Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review
Abstract
1. Introduction
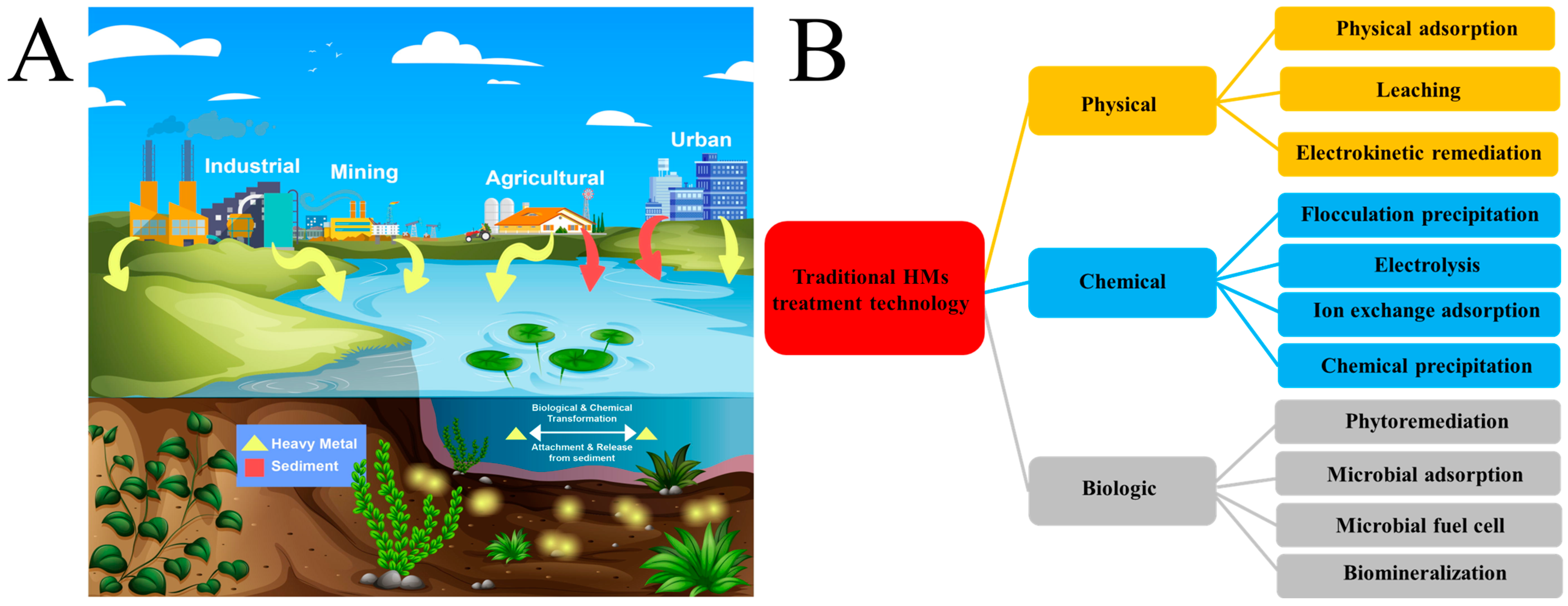
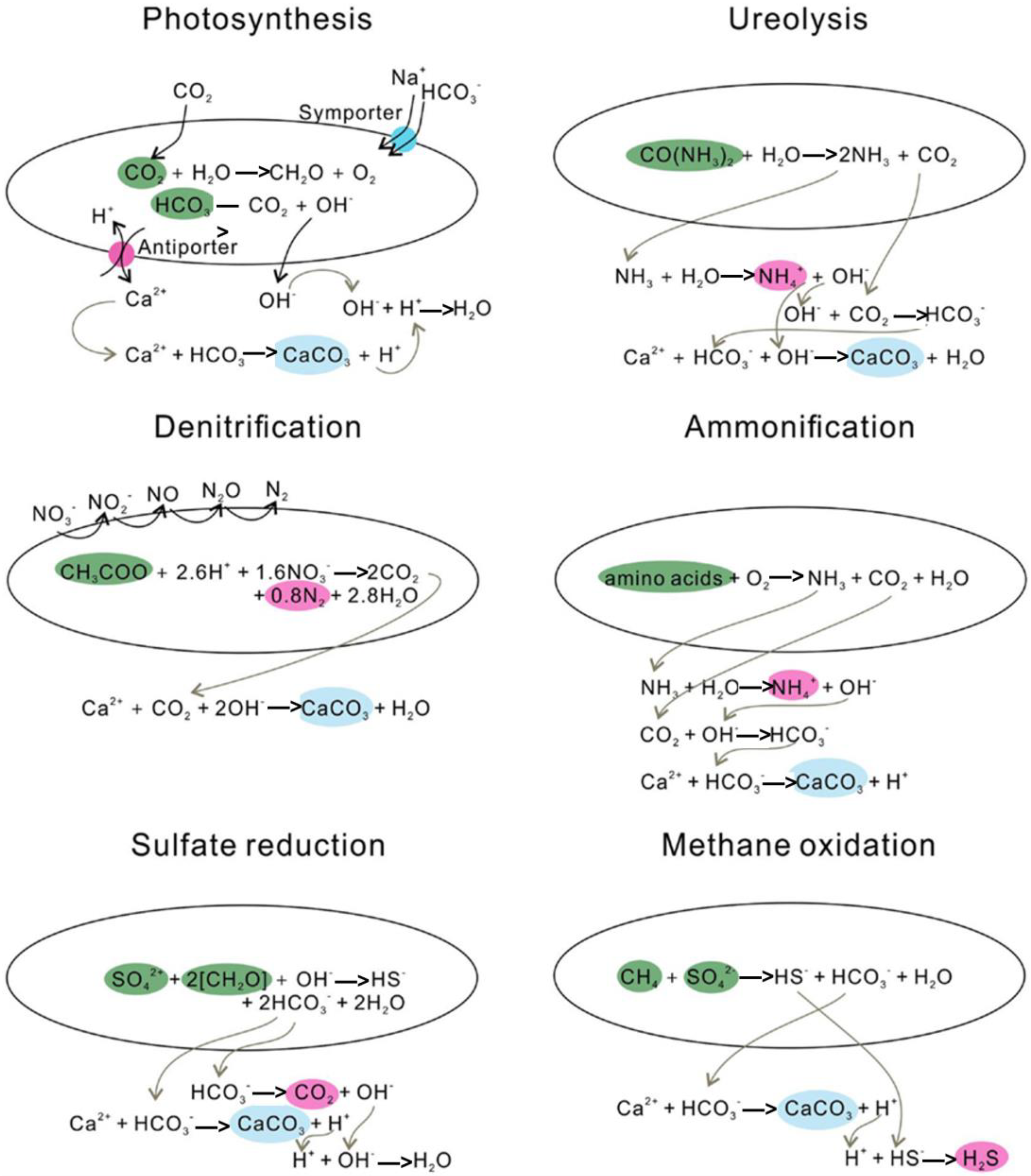
2. Microbial-Induced Carbonate Precipitation
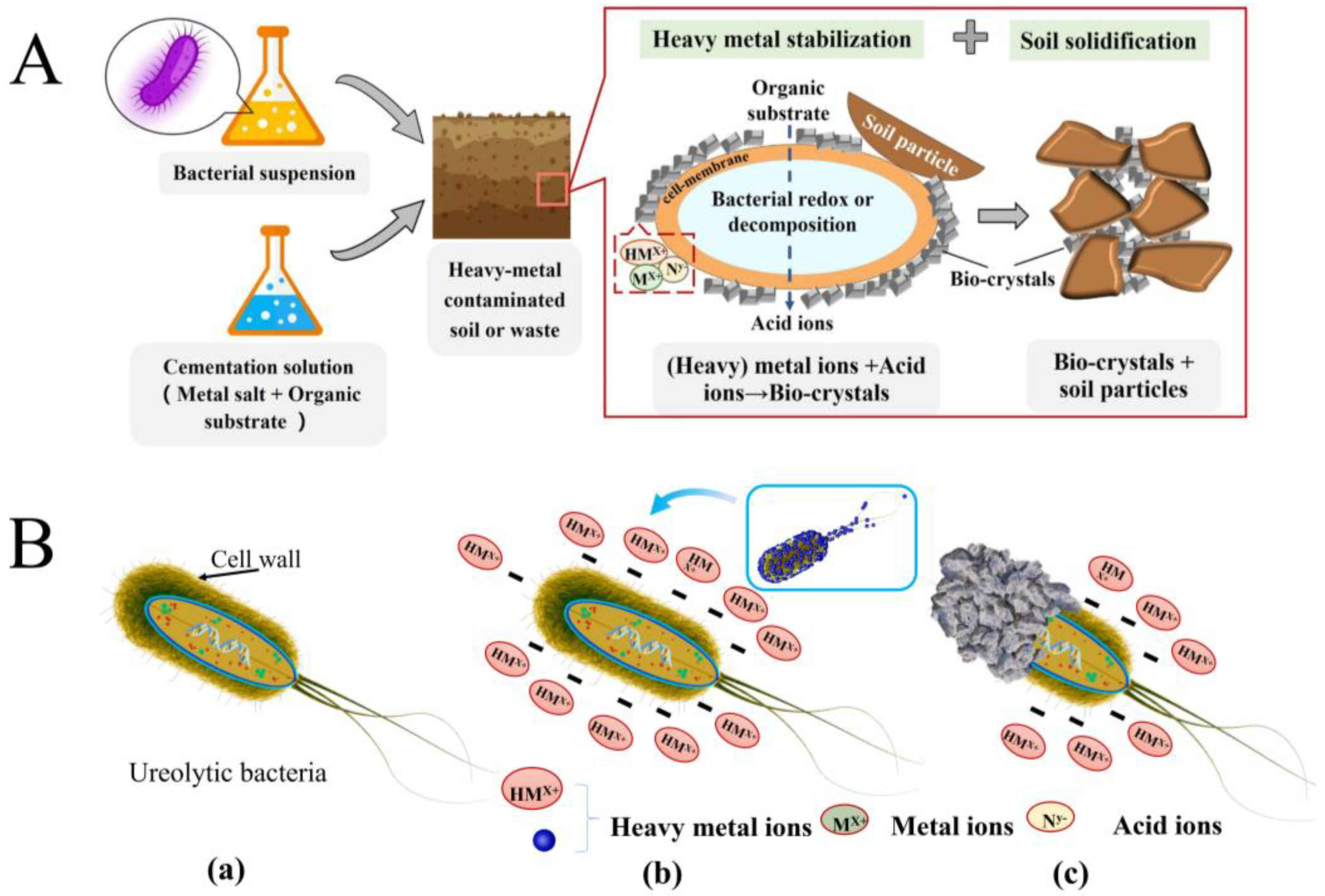
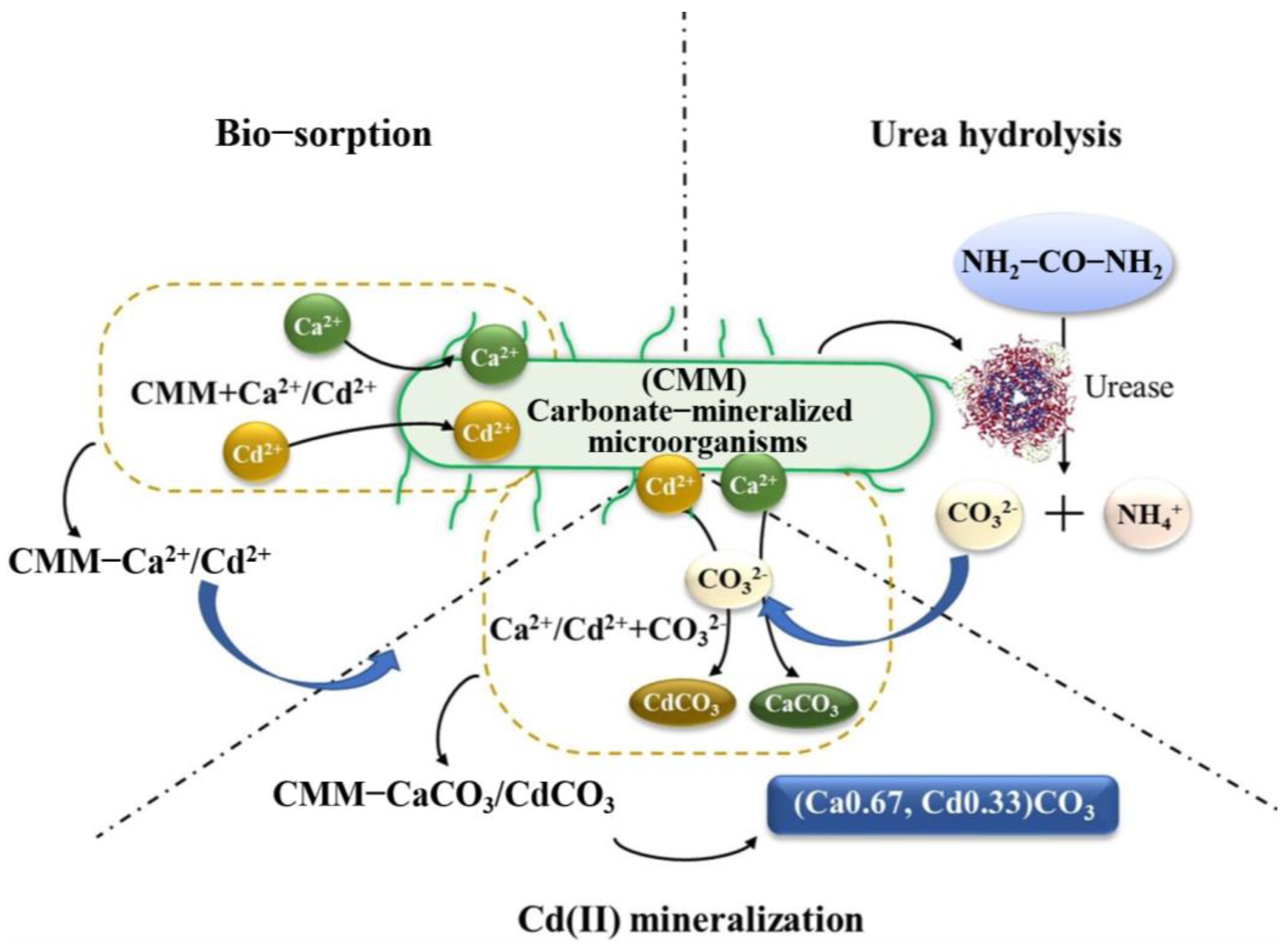
2.1. Mechanism of HM Mineralization with MICP
- (1)
- HM elements (such as lead and cadmium, etc.) can be directly captured by biosorption processes, such as transport across the cell membrane, complexation, ion exchange, rain, and physical adsorption [67,68]. Then, the captured HMs react with the microbially produced carbonate and form the corresponding carbonate precipitates;
