Reuse of Treated Wastewater: Drivers, Regulations, Technologies, Case Studies, and Greater Chicago Area Experiences
Abstract
1. Introduction
2. Drivers for Water Reuse
- Goal 2: Zero hunger
- Goal 3: Good health and well-being
- Goal 6: Clean water and sanitation
- Goal 8: Decent work and economic growth
- Goal 9: Industry, Innovation, and Infrastructure
- Goal 11: Sustainable cities and communities
- Goal 12: Responsible consumption and production
- Goal 14: Life below water
- Goal 17: Partnerships
2.1. Drivers for Water Reuse in the United States
2.2. Drivers for Water Reuse in Greater Chicago Area
3. Guidelines and Regulations for Water Reuse
3.1. Federal Policies and Regulations
3.1.1. USEPA Water Reuse Categories
- Unrestricted urban reuse. The use of reclaimed water for non-potable applications in municipal settings where public access is not restricted.
- Restricted urban reuse. The use of reclaimed water for non-potable applications in municipal settings where public access is controlled or restricted by physical or institutional barriers, such as fencing, advisory signage, or temporal access restriction.
- Agricultural reuse on food crops. The use of reclaimed water to irrigate food crops that are intended for human consumption.
- Agricultural reuse on processed food crops and non-food crops. The use of reclaimed water to irrigate crops that are either processed before human consumption or not consumed by humans.
- Unrestricted Impoundments. The use of reclaimed water in an impoundment in which no limitations are imposed on body-contact water recreation activities (some states categorize snowmaking in this category).
- Restricted Impoundments. The use of reclaimed water in an impoundment where body contact is restricted (some states include fishing and boating in this category).
- Environmental reuse. The use of reclaimed water to create, enhance, sustain, or augment water bodies, including wetlands, aquatic habitats, or stream flow.
- Industrial reuse. The use of reclaimed water in industrial applications and facilities, power production, and extraction of fossil fuels.
- Groundwater recharge. The use of reclaimed water to recharge aquifers that are not used as a potable water source.
- Indirect potable reuse. Augmentation of a drinking water source (surface or groundwater) with reclaimed water followed by an environmental buffer that precedes normal drinking water treatment.
- Direct potable reuse. The introduction of reclaimed water (with or without retention in an engineered storage buffer) directly into a water treatment plant, either collocated or remote from the advanced wastewater treatment system.
3.1.2. USEPA Guidelines on Water Quality for Different Reuse Practices
3.2. State and Local Policies and Regulations
3.2.1. Illinois Environmental Protection Agency
3.2.2. City of Chicago Ordinances
3.2.3. Chicago Metropolitan Agency for Planning (CMAP)
4. Water Reuse Treatment Technologies
- Removal of suspended solids;
- Reducing dissolved chemical concentrations;
- Removal or disinfection of trace organic compounds;
- Stabilization;
- Aesthetics (taste, odor, color correction).
4.1. Non-Potable Reuse Treatment Technologies
4.2. Potable Reuse Treatment Technologies
4.3. Costs of Treatment Technologies
4.4. Water Reuse Distribution Infrastructure
4.5. Water Reuse Planning Model
- Conceptual level planning;
- Preliminary feasibility investigation;
- Facilities planning.
- Performing a market evaluation, i.e., identifying a market for recycled water and specifying the criteria that must be met (e.g., user needs for water quality and pricing);
- Evaluating the current water supply and wastewater facilities and creating some preliminary options that might service the entire market, in parts or in full, while meeting its technical and water quality needs;
- Comparing a wastewater reclamation and reuse option with other non-reclamation facilities, such as wastewater treatment for stream discharge or the construction of a reservoir for water supply;
- Considering technical needs, economics, financial advantages, marketability of recovered water, and other restrictions such as health protection of recycled water.
