Abstract
Fish oils are widely consumed around the world to increase omega-3 fatty acid intake. Due to negative impacts on marine resources and ecosystems from an increasing demand for fish, alternative sustainable sources are under investigation. Squid viscera contains up to 10% oil by mass and is available as a byproduct from squid processing. Squid viscera oil is a source of EPA and DHA and contains the xanthophyll carotenoid astaxanthin, known for its significant anticancer, antioxidant, antidiabetic, and cardiovascular properties. In the raw form, squid viscera oil has a high free fatty acid (FFA) content, so conventional alkaline refining results in low yield and loss of astaxanthin. As a higher-yielding alternative, the current study optimized lipase-catalyzed glycerolysis of squid viscera oil to convert FFA into acylglycerol using a custom-built one-liter immobilized enzyme reactor. To monitor the reaction progress and assess its impact on the oil, we analyzed lipid classes, fatty acid composition and astaxanthin levels. Under optimized conditions, FFA was reduced from 40% to 2.7% in 10 h and 1.7% in 24 h, with no significant effect on EPA and DHA levels, and astaxanthin being retained. Squid viscera presents a safe and sustainable additional source of marine-derived EPA and DHA oil.
1. Introduction
Long-chain omega-3 polyunsaturated fatty acids (PUFAs) including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are beneficial to human health [1]. EPA and DHA are precursors to endogenous anti-inflammatory mediators that are required for optimum health [2]. EPA and DHA are implicated in fetal development, healthy aging, lowering plasma triglycerides and cholesterol, and protection against chronic diseases including type 2 diabetes mellitus, ischemic heart disease, cancer, high blood pressure, depression, Alzheimer’s, and cardiovascular diseases [3,4,5].
EPA and DHA are abundant in the marine environment, including fatty fish and algae, which are the primary sources of these PUFAs in the human diet [6]. Approximately 85% of the world’s population does not consume the recommended levels of EPA and DHA for optimal health, so marine oils are widely used as nutritional supplements and also as starting materials for some pharmaceutical products [7,8,9]. Due to the limited source of fish oil, there is interest in additional sources of EPA and DHA, particularly sustainable sources such as microalgae, plants and fisheries by-products [10,11,12,13]. Fishery by-products can comprise 50% or more of the total fish mass, of which a significant portion is disposed of in landfills with negative environmental and economic impacts.
Squid, or calamari, is a popular seafood widely consumed around the world. Typically, the squid body and tentacles are commercially recovered for consumption, and by-products including viscera are discarded. The viscera consists of approximately 10% oil which is high in omega-3 fatty acids, particularly DHA [14]. Depending on species and harvest location, the oil contains 15 to 20% EPA and 20 to 25% DHA, making it a promising marine source of PUFAs for nutraceuticals or pharmaceuticals [14,15]. Squid oil contains higher DHA levels than standard anchovy oil (180 mg EPA/120 mg DHA), a more balanced ratio of EPA to DHA compared with tuna oil (50 mg EPA/20 mg DHA and considerably higher EPA and DHA than krill oil. Therefore, squid oil offers a high omega-3 alternative to fish and krill oil for nutritional supplement purposes, with further research needed to determine an appropriate dose. From the delivery of DHA perspective, squid oil delivers significantly more DHA than either standard anchovy oil or krill oil per gram of oil. Although some algal oils have high levels of DHA the scale-up of these oils is relatively expensive and, in some cases, involves genetically modified organisms. Also, squid oil is from waste viscera and so offers an environmentally friendly alternative to other oils. In addition to potential use in nutritional supplements, squid oil could be microencapsulated or emulsified for food use with appropriate stabilizers. Squid oil also contains astaxanthin, a xanthophyll carotenoid known for its significant anticancer, antioxidant, antidiabetic and cardiovascular properties [16]. Astaxanthin is added to animal, fish and human foods to impart desirable color or health properties [17,18], hence its presence in squid oil provides a potential health benefit advantage.
Producing a nutritional oil from squid viscera oil requires several processing steps including oil extraction and neutralization of free fatty acids [19]. Depending on the oil properties additional steps of bleaching, winterization and deodorization may be necessary [19]. Conventional neutralization of oils involves alkali deacidification (alkali refining), which can reduce the non-glyceride FFAs to as low as 0.03% [20]. However, the high levels of FFA in squid oil result in low recovery after alkali refining and loss of astaxanthin [20]. However, the removal of FFA is important for stability and sensory acceptance, particularly for nutritional oils [21,22].
Enzymatic glycerolysis of FFAs is an alternative greener method of FFA removal that results in the conversion of FFA to acylglycerol and no loss of yield [20,23,24]. The enzyme process is favored industrially over the chemical-based process for the formation of acylglycerols from FFA in fish oil manufacturing due to the high catalytic efficiency and mild reaction conditions of the enzyme process. Enzymatic acylglycerol formation for fish oil results in higher acylglycerol yields at lower temperatures, less pressure and shorter time than required for the chemical process. In addition, the lipase-catalyzed process can be carried out at close to a 1:1 ratio of glycerol and FFA, whereas the chemical process requires high levels of FFA to drive the reaction forward since it is an equilibrium reaction [25,26]. However, due to the high cost of enzymes, glycerolysis is not viable unless the enzyme can be multiply reused, which requires immobilization of the enzyme. Using immobilized enzymes in glycerolysis offers additional benefits including greater enzyme stability and the elimination of enzyme recovery and purification steps [27,28]. Previous studies have found immobilized enzymes are effective for both vegetable and fish oil esterification reactions, with the enzyme remaining active over multiple cycles [29,30,31,32].
