Abstract
The growing production of biowaste is causing serious environmental concerns, and composting has emerged as an environmentally friendly solution. This approach contributes to the real circular economy of biowaste, avoiding landfill disposal. This process is flexible as it can be carried out on a domestic or industrial scale. This work focused on the formulation and monitoring of two different composting processes (on a laboratory and domestic scale), to recover biowaste from a university canteen and a rural household, as well as evaluating the quality of the final composts. Three different mixtures of canteen food waste (CFW) and olive wood chips (OWC) were tested at lab scale (CFW:OWC 100:0, 40:60, and 60:40%), with two replicates carried out on the second mixture; a single mixture was tested at the domestic experiment (40:60%). The results showed that both processes reached thermophilic temperatures, with a peak of 65 °C on the lab scale and 75 °C recorded in the domestic composting. Reaching thermophilic temperatures is essential in composting, to maximize the rate of organic matter (OM) decomposition and improve compost quality (e.g., stability and maturation). The moisture content (MC) of biowaste proved to be a critical parameter since the high MC of CFW led to the inhibition of the aerobic process in the mixture without OWC (100% of CFW). On the contrary, a large quantity of OWC (60:40%) showed lower biodegradability due to the presence of lignocellulosic compounds. Analysis of the quality of the final compost revealed that although domestic composting was a process with a low level of control, it allowed obtaining quality compost for agronomic applications, similar to that produced on a laboratory scale. All final composts (after 120 days) were stable and mature, according to the oxygen uptake rate (OUR) and the germination index (GI). Indeed, OUR complied with the regulatory limits (15 and 25 mmolO2/kgOM.h) to be considered soil correctives or organic fertilizers, evidencing the stability of the materials. All composts are non-phytotoxic (GI above 80%), meaning that they are suitable for plant growth. The composts produced retained a significant amount of carbon (40–70%), with a high value for returning carbon to the soil in stable OM forms. Thus, when applied to the soil, a significant amount of carbon is carried to this compartment, making a valuable contribution to closing the carbon cycle and avoiding the emission of CO2 into the atmosphere. Overall, it was possible to conclude that biowaste from university canteens and households can be recovered by composting, as long as it is mixed with a bulking agent (such as OWC), which promotes the process and improves the properties of the composts.
1. Introduction
In the European Union (EU), in 2022, the production of waste reached around 2135 Mt, resulting from economic and domestic activities, without accounting for the amount of waste that has been improperly disposed of [1]. The production of municipal solid waste (MSW), which includes biowaste, has been increasing over time due to rapid population growth, large-scale urbanization, and industrial development [2]. In most countries, biowaste represents around 30 to 40% of MSW [3]. Effective collection of organic waste is vital for both environmental and operational reasons, given that a significant portion still ends up in landfills [4]. Sustainable Development Goal (SDG) 12 emphasizes the efficient recycling of organic waste to reduce reliance on fertilizers and promote sustainable agriculture [5]. Recycling organic waste also helps decrease landfill disposal, aiding in closing the nutrient cycle and reducing methane emissions by approximately 62% [6]. Composting can be an alternative to landfill disposal, which aligns with SDG 13 for Climate Action, as it reduces greenhouse gas emissions [5]. Additionally, applying organic fertilizers like compost to soil allows organic carbon from biowaste to directly contribute to reducing carbon dioxide (CO2) emissions by fixing it in the soil [7]. This process aims to mitigate climate change and promote more sustainable waste management practices. Moreover, selective biowaste collection reduces unsorted waste, leading to less contamination and more efficient waste management systems. This not only improves overall management performance but also reduces costs for municipalities or other operators [4].
To face the problem of biowaste, on 30 May 2018, the European Union (EU) approved Directive (EU) 2018/851 of the European Parliament and of the Council amending Directive 2008/98/EC on waste, which aims to promote the circular economy and reduce the environmental impact caused by biowaste, ensuring its importance in global waste management [3,4]. This Directive defines biowaste as “biodegradable waste from gardens and parks, food and kitchen waste from households, restaurants and retail establishments, as well as waste from food processing facilities” [8]. In this way, biowaste comprises all naturally occurring materials from kitchens (whether raw or cooked), gardens, or yards that are biodegradable. Biowaste can also be classified into “Green” or “Brown” based on composition in terms of carbon and nitrogen. “Green” organic waste is rich in nitrogen and generally wet and typically includes food waste, household generated materials, eggshells, coffee grounds, vegetable and fruit remnants, and damp leaves. “Brown” organic waste is rich in carbon and usually dryer materials, as is the case with most garden waste, such as wood chips, dried leaves, and straw [9]. This organic waste can also be categorized according to its source as agriculture and agro-industry, animal waste, food processing and paper industry, forest and wood processing, and municipal waste. Biowaste must be managed with caution since it can be decomposed under aerobic and anaerobic conditions [10].
In this context, the European Green Deal (EGD) is a noteworthy EU initiative aimed at achieving climate neutrality by 2050, aligning with the Paris Agreement. EGD outlines strategies for sustainable growth, circular economy (CE) promotion, biodiversity preservation, and pollution reduction [11,12]. In particular, the Farm to Fork (F2F) strategy is central to the EGD and focuses on sustainable food production and waste reduction along the supply chain [11,12]. Adopting a CE approach, F2F aims to minimize the environmental impact of resource extraction, thereby assisting in the restoration of biodiversity [12]. CE plays a crucial role in biowaste management, starting from food production to waste valorization, including composting as a key technology.
As mentioned above, biowaste represents a significant portion of MSW and it is essential to promote the valorization of this waste (resources) through conversion processes to turn it into useful products [13,14,15]. According to the Portuguese Environmental Agency and the National Statistics Institute, each Portuguese citizen produces an average of 180 kg of food waste per year. The valuable products obtained from biowaste can include the generation of heat and energy, solid or liquid fuels, the creation of biomaterials, and chemical products such as biofertilizers, among others [16]. Nevertheless, the aim is as much as possible to provide an alternative to disposing of biowaste in landfills [15]. The two most common and efficient recycling methods for managing biowaste are composting and anaerobic digestion, which are above the waste hierarchy compared to landfills. Both methods are biological processes that can be implemented sequentially, where the biowaste is initially processed through anaerobic digestion to generate biogas, and the resulting sludge (digestate) can be further stabilized through composting, where a compost with favorable properties for the soil can be produced. Moreover, biowaste can be used as fuel to generate energy through incineration, where there is a reduction of around 80 to 90% in the volume of waste. However, this method emits greenhouse gases (GHGs) and other pollutants into the atmosphere [3,16]. Indeed, the emissions of GHGs are generated in any type of waste treatment, some with more impact than others. Thus, it is important to understand the environmental implications of each treatment process (biological vs. thermal treatment). For example, composting (biological treatment) degrades organic waste under aerobic conditions, generating compost and releasing H2O, CO2, and other gases (e.g., CH4 in minor quantities). CO2 is the main GHG, generated by microbial respiration during the decomposition of organic materials, while CH4 can be emitted if anaerobic conditions are observed within the composting mixture Clique ou toque aqui para introduzir texto. Typically, well-controlled aerobic composting minimizes CH4 emissions. On the other hand, thermal treatment, such as incineration, involves oxidation of waste at high temperatures, generating large emissions of H2O, CO2, some carbon monoxide (CO), and nitrogen oxides (NOx), contributing to global warming and air pollution [16]. According to the literature, the environmental impact in terms of GHGs may be −347 to 2969 kg (CO2,e/ton FW) depending on the multiple parameters of food chains and waste management systems [16].
University canteens generate a significant amount of food waste every day, consisting mainly of food remains resulting from the preparation of meals or not consumed food. If collected properly, this biowaste may be valorized by composting. Some literature has evaluated the environmental and cost impacts of food waste in university canteen from a Life Cycle Perspective (LCA) [17]. This type of study highlighted not only the amount of waste generated in the university canteens but also the GHG emissions and cost of wasted food in the supply chain. However, many questions are still open, and the present study intends to contribute to identifying constraints in this field.