5. Engineering Challenges for Water Reuse
5.1. Sustainable Treatment Technologies
5.2. Resilient Wastewater Treatment
5.3. Treatment of Emerging Contaminants
5.4. Social, Policy, and Economic Barriers
6. Case Studies of Water Reuse
6.1. Selected Case Studies in the U.S.
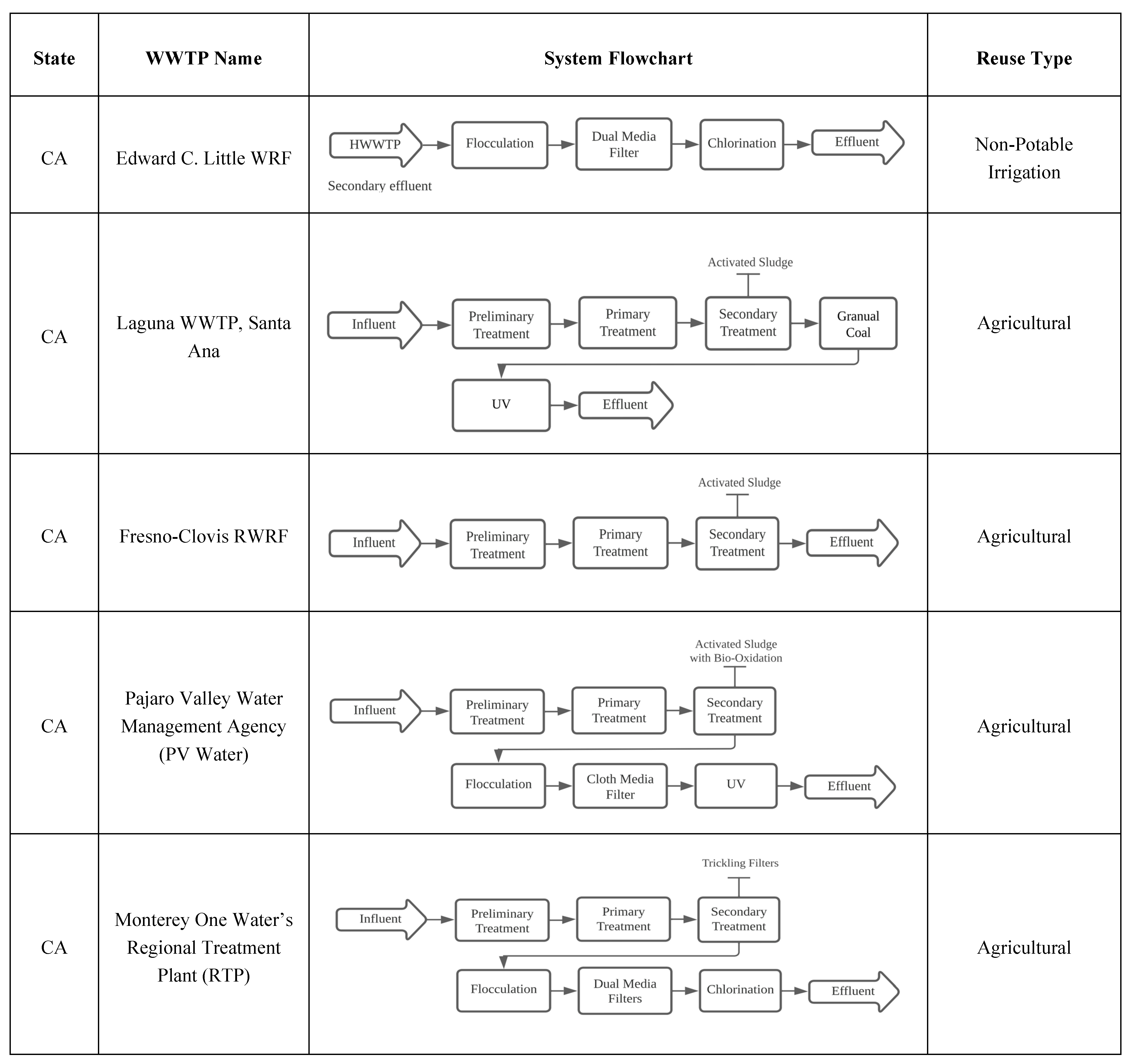
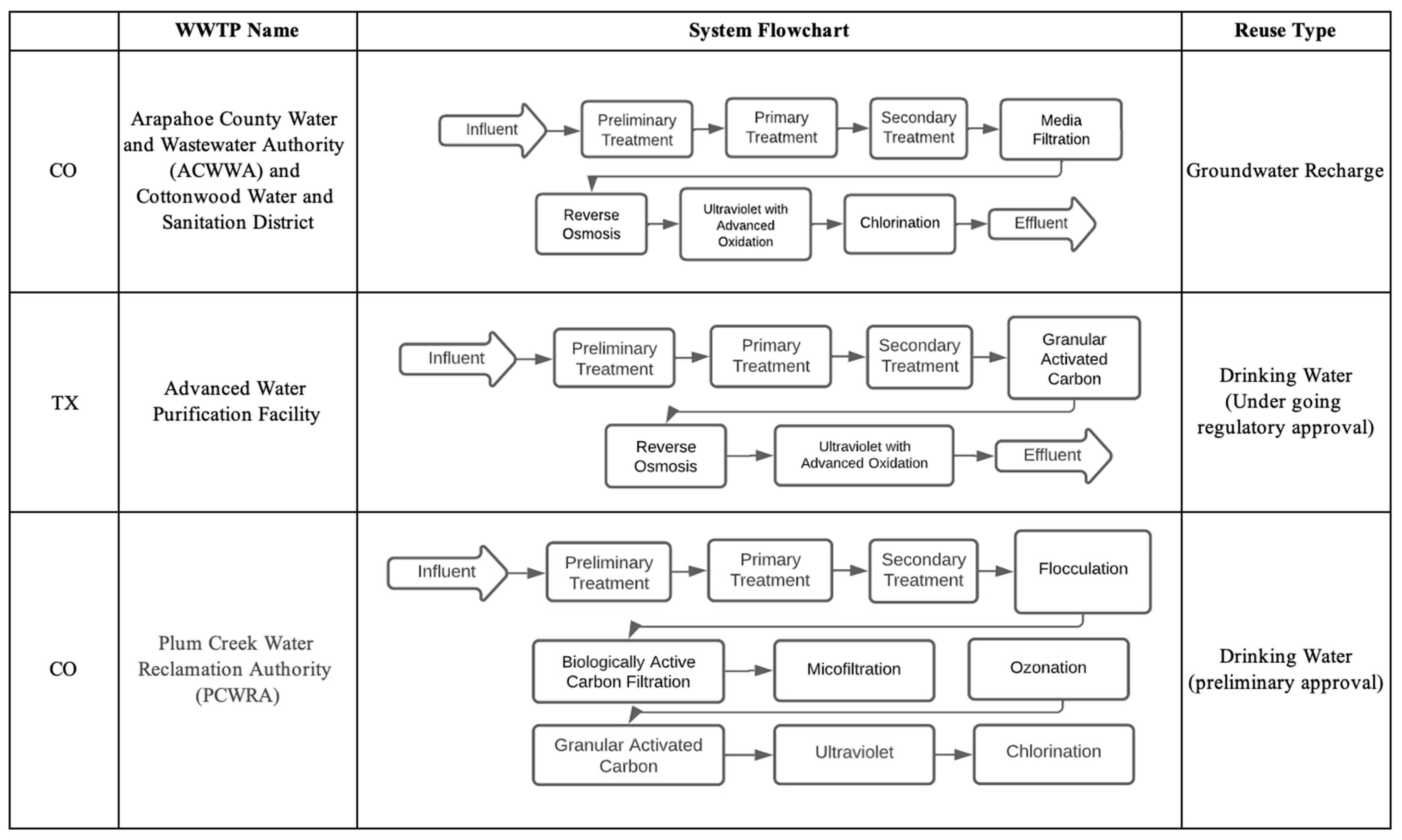
6.1.1. California Case Studies
The Edward C. Little Water Recycling Facility: West Basin Municipal Water District