- (2)
- in the presence of calcium, the microbially induced calcite can also adsorb HMs (such as Cd and Pb) [69], and co-precipitation of CaCO3 and MCO3 (M = Cd, Pb, etc.) can occur during HM removal;
- (3)
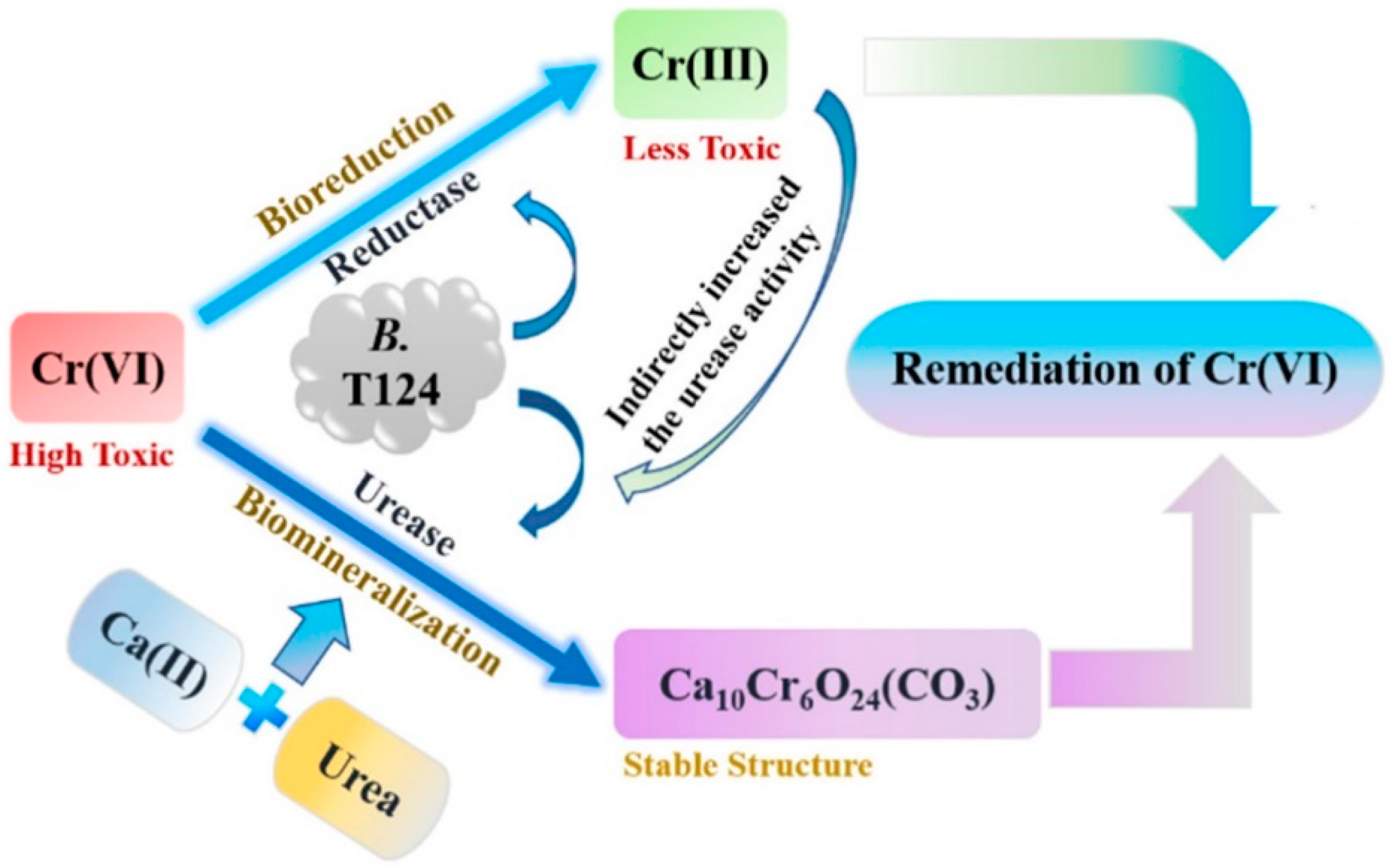
- the MICP process induced by special bacteria that can produce urease and reductase at the same time (such as Bacillus thuringiensis T124) cannot only reduce Cr (VI) but also increase the pH of the microenvironment and make Cr reach the precipitation condition [70]. These proven effective mechanisms provide many references for MICP technology in HM removal.