Composting is an aerobic biological process that stabilizes organic matter through exothermic biochemical reactions to obtain a humified material commonly known as compost [18,19,20]. This process is essential in waste management systems to reduce and reuse the biowaste generated, for example, the waste generated in university canteens and household biowaste [21]. In practice, this process mimics nature by transforming biowaste into a natural fertilizer or soil improver, with benefits to soil conditions [22,23].
The final compost can be considered a soil improver because when applied to the soil it improves its physical and chemical properties. In this way, by applying compost into the soil, a certain amount of organic matter is introduced in the soil that will improve its structure and porosity, making it less susceptible to compaction, and will stimulate biological activity, increasing its water retention capacity [24]. It is important to note that the addition of this product reduces the dependence on chemical fertilizers, since the nutrients present in the compost are released slowly into the soil, making them continuously available to the plants. Moreover, it is possible to reduce the environmental impacts associated with the use of these synthetic fertilizers resulting in fertile soil, as it provides nutrients and water for healthy plant growth [25].
Composting is a process that can be implemented and controlled on the lab scale, industrial scale, as well as domestic scale [26,27]. Continuous monitoring of different parameters is essential (mainly at lab and industrial scales) to ensure the adequate operation of the composting process, such as temperature, aeration, moisture, and oxygen uptake rate [21,28]. However, domestic composting presents additional challenges, such as the fact that society is unaware of the basic aspects of this process and its purpose. Lack of knowledge leads to unnecessary wastage of biowaste and poor reuse of resources. To implement the composting process, one of the obstacles that may arise is achieving thermophilic conditions (>40 °C) [29]. In fact, it is necessary to guarantee a minimum volume of biowaste to achieve self-heating without insulating the composters. Those volumes are rarely available at the domestic scale, and thus, composting is often a mesophilic process at this scale. Moreover, reaching temperatures above 55 °C is essential to guarantee the hygienization of the final compost and a high level of organic matter stabilization, as demonstrated in the study carried out by [30].
Biowaste composting has been studied on a laboratory scale and has proved to be an efficient process to produce a soil amendment [31]. Temperatures above 55 °C are easily reached over a required period of time, and the parameters monitored throughout the process are within the limits established by the literature, such as the case with moisture with values between 60% and 80% and pH in the range of 7 (close to neutrality) [31]. On the other hand, concerning domestic- [26] and laboratory-scale composting as well [19], the use of a bulking agent may be crucial for guaranteeing aerobic conditions in the mixture and the generation of heat to reach thermophilic temperatures. In general, in the case of passive domestic operation, the regular turning of the mixture provides adequate aeration for the biological activity.
Assessing the quality of the final compost is a critical factor, as it must meet the constraints to act as a good organic fertilizer or soil improver. Several parameters can be determined to assess the compost quality, such as carbon-to-nitrogen (C/N) ratio, organic matter content, total N, total P, other relevant elements (e.g., B, Cl, and Na), pH, salinity, phytotoxicity, maturity, stability (e.g., oxygen uptake rate), and other safety parameters (pathogens and potentially toxic heavy metals) [18,31]. Depending on the starting raw material and the compost applications, the quality parameters can be a subset of those. When the compost meets the regulatory requirements, it can be applied to the soil, resulting in an improvement of the physical, chemical, and biological properties of soil, namely in terms of increasing water holding capacity, hydraulic conductivity, and nutrients [24,25,32]. Comparing the compost quality obtained from domestic and industrial composting facilities, it has been observed that in many cases the compost obtained in domestic systems has similar and sometimes better quality than that obtained in industrial facilities [26].
The main objective of this work is to formulate and monitor different composting processes, at the laboratory and domestic scale, using biowaste from a university canteen and biowaste produced in a common rural household, as well as to assess the quality of the final compost produced in both processes. As a bulking agent, to ensure adequate processing of biowaste by composting, olive wood chips were tested in both situations taking into account the regional availability of this material.
2. Materials and Methods
2.1. Materials
In this study, food waste (FW) and olive wood chips (OWCs) were used as starting raw materials for the composting mixtures. In laboratory-scale experiments, FW was obtained from a canteen (CFW) at the University of Coimbra, consisting predominantly of carrots peel, lettuce, and cabbage remaining in meal preparation. In fact, the sample CFW did not entirely represent all canteen biowaste, as only the vegetables used/wasted in meal preparation were used. For the domestic experiment, the domestic food waste (DFW) was collected in a family of 4 people, who live in a rural house and grow vegetables and some fruit trees for their own consumption. DFW was collected over 6 days and consisted predominantly of different waste of cabbage, lettuce, and some carrot and onion peeling.
The CFW was manually reduced to a size of between 2 and 3 cm, to facilitate the sampling and homogenization with OWC. In the case of DFW, a manual gross reduction in size was carried out (between 10 and 15 cm) to simulate what an ordinary citizen would do in this situation. For the preparation of the bulking agent, OWC, olive branches were crushed using a mechanical wood shredder. The materials used in this study (CFW, DFW, and OWC) are shown in Figure 1, before the mixing step. The appearance of OWC is depicted in Figure 1c, where leaves and branches from the olive pruning are well visible. The composition of these materials is given in Table 1.

Figure 1.
(a) CFW used in the laboratory; (b) DFW used at domestic scale; and (c) OWC used in both composting processes as a bulking material.

Table 1.
Characteristics of the materials used in the composting process.
It should be noted that seasonality can be a relevant factor in the composting of rural household biowaste, due to the use of vegetables produced in greater quantities at certain times of the year (e.g., cabbage). In the case of CFW, seasonality issues are not significant, taking into account the type of waste used.
2.2. Composting Experiments
The laboratory composting experiments were conducted in four self-heating reactors (SHRs), with a volume of 106 L each, operating in batch mode. All SHRs were the same size, shape, and other technical characteristics, with the configuration indicated in Figure 2a,b and with more details indicated elsewhere [20]. The reactors were isolated with a rubber-based elastomeric material (Aeroflex MSR) and had two holes in the lid, one for the temperature sensor (located in the geometric center of each reactor) and another for the exit of gas flow (formed during the process and excess of air). The reactors were aerated by introducing compressed air at the bottom of the SHRs. A perforated acrylic plate was installed 0.1 m above the bottom, creating an empty space that allows the passage of air and the collection of leachates.
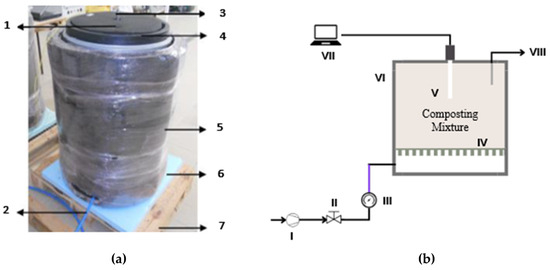
Figure 2.
(a) The self-heating reactor used for laboratory composting and (b) schematic representation of the system associated with each SHR. LEGEND: 1—gas outlet; 2—air entrance; 3—opening for the temperature sensor; 4—SHR lid; 5—thermal insulation material (Aeroflex MSR); 6—extruded polystyrene foam; 7—wood pallet. I—air compressor; II—airflow valve; III—airflow meter; IV—perforated plate for airflow distribution at the bottom of the mixture; V—temperature sensor; VI—thermal insulation; VII—datalogger module; VIII—gas outlet from the composting mixture.
The laboratory composting involved the preparation of four well-homogenized mixtures, according to Table 1. These mixtures were introduced in the four SHRs (hereupon referred to as LR1, LR2, LR3, and LR4). Two of them (LR2 and LR3) are identical, to analyze the replication level of composting over time. Each composition and mass of the mixture to fill a volume of about 100 L are indicated in Table 2.

Table 2.
Composition of the mixtures tested in each reactor (LR1–LR4).