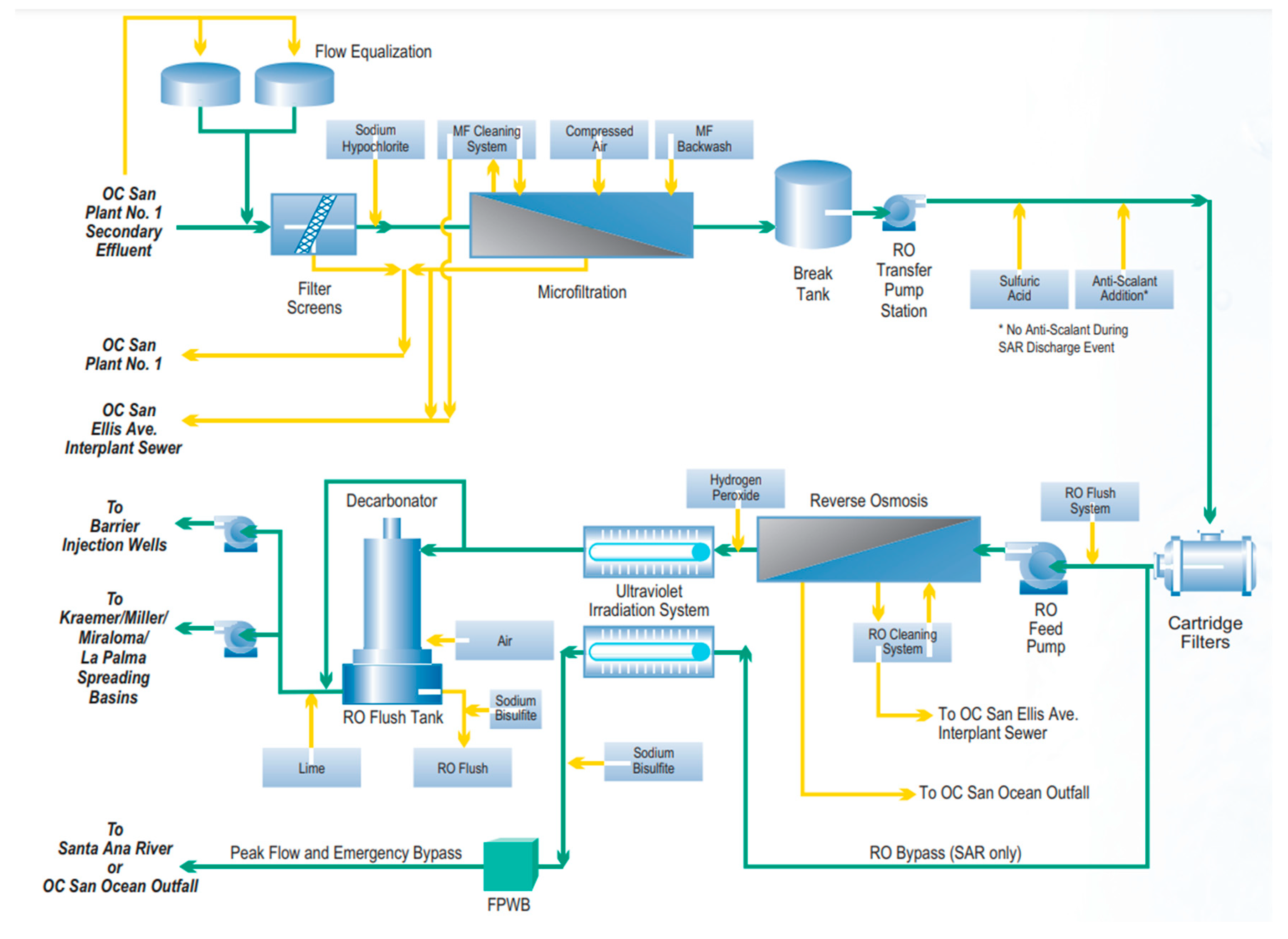
The Groundwater Replenishment System (GWRS): Orange County Water District
6.2. Arizona Case Study
Scottsdale Water Campus: Scottsdale Water Resources Department
6.3. Florida Case Study
St. Petersburg: St. Petersburg Water Resources Department
6.4. International Case Studies
6.4.1. Singapore: NEWater
6.4.2. Cyprus: Suwanu Europe
6.4.3. China: Tianjin Megacity Water Reuse
6.4.4. Israel–Jordan: Advanced Wastewater Treatment Technology for Crop Irrigation
6.4.5. Germany: MULTI-ReUse Project
7. Appraisal of Water Reuse in Greater Chicago Area
- Current trend scenario (CT): assumptions include that the population growth and urban development trends from the past 10–20 years continue, income and price follow historical patterns, geographical distribution of growth based on market forces and expected public policies implementations and increased water efficiency.