This study investigates the use of immobilized lipase for the enzymatic glycerolysis of crude squid viscera oil to produce an alternative to fish oil for omega-3 nutritional applications. A custom-designed one-litre reactor was used to isolate the immobilized enzyme from the stirrer for ease of recycling over multiple cycles. Normal stirring results in the breakdown of the support material and the inability to reuse the enzyme. This work explores the impact of key parameters influencing the reaction and optimizes these for the squid-oil acyglycerolysis process, including choice of enzyme, enzyme amount, substrate ratios, operating temperature, reaction time, mixing speed and removal of water during the reaction. The reaction was optimized for acylglycerol levels while also minimizing damage to sensitive components, particularly EPA, DHA and astaxanthin. The developed process is the first reported one-pot reactor-based lipase re-esterification of natural squid oil that retains the natural triacylglyceride component while converting the FFA component to acylglycerol resulting in no loss of yield and greater than 95% acylglycerol product.
2. Materials and Methods
2.1. Materials
Crude squid viscera oil from the commercial arrow squid (Nototodarus gouldi) was provided by Mantzaris Fisheries Pty Ltd., North Geelong, Australia. Glycerol was purchased from EnviroChem International Pty Ltd., Melbourne, Australia, and immobilized enzyme (Lipozyme RMIM, Novozym 435 and Lipozyme TLIM) was sourced from Novozymes Australia Pty Ltd., North Rocks, Australia. Molecular sieves, HPLC grade hexane, heptane, ethyl acetate and diethyl ether, astaxanthin and phenolphthalein indicator were sourced from Sigma Aldrich, Castle Hill, Australia. Acetone, isopropyl alcohol and methanol were purchased from Chem Supply, Gillman, Australia and toluene from Thermofisher Scientific, Melbourne, Australia. Potassium hydroxide was supplied by Unilab Chemicals and Pharmaceuticals Pvt Ltd., Mumbai, India.
2.2. Design and Working Principle of the One-Liter Reactor
Neutralization of the squid viscera oil was carried out using a custom-built one-liter jacketed reactor. The reactor is jacketed and consists of a reaction chamber at the top (3/4 of reactor volume) with a stirrer placed in the upper reaction chamber and a lower enzyme compartment (1/4 of reactor volume). The two chambers are separated by two layers of stainless-steel mesh which holds the immobilized enzyme and separates it from the stirrer to minimize enzyme support breakdown. An overhead stirrer (IKA RW20 digital, John Morris Scientific, Melbourne, Australia) was used to mix the reactor contents and a peristaltic pump (Masterflex L/S, Cole Parmer Instrument Company, Chatswood, Australia) with PVC tubing was used to recirculate the reaction mixture, enabling either or both to be applied depending on the degree of mixing required. Normally both stirring and recirculation were required for adequate mixing and contact with the enzyme. The temperature was controlled by circulating water through the reaction chamber jacket using a water bath (Temperature RATEK Instrument Pty Ltd., Boronia, Australia). Molecular sieves were loaded into the reaction chamber to remove water as it formed, as an alternative to vacuum if required. To monitor the reaction progress, 5 mL of oil/glycerol mixture was sampled by disconnecting the PVC tubing at the required timepoints. Reaction and reactor operation parameters were optimized to achieve a minimal residual free fatty acid level in the final product (see Table 1 for detailed operating conditions).

Table 1.
Working conditions of the reactor.
2.3. Determination of Lipid Classes by TLC-FID
Lipid class analysis was carried out using TLC-FID (Iatroscan) following a previously described method with minor modifications [33]. Briefly, oil samples were dissolved in heptane (10 mg of oil in 2 mL heptane), then 1 µL from each sample was spotted onto chromarods along the line of origin on the rod holder for separation. The rods were then developed with hexane/diethyl ether/acetic acid (60:17:0.2, v/v/v) for 22 min. The rods were scanned using the TLC-FID (Iatroscan MK6, Iatron Laboratories Inc., Tokyo, Japan) with settings 2.0 L/min air flow rate, 160 mL/min hydrogen flow rate and scan speed 30 s/scan. TLC standards from Nu-Chek Prep were used to identify each lipid class.
