, at p < 0.05). For the concentrations tested, the record of probable deaths caused by ingestion or topical application of flavonoids was at most two deaths, or 16.6% (Table 3 and Table 4).

Table 3.
Larval mortality of Spodoptera frugiperda caused by topical application of flavonoids from barks of Strychnos pseudoquina obtained from the ethyl acetate fraction.

Table 4.
Larval mortality of Spodoptera frugiperda caused by ingestion of flavonoids from barks of Strychnos pseudoquina obtained from the ethyl acetate fraction.
4. Discussion
The crude bark and leaf extracts of S. pseudoquina and extract fractions did not result in repellence of S. frugiperda larvae, i.e., they visited the maize leaf fragments treated with the extracts in the same way as the control treatment (only the solvent used for diluting the extracts and applied similarly to those containing plant extracts). According to Bezerra et al. [30], the absence or insignificant presence of repellent compounds in insecticidal plant extracts ingested by larva is an important aspect. However, parts of maize leaves treated with the crude bark extract were significantly less consumed than those treated with the other treatments. Nevertheless, the low ingestion of maize leaves treated with the crude bark extract was sufficient to result in the highest larval mortality. The low consumption of maize leaves treated with the crude bark extract of S. pseudoquina by larvae was probably due to the presence of phagodeterrent tannins (predominant), alkaloids, and even the different flavonoids. These secondary metabolites are commonly found in S. pseudoquina barks [26]. According to Taiz and Zeiger and El-Aswad et al. [36,37], tannins are phenolic compounds with defense properties produced by plants that are potentially toxic and have antifeedant action for many herbivores.
The bark extracts of S. pseudoquina presented, in general, more pronounced larvicidal effects than the leaf extracts. Treatments with the bark extracts resulted in mortality rates significantly different from the control, which was not found for the treatments with the leaf extracts, mainly the crude leaf extract and its hexane fraction (Figure 4). In this study, we evaluated only the non-volatile compounds of the plant by adsorption chromatography; once the potential larvicidal effect has been verified, future work should be carried out using gas chromatography (GC-MS), since the presence of volatile and semi-volatile bioactive compounds from this plant can mainly affect the behavior of the pest.
The polarity of the solvent used for drag secondary metabolites is an important factor [30,38,39]. This was important mainly for the maize leaves, as the different concentrations of the ethyl acetate fraction of the leaf extracts resulted in significantly different larval mortality levels compared to the hexane fraction (Figure 4E,F). The use of the hexane solvent was indicated by Bezerra et al. and Sousa Neto et al. [30,40], who reported that this solvent is a better extractor of larvicidal compounds for S. frugiperda than ethyl acetate and methanol solvents. However, this was not confirmed, as it resulted in lower larval mortality than the other treatments (Figure 3). In preliminary tests of our work on larval mortality with the hexane leaf extract, very low mortality was observed. Therefore, we chose to change the solvent (methanol) for the bark, which in preliminary tests showed apparently better results.
Considering the situations in which there was a significant correlation between extract concentration and larval mortality, the estimated LC50 values found for the crude bark extract and the ethyl acetate fraction of the leaf extract were relatively similar; considering LC50, the crude bark extract presented a higher toxicity than EABE. Moreover, a lack of dose–response dependence for insects is often reported in the literature, indicating a greater complexity of triggers for deleterious effects on the target [40].
A slight and significant delay in larval stage duration and a slight and not significant increase in adult stage duration was found for the insect when the larva ingested the methanol fraction of the bark extract; however, the total life cycle duration of all treatments presented no significant difference from that in the control (Mann–Whitney, at p < 0.05) (Table 2). Decreases in the total life cycles of insects result in increases in annual generations during crop cycles [41], which is undesirable for pest management.
The S. pseudoquina bark extract and its fractions resulted in inexpressive mortality of S. frugiperda pupae (Mann–Whitney, at p < 0.05) (Table 2). The sex of the insects was verified at the adult stage, considering the number of live insects, which was very low in many treatments; therefore, the differences in sex ratios in the different treatments are not reliable.