2.2. Microorganisms
| Microorganism | Activity (U/mL) | Isolation Site | Isolation Time (Year) | Ref. |
|---|---|---|---|---|
| Bacillus sp. WA | - | Metal-contaminated soil, Shantou, China | 2022 | [99] |
| Acinetobacter sp. H12 | - | Sludge from Qujiang artificial lake, Shaanxi, China | 2021 | [78] |
| Achromobacter sp. L3 | - | Sediments in Datang Industrial Park, Foshan, Guangdong, China | 2021 | [79] |
| Bacillus thuringiensis T124 | 66.4 | Cr-contaminated soils, Jiangsu, China | 2021 | [70] |
| Lysinibacillus sp.; Pseudochrobactrum sp.; Sporosarcina sp. | - | Pyrite station, Sichuan, China | 2021 | [67] |
| Arthrobacter sp. MF-2; Curvibacter sp. HJ-1 | - | Soil, Nanjing Agricultural University, China | 2020 | [81] |
| Bacillus cereus D2 | 194.6 | Ni mine, Hongqiling, Jilin Province, China | 2020 | [83] |
| Enterobacter sp. CJW-1 | - | Paddy soil, Sichuan, China | 2020 | [86] |
| Bacillus lysine | - | Pb-contaminated soil, Hubei, China | 2020 | [85] |
| Sporosarcina luteola | 1082–1120 | Alkaline and acidic tailings soil, Guerrero State, Mexico | 2020 | [89] |
| Sporosarcina pasteurii | 11.08 | Rhizosphere soil, Zanjan, Iran | 2020 | [90] |
| Bacillus amyloliquefaciens HU-48; Bacillus atrophaeus HU-11; Bacillus aryabhattai HU-39; Proteus mirabilis HU-57; Bacillus subtilis HU-34 | 6–13 | Barn horse’s soil, College of Agriculture, Al-Jadriya horsemanship club and Baghdad University, Iraq | 2019 | [82] |
| Bacillus cereus NS4 | - | Industrial soil, Shanghai, China | 2019 | [84] |
| Paecilomyces inflatus; Plectosphaerella cucumerina | - | Perovskite straw stalactites on the concrete ceiling | 2019 | [88] |
| Alcaligenes aquatili | - | Seawater | 2019 | [80] |
| Bacillus cereus P9 | 5–55 | Peat soil, Miri, Malaysia | 2018 | [100] |
2.3. Current Applications of MICP
3. HM Stabilization Using MICP
3.1. Copper
3.2. Cadmium
3.3. Lead
3.4. Nickel
3.5. Zinc
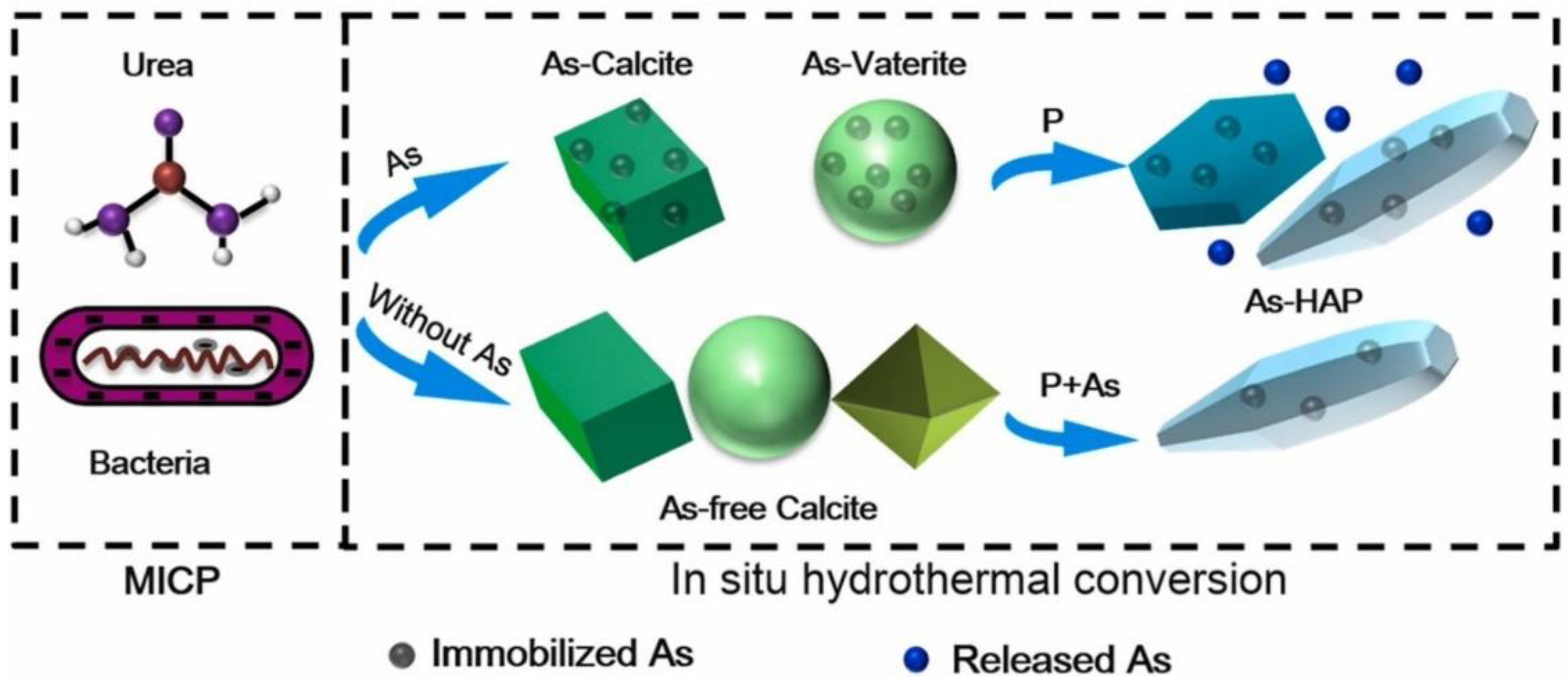
3.6. Arsenic
3.7. Chromium
3.8. Mixed HMs
| HM | Bacterium/Bacterial | Culture Medium | Initial Key Test Condition/Variables | Removal Rate (%) | Advantages/Improvements | Ref. |
|---|---|---|---|---|---|---|
| Cu | Mixed flora: Bacillus genus; Actinobacteria phyla | Nutrient Broth of Urea (NBU) and Calcium Source | The background value in the soil is 14.25 mg/kg; mixed with 25 and 50 mg/kg | More than 90 | Avoid adding UPB bacteria to the soil, and domesticating UPB through in situ biological stimulation. | [114] |
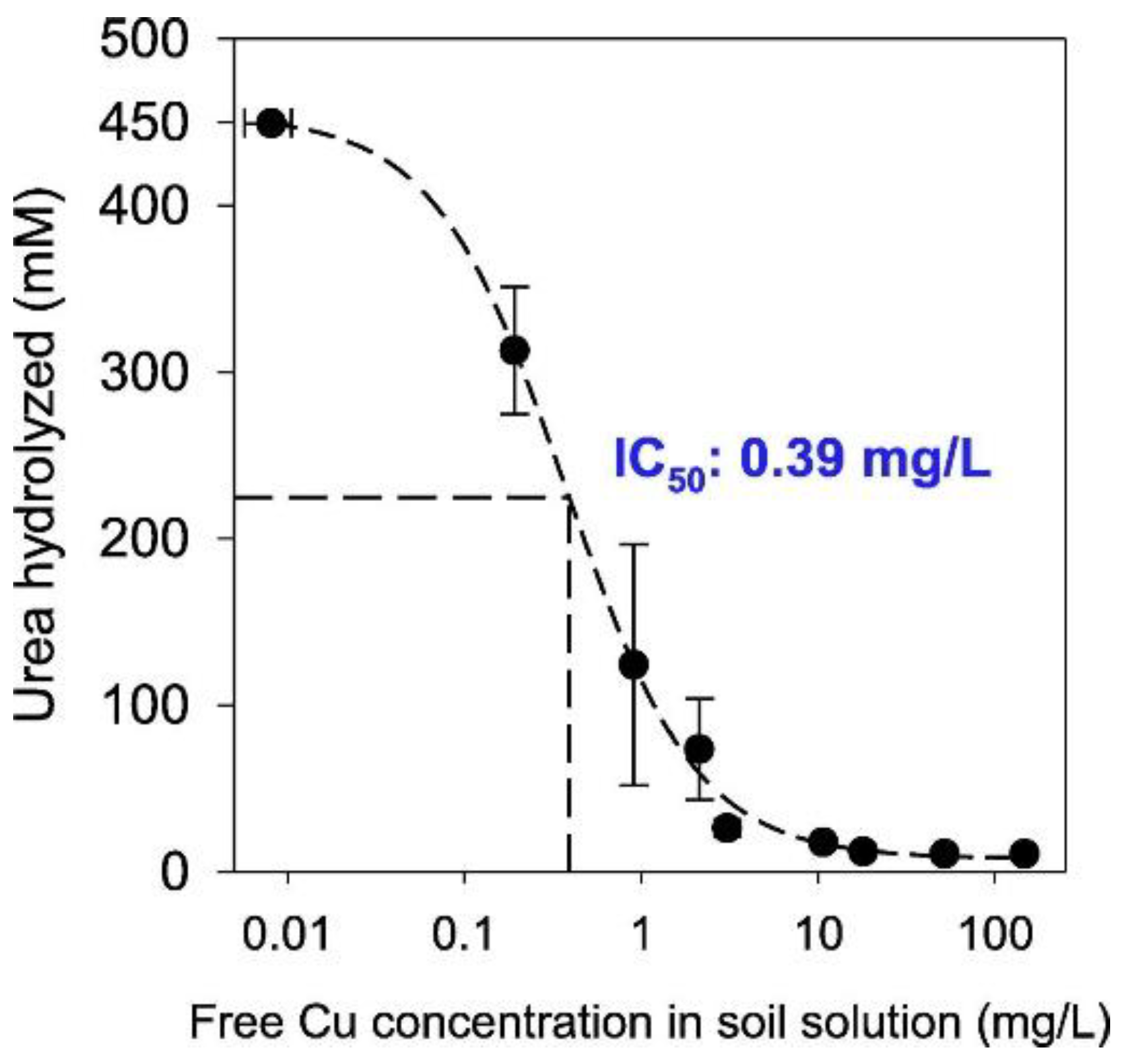
| Sporosarcina pasteurii | Urea, calcium, tryptic soy broth (TSB) | The background value in the soil is 250 mg/kg | - | Relationship between the Cu concentration in the soil solution and the hydrolysis of urea was established, and the IC50 of the free Cu ion concentration in the soil solution was determined to be 0.39 mg/L. | [112] | |
| Sporosarcina pasteurii | NH4-YE medium | 32 mg/L | 10 | Show that the low removal rate of Cu is due to the complexation of Cu2+ with ammonia due to the hydrolysis of urea, and the sequential process of bacterial growth and Cu precipitation decoupling to achieve effective control of urea demand. | [113] | |
| Cd | Achromobacter sp. L3 | Basal media (BM) | 10 mg/L | 100 | Separate the high-efficiency Sulfamethoxazole degradation, and Cd mineralization strains from wastewater at the same time. | [79] |
| Cupriavidus sp. CZW-2; Bacillus sp. CZW-5; Bacillus sp. CZW-9; Bacillus sp. CZW-12 | Lysogeny broth media | 225 mg/L | 80.10, 72.64, 76.70 73.40 | Research on the crystallinity, size, bioavailability, and stability of Cd-mineralized precipitates in the MICP process has been perfected. | [109] | |
| Enterobacter sp. CJW-1 | Luria Broth (LB) liquid medium | 20, 40, 60, 80, 100 mg/L (LB medium); 20 mg/kg (soil) | 99.50, 64.86, 51.83, 40.95 and 10.82; 56.10 | Combination of urea-decomposing bacteria and oyster shell waste may have a better mineralization effect on Cd in the soil. At the same time, it solves the pollution problem of oyster shell waste, the problem of ammonium root treatment, and the economic pressure caused by urea. | [86] | |
| Serratia marcescens (NCIM2919); Enterobacter cloacae EMB19 (MTCC10649) | Nutrient medium containing 25 mM of CaCl2 and 2% urea | 50 mg/L | With calcium (98, 53); without calcium (16, 8) | Calcium–cadmium co-crystallization is beneficial to the removal of Cd, and the addition of calcium can effectively improve the removal rate of cadmium. | [117] | |
| Sporosarcina pasteurii | Nutrient Broth of Urea (NBU) and Calcium Source | 56, 112, and 225 mg/L; 10, 20, 40, and 50 mg/kg (sandy and clay soils) | 99.6, 99.8, and 99.8; Sandy soils: 85.9, 61.1, 74.3, and 80.3. Clay soils: 89.3, 86.6, 76, and 75.6 | Reports on the application of MICP in clay and its research on the immobilization of HMs. The minimum inhibitory concentration (MIC) of bacteria to Cd is 2 mM. | [116] | |
| Mixed flora: Klebsiella pneumoniae; Escherichia coli | Brain heart infusion (BHI) medium | 3710 mg/L | 97.4 ± 1.1 | It proves that there is a carbonate precipitation process induced by microorganisms in anaerobic granular sludge. | [136] | |