- Less resource-intensive scenario (LRI): assumptions include water conservation, decrease in water-intensive activities, lower income and higher water prices in the future, some population shifts to a more urbanized area (Cook and DuPage).
- More resource-intensive scenario (MRI): assumption include less water conservation than indicated by the current trend scenario, increase in water-intensive activities, and some population shifts to western collar counties (Kane, Kendall, and McHenry).
- Lake Michigan water enters the intake crib at depths between 20 and 30 feet.
- The water reaches the intake basin of the purification plant through a tunnel beneath the lakebed.
- The water is filtered by eight moving screens to remove debris.
- For the initial chemical treatment, water is pumped up to 25 feet using low-lift pumps.
- Water is then transferred.
- Water is circulated through mixing basins to initiate the flocculation process.
- Flocculated water is transferred to settling basins, where it sits for hours to allow floc settling.
- Water is filtered through finely graded sand and gravel to provide “natural polishing.”
- The filtered water then flows for final chemical application in a clear well.
- Water flows from water reservoirs to the distribution system.
7.1. Water Reuse Tools and Strategies
- Reducing Fresh Water Usage: The most basic strategy to reduce freshwater usage is to utilize high-efficiency fixtures and appliances wherever practical. Some of accepted toilet/urinal strategies by USEPA are as follows, high-efficiency toilets (HETs), dual flush toilets, and high-efficiency urinals (HEU) [111].
- Rainwater Harvesting System Design: Rainwater harvesting systems can be used on the exterior and/or interior of buildings for irrigation, toilet or urinal flushing, and other non-potable applications. This will significantly reduce potable water utilization and help assist stormwater management. Rainwater harvesting system utilization in offices and institutional buildings can have a significant impact on water conservation since flush fixtures are the main source of potable water demand. The Illinois Plumbing Code does not specify regulations for rainwater harvesting systems (the State has jurisdiction over plumbing issues in Chicago). Therefore, the rainwater harvesting systems should essentially follow some rigorous process to get city and state approvals [111].
- Greywater System Design: The basic strategy of a greywater system for interior building relies on harvesting greywater from the shower, lavatory, and laundry water, filtering and treating the water, and storing it until it is needed to flush toilets and/or urinals. Greywater systems are an excellent fit for buildings with showers, such as residential buildings, because one shower requires nearly the same amount of water as one person’s daily flushing [111].
- Blackwater Systems: Blackwater systems treat wastewater from flush fixtures, which often contain feces and urine, and repurpose it for toilet flushing, irrigation, or fertilization of gardens or farmland. Although blackwater systems may be a feasible option for wastewater treatment, the State of Illinois does not have any aerated or wetland-based blackwater treatment facilities [111].
7.2. Illinois and Greater Chicago Water Reuse Experience
7.3. Three Water Reuse Case Studies in Cook County
7.3.1. Kirie Water Reclamation Plant
7.3.2. O’Brien Water Reclamation Plant
7.3.3. Calumet Water Reclamation Plant
8. Conclusions and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, B.; Asano, T. Water Reuse: An International Survey of Current Practice, Issues and Needs. Water Intell. Online 2015, 7, 9781780401881. [Google Scholar] [CrossRef]
- National Integrated Drought Information System (NIDIS). Drought Status Update for the Midwest: 10 June 2021. Available online: https://www.drought.gov/drought-status-updates/drought-status-update-midwest (accessed on 12 October 2022).