2.4. Fatty Acid Composition by GC-FAMEs
The fatty acid composition of oil samples was determined using gas chromatography-fatty acid methyl esters (GC-FAMEs) with flame ionization detection (FID) as previously described [34]. For each sample 10 mg of oil was dissolved in 1 mL toluene, and 200 μL of internal standard (5 mg/mL methyl nonadecanoate in toluene) and 200 μL of antioxidant (1 mg/mL 2,6-di-tert-butyl-4-methylphenol (butylated hydroxytoluene; BHT in toluene)) were added. To this, 2 mL of acidified methanol (prepared by adding 1 mL acetyl chloride drop wise to 10 mL methanol on ice) was added and mixed with the solutions and left overnight at 50 °C in a sealed tube. After cooling, 5 mL sodium chloride solution (5% m/v) was added. The fatty acid methyl esters were then extracted twice with heptane (5 mL) and the heptane layer was washed with 5 mL potassium bicarbonate solution (2% m/v). Finally, the heptane layer was dried over sodium sulphate and analyzed using an Agilent 6890 gas chromatography with flame ionization detector (FID) equipped with a BPX70 SGE column (30 m, 0.25 mm i.d., 0.25 μm film thickness). The oven was programmed from 140 °C (5 min hold) to 220 °C (5 min hold) at a rate of 4 °C/min for a total run time of 30 min. A volume of 1 μL of each sample was injected with a split ratio of 50:1 (injector temperature, 250 °C). Helium was used as the carrier gas (1.5 mL/min, constant flow) and the detector gases were 30 mL/min hydrogen, 300 mL/min air and 30 mL/min nitrogen. Peak areas were integrated using ChemStation software version A.06.01 and corrected using theoretical relative FID response factors [35]. The GC fatty acids standards used were a mixture of saturated, monounsaturated and polyunsaturated fatty acids ranging from carbon 4 to 24. An internal standard, acetyl chloride and BHT were sourced from Sigma Aldrich, Castle Hill, Australia.
2.5. Determination of Astaxanthin Content by HPLC
The original and esterified squid oils were analyzed for astaxanthin content using HPLC as previously described with minor modifications [36]. Approximately 0.5 g of oil sample was diluted in a mixture of petroleum ether:acetone:water (15:75:10). The sample was then sonicated for 30 min in an ultrasonic bath and centrifuged for 20 min at 4200 rpm. Then 2 mL acetone was added, and the sample centrifuged for an additional 20 min. The supernatant was then filtered through a 0.45 µm syringe filter and 10 µL injected on a reversed-phase column (Luna 5u C18(2) 100 A, 260 × 4.6 mm, Phenomenex Pty. Ltd., Lane Cove West, Australia). An Agilent 1260 Series HPLC system equipped with solvent degasser, quaternary pump, autosampler, thermostated column compartment and diode array detector was used for analysis. Methanol, ethyl acetate and water were used as gradient mobile-phase systems, initially at a ratio of 88:10:2 (methanol/ethyl acetate/water) for 10 min at 0.75 mL/min. Over the next 20 min the flow rate was adjusted to 1.5 mL/min and the mobile phase composition changed to 48:50:2. These conditions were held for an additional 10 min, then the column was re-equilibrated for 15 min for an overall run time of 55 min. Column temperature was maintained at 23 °C. The diode array detector was set to 477 nm for detection of astaxanthin.
The percent loss of astaxanthin due to different operating conditions and time was calculated using Equation (1).
where,
Loss of astaxanthin (%) = [A_original − A_test]/(A_original) × 100
- A_original = Area under peak produced from the original (non-esterified) squid oil
- A_test = Area under peak produced from the esterified squid oil
2.6. Determination of Acid Value
Acid values of the squid oil samples were determined following the AOAC International 2009 titration method. Approximately 0.5 g of each of the samples were measured into a 250 mL conical flask, in which 125 mL of solvent mixture (isopropyl alcohol and toluene, 1:1) was added. The mixture was titrated against 0.1 M potassium hydroxide in presence of 1% phenolphthalein indicator solution in isopropyl alcohol. The titration end point was reached when the solution turned to pink color and persisted for at least 30 s. The acid value was determined using the Equation (2).
where,
Acid value, mg KOH per g of test sample = [(V − B) × M × 56.1]/W
- V = Volume of standard alkali used in the titration, mL
- B = Volume of standard alkali used for the blank titration, mL
- M = Molarity of KOH, the standard alkali
- W = Mass of test sample, g
2.7. Statistical Analysis of Data
Experiments were carried out in triplicate and the average values are reported in the article. The significant difference between the two mean values was determined by using a one-way analysis of variance at a 95% confidence level (p < 0.05). The inbuilt program in Microsoft ® Excel for Microsoft 365 MSO (Version 2303 Build 16.0.16227.20202) was used for this purpose.
3. Results and Discussion
3.1. Crude Squid Viscera Oil
The crude squid viscera oil supplied by Mantzaris Fisheries was characterized for lipid class, EPA and DHA content, and acid value (Table 2). The oil contained 40.3% FFA, 5.5% monoacylglycerols (MAG), 10.1%, diacylglycerols (DAG) and 41.6% triacylglycerols (TAG). It contained approximately 2.6% other lipid classes which may include esters, phospholipids, sterols, and other minor lipid classes, which were similar to previously reported compositions [14]. The EPA and DHA contents of the crude oil were 167.6 mg/g and 167.1 mg/g oil, respectively. The acid value of the supplied oil was 79.6 mg KOH/g of the sample. Astaxanthin content of the squid viscera oil was 22.5 mg/kg.

Table 2.
Percent lipid classes and omega-3 fatty acids content in the crude squid viscera oil.
3.2. Optimizing the Enzymatic Glycerolysis of Squid Viscera Oil
3.2.1. Selection of Enzyme
Three commercially available immobilized lipases were trialed for the enzymatic glycerolysis of squid viscera oil: Lipozyme TLIM from Thermomyces lanuginosus, Lipozyme RMIM from Rhizormucor miehei and Novozym 435 from Candida antarctica. Each of these enzymes complies with the recommended food grade specifications of the joint FAO/WHO expert committee on Food Additives (JEFCA) and the Food Chemical Codex (FCC). The three enzymes were compared by stirring under a vacuum in a glass bottle for 24 h at 60 °C using a 2:1 crude oil to glycerol ratio, 1% m/v enzyme, and 1% m/v molecular sieves. Lipozyme RMIM and Novozym 435 both provided greater than 98% conversion of FFA’s to acylglycerol, compared to only a 4.4% conversion for Lipozyme TLIM (Figure 1).