| Urease-producing consortium (UPC): Sporosarcina; norank_f_Bacillaceae; unclassified_f_Bacillaceae | NBU media | 100 mg/L | 92.87 | Construct a bacterial consortium with high urease activity that can adapt to different environmental conditions; characterize the urease gene and bacterial community succession during the adaptation process. | [101] | |
| Cr (VI) | Bacillus thuringiensis T124 | LB medium | The background value is 39.89 mg/kg; mixed with 100 mg/L | - | By screening Bacillus thuringiensis T124, which produces reductase and urease, it first reduces Cr(VI) to a state of low toxicity and then undergoes stable mineralization. | [70] |
| Pb | Sporosarcina pasteurii | NH4-YE medium | 2072, 4144, 6216, 8288, and 10,360 mg/L | - | A hypothetical multilayer precipitation structure is proposed; it is found that a lead concentration of 30 mM is the marginal value that Sporosarcina pasteurii (OD600 = 1.5) can tolerate. | [121] |
| Bacillus sphaericus; Sporosarcina pasteurii | BG11 broth and nutrient broth | Around 225 mg/L | 94–99.2 | Use cyanobacteria and bacteria to enhance the strength of artificially polluted sand and immobilize pollutants. | [120] | |
| Lysinibacillus fusiformis | LB medium | 200 mg/L | pH = 3, <40; pH = 4, around 90 | The acidophilic UPB that can adjust the pH value of the solution is screened out, and then the Pb in the acid wastewater can be removed. | [85] | |
| Ni | Bacillus cereus NS4 | NBU media | 50 and 100 mg/L | 89 and 66 | Apply biochar to the MICP process to remediate Ni. | [78] |
| Bacillus cereus D2 | Nutrient broth (NB) medium | 50 mg/L | 73.47 | Bacillus cereus D2, which is resistant to low temperatures (10 °C), was selected, which can mineralize Ni in low-temperature areas. | ||
| Acinetobacter sp. H12 | Basal medium | 3 mg/L | 56.67 | Use denitrifying bacteria Acinetobacter sp. H12 to study its ability to remove F− and Ni2+. | [78] | |
| Cd and Pb | Micrococcus sp. NCTC-1716 | Urease broth medium | ½ MIC of each HM | Cd: 60.66; Pb: 97.20 | Select Micrococcus sp. NCTC-1716 from calcareous soil proved to be useful for HMs removal. | [137] |
| Cd and Ni | Bacillus and Proteus species | NB medium | 500 mg/L of each | Cd:96; Ni:89 | The precipitation of metal carbonates induced by microorganisms with and without calcium precipitation is reported. | [82] |
| Cu, Zn, Ni, and Cd | Sporosarcina kp-4; Sporosarcina kp-22 | BPU fluid medium | 160 mg/L of each | 75.10, 98.03, 59.46 and 96.18 | Explore the precipitation modes of different HMs and the role of bacteria in biomineralization. | [67] |
| Pb and Hg | Proteus mirabilis 10B | Basal medium | 350 mg/L of each | Aerobic: (Pb: 95.2 and Hg: 91.1); Anaerobic (Pb: 92 and Hg: 88.3) | Proteus mirabilis 10B was first used to reduce nitrate, which drives calcium carbonate precipitation to remediate Pb and Hg in aerobic and anaerobic conditions. | [119] |
| Pb and Cu | Paecilomyces inflatus; Plectosphaerella cucumerina | NB medium | 100 mg/L | Pb: 100 and 100; Cu: 13 and 10 | Isolated fungal strains can induce carbonate precipitation. | [88] |
| Pb, Zn, and Cd | Sporosarcina pasteurii | Nutrient solution recommended by German strain Center | 342 mg/kg; 6.79 mg/kg; 235 mg/kg | Pb: 33.3–85.9; Zn: 21.4–66.0; Cd: 13.6–29.9 | It is proposed to pre-mix the required UPB, nutrient substrate, and calcium salt into the contaminated soil through a mixing method to realize the one-time efficient solidification of HM ions in the soil. | [97] |
| Pb, Cd, and Zn | Stenotrophomonas rhizophila A323; Variovorax boronicumulans C113; Sporosarcina pasteurii DSM33 | BPU broth medium | 20,720, 62,160 and 103,600 mg/L; 11,240, 33,720 and 56,200 mg/L; 6540, 19,620, and 32,700 mg/L | Pb: 96.25, 95.93 and 98.71; Cd: 71.3, 73.45 and 97.15; Zn: 63.91, 73.81, and 94.83 | Stenotrophomonas rhizophila and boronicumulans ariovorax were firstly reported as ureolytic bacteria for metal biomineralization. | [90] |
| Mn, Pb, Cd, Sr, Ba, and Zn | Sporosarcina luteola UB3; Sporosarcina luteola UB5 | NB medium NH4-YE medium | - | - | Evaluate its ability to produce bio-carbonate, reduce porosity and permeability, and fix biologically effective toxic elements. | [89] |
| As, Cd, Mn, Ni, Ba, and Sr | Ureolytic bacterial consortium: Sporosarcina; Arthrobacter | NB medium | 1.091 mg/L, 0.011 mg/L, 0.4623 mg/L, 0.041 mg/L, 1.074 mg/L and 342 mg/L, respectively | As, Cd, Mn, Ni: 100; Ba: 92.2; Sr: 94.2 | MICP is applied to the shale oil and gas industry to remove calcium in the produced water to reduce the possibility of clogging the wellbore and damaging the formation. | [135] |
4. Challenges and Prospects of MICP in Removing HMs
4.1. Improvement of HM Removal
4.2. Long-Term Stability of MICP-Treated Soil
4.3. Large-Scale Practical Application of MICP in Contaminated Soil
- (1)
- During the process of MICP, urea will produce not only carbonate but also ammonium during the decomposition process. Therefore, determining the optimal substrate balance (e.g., urea and Ca) for various MICP applications to optimize the precipitation efficiency of HMs, may increase economic feasibility and reduce the production of harmful by-products.