The highest concentrations of the ethyl acetate bark extract resulted in a faster larval mortality of S. frugiperda compared to the crude bark extract and its methanol fraction. According to Lopes et al. [42], this fast larvicidal action should be considered in insect pest management programs. According to Lazarević et al. [43], this expressive initial mortality is connected to the insect response capacity to oxidative stresses, which can lead to damage at a macromolecular level in their cells.
The fast larval mortality found for EABE was indicative for the second step of the study, in which the larvicidal effects of the flavonoids 3-O-methylquercetin and strychobiflavone, obtained from this bark extract fraction, were evaluated for the S. frugiperda population. Larvicidal effects of flavonoids extracted from plants have been reported, mainly as mosquitocides for human disease vectors [44,45,46].
Flavonoids are secondary plant metabolites characterized by a 2-fenil-benzil-γ-pirona, which is ubiquitously found throughout the plant kingdom [47]. According to Ferreira et al. [48], flavonoids and alkaloids in plant extracts are probably responsible for hindering insect feeding and digestion by releasing free radicals that are harmful to them. Indirectly, proanthocyanidins (condensed tannins), commonly found in S. pseudoquina [49], are structural derivatives of certain flavonols [50]. According to Ayres et al. [51], proanthocyanidins are mainly associated with rapid insect death rather than inhibition of digestibility. In the present study, the flavonoids tested by contact or ingestion did not show a significant larvicidal effect, denoting an indirect deleterious action of these compounds on the pest or a synergistic effect with other plant bioactive compounds. It is important to highlight that our study only evaluated the larvicidal effects of isolated flavonoids. However, deleterious changes due to these bioactive compounds that did not cause death may have occurred and, therefore, should be investigated in future work. Also, other bioactive compounds from this plant must be isolated, identified, and tested for their insecticidal effects on this important pest.
5. Conclusions
The pronounced larvicidal effect of extracts of S. pseudoquina on Spodoptera frugiperda indicates that this plant has an insecticidal potential that can be explored in integrated pest management programs. The deleterious effect on S. frugiperda was more expressive when using bark extract, as well as its fraction obtained by ethyl acetate. The flavonoids 3-O-methylquercetin and strychnobiflavone, isolated from the bark extract, did not show a significant larvicidal effect; however, they may potentially show indirect deleterious effects or larvicidal effects in synergy with other plant bioactive compounds.
Author Contributions