- Tollefson, J. Climate Change Is Hitting the Planet Faster Than Scientists Originally Thought. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Cameselle, C.; Adams, J.A. Sustainable Engineering: Drivers, Metrics, Tools, Engineering Practices, and Applications; John Wiley & Sons, Incorporated: Newark, NJ, USA, 2019. [Google Scholar]
- Hallegatte, S.; Green, C.; Nicholls, R.J.; Corfee-Morlot, J. Future Flood Losses in Major Coastal Cities. Nat. Clim. Chang. 2013, 3, 802–806. [Google Scholar] [CrossRef]
- United Nations (UN). Sustainable Development Goal 6—Synthesis Report on Water and Sanitation. Available online: https://sustainabledevelopment.un.org/content/documents/19901SDG6_SR2018_web_3.pdf (accessed on 12 October 2022).
- Global Water Security and Sanitation Partnership (GWSP). Towards a Sustainable Water Future—Sustainable Development Goals: A Water Perspective. Available online: https://collections.unu.edu/eserv/UNU:3377/GWSPConference.pdf (accessed on 12 October 2022).
- United Nations Educational, Scientific and Cultural Organization (UNESCO). The United Nations World Water Development Report: Water for a Sustainable Development. UNESCO-WWAP. Available online: http://unesdoc.unesco.org/images/0023/002318/231823E.pdf (accessed on 10 June 2022).
- Bhaduri, A.; Bogardi, J.; Siddiqi, A.; Voigt, H.; Vörösmarty, C.; Pahl-Wostl, C.; Bunn, S.E.; Shrivastava, P.; Lawford, R.; Foster, S.; et al. Achieving Sustainable Development Goals from a Water Perspective. Front. Environ. Sci. 2016, 4, 64. [Google Scholar] [CrossRef]
- Schramm, E.; Becker, D.; Fischer, M. Advanced Processed Wastewater for Different Uses: Constellations Favouring Future Implementation of a Multimodal Water Reuse Concept. J. Water Reuse Desalin. 2020, 10, 284–300. [Google Scholar] [CrossRef]
- National Centers for Environmental Information (NCEI). October 2022 Drought Report. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/drought/202210 (accessed on 1 November 2022).
- Office of Environmental Health Hazard Assessment (OEHHA). The Human Right to Water in California. Available online: https://oehha.ca.gov/water/report/human-right-water-california (accessed on 28 January 2023).
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Dieter, C.A.; Maupin, M.A.; Caldwell, R.R.; Harris, M.A.; Ivahnenko, T.I.; Lovelace, J.K.; Barber, N.L.; Linsey, K.S. Estimated Use of Water in the United States in 2015. Circular 2018, 1, 7–8. [Google Scholar] [CrossRef]
- Konikow, L.F. Groundwater Depletion in the United States (1900−2008). Sci. Investig. Rep. 2013, 1, 6–7. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). National Water Reuse Action Plan Draft—EPA. Available online: https://www.epa.gov/sites/default/files/2019-09/documents/water-reuse-action-plan-draft-2019.pdf (accessed on 12 October 2022).
- Asano, T. Water Reuse: Issues, Technology, and Applications; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). 2004 Guidelines for Water Reuse, EPA/625/R-04/108. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2004-guidelines-water-reuse.pdf (accessed on 28 January 2022).
- The City of Joliet. City of Joliet Alternative Water Source Study—Phase 1. Joliet. Available online: https://docs.wixstatic.com/ugd/38f500_56d76d20806543cebeabc1b6a631785c.pdf (accessed on 8 October 2022).
- City of Chicago. Chicago City Council Approves Revised Joliet Water Deal Ensuring Millions in Future Revenue. Available online: https://www.chicago.gov/content/dam/city/depts/mayor/Press%20Room/Press%20Releases/2021/February/JolietWaterDeal.pdf (accessed on 8 October 2022).