- (2)
- The MICP process is more complicated than simple chemical processes and controlled by many factors, such as microbial tolerance, pH, and temperature. Compared with MICP based on suspension cells, MICP based on protective materials has greater potential.
- (3)
- The nutritional source of laboratory-grade MICP is economically limited in practical applications. Urea-containing wastewater produced by fertilizer plants, the urea synthesis industry, or human activities (sewage) seems to be the simplest solution [149,150]. Seeking more low-cost sources of nutrition can make the application of MICP more economical.
- (4)
- Due to the influence of the traditional mindset, the behavior of adding microorganisms (albeit harmless microorganisms) into the environment is not accepted by most people. Therefore, in situ simulation to induce bacteria with urease decomposition is an idea worth considering.
- (5)
- Carry out large-scale experiments and construct mathematical models related to the large-scale experiment process (e.g., urea decomposition and growth kinetics, precipitation kinetics, crystal growth, and microbial–mineral interactions, etc.), which can help evaluate the transportation process—important in controlling MICP for engineering field applications.
- (6)
- Develop induced polarization (geophysical method) detection tools to detect and evaluate the propagation of MICP in the treatment area in space and time.
4.4. Efficient Removal of Heavy Metals from Contaminated Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UPB | Urease-producing bacteria |
| EPS | Extracellular polymers |
| SAR | Simulated acid rain |
| MICP | Microbial-induced carbonate precipitation |
| HM | Heavy metal |
| IC50 | Half-maximal inhibition concentration |
| UPC | Urease-producing consortium |
| NBU | Nutrient broth of urea |
| TSB | Tryptic soy broth |
| BM | Basal media |
| LB | Luria broth |
| MIC | Minimum inhibitory concentration |
| BHI | Brain heart infusion |
| NB | Nutrient broth |
| FT | Freeze–thaw |
| CMM | Carbonate-mineralized microorganisms |
References
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Americo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A review on global metal accumulators-mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. Int. 2019, 26, 26449–26471. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, P.-A.; Staldvik, F.J.; Lukin, A.A.; Kashulin, N.A.; Popova, O.A.; Reshetnikov, Y.S. Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Sci. Total Environ. 1997, 201, 211–224. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Xu, J. Heavy metal pollution in the East China Sea: A review. Mar. Pollut. Bull. 2020, 159, 111473. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Jiang, X.; Wang, K.; Xia, J.; Jiao, W.; Niu, Y.; Yu, H. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020, 700, 134509. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Minasse, F.A.; Guilherme, M.R.; Reis, A.V.; Muniz, E.C.; Nozaki, J. Novel adsorbent based on silkworm chrysalides for removal of heavy metals from wastewaters. J. Colloid Interface Sci. 2006, 301, 479–487. [Google Scholar] [CrossRef]
- Harada, M.; Miyagawa, K.; Honma, Y.; Hiura, M.; Shibata, M.; Matsuhashi, T.; Abe, S.; Harada, R.; Tabaru, A. Excess copper chelating therapy for Wilson disease induces anemia and liver dysfunction. Intern. Med. 2011, 50, 1461–1464. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Farrag, K.; Elbastamy, E.; Ramadan, A. Health Risk Assessment of Heavy Metals in Irrigated Agricultural Crops, El-Saff Wastewater Canal, Egypt. CLEAN-Soil Air Water 2016, 44, 1174–1183. [Google Scholar] [CrossRef]
- Yang, L.; Liu, B.; Lu, Y.; Lu, F.; Wu, X.; You, W.; Huang, B. Bioavailability of cadmium to celery (Apium graveolens L.) grown in acidic and Cd-contaminated greenhouse soil as affected by the application of hydroxyapatite with different particle sizes. Chemosphere 2020, 240, 124916. [Google Scholar] [CrossRef] [PubMed]
- Naseem, R.; Tahir, S.S. Removal of Pb(II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res. 2001, 35, 3982–3986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, L.; Meng, Z.; Yu, A.; Guo, L.; Wang, H. A sensitive fluorescence anisotropy method for detection of lead (II) ion by a G-quadruplex-inducible DNA aptamer. Anal. Chim. Acta 2014, 812, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Langford, N.J.; Ferner, R.E. Toxicity of mercury. J. Hum. Hypertens. 1999, 13, 651–656. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Guo, G.; Yan, Z. Status and environmental management of soil mercury pollution in China: A review. J. Environ. Manag. 2021, 277, 111442. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Oyaro, N.; Ogendi, J.; Murago, E.N.; Gitonga, E. The contents of Pb, Cu, Zn and Cd in meat in Nairobi, Kenya. J. Food Agric. Environ. 2007, 5, 119–121. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Simões, B.D.F.; Lino, C.; Pena, A. Exposure to nickel through commercial premade baby foods: Is there any risk? J. Food Compos. Anal. 2020, 92, 103541. [Google Scholar] [CrossRef]
- Babaahmadifooladi, M.; Jacxsens, L.; Van de Wiele, T.; Laing, G.D. Gap analysis of nickel bioaccessibility and bioavailability in different food matrices and its impact on the nickel exposure assessment. Food Res. Int. 2020, 129, 108866. [Google Scholar] [CrossRef]
- Wang, M.; Wu, S.; Guo, J.; Liao, Z.; Yang, Y.; Chen, F.; Zhu, R. Immobilization and migration of arsenic during the conversion of microbially induced calcium carbonate to hydroxylapatite. J. Hazard. Mater. 2021, 412, 125261. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.F.; Abdul Karim, N.; Ahmad, W.A.; Zakaria, Z.A. Chromate detoxification using combination of ChromeBac™ system and immobilized chromate reductase beads. Int. Biodeterior. Biodegrad. 2016, 113, 238–243. [Google Scholar] [CrossRef]
- Shadreck, M.; Mugadza, T. Chromium, an essential nutrient and pollutant: A review. Afr. J. Pure Appl. Chem. 2013, 7, 310–317. [Google Scholar]
- El-sayed, M.E.A. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, F.J.; Zhou, Y.; Zhang, H.J.; Guo, G.L.; Tian, Y. Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 2020, 255, 126690. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; He, X.; Liu, Y. Optimization analysis and mechanism exploration on the removal of cadmium from contaminated soil by electrokinetic remediation. Sep. Purif. Technol. 2020, 250, 117180. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Yang, P.P.; Liu, Y.Y.; Wang, H.Y.; Chen, J.F.; Tang, M.Z.; Wang, R.J.; Wei, Y.S.; Yang, Y.W. The treatment of Cr (VI) containing wastewater by Fe/C micro-electrolysis system. Fresenius Environ. Bull. 2020, 29, 3698–3702. [Google Scholar]
- Jumina; Priastomo, Y.; Setiawan, H.R.; Mutmainah; Kurniawan, Y.S.; Ohto, K. Simultaneous removal of lead(II), chromium(III), and copper(II) heavy metal ions through an adsorption process using C-phenylcalix[4]pyrogallolarene material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process. Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Xu, S.; Xing, Y.; Liu, S.; Hao, X.; Chen, W.; Huang, Q. Characterization of Cd2+ biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China. Chemosphere 2020, 240, 124893. [Google Scholar] [CrossRef] [PubMed]
- Parkhey, P.; Sahu, R. Microfluidic microbial fuel cells: Recent advancements and future prospects. Int. J. Hydrogen Energy 2020, 46, 3105–3123. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C.S. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review. Sci. Total Environ. 2020, 746, 140967. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.; Ren, X.; Yi, H.; Cheng, M.; et al. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Torres-Aravena, A.E.; Duarte-Nass, C.; Azocar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can microbially induced calcite precipitation (MICP) through a ureolytic pathway be successfully applied for removing heavy metals from wastewaters? Crystals 2018, 8, 438. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, D.; Li, X.; Xue, Y. Biomineralization of lead in wastewater: Bacterial reutilization and metal recovery. J. Hazard. Mater. 2022, 421, 126765. [Google Scholar] [CrossRef]