Conceptualization, M.S.A., A.C.S.M. and T.A.A.S.; methodology, T.A.A.S., F.G.J. and C.K.G.S.; software, T.A.A.S. and E.C.R.; validation, M.S.A. and T.A.A.S.; formal analysis, A.C.S.M., T.A.A.S. and M.S.A.; investigation, T.A.A.S. and C.K.G.S.; resources, M.S.A. and A.C.S.M.; data curation, T.A.A.S., E.C.R. and M.S.A.; writing—original draft preparation, T.A.A.S. and M.S.A.; writing—review and editing, M.S.A., F.G.J. and T.A.A.S.; visualization, M.S.A. and T.A.A.S.; supervision, M.S.A. and A.C.S.M.; project administration, M.S.A.; funding acquisition, M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was carried out with support from the Universidade Estadual de Goiás (UEG), call Pró-Programas (no. 21/2022), and call Bioinsumos (32/2022 and 32/2023; SEI Process no. 202200020023142).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, for the master’s fellowship to the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Y.C.; Chen, D.F.; Yang, M.F.; Liu, J.F. The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.M.; Aguado-Pedraza, A.J.; Viñuela, E.; Rodríguez-Enríquez, C.L.; Lobit, P.; Gómez, B.; Pineda, S. Effects of ethanolic extracts of Argemone ochroleuca (Papaveraceae) on the food consumption and development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Fla. Entomol. 2017, 100, 339–345. [Google Scholar] [CrossRef]
- Ren, Q.; Haseeb, M.; Fan, J.; Wu, P.; Tian, T.; Zhang, R. Functional response, and intraspecific competition in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 806. [Google Scholar] [CrossRef]
- Vassallo, C.N.; Bunge, F.F.; Signorini, A.M.; Valverde-Garcia, P.; Rule, D.; Babcock, J. Monitoring the evolution of resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) to the Cry1F Protein in Argentina. J. Econ. Entomol. 2019, 112, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Embrapa. Cultivo do Milho: Pragas da Fase Vegetativa e Reprodutiva; Empresa Brasileira de Pesquisa Agropecuária—Centro Nacional de Pesquisa de Milho e Sorgo: Sete Lagoas, Brazil, 2002. [Google Scholar]
- Horikoshi, R.J.; Vertuan, H.; Castro, A.A.; Morrell, K.; Griffith, C.; Evans, A.; Tan, J.; Assimwe, P.; Anderson, H.; José, M.O.M.A.; et al. A new generation of Bt maize for control of fall armyworm (Spodoptera frugiperda). Pest Manag. Sci. 2021, 77, 3727–3736. [Google Scholar] [CrossRef]
- Kuzhuppillymyal-Prabhakarankutty, L.; Ferreira-Rivero, F.H.; Tamez-Guerra, P.; Gomes-Flores, R.; Rodríguez-Padilha, M.C.; Ramos-Ek, M.J. Effect of Beauveria bassiana-seed treatment on Zea mays L. response against Spodoptera frugiperda. Appl. Sci. 2021, 11, 2887. [Google Scholar] [CrossRef]
- Leão, R.M.; Cruz, J.V.S.; Ramos, V.M.; Almeida, V.T.; Gorni, P.H.; Camargo, R.S.; Pacheco, A.C.; Lima, L.V.; Forti, L.C. Secondary metabolites of Asclepias curassavica (Apocynaceae) and its effects on food preference and mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae). Emir. J. Food Agric. 2020, 32, 583–590. [Google Scholar] [CrossRef]
- Trindade, R.C.P.; Ferreira, E.S.; Gomes, I.B.; Silva, L.; San’Ana, A.E.G.; Broglio, S.M.F.; Silva, M.S. Extratos aquosos de inhame (Dioscorea rotundata Poirr.) e de mastruz (Chenopodium ambrosioides L.) no desenvolvimento da lagarta-do-cartucho-do-milho Spodoptera frugiperda (J.E. Smith, 1797). Rev. Bras. Plan. Med. 2015, 17, 291–296. [Google Scholar] [CrossRef]
- Nogueira, L.; Costa, E.N.; Belo, M.M.D.; Diniz, J.F.S.; Ribeiro, Z.A.; Boiça Júnior, A.L. Oviposition preference and antibiosis to Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazilian maize landraces. J. Econ. Entomol. 2019, 20, 939–947. [Google Scholar] [CrossRef]