Before starting the composting process, it was necessary to collect CFW from the canteen and then reduce the particle size in the laboratory in order to facilitate sampling during the monitoring of composting. However, the daily amount of CFW collected in the canteen was variable and the ability to adjust the size in the laboratory was limited. For these reasons, CFW had to be preserved in the refrigerator before being introduced into the reactors to begin the composting process. In particular, due to the high amount of CFW required for the LR4 experiment, as can be observed in Table 2, this reactor was filled in two phases: 43.0 kg at first and 4.7 kg the next day to complete the initial mass required. During the composting experiments, the temperature inside the mixture and the oxygen concentration at the reactor outlet were monitored. The temperature was continuously monitored using a sensor (datalogger module—Virtual HMI CSMSTRSX, Redlion), recording data at 10 min intervals. The oxygen concentration at the outlet flow was monitored using a sensor (GAS DATA—GMF 406) by inserting the probe into the lid hole in the reactor, and the readings were performed four times a day. During the experiments, the airflow rates were adjusted to guarantee aerobic conditions but without excessive aeration (oxygen concentration in the range of 5–15%). Thus, LR1–LR4 were aerated with an initial flow in the range of 1.32 to 2.47 mL/min.kgVS, decreasing the flow over the composting time due to the lower oxygen consumption per kg VS.
In addition, other parameters were monitored over time, such as moisture content (MC), organic matter (OM), pH, electrical conductivity (EC), and C/N ratio. For that, representative samples were collected on the 1st, 5th, 12th, 19th, 26th, and 30th days of operation. During this period, the homogenization of the mixture was ensured once a week by rolling the reactors on their axial axis. After the 30th day, the mixtures were removed from the reactors and placed in a pile in open air conditions at room temperature, beginning the maturation phase. Two additional samples were taken during this phase (60th and 90th days). The collected samples were stored in refrigerated conditions until further analysis.
For the domestic composting experiments, a wooden composter was built, as shown in Figure 3, with a capacity of around 346 L. At the bottom of the vessel, an opening was made in the center to allow the drainage of the leachate eventually produced. A lid was placed on top of the composter to minimize the influence of variability of the ambient conditions (e.g., solar radiation and precipitation). To facilitate air circulation by natural convection, around 150 holes with 8 mm diameter were made on the sides of the composting container.
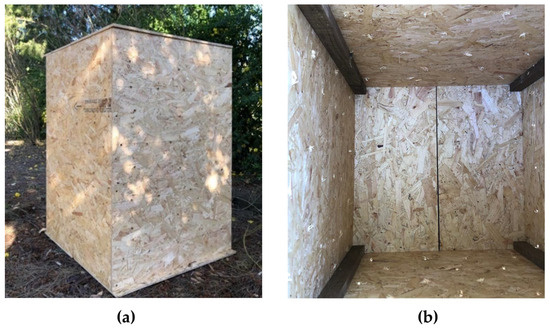
Figure 3.
(a) Composter used for domestic composting and (b) view inside the composter.
Due to the significant initial amount of DFW needed to fill the domestic composter, this experiment was split into two phases. In the first phase, the fraction of the biowaste and bulking agent (about 58.85 kg) was placed in the composter (as a homogenized mixture of DFW and OWC). The second phase began after 15 days, by adding 45.62 kg of a similar mixture. The total mass composted was 104.47 kg, which comprehends 62.68 kg of DFW (60%) and 37.32 kg of OWC (40%). Throughout the process, the height of the mixture was recorded once a day, and the temperature was monitored with a digital thermometer placed in the geometric center of the mixture. The parameters monitored over time in domestic composting were similar to laboratory composting, except for the oxygen concentration, which was not measured in this case. The samples for domestic composting were taken on the following days: 1st, 5th, 19th, 26th, 33rd, 40th, and 45th. After 45 days, the mixture was removed from the composter and placed in piles in the open air to initiate the maturation phase. The samples collected during the maturation phase were taken on the 75th and 105th days. On the sampling days, the mixture was turned to ensure homogenization.
2.3. Analytical Methods
The MC was determined using the gravimetry technique, with three replicates for each analysis (as well as for all the other parameters). The crucibles containing a representative sample were placed into an oven at 105 °C for 24 h. The total solid (TS) content corresponds to the dry mass of the samples (%) and was determined by the difference between the mass sample (100%) and its MC (%). The OM content was determined as volatile solids (VS) by calcination of the dry sample at a constant temperature of 550 °C for a period of 2 h. The pH and electrical conductivity (EC) were determined using specific electrodes applied to the aqueous extracts obtained at a liquid-to-solid ratio of 10 mL/g. The suspensions were placed in an orbital shaker at constant rotation for 2 h. After centrifugation to separate the supernatant, pH and EC were measured with a Multi-parameter Analyzer Consort C1020. The elemental analysis, namely, C and N content, was determined with Elemental Analyzer NA 2500, after drying and milling the samples as a fine powder. The oxygen uptake rate (OURp), gO2/kgVS.h, over the composting process, was determined by Equation (1) [27]:
where Q (L/h) is the airflow rate, C_O2 (mL/L) is the O2 in the inlet (i) and the outlet (o) airflow, 1000 is the conversion factor from mL to L, Vg (L/mol) the volume of one mole of gas at inlet conditions (P, T), 31.98 g/mol is the molecular weight of O2, and vs. (kg) is the mass of the OM of the composting mixture.
The quality of the final composts was analyzed on day 120, regarding the respirometry and GI. The respirometric analyses for testing the final compost in an aqueous matrix were carried out following the standard EN 16087-1: Soil improvers and growing media—Determination of the aerobic biological activity—Part 1: Oxygen uptake rate. The equipment used for this test was the Oxitop®, which consists of a hermetically sealed vessel with a capacity of 1 L, equipped with a sensor at the top to measure the drop in pressure over time due to oxygen consumption. Initially, different solutions were prepared, such as a pH buffer, a solution of micronutrients and macronutrients, a nutrient solution, and a nitrification inhibitor. Then, the pressure drop profiles were recorded over time, and the oxygen consumption was determined as the respirometric activity, RA, expressed in mmolO2/kgOM, according to Equation (2) [33]:
where ΔP is the pressure variation (kPa), R is the universal gas constant (mL.kPa/K.mol), T is the temperature (°C), W is the sample weight (kg), DM is the dry matter content of the sample (%), OM is the organic matter content (%), and Vgas is the gas volume in the vessel (mL). Afterward, the OURf measured in the final compost is calculated by dividing the RA value by 72 h (after the pressure inside the vessel attained 0 kPa).
Based on the EN 10687-1 standard, it is also possible to establish the maximum limits that OURf must comply with for compost to be considered an organic fertilizer (25 mmolO2/kgOM.h) or an organic soil improver (15 mmolO2/kgOM.h).
The overall yield of compost produced, on a dry basis, , was determined by Equation (3):
where MTSf is the mass of total solids in the mixture at the end of the composting process (kg) and MTSi is the mass of total solids in the mixture at the start of the process (kg).
The overall yield of carbon retained in the final compost, FCretained, on a dry basis, was determined by Equation (4):
where CMf is the total mass of carbon, on a dry basis, at the end of the composting process (kg) and CMi is the total mass of carbon at the beginning of the process (kg).
The phytotoxicity was assessed based on the germination index (GI), where seed germination (Lepidium Sativum L.) was tested in aqueous extracts using petri dishes. For obtaining those extracts, suspensions of the testing solid materials (L/S 10 mL/g) were agitated on an orbital shaker for 24 h. The suspensions were centrifuged at 400 rpm for 20 min and the supernatant, 5 mL, was poured into the petri dishes, where 10 seeds were placed over a filter paper. The petri dishes were placed 48 h in an oven at 25 °C at dark conditions. The germination of Lepidium Sativum L. seeds was evaluated by measuring the number of seeds germinated and the root growth. The same measurements were conducted in control experiments (with deionized water). The GI (%) was calculated based on the relative seed germination (%) and the relative root growth (%), according to Equation (5) [27]:
where ANGS is the average number of germinated seeds, ARL is the average root length (cm), (a) the test with the compost sample, and (b) the test with distilled water. Table 3 shows the phytotoxicity scale used in terms of GI to classify the composts produced in this work.

Table 3.
Classification of phytotoxicity in the compost generated [34].
3. Results and Discussion
3.1. Characterization of Mixtures
In this study, mixtures composed of CFW and OWC at the lab scale and DFW and OWC at the domestic scale were tested. Four mixtures were processed in self-heating reactors—LR1, LR2, LR3, and LR4. The mixture of the domestic reactor (DR), similar to LR2 and LR3, was tested on a domestic scale. Table 4 shows the main characteristics (mean ± standard deviation) of the initial mixtures.

Table 4.