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. JEnvS 2023, 125, 662–677. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Pan, H.; Tang, R. Biomineralization inspired crystal growth for biomimetic materials preparation. J. Cryst. Growth 2023, 603, 127029. [Google Scholar] [CrossRef]
- Dhami, N.K.; Quirin, M.E.C.; Mukherjee, A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol. Eng. 2017, 103, 106–117. [Google Scholar] [CrossRef]
- Gorlich, S.; Pawolski, D.; Zlotnikov, I.; Kroger, N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol. 2019, 2, 245. [Google Scholar] [CrossRef]
- Aizenberg, J.; Lambert, G.; Weiner, S.; Addadi, L. Factors involved in the formation of amorphous and crystalline calcium carbonate: A study of an ascidian skeleton. J. Am. Chem. Soc. 2002, 124, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vuola, J.; Göransson, H.; Böhling, T.; Asko-Seljavaara, S. Bone marrow induced osteogenesis in hydroxyapatite and calcium carbonate implants. Biomaterials 1996, 17, 1761–1766. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotech. 2016, 4, 4. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Yin, H.; Liang, Y.; Liu, H.; Miao, B.; Peng, Q.; Meng, D.; Wang, S.; Yang, J.; et al. The utilization of biomineralization technique based on microbial induced phosphate precipitation in remediation of potentially toxic ions contaminated soil: A mini review. Ecotoxicol. Environ. Saf. 2020, 191, 110009. [Google Scholar] [CrossRef]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, mechanisms and environmental implications of microbial biomineralization. C.R. Geosci. 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, Z.; Lyu, Q.; Cheng, Y.; Huan, C.; Jiang, X.; Yan, Z.; Tan, Z. Microbiologically induced calcite precipitation for in situ stabilization of heavy metals contributes to land application of sewage sludge. J. Hazard. Mater. 2023, 441, 129866. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Baumgartner, L.K.; Reid, R.P.; Dupraz, C.; Decho, A.W.; Buckley, D.H.; Spear, J.R.; Przekop, K.M.; Visscher, P.T. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment. Geol. 2006, 185, 131–145. [Google Scholar] [CrossRef]
- González-Muñoz, M.T.; Rodriguez-Navarro, C.; Martínez-Ruiz, F.; Arias, J.M.; Merroun, M.L.; Rodriguez-Gallego, M. Bacterial biomineralization: New insights fromMyxococcus-induced mineral precipitation. Geo.Soc. London Special Pub. 2010, 336, 31–50. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Belie, N.D.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar] [CrossRef]
- Zhang, S. The relationship between organoclastic sulfate reduction and carbonate precipitation/dissolution in marine sediments. Mar. Geol. 2020, 428, 106284. [Google Scholar] [CrossRef]
- Gong, S.; Hu, Y.; Li, N.; Feng, D.; Liang, Q.; Tong, H.; Peng, Y.; Tao, J.; Chen, D. Environmental controls on sulfur isotopic compositions of sulfide minerals in seep carbonates from the South China Sea. J. Asian Earth Sci. 2018, 168, 96–105. [Google Scholar] [CrossRef]
- van Verseveld, C.J.W.; Gebert, J. Effect of compaction and soil moisture on the effective permeability of sands for use in methane oxidation systems. Waste Manag. 2020, 107, 44–53. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef]
- Kumari, D.; Qian, X.-Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced carbonate precipitation for immobilization of toxic metals. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [PubMed]
- Dick, J.; De Windt, W.; De Graef, B.; Saveyn, H.; Van der Meeren, P.; De Belie, N.; Verstraete, W. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Dideriksen, K.; Spangler, L.H.; Cunningham, A.B.; Gerlach, R. Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 2010, 44, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria and the precipitation patterns exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef]
- Liu, R.; Guan, Y.; Chen, L.; Lian, B. Adsorption and Desorption Characteristics of Cd2+ and Pb2+ by Micro and Nano-sized Biogenic CaCO3. Front. Microbiol. 2018, 9, 41. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, D.; Jiang, Y. Effect of the reduction–mineralization synergistic mechanism of Bacillus on the remediation of hexavalent chromium. Sci. Total Environ. 2021, 777, 146190. [Google Scholar] [CrossRef]
- Donnison, L.M.; Griffith, G.S.; Hedger, J.; Hobbs, P.J.; Bardgett, R.D. Management influences on soil microbial communities and their function in botanically diverse haymeadows of northern England and Wales. Soil Biol. Biochem. 2000, 32, 253–263. [Google Scholar] [CrossRef]
- Alizadeh, H.; Kandula, D.R.W.; Hampton, J.G.; Stewart, A.; Leung, D.W.M.; Edwards, Y.; Smith, C. Urease producing microorganisms under dairy pasture management in soils across New Zealand. Geoderma Reg. 2017, 11, 78–85. [Google Scholar] [CrossRef]
- Hasan, H.A.H. Ureolytic microorganisms and soil fertility: A review. Commun. Soil Sci. Plant Anal. 2008, 31, 2565–2589. [Google Scholar] [CrossRef]
- Lee, M.N.; Park, H.D. Isolation and Characterization of Acidophilic Yeasts Producing Urease from Korean Traditional Nuruk. Korean J. Food Preserv. 2012, 19, 308–314. [Google Scholar] [CrossRef]
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Han, S.-H.; Nam, I.-H.; So, J.-S. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol. Eng. 2015, 74, 402–407. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Williams, B.C.; Crawford, R.L. Urease Activity of Ureolytic Bacteria Isolated from Six Soils in which Calcite was Precipitated by Indigenous Bacteria. Geomicrobiol. J. 2012, 29, 389–395. [Google Scholar] [CrossRef]
- Wu, Z.; Su, J.; Ali, A.; Hu, X.; Wang, Z. Study on the simultaneous removal of fluoride, heavy metals and nitrate by calcium precipitating strain Acinetobacter sp. H12. J. Hazard. Mater. 2021, 405, 124255. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y. Application of a heavy metal-resistant Achromobacter sp. for the simultaneous immobilization of cadmium and degradation of sulfamethoxazole from wastewater. J. Hazard. Mater. 2021, 402, 124032. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, Z.; Guo, N.; Liu, T. One-Step Removal of Calcium, Magnesium, and Nickel in Desalination by Alcaligenes aquatilis via Biomineralization. Crystals 2019, 9, 633. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Lyu, J.; Li, F. Comparison of carbonate precipitation induced by Curvibacter sp. HJ-1 and Arthrobacter sp. MF-2: Further insight into the biomineralization process. J. Struct. Biol. 2020, 212, 107609. [Google Scholar] [CrossRef] [PubMed]
- Khadim, H.J.; Ammar, S.H.; Ebrahim, S.E. Biomineralization based remediation of cadmium and nickel contaminated wastewater by ureolytic bacteria isolated from barn horses soil. Environ. Technol. Innov. 2019, 14, 100315. [Google Scholar] [CrossRef]
- Do, H.; Wang, Y.; Long, Z.; Ketehouli, T.; Li, X.; Zhao, Z.; Li, M. A psychrotolerant Ni-resistant Bacillus cereus D2 induces carbonate precipitation of nickel at low temperature. Ecotoxicol. Environ. Saf. 2020, 198, 110672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kumari, D.; Fang, C.; Achal, V. Combining the microbial calcite precipitation process with biochar in order to improve nickel remediation. Appl. Geochem. 2019, 103, 68–71. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, Y.; Zhang, J.; Hu, X. Removal of lead from acidic wastewater by bio-mineralized bacteria with pH self-regulation. Chemosphere 2020, 241, 125041. [Google Scholar] [CrossRef]
- Peng, D.; Qiao, S.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.; Wu, B.; Xu, H. Performance of microbial induced carbonate precipitation for immobilizing Cd in water and soil. J. Hazard. Mater. 2020, 400, 123116. [Google Scholar] [CrossRef]
- Xu, G.; Li, D.; Jiao, B.; Li, D.; Yin, Y.; Lun, L.; Zhao, Z.; Li, S. Biomineralization of a calcifying ureolytic bacterium Microbacterium sp. GM-1. Electron. J. Biotechnol. 2017, 25, 21–27. [Google Scholar] [CrossRef]
- Pasquale, V.; Fiore, S.; Hlayem, D.; Lettino, A.; Huertas, F.J.; Chianese, E.; Dumontet, S. Biomineralization of carbonates induced by the fungi Paecilomyces inflatus and Plectosphaerella cucumerina. Int. Biodeterior. Biodegrad. 2019, 140, 57–66. [Google Scholar] [CrossRef]