- Michelotto, M.D.; Crosariol Neto, J.; Pirota, M.Z.; Duarte, A.P.; Freitas, R.S.; Finoto, E.L. Efficacy of transgenic mayze insecticide treatment to control fall armyworm in late-season maize in São Paulo state, Brazil. Ciênc. Agrotec. 2017, 41, 128–138. [Google Scholar] [CrossRef]
- Silva, G.; Rodríguez, J.C.; Blanco, C.A.; Lagunes, A. Bioactivity of a water extract of boldus (Peumus boldus Molina) against Spodoptera frugiperda (J.E. Smith) and Helicoverpa zea Boddie (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2013, 73, 135–141. [Google Scholar] [CrossRef]
- Oliveira, E.R.; Alves, D.; Carvalho, G.A.; Oliveira, B.M.R.G.; Smail, A.; Bertolucci, S.K. Toxicity of Cymbopogon flexuosus essential oil and citral for Spodoptera frugiperda. Ciên. Agrotec. 2018, 42, 408–419. [Google Scholar] [CrossRef]
- Green, P.W.C.; Belmain, S.R.; Ndakidem, P.A.; Farrell, I.W.; Stevenson, P.C. Insecticidal activity of Tithonia diversifolia and Vernonia amygdalina. Ind. Crops Prod. 2017, 110, 15–21. [Google Scholar] [CrossRef]
- Spletozer, A.G.; Santos, C.R.; Sanches, L.A.; Garlet, J. Plantas com potencial inseticida: Enfoque em espécies amazônicas. Ciênc. Florest. 2021, 31, 974–997. [Google Scholar] [CrossRef]
- Vieira, R.S.; Potta, A.; Souza, A.P.; Bogo, D. Cerrado plants with larvicide activity against Aedes aegypti. Ens. Ciênc. 2023, 27, 222–230. [Google Scholar] [CrossRef]
- Martins, M.M.; Dias, A.C.A.; Facundo, V.A.; Lima, R.A.; Meneguetti, D.U.O. Larvicidal activity of Maytenus guianensis (Celastraceae) against Aedes aegypti (Diptera: Culicidae). Rev. Soc. Bras. Med. Trop. 2021, 54, e0835. [Google Scholar] [CrossRef] [PubMed]
- BFG-The Brazil Flora Group. Growing knowledge: An overview of seed plant diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Pinheiro, H.S.; Giacomin, L.L.; Reis, I.M.S.; Baratto, L.C. Avaliação do desenvolvimento e da produção de flavonoides de Kalanchoe pinnata (Lam.) Pers. (Crassulaceae) em diferentes condições de luz e nutrição. Rev. Fitos 2016, 10, 375–547. [Google Scholar] [CrossRef][Green Version]
- Adebowale, A.; Lamb, J.M.; Nicholas, A.; Naidoo, Y. Molecular systematics of southern African monkey orange Strychnos L. (Loganiaceae). Kew Bull. 2016, 71, 17. [Google Scholar] [CrossRef]
- Setubal, R.B.; Frasier, C.L.; Molina, J.; Torke, B.M.; Forzza, R.C.; Struwe, L. A toxic story: Phylogeny and classification of Strychnos L. (Loganiaceae). Syst. Bot. 2021, 46, 639–655. [Google Scholar] [CrossRef]
- Côrtes, M.A.; França, E.L.; Reinaque, A.P.B.; Scherer, E.F.; Honório-França, A.C. Imunomodulação de fagócitos do sangue humano pelo extrato de Strychnos Pseudoquina ST. HILL adsorvido em microesferas de polietilenoglicol. Polímeros 2013, 23, 402–409. [Google Scholar] [CrossRef]
- Nicoletti, M.; Goulart, M.O.F.; Lima, R.A.; Goulart, A.E.; Monache, F.D.; Bettolo, B.M. Flavonoids and alkaloids from Strychnos pseudoquina. J. Nat. Prod. 1984, 47, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Lage, P.S.; Chávez-Fumagalli, M.A.; Mesquita, J.T.; Mata, L.M.; Fernandes, S.O.A.; Cardoso, V.N.; Soto, M.; Tavares, C.A.P.; Leite, J.P.V.; Tempone, A.G.; et al. Antileishmanial activity and evaluation of the mechanism of action of strychnobiflavone flavonoid isolated from Strychnos pseudoquina against Leishmania infantum. Parasitol. Res. 2015, 114, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