Characteristics of the initial mixtures tested in composting at the lab (LR1 to LR4) and domestic scale (DR).
The mixtures LR2, LR3, and DR contain a higher MC than LR1, while mixture LR4 reveals a very high MC (92.93%). The differences in MC are explained by the fact that LR1 contains 60% of OWC as a bulking agent (characterized by 37.97% of MC), while LR2, LR3, and DR comprise only 40% of OWC. The very high MC in mixture LR4 proved not to be suitable for the composting process [34].
Regarding OM (measured as VS), it is evident that high values (>89%) were observed in all mixtures due to the biological nature of the substrates. Biodegradable OM content plays a crucial role in the composting process since microorganisms require carbon as a source of energy and other nutrients (e.g., nitrogen) to grow and speed up the process until the mixtures are stabilized. This parameter is directly related to the efficiency of composting and can also influence the pH and MC levels in the compost pile [35].
Concerning the pH, a slightly acidic condition is observed in all cases, in particular for LR4. In fact, the pH values of the materials to be composted affect microbial activity and can inhibit the process. Typically, pH should be between 5 and 9 [36]. High pH values (close to 10) cause a latency period, while low pH values (below 5) slow down the temperature rise and inhibit the growth of thermophilic bacteria and actinomycetes [37]. As indicated in Table 1, both CFW and DFW have a pH below 5, probably because hydrolysis and organic acid formation occur rapidly in these types of biodegradable substrates. Also, for this reason, the addition of OWC (pH 5.54) is relevant to increase the pH of initial mixtures. For LR4, this correction did not occur since the mixture is only made up of CFW with a pH of 4.29. This fact also contributes to the failure of composting based solely on CFW.
The C/N ratios of the mixtures are within a favorable starting point, while the DR mixture presents a slightly lower value [38]. The optimal ratio for C/N in composting is typically around 25 to 30 parts of C to 1 part of N (by mass) [38]. This ratio ensures that carbon found in C-rich materials predominates in the mixture. Indeed, at lower ratios, N is supplied in excess and will be lost as ammonia gas (creating bad odors), while higher C/N ratios mean that N is scarce for optimal growth of microorganisms (mixture does not heat up and degradation of OM occurs at a slow rate). The low C/N ratio in the case of DR explains the fast heating up of the material [39]. To address this imbalance, OWC was used as a C-rich agent. Moreover, OWC was used as a bulking agent in the lab and domestic composting processes, due to its low MC, low density (high interparticle porosity), and adequate particle size to provide structural support and maintain air spaces within the composting matrix. On the contrary, food waste has a high MC, affecting proper aeration (anaerobic zones appear) and promoting the formation of leachate, as well as high amounts of nitrogen (low C/N ratio). By adding OWC, excess water from CFW and DFW was absorbed and free air space remained adequate for ensuring aerobic conditions, improving microbial activity [19]. The addition of this bulking agent was crucial to promote adequate aeration, since ventilation through empty spaces is easier [40]. Also, for this reason, composting LR4 failed (as discussed in the next section), because no bulking agent was used in this experiment.
3.2. Lab-Scale Composting
It should be noted that the beginning of the composting laboratory experiments was quite demanding in logistical terms due to the high quantities of materials to be processed to obtain homogeneous mixtures and completely fill the four SHRs. Globally, LR1 to LR4 required 27.7, 29.7, 29.6, and 47.7 kg of homogenous mixtures (Table 2), respectively. These composting experiments were monitored over time, in terms of the most relevant process variables. Figure 4a,b illustrates the temperature profiles over time in the reactors containing mixtures made up of CFW and OWC (LR1 to LR3) and only CFW (mixture LR4). A rapid temperature rise can be observed, reaching the maximum values of 58 °C in LR1, 61 °C in LR2, and 65 °C in LR3 between the 1st and the 2nd days. These high-temperature values were maintained for a few days, followed by a gradual decrease until ambient temperature. This behavior is in line with the typical phases of the composting process [19,41]. The processes began with the mesophilic phase, which lasted less than one day, characterized by temperatures below 40 °C. It is in this phase that microorganisms multiply rapidly, due to the presence of easily degradable OM. Thus, this phase is very active, with intensive heat production, increasing the compost temperature to values that inhibit the activity of mesophilic microorganisms [42]. The thermophilic phase (temperatures > 40 °C) appears, having remained for 15 days in the LR2 and LR3 mixtures and 20 days in the LR1 mixture. This increase in temperature is due to the maximum decomposition of OM, which is fundamental for the quality of the compost, since high temperatures favor the elimination of pathogens and also destroy weed seeds. However, temperatures should not exceed 65 °C; in this way, the organisms are eliminated and composting can stop. To counteract this increase, the mixture can be remixed or the aeration flow rate increased. The second mesophilic phase reappeared due to the decrease in the amount of biodegradable matter present in the mixtures [34]. As temperatures approached ambient conditions, the maturation phase was developed to complete the humification of the composts [39]. This phase is essential in the composting process because it is during this period that the compost undergoes its final maturation, acquiring properties suitable for use in soil applications. The maturation phase lasted 2 months, during which the mixtures were left outside reactors. Monitoring the maturation phase involved checking the appearance of the compost (namely, the MC, color, and growth of fungi) and the odor (gradually reaching a phase in which a pleasant earthy smell is released). The temperature of the compost at this phase is expected to gradually decrease until it reaches ambient temperature. This parameter indicates that microbial activity is low (intense exothermic decomposition processes are no longer relevant).
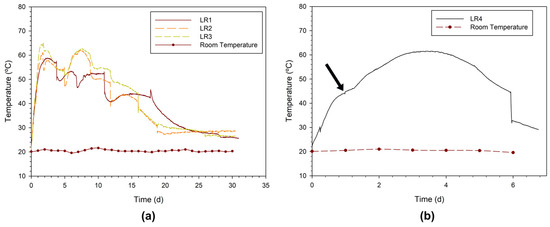
Figure 4.
Temperature profiles for reactors (a) LR1, LR2, and LR3; (b) LR4 (the arrow ↓ indicates the moment when the reactor was completely filled).
It is important to note that throughout the experiment, there was a periodic necessity to open the reactors to collect samples and homogenize the mixtures, which influenced the process and resulted in perturbations in the temperature profiles as can be observed in Figure 4.
Figure 4b depicts the temperature profile in LR4 observed using a single substrate (CFW), which means without any bulking agent. The evolution of the temperature reached a maximum value on the 3rd day, of about 61 °C. In this case, the initial mixture contains a very high MC (92.93%) as observed in Section 3.1, due to the intrinsic moisture of the raw material used (fresh peels of fruits and vegetables) plus due to the washing procedure before preparing meals. The reactor filling phase took place in two phases (43.0 kg on the 1st day and 4.7 kg on the 2nd day), while the temperature increased during this period, reaching 61 °C. However, the progressive degradation of the OM associated with the microbiologic activity led to liquefaction of the mixture (with intense production of leachate), which also filled the empty space between the materials and the bottom of the SHR. Under this condition, oxygen aeration was incipient, because of the low solubility of O2 in aqueous solutions, and anaerobic conditions prevailed inside the mixture. The liquefaction of the CFW led to the release of unpleasant odors (mostly ammonia), especially on the 5th day of the experiment. On this day, the recorded temperature decreased because the sensor was not submerged in the mixture but in the headspace. This fact was due to a significant decrease in the volume of the mixture. Because of these problems, it was decided to stop the experiment, as it is clear that aerobic composting of CFW is not a good starting point. Table S1 in the Supplementary Information shows a sequence of images from days 0, 5, 12, 19, 26, 30, 60, and 90 to visualize the evolution of the mixtures throughout the composting process in the laboratory (inside and outside the reactors). It can be seen that the CFW in LR4 suffered partial liquefaction over the 5 days that it remained in the reactor.