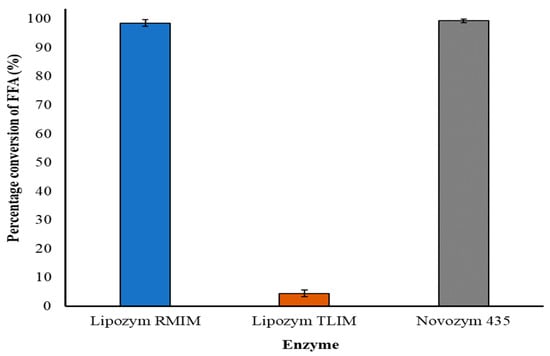
Figure 1.
Comparison of the effectiveness of three lipases to convert FFA to acylglycerol for crude squid oil, using conditions of 2:1 crude oil to glycerol ratio, 1% m/v enzyme, 1% m/v molecular sieves at 60 °C for 24 h. Results are mean values of three replicates.
To select between Lipozym RMIM and Novozym 435 we used the glass bottle reaction system to compare the effect of temperature, molecular sieves, and reaction time on the degree of acylglycerol formation with each enzyme. Novozym 435 gave a more complete reaction in the absence of molecular sieves, however, Lipozym RMIM reacted more effectively at the lower temperatures of 40 and 50 °C, so the latter was selected for a more energy-efficient reaction.
3.2.2. Removal of Water Formed during the Reaction
The glycerolysis of squid viscera oil releases water as a reaction product, which hinders the reaction progress and needs to be removed as the reaction progresses, to drive the equilibrium reaction forward [20]. Two methods were investigated for the removal of water, vacuum, and molecular sieves. At the 1-L scale, molecular sieves were the more effective, although it is the less scalable option [24,37,38]. We compared the effect of adding up to 2% m/v low-cost inert aluminosilicate beads, either before or during the reaction (detailed in Table 1). The other parameters were selected based on the preliminary enzyme screening experiments: stirring at 400 rpm in the custom-designed reactor at 50 °C, 3:1 oil to glycerol and 1% m/v Lipozym RMIM enzyme. A reaction time of 24 h was selected for all experiments to ensure reaction completion. The experimental data (Figure 2A) show that the addition of molecular sieves resulted in a faster reaction and a 2% improvement in FFA after 8 h and 1.5% after 24 h, irrespective of the quantity added. Despite the relatively minor improvement, we elected to continue using 1% m/v molecular sieves for the remaining optimization experiments.
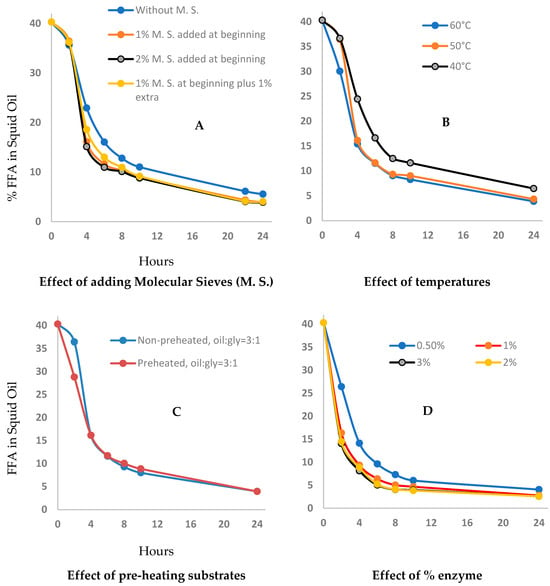
Figure 2.
Optimization of (A) molecular sieves (B) reaction temperature (C) pre-heating of substrate and (D) percent enzyme for esterification process.
3.2.3. Effect of Reaction Temperature
Reaction temperature influences kinetics, enzyme longevity, energy costs and hence environmental impact for the FFA to acylglycerol conversion process. With these factors in mind, we compared reaction rate and yield at 40, 50 and 60 °C (starting from room temperature, Figure 2B), with stirring at 400 rpm, 1% m/v molecular sieves, 3:1 oil to glycerol and 1% m/v enzyme. At 40 °C the conversion rate of FFA to acylglycerol (TAG, DAG and MAG) was slower than at 50 °C and 60 °C. Although the reaction at 60 °C started earlier than at 50 °C, there were no significant differences between these two temperatures from 4 h onwards. After 8 h at 40 °C there was 11.6% FFA remaining, compared to 9% at the two higher temperatures and at 24 h the 40 °C reaction still had a 2% higher residual FFA content. Consequently, we selected 50 °C as the optimum temperature for the enzymatic esterification of squid oil to minimize damage to the enzyme and energy usage, while maximizing product quality. This temperature is consistent with previous work for lipase-catalyzed esterification of fish and microalgae oils [39].