- Cuaxinque-Flores, G.; Aguirre-Noyola, J.L.; Hernandez-Flores, G.; Martinez-Romero, E.; Romero-Ramirez, Y.; Talavera-Mendoza, O. Bioimmobilization of toxic metals by precipitation of carbonates using Sporosarcina luteola: An in vitro study and application to sulfide-bearing tailings. Sci. Total Environ. 2020, 724, 138124. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A. Urease active bioslurry: A novel soil improvement approach based on microbially induced carbonate precipitation. Can. Geotech. J. 2016, 53, 1376–1385. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.-S.; Xie, Y.-H.; Yin, L.-Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2020, 264, 105389. [Google Scholar] [CrossRef]
- Naveed, M.; Duan, J.; Uddin, S.; Suleman, M.; Hui, Y.; Li, H. Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil. Ecol. Eng. 2020, 153, 105885. [Google Scholar] [CrossRef]
- Dikshit, R.; Dey, A.; Gupta, N.; Varma, S.C.; Venugopal, I.; Viswanathan, K.; Kumar, A. Space bricks: From LSS to machinable structures via MICP. Ceram. Int. 2020, 47, 14892–14898. [Google Scholar] [CrossRef]
- Cheng, L.; Kobayashi, T.; Shahin, M.A. Microbially induced calcite precipitation for production of “bio-bricks” treated at partial saturation condition. Constr. Build. Mater. 2020, 231, 117095. [Google Scholar] [CrossRef]
- Lambert, S.E.; Randall, D.G. Manufacturing bio-bricks using microbial induced calcium carbonate precipitation and human urine. Water Res. 2019, 160, 158–166. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2020, 166, 107856. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp. Chemosphere 2012, 89, 764–768. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Achal, V. Biochemical composite material using corncob powder as a carrier material for ureolytic bacteria in soil cadmium immobilization. Sci. Total Environ. 2022, 802, 149802. [Google Scholar] [CrossRef]
- Phang, I.R.K.; Chan, Y.S.; Wong, K.S.; Lau, S.Y. Isolation and characterization of urease-producing bacteria from tropical peat. Biocatal. Agric. Biotechnol. 2018, 13, 168–175. [Google Scholar] [CrossRef]
- Yin, T.; Lin, H.; Dong, Y.; Li, B.; He, Y.; Liu, C.; Chen, X. A novel constructed carbonate-mineralized functional bacterial consortium for high-efficiency cadmium biomineralization. J. Hazard. Mater. 2020, 401, 123269. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Stehmeier, L.G.; Kantzas, A.; Mourits, F.M. Mourits. Bacteriogenic Mineral Plugging. J. Can. Pet. Technol. 1997, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Stocksfischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Nephrol. Dial. Transplant. 1999, 26, 3640–3645. [Google Scholar]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Gonzalez-Munoz, M.T. Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: Implications for stone conservation. Appl. Environ. Microbiol. 2012, 78, 4017–4029. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using microorganisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially Induced Carbonate Precipitation Techniques for the Remediation of Heavy Metal and Trace Element–Polluted Soils and Water. Water Air Soil Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Fang, L.; Niu, Q.; Cheng, L.; Jiang, J.; Yu, Y.-Y.; Chu, J.; Achal, V.; You, T. Ca-mediated alleviation of Cd2+ induced toxicity and improved Cd2+ biomineralization by Sporosarcina pasteurii. Sci. Total Environ. 2021, 787, 147627. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.; Lee, D.-J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wang, H.; Tang, D.; Huang, J.; Sun, Y. Study on the remediation of Cd pollution by the biomineralization of urease-producing bacteria. Int. J. Environ. Res. Public. Health 2019, 16, 268. [Google Scholar] [CrossRef]
- Kang, C.H.; Shin, Y.; Anbu, P.; Nam, I.H.; So, J.S. Biosequestration of copper by bacteria isolated from an abandoned mine by using microbially induced calcite precipitation. J. Gen. Appl. Microbiol. 2016, 62, 206–212. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H.; Yang, Z. Biomineralization of carbonate by Terrabacter Tumescens for heavy metal removal and biogrouting applications. J. Environ. Eng. 2016, 142, C4015005. [Google Scholar] [CrossRef]
- Chung, H.; Kim, S.H.; Nam, K. Inhibition of urea hydrolysis by free Cu concentration of soil solution in microbially induced calcium carbonate precipitation. Sci. Total Environ. 2020, 740, 140194. [Google Scholar] [CrossRef]
- Duarte-Nass, C.; Rebolledo, K.; Valenzuela, T.; Kopp, M.; Jeison, D.; Rivas, M.; Azocar, L.; Torres-Aravena, A.; Ciudad, G. Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: Challenges to future industrial application. J. Environ. Manag. 2020, 256, 109938. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Achal, V. Biostimulation of carbonate precipitation process in soil for copper immobilization. J. Hazard. Mater. 2019, 368, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wu, S.; Zhou, Z.; Wang, G. Microbial Cd(II) and Cr(VI) resistance mechanisms and application in bioremediation. J. Hazard. Mater. 2021, 401, 123685. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Abduolrahimi, S.; Forghani, A.; Farhangi, M.B. Bioremediation of cadmium in a sandy and a clay soil by microbially induced calcium carbonate precipitation after one week incubation. Arid Land Res. Manag. 2020, 34, 319–335. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Naik, S.N.; Khare, S.K. Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium (II). J. Environ. Manag. 2018, 215, 143–152. [Google Scholar] [CrossRef]
- Wu, Z.D.; Zheng, R.K.; Liu, G.; Liu, R.; Wu, S.M.; Sun, C.M. Calcium protects bacteria against cadmium stress via reducing nitric oxide production and increasing iron acquisition. Environ. Microbiol. 2020, 23, 3541–3553. [Google Scholar] [CrossRef]
- Eltarahony, M.; Zaki, S.; Abd-El-Haleem, D. Aerobic and anaerobic removal of lead and mercury via calcium carbonate precipitation mediated by statistically optimized nitrate reductases. Sci. Rep. 2020, 10, 4029. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N.; Reddy Krishna, R. Strength Enhancement and Lead Immobilization of Sand Using Consortia of Bacteria and Blue-Green Algae. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020049. [Google Scholar] [CrossRef]
- Jiang, N.J.; Liu, R.; Du, Y.J.; Bi, Y.Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Sivulka, D.J.; Seilkop, S.K. Reconstruction of historical exposures in the US nickel alloy industry and the implications for carcinogenic hazard and risk assessments. Regul. Toxicol. Pharmacol. 2009, 53, 174–185. [Google Scholar] [CrossRef]
- Cheng, G.; Sun, M.; Lu, J.; Ge, X.; Zhang, H.; Xu, X.; Lou, L.; Lin, Q. Role of biochar in biodegradation of nonylphenol in sediment: Increasing microbial activity versus decreasing bioavailability. Sci. Rep. 2017, 7, 4726. [Google Scholar] [CrossRef]
- Mossa, A.W.; Young, S.D.; Crout, N.M.J. Zinc uptake and phyto-toxicity: Comparing intensity- and capacity-based drivers. Sci. Total Environ. 2020, 699, 134314. [Google Scholar] [CrossRef]
- Gozzard, E.; Mayes, W.M.; Potter, H.A.; Jarvis, A.P. Seasonal and spatial variation of diffuse (non-point) source zinc pollution in a historically metal mined river catchment, UK. Environ. Pollut. 2011, 159, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, J.; Choi, M.M.; Chen, Y.; Chen, H.; Mohammad, R.; Zhuang, R.; Chen, H.; Wang, F.; Maskow, T.; et al. A combination method to study microbial communities and activities in zinc contaminated soil. J. Hazard. Mater. 2009, 169, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Noma, T.; Araki, N.; Yamaguchi, T.; Kusube, M.; Hayashi, K. Isolation of Acinetobacter sp. strain WKDN with capacity for aerobic denitrification and CaCO3 biomineralization and its potential application in dissolved Zn removal. Desalin. Water Treat. 2020, 194, 172–179. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Borg, S.; Liu, W.; Pearce, M.; Cleverley, J.; MacRae, C. Complex mineral zoning patterns caused by ultra-local equilibrium at reaction interfaces. Geology 2014, 42, 415–418. [Google Scholar] [CrossRef]
- Race, M.; Marotta, R.; Fabbricino, M.; Pirozzi, F.; Andreozzi, R.; Guida, M.; Siciliano, A. Assessment of optimal conditions for the restoration and recovery of agricultural soil. J. Hazard. Mater. 2019, 373, 801–809. [Google Scholar] [CrossRef]