To ensure an aerobic environment in the composting process, the mixtures were aerated with air to provide oxygen. The flow of air introduced into the reactors was regulated based on the concentration of oxygen in the outlet gas. This control aimed to maintain a minimum volume of 5–15% oxygen in the interparticle spaces [43]. Supplementary Information (Figure S1) shows the oxygen concentration (%) and CO2 (%) profiles at the outlet flow of the reactors during the composting process. These profiles show that the concentration of O2 is inversely related to the concentration of CO2 produced due to the biological oxidation of OM, which is an exothermal reaction. Indeed, aerobic microorganisms consume O2 and nutrients and produce CO2 and H2O plus other minor gases (e.g., NH3) [44]. It is important to note that during the thermophilic phase, CO2 concentration was mostly higher than 5%, while in the second mesophilic (after the first 15 days), CO2 concentration is low (<5%) as the oxidation reactions are less intense [45]. For example, in reactor LR1, there was a decrease from 4.2% to 2.6% CO2 between days 20 and 34 (corresponding to an OURp of 0.055 and 0.006 gO2/kgVS.h, respectively).
Based on the O2 profiles (Figure S1 in the Supplementary Information), it was possible to determine the OURp (Figure S2 in the Supplementary Information). At the beginning of the process, there was a significant increase in the OURp on the first day in reactors LR2 and LR3, reaching maximum values of 17.87 gO2/kgOM.h and 24.61 gO2/kgOM.h, respectively. The high OURp reflects the intense microbial activity taking place during the process [27]. After the thermophilic phase, the OURp decreases reflecting the lower aerobic activity due to low biodegradable organic compounds at this stage. The final values measured in LR1, LR2, and LR3 were 0.04, 0.05, and 0.05 gO2/kgOM.h, respectively. These observations are in line with the findings of other authors [46], who indicated that from day 21, OURp values reached approximately 0.11 gO2/kgOM.h. Another indicator that is easy to monitor through weighing is the decrease in mass in the reactors, which is due to the degradation of OM and conversion to CO2 and H2O (Figure S3 in the Supplementary Information). After the process was completed, there was a reduction on a wet basis from 27.7 to 21.0 kg (equivalent to an overall yield of compost produced, on a dry basis, of 91.3%TS) in LR1, from 29.7 to 16.4 kg (yield of 62.9%TS) in LR2, and from 29.6 to 20.3 kg (yield of 78%TS) in LR3. During the period inside the reactors, approximately 500 g of mixture was taken for monitoring purposes in each case.
According to Figure 5a, the MC profiles remained relatively constant during the time the mixtures were inside the SHRs, from day 1 to day 33. Mixture LR1 was characterized by an MC near 60%, whereas LR2 and LR3 contained between 70% and 80% due to the prevalence of CFW in their composition. As aforementioned, high MC in the mixture may hinder the flow of oxygen, thus favoring the appearance of anaerobic zones [34]. After the mixtures were removed from the reactors, the MC decreased due to evaporation loss to the surrounding environment. The OM content was quantified through volatile solids, and according to Figure 5b, this parameter remained above 85% for all samples analyzed, while at the beginning, the OM was around 92% in the three mixtures. The pH plays a crucial role in this process, as it provides information on the state of decomposition of the OM and influences the activity of the microorganisms [21]. Figure 5c shows that initially, the mixtures had a low pH (around 5), whereas as the composting progressed, it gradually increased. On the 90th day, the compost pH is in the range of 8–8.5, which reflects the process of maturation and stabilization of the materials [47]. Regarding the electrical conductivity (EC), Figure 5d shows a gradual evolution of this parameter over time. At the end of the process, the EC was 2.85 mS/cm for reactor LR1, 4.87 mS/cm for LR2, and 4.58 mS/cm for LR3. EC (related to salinity) is proportional to the concentration of ions present in the solution obtained by extracting the compost and is a common indicator of quality [48]. The salinity may play a role if a high load of compost is applied to the soil by hindering growth or the germination of seeds. The levels of EC measured in this study do not compromise its application since the criterion of quality commonly is that EC should be lower than 4–5 mS/cm [48].
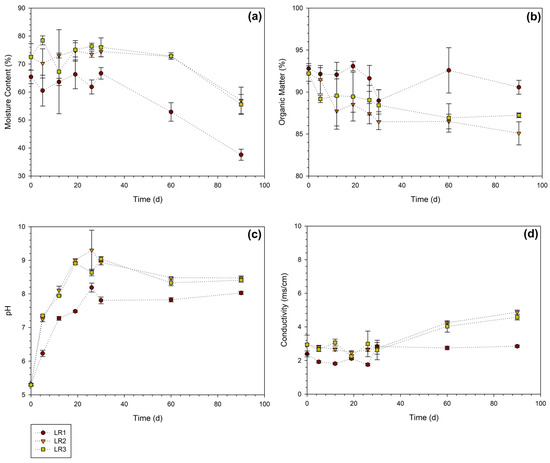
Figure 5.
Variation in parameters throughout the laboratory composting process: (a) MC, (b) OM content, (c) pH, and (d) electrical conductivity.
The experience of composting on a laboratory scale provided valuable lessons regarding the optimization of the process and possible scale-up. In particular, the high moisture in CFW requires adequate management to ensure the success of the process. In this case, the risk of dryness is low whereas the excess moisture can lead to leachate formation, which complicates the operation of the process regarding the microbial activity and the efficient decomposition of organic waste. A satisfactory aeration is required to avoid the formation of anaerobic zones in the mixture, which can be challenging if a bulk agent is not used, which results in incomplete decomposition and release of unpleasant odors. The C/N ratio is typically low if CFW is the only substrate, and thus, a correction should be required. The bulking agent may help in this regard since they are commonly C-rich materials. If this adjustment is not made, there is a risk of fast decomposition and intensive ammonia release. The particle size of CFW may also require some adjustment to facilitate homogenization with bulking material, while the size reduction should not be excessive so as not to impair aeration.
3.3. Domestic Composting
In the domestic composting experiment, a mixture with a similar composition to reactors LR2 and LR3 was tested to compare the two processes. In this case, also OWC was used as bulking material, while other similar materials (other shredded branches, straw, and wood chips) available nearby may be used to achieve a similar effect. Figure 6a shows the temperature profile achieved after 58.85 kg of the mixture was introduced in the composter, where it can be observed that the temperature increased gradually until 51 °C (on day 9). However, as with lab experiments, the temperature decreases as biodegradable matter decomposes. Moreover, it should be mentioned that during the day, the ambient temperature reached 30 °C or more, while at night it dropped to around 15 °C, with episodes of some precipitation. Then, on the 15th day of the experiment, 29.25 kg was added to the domestic composter (this event is marked in Figure 6a,b with an arrow). As a result, the temperature rose again, reaching in this second phase a maximum of 74.8 °C. There was then a gradual temperature decrease until reaching the ambient temperature. Thus, taking into consideration that the vessel was filled through two additions, the global temperature profile was according to the expected behavior. This temperature rise is essential to destroy possible pathogenic organisms present in the mixture and increase the biological degradation rate until the maturation phase [39].
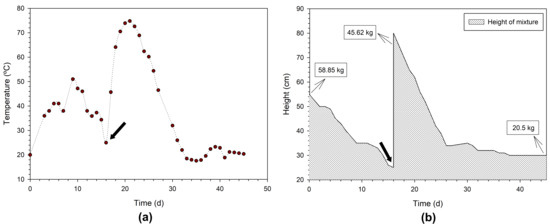
Figure 6.
Monitoring of domestic composting: (a) temperature profile and (b) mixture height variation over time (the arrows ↓ indicate the moment of the second addition of biowaste to the composter).
Figure 6b illustrates the evolution of the height of the mixture in the reactor over time, showing that the height gradually decreased after each addition of the biowaste to the reactor. The second addition of DFW aimed to take advantage of the reactor volume to produce more compost at the end and also ensure that thermophilic conditions lasted longer inside the reactor. In the end, from the total mass introduced (104.47 kg on a wet basis), only 20.50 kg (on a wet basis) remained as final compost, which corresponds to a yield of 44.8% TS (dry basis).