3.2.4. Influence of Pre-Heating Substrates
From Figure 2B, we can see an initial lag in the reaction rates at 40 °C and 50 °C compared to the trial at 60 °C. Given each trial commenced at room temperature the enzyme activity is initially low until sufficient heat is transferred to the reaction mixture from the jacketed reactor, which occurs more rapidly at 60 °C. To avoid the initial lag in reaction rate we compared the effect of pre-heating the substrate to the optimized temperature of 50 °C, with the additional parameters held consistent: stirring at 400 rpm, 50 °C, 3:1 oil to glycerol and 1% m/v enzyme (Figure 2C). Despite an initial increase in the rate of reaction gained from pre-heating the substrates, the results show an almost identical FFA level remaining in the product from 4 h into the reaction. For further trials we elected to retain the pre-heating step, to remove any possible variation from differences in the starting room temperature.
3.2.5. Effect of Immobilised Enzyme Ratio
An enzyme loading from 0.5% w/v to 3% w/v was compared for the glycerolysis reaction (Figure 2D), with consistent parameters of stirring at 400 rpm, 50 °C with pre-heated reactants, 3:1 oil to glycerol and 1% m/v molecular sieves. At 0.5% w/v the conversion of FFA to acylglycerol was slower compared to the higher enzyme quantities, among which there was no significant difference (4 to 5% FFA after 8 h and 2.6 to 2.8% of the residual FFA after 24 h). We selected 1% m/v as the optimized enzyme level, balancing cost and yield, which is consistent with results from a previous study using sardine oil [20].
3.2.6. Substrate Ratio
To optimize the substrate ratio, we compared crude oil to glycerol ratios of 4:1, 3:1 and 2:1 for the effect on the rate and extent of FFA conversion. Consistent parameters were selected of stirring at 400 rpm, 50 °C with pre-heated reactants, 1% m/v enzyme and 1% m/v molecular sieves. A 1:1 oil to glycerol was also trialed but resulted in a mixture too viscous to mix effectively. Our results presented in Figure 3A show that the highest substrate ratio of 4:1 oil–glycerol resulted in a slower rate of reaction and final product with 5% more FFA after 8 h and 3% more FFA after 24 h, compared to both 3:1 and 2:1 ratios. This finding is consistent with a similar study indicating that a glycerol/PUFA 1.2:3 ratio was optimum for lipase-catalyzed esterification [39].
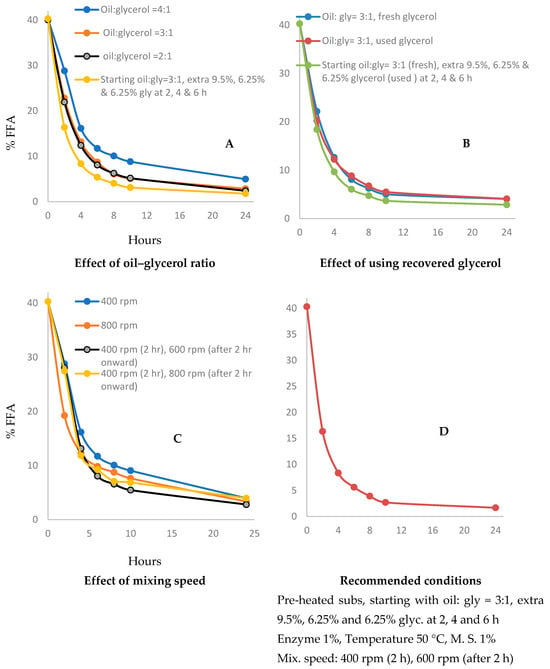
Figure 3.
Effect of (A) oil to glycerol ratio (B) reusing of glycerol and (C) mixing speed for esterification process, (D) recommended conditions for the best performance of the lab-scale reactor.
We also compared the reaction progression as extra glycerol was added at different time points, with the aim of driving the esterification reaction forward faster. The reaction started with a 3:1 oil-to-glycerol ratio, and an extra 9.5% (75 mL) was added at 2 h and an extra 6.25% (50 mL) after 4 and 6 h. These conditions resulted in an increased reaction rate, with residual FFA at 4% within 8 h and 1.8% in 24 h. However, a significant amount of glycerol remains unreacted requiring removal at the completion of the reaction. This was achieved through phase separation and centrifugation of the glycerol and oil product.
3.2.7. Recovery and Reutilization of Glycerol
To reduce the cost of substrates for the glycerolysis reaction, we trialed recovering unreacted glycerol and reusing it in a subsequent trial. We compared reactions using a 3:1 oil-to-glycerol ratio with recovered glycerol only to a 3:1 oil-to-glycerol ratio starting with fresh material and adding recovered glycerol at 2-, 4- and 6-h timepoints (Figure 3B). In both cases, our reaction data show a reaction rate and degree of FFA neutralization comparable to using fresh substrate only. We concluded that using fresh glycerol, recovered glycerol or a combination of both is suitable to reduce input material costs.
3.2.8. Reaction Mixing Speed
To further increase kinetics and reduce the reaction time for commercial viability we studied the effect of mixing speed in the 1 L vessel. Five different mixing speeds of 400, 500, 600, 700 and 800 rpm were compared under conditions of 1% (m/v) enzyme, 1% (m/v) molecular sieves, oil to glycerol ratio 3:1, temperature of 50 °C with pre-heated reactants (Figure 3C). The reaction rate was slowest at 400 rpm and highest at 800 rpm, although after 6 h the difference was within 2%, and within 1.25% after 8 h. We also compared the effect of increasing the mixing speed to 600 or 800 rpm after the first two hours and found both trials significantly increased the percent FFA conversion. Using a low speed (400 rpm) for the first 2 h and then to moderate high (600 rpm) for the remainder of the reaction showed the most complete neutralization between 6 and 24 h. Previous studies indicated mixing speed ranges from 300 to 700 rpm were suitable for maximum glycerolysis of conjugated linoleic acid catalyzed by immobilized enzyme [40,41].