- US Army Corps of Engineers. LRC WM Annual Report 2021—United States Army. Lake Michigan Diversion Accounting Program. Available online: https://www.lrc.usace.army.mil/Portals/36/docs/divacct/annual/LRC_WM_Annual_Report_2021.pdf (accessed on 20 November 2022).
- Hartley, T.W. Public Perception and Participation in Water Reuse. Desalination 2006, 187, 115–126. [Google Scholar] [CrossRef]
- Lazarova, V. Water Reuse: A Pillar of the Circular Water Economy. Resour. Recovery Water 2022, 1, 61–98. [Google Scholar] [CrossRef]
- Fekete, B.M.; Bogárdi, J.J. Role of Engineering in Sustainable Water Management. Earth Perspect. 2015, 2, 2. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). 2012 Guidelines for Water Reuse; EPA/600/R-12/618; U.S. Environmental Protection Agency (EPA): Washington, DC, USA. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2012-guidelines-water-reuse.pdf (accessed on 28 January 2022).
- Illinois Environmental Protection Agency (IEPA). Bureau of Water. Illinois.gov. Available online: https://www2.illinois.gov/epa/topics/water-quality/Pages/default.aspx (accessed on 12 October 2022).
- Anderson, P.R.; Meng, Y.; Assessing Opportunities for Municipal Wastewater Reuse in The Metropolitan Chicago Area. Illinois Sustainable Technology Centre. Available online: https://hdl.handle.net/2142/27738 (accessed on 2 February 2022).
- Illinois Pollution Control Board (IPCB). Title 35: Environmental Protection, Subtitle C: Water Pollution. Title 35 Procedural and Environmental Rules. Available online: https://pcb.illinois.gov/SLR/IPCBandIEPAEnvironmentalRegulationsTitle35 (accessed on 2 February 2022).
- America Legal Publishing. Title 11 Utilities and Environmental Protection. Municipal Code of Chicago. Available online: https://codelibrary.amlegal.com/codes/chicago/latest/chicago_il/0-0-0-2653760 (accessed on 2 February 2022).
- Chicago Metropolitan Agency for Planning (CMAP). About CMAP. CMAP. Available online: https://www.cmap.illinois.gov/about/ (accessed on 3 February 2022).
- Chicago Metropolitan Agency for Planning (CMAP). Northeastern Illinois Regional Water Supply/Demand Plan. Available online: https://www.cmap.illinois.gov/documents/10180/14452/NE+IL+Regional+Water+Supply+Demand+Plan.pdf/26911cec-866e-4253-8d99-ef39c5653757 (accessed on 3 February 2022).
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Water Environment Federation (WEF). The Water Reuse Roadmap. Available online: https://ebookcentral-proquest-com.proxy.cc.uic.edu/lib/uic/detail.action?docID=6186983 (accessed on 1 March 2022).
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F. Wastewater Engineering Treatment and Resource Recovery; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Tricas, M.; Albert, R.; Bastian, R.; Nappier, S.; Regli, S.; Kasparek, L.; Gorke, R. 2017 Potable Reuse Compendium; United States Environmental Protection Agency: Washington, DC, USA, 2018. [CrossRef]
- Berefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice-Hall: Hoboken, NJ, USA, 1982. [Google Scholar]
- Henze, M.; Harremoes, P.; Arvin, E.; la Cour Jansen, J. Wastewater Treatment. Biological and Chemical Processes; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Sonune, A.; Ghate, R. Developments in Wastewater Treatment Methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Treatment of Pulp and Paper Mill Wastewater—A Review. Sci. Total Environ. 2004, 333, 37–58. [Google Scholar] [CrossRef]
- Parsons, S. (Ed.) Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004. [Google Scholar]
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of Industrial Effluents—Available Methods and Emerging Technologies—A Review. Environ. Sci. Bio/Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.T. Rice Husk as A Potentially Low-Cost Biosorbent for Heavy Metal and Dye Removal: An Overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents In Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation In Water And Wastewater Treatment. IWA Publishing: London, UK, 2006. [Google Scholar]