According to Figure 7a, the MC remained relatively constant during the time the mixture was in the reactor, with an MC of approximately 65%. However, it is important to note that the amount of moisture in domestic composting is lower than the one previously observed in laboratory experiments since the DFW used contained only its intrinsic moisture from biowaste. Typically, the optimal range of MC in composting is between 45% and 65% [47]. When the mixture was removed from the reactor and placed in an external pile, the MC dropped significantly due to water losses through evaporation due to the ambient conditions. In this case, it was necessary to add water three times (on the 47th, 50th, and 57th days) because the compost had undesirable levels of dryness. MC correction was based on visual inspection and the practical rule of “holding the sample in hand and not getting it wet”. Figure 7b shows the variation in the OM content of the mixture, which was around 93%TS at the start of the process. After the initial decrease in OM, a sudden increase was observed between the 12th and 19th days, because of the introduction of more biowaste on the 15th day. The OM stabilized slightly above 85% from the 45th day onward. In fact, in this phase, the activity of microorganisms is very small (temperature close to the ambient conditions, Figure 6a) because only the biorefractory organic matter remains in the process. Figure 7c illustrates the typical pattern of pH evolution during the composting process, starting as acidic and then increasing over time (this pattern is observed twice due to the second addition of DFW). Initially, the mixture has a pH close to 6, and during the thermophilic phase, the pH increases to alkaline values, between 7.5 and 9, due to the release of ammonia. Typically, as the composting evolves, the pH changes to alkaline conditions during the stabilization and maturation processes [47]. Figure 7d depicts the profile of EC over time. The effect of the addition of biowaste is well observed in this parameter, reaching 6.24 mS/cm, while in the end, the value tends to 5 mS/cm. In fact, the addition of OM on the 15th day caused an elevation in the temperature of the reactor (Figure 6a), which led to higher OM degradation and consequent formation of organic acids, causing a decrease in pH (Figure 7c) and an increase in EC (Figure 7d).
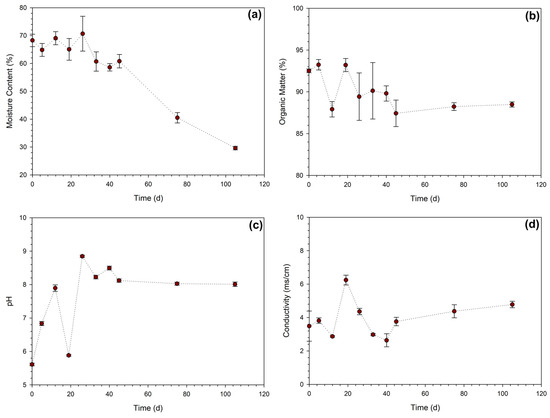
Figure 7.
Evolution of parameters analyzed throughout the domestic composting process: (a) MC, (b) OM content, (c) pH, and (d) electrical conductivity.
Table S2 in the Supplementary Information shows the physical appearance of the domestic composting on the 1st, 12th, and 45th days. It can be seen that over time the mixture acquires a dark color, probably due to the humification process, which leads to the accumulation of dark-colored amorphous substances.
In domestic composting, the release of bad odors is a relevant issue. Although not specifically addressed in this study, it should be noted that to avoid unpleasant odors, the application of dry biomass coverings or a small layer of earth is recommended. The C/N ratio must not be too low (to avoid the release of ammonia gas); therefore, the “green” and “brown” materials must be in adequate proportion (for example, an equal volume of each). Adequate aeration also plays a role, since anaerobic conditions promote the release of malodorous molecules (e.g., volatile fatty acids, NH3 or H2S). In any case, the composting reactor must be located away from the house.
3.4. Compost Quality
The assessment of the quality of the final compost produced in the experiments carried out in the laboratory (compost LC1, LC2, and LC3) and the domestic experiment (compost DC) is crucial, to determine whether they could be used as an organic fertilizer and/or as a soil improver. On day 120th of the experiments, each compost was analyzed for the quality parameters shown in Table 5, which summarizes the main properties used to assess the quality of the composts obtained in this work.

Table 5.
Parameters that the final compost must comply with to be considered an organic fertilizer.
In this regard, respirometric tests were performed (following the standard EN 10687-1) to assess the biological stability of the final composts. Each sample was tested in duplicate, and the results of the pressure drop profile were consistent among them (Figure S4 in the Supplementary Information). From these data, the oxygen uptake rate at the end of the composting process (OURf) was calculated (dividing RA obtained through Equation (2) by 72 h) as mentioned in Section 2.3, and the results are summarized in Figure 8. It can be seen that the OUR values measured for all composts are in the range of 10.31 mmolO2/kgOM.h to 13.98 mmolO2/kgOM.h. Considering the limits established in standard EN 10687-1—for compost to be considered an organic fertilizer (limit L1–25 mmolO2/kgOM.h) or a soil improver (limit L2–15 mmolO2/kgOM.h), it can be concluded that all composts obtained in this study can be used for both specific purposes. Also, in the literature, it has been mentioned that if a compost presents an OURf below 15 mmolO2/kgOM.h it can be considered stable, as it provokes low consumption of oxygen [49]. Composting of other materials (e.g., pig manure and a variety of bulking agents) can start with an oxygen uptake rate in the range of 40–50 mmolO2/kgOM.h., while after 2 months of composting, there is a decrease to values below 15 mmolO2/kgOM.h, indicating “stability” of the final composts [50].
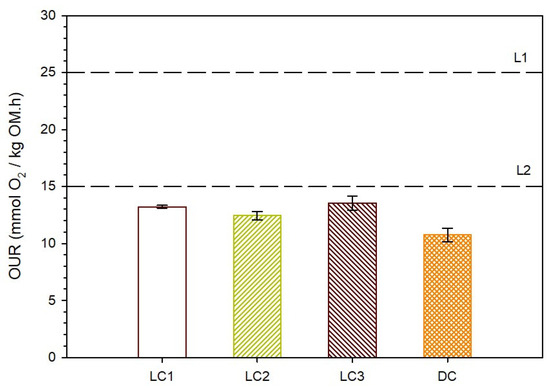
Figure 8.
Oxygen uptake rate for each final compost and stability limit (L1 and L2).
The phytotoxicity tests were then carried out and the GI was determined, and these results are shown in Table 5. For the classification of composts according to phytotoxicity, the criteria indicated in Table 3 (Section 2) should be used. The values obtained were all higher than 80%, which indicates that the composts are classified as not phytotoxic and can be applied in the soil without compromising seed germination and growth. Overall, according to the criteria established for the final compost to be considered an organic fertilizer, in accordance with Portuguese Decree-Law no. 103/2015, all the parameters analyzed fulfill the limits prescribed. These limits are shown in Table 5, as well as the values of each parameter analyzed for each compost. In fact, the OM remains high in the final composts since starting substrates also contain significant quantities of OM, including nutrients, such as nitrogen, which is essential for plant growth. It should be noted that composts with high OM contents tend to improve the structure of the soil, increasing its capacity to retain water and nutrients [24].
The MC plays a fundamental role in the maturation of the compost and, consequently, its quality, since a high MC can slow down the maturation process, while a lack of moisture can interrupt the microbial activity necessary for complete decomposition. Moreover, moisture influences the density and texture of the final compost. It is therefore essential that the MC complies with the limit imposed by law, which is verified in the final composts studied, ensuring stability during storage until application as organic fertilizer.
Finally, the remaining carbon and nitrogen are measured in the final composts, and the observed C/N ratios are reported in Table 5. If these values are compared to the initial ones (reported in Table 4), it can be concluded that a significant reduction occurred in all cases, as expected. In fact, during the composting process, the rate of carbon consumption is higher (source of energy for microbial activity and for building microbial cells) than nitrogen (required for protein synthesis), and thus, the C/N ratio decreases. Carbon is lost from the mixture in decomposition as CO2, and some nitrogen can also be lost as NH3. Nevertheless, it is relevant to determine the quantity of carbon retained in the final composts when compared to the initial quantity. In this regard, the parameter FCretained was calculated on a dry basis. Therefore, it can be concluded that LC1 retained 71% of C, composts LC2 and LC3 (replicates) retained 49–57% of C, and DC retained about 40%. These results are a consequence of LC1 containing more OWC (60%) when compared to LC2, LC3, and DC (40%), and because OWC contains not only higher carbon content (47.96%TS) when compared to CFW (45.00%TS) but also presents lower biodegradability due to its lignocellulosic nature. Furthermore, these results showed that the compost may convey a significant amount of carbon when applied as soil conditioners and may give a valuable contribution to closing the loop of the carbon, avoiding the emission of CO2 to the atmosphere. It is also important to note that the decrease in the C/N ratio directly affects the nutrient cycles in soil when compost is applied to it, as this reduction has significant implications for the availability of nitrogen and other essential nutrients for plants. In fact, when this ratio decreases, it means that the compost has become rich in nitrogen and that this element is more readily available in mineral form (nitrate and ammonia), which is easily absorbed by plants. So, when compost is applied to the soil, the available nutrients, including nitrogen, are released slowly as the compost continues to decompose, contributing to the fertility and health of the plants and soil. Regular application of mature and stable compost is good practice for improving soil quality and promoting healthier and more resilient agricultural systems.