3.2.9. Optimised Glycerolysis Conditions
Figure 3D shows optimized reaction conditions for the glycerolysis reaction, where the pre-heated substrate of 3:1 oil/glycerol was reacted with an extra 175 mL (22% of the starting substrate volume) of glycerol being added progressively at 2,4 and 6 h (75 mL, 50 mL and 50 mL, respectively). The mixing speed started at 400 rpm and increased to 600 rpm after two hours. The reaction temperature was 50 °C with preheated substrates, 1% (m/v) enzyme and 1% (m/v) molecular sieves. Under these conditions, a reaction time of 10 h was sufficient to reduce FFA levels to 2.7%.
3.3. Product Characterisation
The neutralized squid viscera oil was analyzed for lipid classes, fatty acid compositions, acid values and astaxanthin content to confirm its suitability as a nutritional lipid.
3.3.1. Lipid Classes
The changes in lipid classes during the glycerolysis reaction of squid oil under optimized conditions are shown in Table 3. As shown, after 24 h of reaction FFA was reduced from 40.3% to 1.8%, DAG increased from 10.1% to 48.8% and MAG increased from 5.5% to 25.9%. Total glycerides increased from 57.2% to 96.3% over the course of the reaction. The unidentified lipids reduced from 2.6 to 1.9%. The low levels of FFA achieved compare favorably with levels achieved in fish oil re-esterification and also when lipases have been applied to reduce fish oil acidity [25,42].

Table 3.
Changes of lipid classes with reaction time under optimized conditions.
3.3.2. Fatty Acid Compositions
Fatty acid analysis was carried out using GC-FAMES (Table 4), which showed the squid oil had 45.8% PUFA, 26.4% MUFA and 25.0% SFA. These were unchained from the starting material within experimental error and consistent with literature fatty acid compositions for squid oil [43]. Individual fatty acids were essentially unchanged after glycerolysis. These results compare favorably with fish oil re-esterification, particularly chemical methods that result in some polymerization of PUFA [26].

Table 4.
Fatty acid compositions of the squid oil before and after esterification.
3.3.3. Astaxanthin Content after Glycerolysis
Astaxanthin content was compared for the crude input squid oil and product prepared under optimized conditions, with fresh and recovered glycerol (Figure 4A). After a reaction time of 24 h the astaxanthin content was reduced by approximately 50% showing some loss of this oxidatively sensitive carotenoid. To further investigate this partial degradation, we analyzed the change in astaxanthin content over time using the optimized conditions set (Figure 4B). Significantly, within the first 10 h, there was only a 12% reduction in astaxanthin, while the glycerolysis reaction was mostly complete. High astaxanthin degradation is observed after 24- and 48-h reactions, with the change modelled by a 2nd-order polynomial trendline with a 0.965 R2 value. Consequently, we recommend a reaction time of 10 h or less to achieve the optimum balance between neutralization and retention of EPA, DHA and astaxanthin. Oils containing EPA and DHA such as fish oil, algal oil and squid oil normally require added antioxidants for stabilization. The presence of astaxanthin, a natural antioxidant, appears to be important for stabilizing the oil and so its retention is not only important for potential health benefits, but also for oil stability.
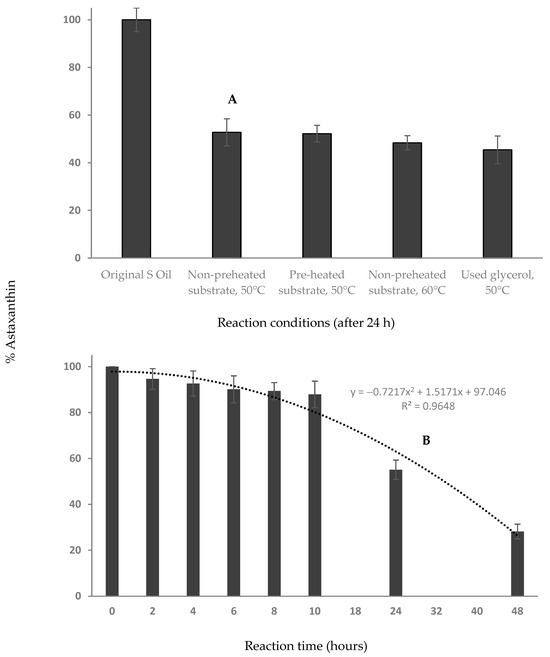
Figure 4.
Effect of (A) different reaction conditions (B) reaction time (under optimized conditions) on the astaxanthin content of squid oil during esterification process.
3.3.4. Acid Value
The acid value of an oil is used commercially as a measure of free fatty acid levels [42,44]. Consistent with the lipid class data obtained from TLC-FID, acid values of the squid oil decreased as the reaction progressed (Figure 5), from an initial acid value of 79.62 mg KOH/g oil to 6.78 mg KOH/g oil after 10 h and 3.54 mg KOH/g oil after 24 h.