- Boff, L.; Silva, I.T.; Argenta, D.F.; Farias, L.M.; Alvarenga, L.F.; Pádua, R.M.; Braga, F.C.; Leite, J.P.V.; Kratz, J.M.; Simões, C.M.O. Strychnos pseudoquina A. St. Hil.: A Brazilian medicinal plant with promising in vitro antiherpes activity. J. Appl. Microbiol. 2016, 121, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Miranda, L.L.; Altoé, L.S.; Novaes, R.R.; Zanuncio, V.V.; Leite, J.P.V.; Gonçalves, R.V. Strychnos pseudoquina modulates the morphological reorganization of the scar tissue of second intention cutaneous wounds in rats. PLoS ONE 2018, 13, e0195786. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, D.C.; Nunes, L.G.; Farias, L.M.; Duarte, M.G.R.; Carvalho, A.F.; Fietto, L.G.; Leite, J.P.V. Assessment of the phenolic content, mutagenicity and genotoxicity of ethanolic extracts of stem bark and leaves from Strychnos pseudoquina A. St.-hil. Drug Chem. Toxicol. 2020, 43, 539–545. [Google Scholar] [CrossRef]
- Bonamin, F.; Moraes, T.M.; Kushima, H.; Silva, M.A.; Rozza, A.L.; Pellizzon, C.H.; Bauab, T.M.; Rocha, L.R.M.; Vilegas, W.; Hiruma-Lima, C.A. Can a Strychnos species be used as antiulcer agent? Ulcer healing action from alkaloid fraction of Strychnos pseudoquina St. Hil. (Loganiaceae). J. Ethnopharmacol. 2011, 138, 47–52. [Google Scholar] [CrossRef]
- Silva, M.A.; Rafacho, B.P.; Hiruma-Lima, C.A.; Rocha, L.R.; Santos, L.C.; Sannomiya, M.; Souza-Brito, A.R.; Vilegas, W. Evaluation of Strychnos pseudoquina ST.HIL. leaves extract on gastrointestinal activity in mice. Chem. Pharm. Bull. 2005, 53, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, E.F.B.; Menezes, A.C.S.; Almeida, A.C.S.; Jesus, F.G.; Araújo, M.S. Toxicity of Machaerium opacum (Fabaceae) leaf extracts against fall armyworm (Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). J. Agric. Sci. 2019, 11, 292–301. [Google Scholar] [CrossRef]
- Leite, J.P.V.; Xavier, A.A.; Batista, D.S.; Vital, C.E.; Ramos, H.J.O.; Otoni, W.C. Embryo culture, callus induction, and flavonoid profile of Strychnos pseudoquina A. St.-Hil., an important medicinal species from the Brazilian Cerrado biome. Plant Cell Tissue Organ Cult. 2021, 145, 579–589. [Google Scholar] [CrossRef]
- Kasten Junior, P.; Precetti, A.A.C.M.; Parra, J.R.P. Dados biológicos comparativos de Spodoptera frugiperda (J.E. Smith, 1797) em duas dietas artificiais e substrato natural. Rev. Agric. 1978, 53, 68–78. [Google Scholar]
- Pires Junior, W.; Arini, L.E.S.; Menezes, A.C.S.; Jesus, F.G.; Rocha, E.C.; Araújo, M.S. Toxic effects of the crude methanolic extract of Austroplenckia populnea leaves and fractions on the growth of Spodoptera frugiperda. Acta Agrobot. 2023, 76, 169582. [Google Scholar] [CrossRef]
- Santos, C.K.G. Avaliação da Atividade Citotóxica da Fração Acetato de Etila das Cascas do Caule de Strychnos pseudoquina (Loganiaceae). Magister Scientiae, State University of Goiás: Anápolis, Brazil, 2022. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org/ (accessed on 10 January 2022).