Globally, the composting process proved to be very flexible as it can be carried out at the domestic and lab scale with the production of composts with quality for use as organic fertilizers. The scale-up from a lab scale of canteen biowaste to an industrial scale must deal with logistic issues (e.g., transport, storage, leachate production, and bad odors release). In particular, this study proved the need to use a bulking material to process canteen biowaste (and household residue), which should be as much as possible available in the region (also due to logistic issues). The aeration must be conducted in a way that aerobic conditions inside the mixture must be guaranteed (5–15%). Excessive aeration is not advisable because of energy costs and the removal of moisture and heat.
4. Conclusions
Several composting experiments were carried out, in the laboratory and at the domestic scale, aiming at valorizing biowaste as stable compost and avoiding landfill disposal. For that, a bulking agent was necessary, and in this study, OWC was used as an available local material. In fact, at the lab scale, it was observed that biowaste from a university canteen contains a very high MC, which hinders the composting process. This high water content presents a major challenge because a very wet mixture can lead to compaction, leachate formation, difficulties in aeration, and bad odors due to anaerobic activity. In this regard, the right balance between wet and dry biowaste (bulking agent) in the mixture was relevant to guarantee proper composting operation. The air flowrate also played a role in controlling MC in the mixture. Regarding temperature, this variable increased until reaching the thermophilic phase (>40 °C), with LR1 lasting around 20 days, LR2 and LR3 about 15 days, and DR around 14 days (including the second addition of DFW). Temperatures above 55 °C were observed in all reactors, which corresponds to levels suitable for eliminating pathogens and weed seeds. After the thermophilic phase, a decrease in temperature was observed to ambient conditions, and the maturation phase occurred. In all cases, except LR4, regular evolution of the monitoring parameters was observed and final composts with good properties were obtained, allowing further application as soil conditioners. Indeed, the analysis of the quality of the final composts revealed that the most relevant properties meet the legal requirements, particularly with regard to the GI, which indicates that the composts have reached a good level of maturity, favoring plant growth. Also, the OUR indicates that all composts are stable and in good condition for soil applications. The analysis of the carbon retained in the final compost revealed that more than 50% was retained in lab experiments and about 40% in domestic scale. These results showed that the compost can retain a significant amount of carbon in stabilized OM, with added value when applied as a soil conditioner.
Although composting mimics nature, there are still several factors that make it difficult to implement even on a domestic scale. To encourage people to adopt composting to manage biowaste, specific policy measures could be enforced to support domestic and community composting, namely, education programs, the creation of infrastructures, friendly regulations, and even financial incentives. Subsidies or discounts for the purchase of the necessary equipment (e.g., domestic composters) and tax incentives for families could have a positive effect. Additionally, door-to-door organic biowaste collection programs or the creation of drop-off points for the community could boost composting practices. In this way, this study demonstrates once again (like in other references) that the recovery of biowaste produced in a university canteen or domestic residences can be valorized instead of wasted in landfills. This strategy is fully aligned with circular economy approaches, producing a stable compost with beneficial properties for the soil. Furthermore, in future investigations, it would be relevant to address the impact of composting on managing different types of biowaste and the effect of the compost produced on the bioremediation of contaminated soils.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su16114368/s1, Figure S1: Gas concentration at the outlet of reactors (a) LR1, (b) LR2, and (c) LR3; Figure S2: OURp evolution throughout the laboratory composting process; Figure S3: Weight of mixtures inside reactors LR1, LR2, and LR3 during the laboratory composting process; Figure S4: Pressure (P) drop for different composts (a) LC1, (b) LC2, and (c) LC3 and DC; Table S1: Physical appearance of the mixtures over the laboratory composting period; Table S2: Physical appearance of mixture from domestic composting.
Author Contributions
C.F.T.B. and R.P.R. performed the experimental assays. C.F.T.B. wrote the manuscript with the support of coauthors. All authors contributed to the analysis and interpretation of the results. M.J.Q. supervised the work and reviewed and edited the text. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through the Programa Operacional Regional do Centro (CENTRO2020) CENTRO-01-0145-FEDER-000014 and the Strategic Project of CERES (UIDB/00102/2020), https://doi.org/10.54499/UIDB/00102/2020, which is financed by the FCT through national funds. Rafaela P. Rodrigues was funded by the Fundação para a Ciência e Tecnologia (FCT) through the PhD grant (SFRH/BD/145694/2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Rafaela P. Rodrigues acknowledges the Fundação para a Ciência e Tecnologia (FCT) for the PhD grant (SFRH/145694/2019).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eurostat. Waste Statistics: Statistics Explained. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/SEPDF/cache/1183.pdf (accessed on 24 October 2023).
- Triassi, M.; Simone, B.; Montuori, P.; Russo, I.; Rosa, E.; Duca, F.; Crivaro, C.; Cerullo, V.; Pontillo, P.; Díez, S. Determination of Residual Municipal Solid Waste Composition from Rural and Urban Areas: A Step toward the Optimization of a Waste Management System for Efficient Material Recovery. Sustainability 2023, 15, 13378. [Google Scholar] [CrossRef]
- Interreg Europe. The Biowaste Management Challenge: A Policy Brief from the Policy Learning Platform on Environment and Resource Efficiency. 2021. Interreg Europe Report. Available online: https://www.interregeurope.eu/sites/default/files/2022-04/Biowaste%20challenge.pdf (accessed on 19 October 2023).
- Wanderley, T.; McQuibban, J.; Mörsen, T. Como Fazer uma Melhor Recolha de Biorresíduos. 2022. Zero Waste Europe Report. Available online: https://zerowastecities.eu/wp-content/uploads/2022/11/Biowaste-Guide_PT.pdf (accessed on 25 October 2023).
- Jones, P.; Wynn, M.; Hillier, D.; Comfort, D. The Sustainable Development Goals and Information and Communication Technologies. Indones. J. Sustain. Account. Manag. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- GAIA. Zero Waste to Zero Emissions—How Reducing Waste Is a Climate Gamechanger. 2022. GAIA Report. Available online: https://www.no-burn.org/resources/zero-waste-to-zero-emissions-how-reducing-waste-is-a-climate-gamechanger/ (accessed on 18 October 2023).
- Confalonieri, A. ECN Position Paper ECN Position Paper on the Role of Organic Waste derived Soil Improvers and Organic Fertilizers within Carbon Farming Initiative. 2022. Available online: https://www.compostnetwork.info/download/the-role-of-organic-waste-derived-soil-improvers-and-organic-fertilizers-within-carbon-farming-initiative/ (accessed on 20 October 2023).
- EEA. Bio-Waste in Europe—Turning Challenges into Opportunities. 2020. European Environment Agency Report. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 19 October 2023).
- CML. Guia Prático de Compostagem. 2018. Câmara Municipal de Lisboa Report. Available online: https://lisboaacompostar.cm-lisboa.pt/pls/OKUL/r/wks_dmhu/178/files/static/v157/guia_lc.pdf (accessed on 26 October 2023).
- Chattopadhyay, S.; Singha, R. Bio-Waste: An Introduction to its Management. Agric. Food E-Newsl. 2022, 4, 379–381. Available online: https://www.researchgate.net/publication/365210713 (accessed on 19 October 2023).
- Wesseler, J. The EU’s farm-to-fork strategy: An assessment from the perspective of agricultural economics. Appl. Econ. Perspect. Policy 2022, 44, 1826–1843. [Google Scholar] [CrossRef]
- EC. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System. 2020. European Commission Report. Available online: https://food.ec.europa.eu/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 19 October 2023).