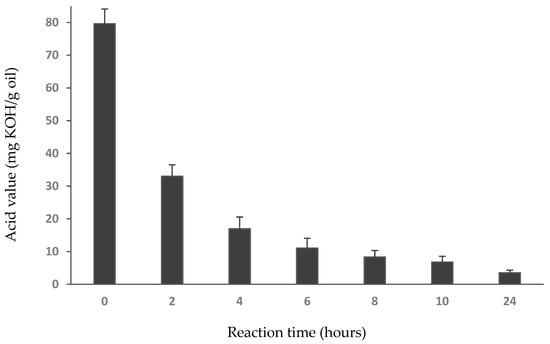
Figure 5.
Decrease of Acid Value of squid oil with increasing esterification time.
4. Conclusions
A custom-built one-liter reactor was used to optimize the lipase-catalyzed neutralization of a marine oil rich in the omega-3 fatty acids EPA and DHA derived from squid waste to produce a nutritional oil. Optimized conditions include using 1% m/v lipozyme RMIM, and 1% m/v molecular sieves, commencing with a 3:1 oil-to-glycerol ratio and adding additional glycerol at 2, 4 and 6 h. The reaction proceeded faster at 50 °C, when reactants were pre-heated separately and the stirring speed was 400 rpm for the first 2 h, before being increased to 600 rpm. Under the optimized conditions, greater than 93% of the free fatty acids were converted to acylglycerol in 10 h and 96% in 24 h, with a corresponding decrease in acid values. Dietary significant PUFAs including EPA and DHA were unchanged after the reaction, at 46.5 ± 1.0%. Astaxanthin was mostly retained after 10 h of reaction (88% retained) but decreased significantly after 24 h reaction (50%). This study shows that high FFA-containing oils, particularly squid viscera oil, can be effectively converted to high acylglycerol oils using an immobilized lipase reactor system, with retention of EPA, DHA and astaxanthin.
Author Contributions
M.A.H., B.J.H. and C.J.B. designed the experiments and confirmed the experimental protocols. C.J.B., T.O.A. and M.S. designed and built the lab-scale reactor. M.A.H., T.O.A. and B.J.H. conducted the laboratory experiments. M.A.H. and B.J.H. prepared the draft of the manuscript. All co-authors contributed by editing and checking. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the State Government of Victoria, Australia under the Recycling Victoria Circular Economy Innovation Fund Round One (2021) and Mantzaris Fisheries Pty Ltd., Geelong Australia.
Data Availability Statement
The original data presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Moninder Sachar was employed by Mantzaris Fisheries. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dunstan, J.A.; Mitoulas, L.R.; Dixon, G.; Doherty, D.A.; Hartmann, P.E.; Simmer, K.; Prescott, S.L. The Effects of Fish Oil Supplementation in Pregnancy on Breast Milk Fatty Acid Composition Over the Course of Lactation: A Randomized Controlled Trial. Pediatr. Res. 2007, 62, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Taati, M.M.; Shabanpour, B.; Ojagh, M. Investigation on fish oil extraction by enzyme extraction and wet reduction methods and quality analysis. AACL Bioflux 2018, 11, 83–90. [Google Scholar]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Nielsen, A.B. Plasma lipid and lipoprotein pattern in greenlandic west-coast eskimos. Lancet 1971, 297, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Hatcher, L. The effect of lean fish consumption on triglyceride levels. Phys. Sport 2009, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Renata, M.; Shahab, K.; Peilin, S.; Saman, F.; Stephen, L.; Kathryn, G.A.; Rebecca, E.E.; John, P.; Majid, E.; Dariush, M. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ Br. Med. J. 2014, 348, g2272. [Google Scholar]
- Rizliya, V.; Mendis, E. Biological, Physical, and Chemical Properties of Fish Oil and Industrial Applications, in Seafood Processing By-Products: Trends and Applications; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 285–313. [Google Scholar]
- Bartek, L.; Strid, I.; Henryson, K.; Junne, S.; Rasi, S.; Eriksson, M. Life cycle assessment of fish oil substitute produced by microalgae using food waste. Sustain. Prod. Consum. 2021, 27, 2002–2021. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Yi, M.; You, Y.; Zhang, Y.; Wu, G.; Karrar, E.; Zhang, L.; Zhang, H.; Jin, Q.; Wang, X. Highly valuable fish oil: Formation process, enrichment, subsequent utilization, and storage of eicosapentaenoic acid ethyl esters. Molecules 2023, 23, 672. [Google Scholar] [CrossRef]
- Neff, L.M.; Culiner, J.; Cunningham-Rundles, S.; Seidman, C.; Meehan, D.; Maturi, J.; Wittkowski, K.M.; Levine, B.; Breslow, J.L. Algal Docosahexaenoic Acid Affects Plasma Lipoprotein Particle Size Distribution in Overweight and Obese Adults. J. Nutr. 2011, 141, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Finco AM, D.O.; Mamani LD, G.; Carvalho JC, D.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Eun-mi, K. Characteristics of Squid Viscera Oil. Korean J. Fish. Aquat. Sci. 1997, 30, 595–600. [Google Scholar]
- Kang, K.-Y.; Ahn, D.-H.; Wilkinson, G.T.; Chun, B.-S. Extraction of lipids and cholesterol from squid oil with supercritical carbon dioxide. Korean J. Chem. Eng. 2005, 22, 399–405. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Kolanowski, W.; Laufenberg, G. Enrichment of food products with polyunsaturated fatty acids by fish oil addition. Eur. Food Res. Technol. 2006, 222, 472–477. [Google Scholar] [CrossRef]