- Bai, J.; Xun, P.; Morris, S.; Jacobs, D.R.; Liu, K.; He, K. Chromium exposure and incidence of metabolic syndrome among American young adults over a 23-year follow-up: The CARDIA Trace Element Study. Sci. Rep. 2015, 5, 15606. [Google Scholar] [CrossRef]
- Sheta, M.; Yousry, B.; Zattot, A.; Taha, N.A. Optimization of Chitosan Surface Response Methodology (Natural and Commercial) Used for Chromium Ion Removal from Wastewater across Different Parameters. Sustainability 2021, 13, 13494. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, H.; Xu, P.; Zhang, Y. Biomineralization of hypersaline produced water using microbially induced calcite precipitation. Water Res. 2021, 190, 116753. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.M.; Rivera-Hernández, M.; Álvarez, L.H.; Acosta-Rodríguez, I.; Ruíz, F.; Compeán-García, V.D. Biosynthesis and characterization of cadmium carbonate crystals by anaerobic granular sludge capable of precipitate cadmium. Mater. Chem. Phys. 2020, 246, 122797. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Rom. Biotech. Lett. 2019, 24, 147–153. [Google Scholar] [CrossRef]
- Sun, Z.H.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Yang, Y. Complex electronic waste treatment-An effective process to selectively recover copper with solutions containing different ammonium salts. Waste Manag. 2016, 57, 140–148. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Da Silva, F.B.; Boon, N.; Verstraete, W.; De Belie, N. Screening of bacteria and concrete compatible protection materials. Constr. Build. Mater. 2015, 88, 196–203. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gomez del Pulgar, E.-M.; Fabra, M.J.; Gómez-Mascaraque, L.G.; Benítez-Páez, A.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Agarose-based freeze-dried capsules prepared by the oil-induced biphasic hydrogel particle formation approach for the protection of sensitive probiotic bacteria. Food Hydrocoll. 2019, 87, 487–496. [Google Scholar] [CrossRef]
- Liu, S.; Wen, K.; Armwood, C.; Bu, C.; Li, C.; Amini, F.; Li, L. Enhancement of MICP-Treated Sandy Soils against Environmental Deterioration. J. Mater. Civ. Eng. 2019, 31, 04019294. [Google Scholar] [CrossRef]
- Chen, X.; Achal, V. Effect of simulated acid rain on the stability of calcium carbonate immobilized by microbial carbonate precipitation. J. Environ. Manag. 2020, 264, 110419. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. A feasible scale-up production of Sporosarcina pasteurii using custom-built stirred tank reactor for in-situ soil biocementation. Biocatal. Agric. Biotechnol. 2020, 24, 101544. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Ghose, R.; van der Linden, T.J.; van der Star, W.R.; van Loosdrecht, M.C. Quantifying biomediated ground improvement by ureolysis: Large-scale biogrout experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- van Paassen, L.A. Bio-mediated ground improvement: From laboratory experiment to pilot applications. Adv. Geotech. Eng. 2011, 13, 4099–4108. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; deVlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. 2015, 168, 206–216. [Google Scholar] [CrossRef]
- Barmaki, M.M.; Rahimpour, M.R.; Jahanmiri, A. Treatment of wastewater polluted with urea by counter-current thermal hydrolysis in an industrial urea plant. Sep. Purif. Technol. 2009, 66, 492–503. [Google Scholar] [CrossRef]
- Matijašević, L.; Dejanović, I.; Lisac, H. Treatment of wastewater generated by urea production. Resour. Conserv. Recycl. 2010, 54, 149–154. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Guan, C.Y.; Tseng, Y.H.; Tsang, D.C.W.; Hu, A.; Yu, C.P. Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J. Hazard. Mater. 2019, 365, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
| HMs | Permissible Limit (mg/L) | Sources | Symptoms/Disease | Severity | Ref. | |
|---|---|---|---|---|---|---|
| Agency | Limits | |||||
| Cu (II) | WHO a | - | Textile, paper, paint manufacturing, leather tanning, battery manufacturing, automobile radiator manufacturing, and agricultural resources (wide use of chemical fertilizers and fungicides), etc. | Vomiting, anemia, cramps, convulsions, and even death, etc. | Average | [7,8,9,10] |
| FAO b | 0.2 | |||||
| EPA c | 1.3 | |||||
| Cd (II) | WHO | 0.003 | Galvanizing and electroplating, manufacturing of batteries, electrical conductors, alloys, pigments, and plastics, stabilization of phosphate fertilizers, etc. | Damage to kidneys, gill epithelium, cardiovascular system, and musculoskeletal system, and even death, etc. | High | [9,10,11] |
| FAO | 0.01 | |||||
| EPA | 0.005 | |||||
| Pb (II) | WHO | 0.001 | Lead mining, smelting, coal combustion, use of lead-based paint and lead-containing pipes in water supply systems, food canned solder, ceramic glazes, lead-containing batteries, and cosmetics, etc. | Damage to the kidneys, central nervous system, liver and reproductive system, basic cell processes, and brain function. Symptoms of poisoning are anemia, insomnia, headache, dizziness, irritability, muscle weakness, hallucinations, and kidney damage. | High | [9,10,12,13] |
| FAO | 0.01 | |||||
| EPA | 0.015 | |||||
| Hg (II) | WHO | 0.001 | Natural activities, such as degassing of the earth’s crust, emissions of volcanoes, evaporation of the ocean, gold mining and refining, coal-fired power plants, etc. | Cause kidney dysfunction, nervous system problems, sleep disorders, hearing loss, impaired reproductive function, heart problems, etc. | High | [9,14,15] |
| FAO | - | |||||
| EPA | 0.002 | |||||
| Zn (II) | WHO | - | Rock, mineral, steel production and coal or waste burning, etc. | Cause stomach cramps, skin irritation, vomiting, nausea, anemia, etc. | Low | [10,16,17] |
| FAO | 2 | |||||
| EPA | - | |||||
| Ni (II) | WHO | 0.02 | Electroplating, batteries, electronic equipment, alloys (such as stainless steel), pesticides, fertilizers, and herbicides, etc. | Allergies, kidney and liver diseases, infertility, dermatitis, stomach pain, gingivitis, migraine, insomnia, and nausea, etc. | High | [9,10,18,19] |
| FAO | 0.2 | |||||
| EPA | 0.1 | |||||
| As (III), As(V) | WHO | - | Coal-fired power plants, mining, smelting, agricultural pesticides, and volcanic activities, etc. | Cause cardiovascular disease, chronic bronchitis, liver and kidney damage, etc. | High | [20,21] |
| FAO | - | |||||
| EPA | - | |||||
| Cr (VI), Cr (III) | WHO | 0.005 | Alloy manufacturing, dyes and pigments, electroplating, metal finishing, petroleum refining, leather tanning, wood preservation, corrosion inhibitors in conventional and nuclear power plants, etc. | Damage the liver and kidneys, cause skin lesions or rashes, and be mutagenic, carcinogenic, and teratogenic. | High | [9,10,22,23] |
| FAO | 0.1 | |||||
| EPA | 0.1 | |||||
| Types | HMs Source | Morphology of Precipitation | Size (µm) | Ref. |
|---|---|---|---|---|
| As | NaAsO2, Na2HAsO4 | Rhombus, spherical, or double pyramid | 0.2–2 | [20] |
| Cd | CdSO4, CdCl2 | Sparsely soluble, irregular shape, sphere | 0.5–50 | [82,108,109] |
| Cr | K2Cr2O7 | Sphere | Approximately 0.1 | [70] |
| Cu | CuCl2 | Sphere | 5–10 | [110] |
| Mn | MnCl2 | Sphere | <3 | [89] |
| Ni | NiCl2 | Irregular shape, rhombus | 10–40 | [82] |
| Pb | PbCl2 | Sphere, platy | 5–50 | [89,111] |
| Zn | ZnCl2 | Acicular, fibro-radial | 1–100 | [89,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fang, L.; Dapaah, M.F.; Niu, Q.; Cheng, L. Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review. Sustainability 2023, 15, 7622. https://doi.org/10.3390/su15097622
Wang S, Fang L, Dapaah MF, Niu Q, Cheng L. Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review. Sustainability. 2023; 15(9):7622. https://doi.org/10.3390/su15097622
Chicago/Turabian StyleWang, Sheng, Longyang Fang, Malcom Frimpong Dapaah, Qijian Niu, and Liang Cheng. 2023. "Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review" Sustainability 15, no. 9: 7622. https://doi.org/10.3390/su15097622
APA StyleWang, S., Fang, L., Dapaah, M. F., Niu, Q., & Cheng, L. (2023). Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review. Sustainability, 15(9), 7622. https://doi.org/10.3390/su15097622