- Bhatia, S.K.; Otari, S.V.; Jeon, J.M.; Gurav, R.; Choi, Y.K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour Technol. 2021, 326, 124733. [Google Scholar] [CrossRef] [PubMed]
- Pavlas, M.; Dvořáček, J.; Pitschke, T.; Peche, R. Biowaste Treatment and Waste-to-Energy—Environmental Benefits. Energies 2020, 13, 1994. [Google Scholar] [CrossRef]
- Arancon, R.A.D.; Lin, C.S.K.; Chan, K.M.; Kwan, T.H.; Luque, R. Advances on waste valorization: New horizons for a more sustainable society. Energy Sci. Eng. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A review on the challenges and choices for food waste valorization: Environmental and economic impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Wang, L.; Jin, B. Environmental and Cost Impacts of Food Waste in University Canteen from a Life Cycle Perspective. Energies 2021, 14, 5907. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Soares, M.A.R.; Quina, M.J.; Quinta-Ferreira, R. Prediction of free air space in initial composting mixtures by a statistical design approach. J. Environ. Manag. 2013, 128, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.A.R.; Andrade, S.R.; Martins, R.C.; Quina, M.J.; Quinta-Ferreira, R.M. Organic biowastes blend selection for composting industrial eggshell by-product: Experimental and statistical mixture design. Water Sci. Technol. 2012, 65, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cheruiyot, N.K.; Bui, X.T.; Ngo, H.H. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Kopaei, H.R.; Nooripoor, M.; Karami, A.; Petrescu-Mag, R.M.; Petrescu, D.C. Drivers of residents’ home composting intention: Integrating the theory of planned behavior, the norm activation model, and the moderating role of composting knowledge. Sustainability 2021, 13, 6826. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Ribeiro, A.A.; Marques, A.P.; Costa, I.H.; Magalhães, M.C. Feasibility study on windrow co-composting to recycle industrial eggshell waste. Waste Biomass Valorization 2014, 5, 87–95. [Google Scholar] [CrossRef]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The effects of compost incorporation on soil physical properties in urban soils—A concise review. J. Environ. Manag. 2020, 261, 110209. [Google Scholar] [CrossRef] [PubMed]
- Cahyono, P.; Loekito, S.; Wiharso, D.; Rahmat, A.; Nishimura, N.; Senge, M. Effects of compost on soil properties and yield of pineapple (Ananas comusus L. Merr.) on red acid soil, Lampung, Indonesia. Int. J. GEOMATE 2020, 19, 33–39. [Google Scholar] [CrossRef]
- Barrena, R.; Sánchez, A. Home Composting: A Review of Scientific Advances. Eng. Proc. 2022, 19, 35. [Google Scholar] [CrossRef]
- Soares, M.A.R.; Quina, M.M.J.; Quinta-Ferreira, R.M. Co-composting of eggshell waste in self-heating reactors: Monitoring and end product quality. Bioresour. Technol. 2013, 148, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.R.; Quina, M.J.; Quinta-Ferreira, R. Influence of N-rich material in valorization of industrial eggshell by co-composting. Environ. Technol. 2016, 37, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Torrijos, V.; Calvo Dopico, D.; Soto, M. Integration of food waste composting and vegetable gardens in a university campus. J. Clean. Prod. 2021, 315, 128175. [Google Scholar] [CrossRef]
- Tatàno, F.; Pagliaro, G.; Giovanni, P.; Floriani, E.; Mangani, F. Biowaste home composting: Experimental process monitoring and quality control. Waste Manag. 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.A.R.; Quina, M.J.; Reis, M.S.; Quinta-Ferreira, R. Assessment of co-composting process with high load of an inorganic industrial waste. Waste Manag. 2017, 59, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, A.; Scelza, R.; Scotti, R.; Rao, M.A.; Jedidi, N.; Gianfreda, L.; Abdelly, C. The effect of compost and sewage sludge on soil biologic activities in salt affected soil. Rev. Cienc. Suelo Nutr. Veg. 2010, 10, 40–47. [Google Scholar] [CrossRef]
- Santos, A.F.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Agronomic valorization of sewage sludge: The potential of thermal drying to achieve sanitation and biological stability. Sustain. Chem. Pharm. 2022, 27, 100646. [Google Scholar] [CrossRef]
- Trautmann, N.M.; Krasny, M.E. Composting in the Classroom: Scientific Inquiry for High School Students. 1997. Available online: https://www.researchgate.net/publication/237413430 (accessed on 11 October 2023).
- Batista, J.G.F.; Batista, E.R.B. Compostagem—Utilização de Compostos em Horticultura; Universidade dos Açores: Angra do Heroísmo, Açores, 2017; p. 252. [Google Scholar]
- Rodrigues, M.S.; Silva, F.C.; Barreira, L.P.; Kovacs, A. Compostagem: Reciclagem de resíduos sólidos orgânicos. In Gestão de Resíduos na Agricultura e Agroindústria; Spadotto, C.A., Ribeiro, W., Eds.; FEPAF: Botucatu, Brazil, 2006; pp. 63–94. [Google Scholar]
- Nakasaki, K.; Ohtaki, A.; Takano, H. Biodegradable plastic reduces ammonia emission during composting. Polym. Degrad. Stab. 2000, 70, 185–188. [Google Scholar] [CrossRef]
- Azis, F.A.; Choo, M.; Suhaimi, H.; Abas, P.E. The Effect of Initial Carbon to Nitrogen Ratio on Kitchen Waste Composting Maturity. Sustainability 2023, 15, 6191. [Google Scholar] [CrossRef]
- Berticelli, R.; Decesaro, A.; Magro, F.; Colla, L.M. Compostagem como alternativa de biorremediação de áreas contaminadas. CIATEC—UPF 2016, 8, 12–28. [Google Scholar] [CrossRef]
- Neves, L.; Ferreira, V.; Oliveira, R. Co-composting cow manure with food waste: The influence of lipids content. World Acad. Sci. Eng. Technol. 2009, 58, 986–991. [Google Scholar]
- Parihar, P.; Sharma, S. Composting: A Better Alternative of Chemical Fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2021, 795, 012038. [Google Scholar] [CrossRef]
- Tiquia, S.M. Microbiological parameters as indicators of compost maturity. J. Appl. Microbiol. 2005, 99, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Puyuelo, B.; Gea, T.; Sánchez, A. A new control strategy for the composting process based on the oxygen uptake rate. Chem. Eng. J. 2010, 165, 161–169. [Google Scholar] [CrossRef]
- Ho, T.T.K.; Tra, V.T.; Le, T.H.; Nguyen, N.K.Q.; Tran, C.S.; Nguyen, P.T.; Vo, T.D.H.; Thai, V.N.; Bui, X.T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Szanto, G.L.; Hamelers, H.V.M.; Rulkens, W.H.; Veeken, A.H.M. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 2007, 98, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Gea, T.; Barrena, R.; Artola, A.; Sánchez, A. Monitoring the biological activity of the composting process: Oxygen uptake rate (OUR), respirometric index (RI), and respiratory quotient (RQ). Biotechnol. Bioeng. 2004, 88, 520–527. [Google Scholar] [CrossRef]
- Jiménez, E.I.; Garcia, V.P. Evaluation of City Refuse Compost Maturity: A Review. Biol. Wastes 1989, 27, 115–142. [Google Scholar] [CrossRef]
- Irvan; Husaini, T.; Trisakti, B.; Batubara, F.; Daimon, H. Composting of empty fruit bunches in the tower composter—Effect of air intake holes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012066. [Google Scholar] [CrossRef]
- Amery, F.; Vandaele, E.; Körner, I.; Loades, K.; Viaene, J.; Vandecasteele, B.; Willekens, K. SOILCOM Report Number 5.1. Compost Quality Indicators. 2020, pp. 1–23. Available online: https://northsearegion.eu/media/15220/soilcom-report-1-compost-quality-indicators.pdf (accessed on 5 November 2023).
- Nolan, T.; Troy, S.M.; Healy, M.G.; Kwapinski, W.; Leahy, J.J.; Lawlor, P.G. Characterization of compost produced from separated pig manure and a variety of bulking agents at low initial C/N ratios. Bioresour. Technol. 2011, 102, 7131–7138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).