- Mariem, K.; Fatima, B. Reduction of Free Fatty Acid Content of Crude Sardine Oil by Enzymatic Esterification at Laboratory Scale. Int. J. Biol. Chem. 2017, 11, 23–29. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. How the brain fights fatty acids’ toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Wee, C.; Lee, J.; Kim, B.H.; Kim, H.R.; Kim, I.H. Concentration of docosahexaenoic acid from tuna oil by a combination of solvent crystallization and lipase-catalyzed ethanolysis. J. Am. Oil Chem. Soc. 2024, 1–9. [Google Scholar] [CrossRef]
- Moreno-Perez, S.; Luna, P.; Señorans, F.; Guisan, J.; Fernandez-Lorente, G. Enzymatic synthesis of triacylglycerols of docosahexaenoic acid: Transesterification of its ethyl esters with glycerol. Food Chem. 2015, 187, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Señoráns, F.J. Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: A review. New Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Angulo, B.; Fraile, J.M.; Gil, L.; Herrerias, C. Comparison of Chemical and Enzymatic Methods for the Transesterification of Waste Fish Oil Fatty Ethyl Esters with Different Alcohols. ACS Omega 2020, 5, 1479–1487. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, X.H. 2.23-Enzyme Bioreactors, in Comprehensive Biotechnology, 2nd ed.; Murray, M.-Y., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 319–329. [Google Scholar]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Lipase-catalysed incorporation of EPA into emu oil: Formation and characterisation of new structured lipids. J. Funct. Foods 2015, 19, 801–809. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- García, H.S.; Baeza-Jiménez, R.; Miranda, K.; Otero, C. Lipase-catalyzed glycerolysis of fish oil to obtain diacylglycerols. Grasas Aceites 2013, 64, 237–242. [Google Scholar] [CrossRef][Green Version]
- Mustafa, A.; Ramadan, R.; Niikura, F.; Inayat, A.; Hafez, H. Highly selective synthesis of glyceryl monostearate via lipase catalyzed esterification of triple pressed stearic acid and glycerin. Sustain. Energy Technol. Assess. 2023, 57, 103200. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J. Lipid profiles, in vitro digestion and oxidative stability of mutton bird oil. J. Food Sci. Technol. 2016, 53, 1230–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Craske, J.D.; Bannon, C.D. Gas liquid chromatography analysis of the fatty acid composition of fats and oils: A total system for high accuracy. J. Am. Oil Chem. Soc. 1987, 64, 1413–1417. [Google Scholar] [CrossRef]
- Armenta, R.E.; Burja, A.; Radianingtyas, H.; Barrow, C.J. Critical Assessment of Various Techniques for the Extraction of Carotenoids and Co-enzyme Q10 from the Thraustochytrid Strain ONC-T18. J. Agric. Food Chem. 2006, 54, 9752–9758. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Zhao, Q.; Zhang, Q.; Zhao, M. Fast synthesis of 1,3-DAG by Lecitase® Ultra-catalyzed esterification in solvent-free system. Eur. J. Lipid Sci. Technol. 2011, 113, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Du, Z.; Yao, Y.; Li, R.; Wu, D. Effect of Molecular Sieves on Lipase-Catalyzed Esterification of Rutin with Stearic Acid. J. Agric. Food Chem. 2006, 54, 6219–6225. [Google Scholar] [CrossRef] [PubMed]
- Robles Medina, A.; Esteban Cerdán, L.; Giménez Giménez, A.; Camacho Páez, B.; Ibáñez González, M.J.; Molina Grima, E. Lipase-catalyzed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. In Progress in Industrial Microbiology; Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 379–391. [Google Scholar]
- Ravelo, M.; Esteban, J.; Ladero, M.; García-Ochoa, F. Enzymatic synthesis of ibuprofen monoglycerides catalyzed by free Candida antarctica lipase B in a toluene–glycerol biphasic medium. RSC Adv. 2016, 6, 69658–69669. [Google Scholar] [CrossRef]
- Kim, I.-H.; Lee, S.-M. Synthesis of Diacylglycerols Containing CLA by Lipase-Catalyzed Esterification. J. Food Sci. 2006, 71, C378–C382. [Google Scholar] [CrossRef]
- Mata, T.M.; Correia, D.; Andrade, S.; Casal, S.; Ferreira, I.M.P.L.V.O.; Matos, E.; Martins, A.A.; Caetano, N.S. Fish oil enzymatic esterification for acidity reduction. Waste Biomass Valorization 2020, 11, 1131–1141. [Google Scholar] [CrossRef]
- Asadpour, Y.A. Squid (Loligo loligo): The new source to extract omega-3 and omega-6 rich marine oils. Iran. J. Fish. Sci. 2016, 15, 100–107. [Google Scholar]
- Haq, M.; Park, S.-K.; Kim, M.-J.; Cho, Y.-J.; Chun, B.-S. Modifications of Atlantic salmon by-product oil for obtaining different ω-3 polyunsaturated fatty acids concentrates: An approach to comparative analysis. J. Food Drug Anal. 2018, 26, 545–556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).