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd ed.; Artmed: Porto Alegre, Brazil, 2009. [Google Scholar]
- El-Aswad, A.F.; Aisu, J.; Khalifa, M.H. Biological activity of tannins extracts from processed Camellia sinensis (black and green tea), Vicia faba and Urtica dioica and Allium cepa essential oil on three economic insects. J. Plant Dis. Prot. 2022, 130, 495–508. [Google Scholar] [CrossRef]
- Sarmento, N.C.; Worachartcheewan, A.; Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial, antioxidant and anticancer activities of Strychnos lucida R. Br. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 122–127. [Google Scholar] [CrossRef]
- Ibrahim, H.; Uttu, A.J.; Sallau, M.S.; Iyun, O.R.A. Gas chromatography–mass spectrometry (GC–MS) analysis of ethyl acetate root bark extract of Strychnos innocua (Delile). Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 65. [Google Scholar] [CrossRef]
- Neto, M.d.S.; da Silva, F.C.; Almeida, A.C.D.S.; Menezes, A.C.S.; Araujo, M.D.S.; de Jesus, F.G. Toxicity of Andira paniculata (Fabaceae) extracts to Helicoverpa armigera (Lepidoptera: Noctuidae). J. Agric. Sci. 2018, 10, 246–271. [Google Scholar]
- Boiça Júnior, A.L.; Souza, B.H.S.; Lopes, G.S.; Costa, E.N.; Moraes, R.F.O.; Eduardo, W.I. Atualidade em resistência de plantas a insetos. In Tópicos em Entomologia Agrícola–IV; Busoli, A.C., Alencar, J.R.C.C., Fraga, D.F., Souza, B.H.S., Grigolli, J.F.J., Eds.; Maria de Lurdes Brandel: Jaboticabal, Brazil, 2013; pp. 207–244. [Google Scholar]
- Lopes, G.S.; Silva, L.B.; Carneiro, E.; Silva Filho, M.L.; Souza, J.S.N.; Almeida, F.A.; Pavan, B.E. Potencial inseticida do extrato etanólico de Anadenanthera macrocarpa (Benth.) em lepidópteros-praga. Nativa 2019, 7, 668–674. [Google Scholar] [CrossRef]
- Lazarević, J.; Jevremocić, S.; Costić, I.; Vuleta, A.; Jovanović, S.M.; Jovanović, D.Š. Toxic, oviposition deterrent and oxidative stress effects of Thymus vulgaris essential oil against Acanthoscelides obtectus. Insects 2020, 11, 563. [Google Scholar] [CrossRef]
- Rajkumar, S.; Jebanesan, A. Bioactivity of flavonoid compounds from Poncirus trifoliata L. (Family: Rutaceae) against the dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2008, 104, 19–25. [Google Scholar] [CrossRef]
- Gautam, K.; Kumar, P.; Poonia, S. Larvicidal activity and GC-MS analysis of flavonoids of Vitex negundo and Andrographis paniculata against two vector mosquitões Anopheles stephensi and Aedes aegypti. J. Vector Borne Dis. 2013, 50, 171–178. [Google Scholar] [CrossRef]
- Inaba, K.; Ebihara, K.; Senda, M.; Yoshino, R.; Sakuma, C.; Koiwai, K.; Takaia, D.; Watanabe, C.; Watanabe, A.; Kawashima, Y.; et al. Molecular action of larvicidal flavonoids on ecdysteroidogenic glutathione S-transferase Noppera-bo in Aedes aegypti. BMC Biol. 2022, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.A.; Souza, S.A.; Domingues, A.; Silva, M.M.M.; Padial, I.M.P.M.; Carvalho, E.M.; Cardoso, C.A.L.; Silva, S.V.; Mussury, R.M. Phytochemical screening and bioactivity of Ludwigia spp. in the control of Plutella xylostella (Lepidoptera: Plutellidae). Insects 2020, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.G. Prospecção Fitoquímica e Avaliação de Mutagenicidade In Vitro de três Espécies Vegetais: Strychnos pseudoquina A. St.-Hil., Coutarea hexandra (Jacq.) K. Schum e Bathysa cuspidata (A. St.-Hil.) Hook; Magister Scientiae, Federal University of Viçosa: Viçosa, Brazil, 2008. [Google Scholar]
- Couto, L.C.; Scádua, F.P.; Codeiro, S.A.; Rodrigues, C.C. Taninos Vegetais ou Polifenóis; UFVJM: Diamantina, Brazil, 2021. [Google Scholar]
- Ayres, M.P.; Clausen, T.P.; Maclean, S.F.; Redman, A.M.; Reichardt, P.B. Diversity of structure and anti-herbivore activity in condensed tannis. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).