- Crini, G.; Montiel, A.J.; Badot, P.M. Traitement Et Épuration Des Eaux Industrielles Polluées: Procédés Membranaires, Bioadsorption Et Oxydation Chimique; Presses Universitaires de Franche-Comté: Besançon, France, 2007; Volume 352. [Google Scholar]
- Crini, G.; Badot, P.M. (Eds.) Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents For Pollutant Removal From Wastewaters; Presses Universitaires de Franche-Comté: Besançon, France, 2010. [Google Scholar]
- Cox, M.; Négré, P.; Yurramendi, L. Industrial Liquid Effluents; INASMET Tecnalia: San Sebastian, Spain, 2007; Volume 283. [Google Scholar]
- Mohan, D.; Pittman, C.U., Jr. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid Treatment Systems for Dye Wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Irradiation Treatment of Azo Dye Containing Wastewater: An Overview. Radiat. Phys. Chem. 2008, 77, 225–244. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sanghi, R. (Eds.) Advances In Water Treatment and Pollution Prevention; Springer Science+Business Media: Berlin, Germany, 2012. [Google Scholar]
- Rathoure, A.K. (Ed.) Toxicity and Waste Management Using Bioremediation; IGI Global: Hershey, PA, USA, 2015. [Google Scholar]
- Morin-Crini, N.; Crini, G.; Roy, L. Eaux industrielles contaminées. PUFC Besanço 2017, 513, 37–47. [Google Scholar]
- WateReuse Research Foundation; American Water Works Association; Water Environment Federation; National Water Research Institute. Framework for Direct Potable Reuse; WateReuse Research Foundation: Alexandria, VA, USA, 2015. [Google Scholar]
- American Water Works Association (AWWA). Water Reuse Cost Allocations and Pricing Survey; AWWA: Denver, CO, USA, 2019. [Google Scholar]
- National Research Council (NRC). Water Reuse: Potential for Expanding the Nation’s Water Supply Through Reuse of Municipal Wastewater; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Asano, T.; Mills, R.A. Planning and Analysis for Water Reuse Projects. J.-Am. Water Work. Assoc. 1990, 82, 38–47. [Google Scholar] [CrossRef]
- Chaubey, M. Wastewater Treatment Technologies; Wiley-Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Pamidimukkala, A.; Kermanshachi, S.; Adepu, N.; Safapour, E. Resilience in Water Infrastructures: A Review of Challenges and Adoption Strategies. Sustainability 2021, 13, 12986. [Google Scholar] [CrossRef]
- Rout, P.R.; Dash, R.R.; Bhunia, P. Development of An Integrated System for The Treatment Of Rural Domestic Wastewater: Emphasis On Nutrient Removal. RSC Adv. 2016, 6, 49236–49249. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the Sorption and Biotransformation of Organic Micropollutants in Innovative Biological Wastewater Treatment Technologies. Sci. Total Environ. 2018, 615, 297–306. [Google Scholar] [CrossRef]
- Roccaro, P. Treatment Processes for Municipal Wastewater Reclamation: The Challenges of Emerging Contaminants and Direct Potable Reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 46–54. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.; Silva, A.M.T. Environmental Impact Assessment of Advanced Urban Wastewater Treatment Technologies for the Removal of Priority Substances and Contaminants of Emerging Concern: A Review. J. Clean. Prod. 2020, 261, 121078. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Acero, J.L.; Javier Benitez, F.; Real, F.J.; Teva, F. Coupling of Adsorption, Coagulation, and Ultrafiltration Processes for the Removal of Emerging Contaminants in A Secondary Effluent. Chem. Eng. J. 2012, 210, 1–8. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Al-Huwaidi, J.S.; Al-Obaidi, M.A.; Jarullah, A.T.; Kara-Zaïtri, C.; Mujtaba, I.M. Modeling and Simulation of a Hybrid System of Trickle Bed Reactor and Multistage Reverse Osmosis Process for the Removal of Phenol from Wastewater. Comput. Chem. Eng. 2021, 153, 107452. [Google Scholar] [CrossRef]
- Lee, K.; Jepson, W. Drivers and Barriers to Urban Water Reuse: A systematic review. Water Secur. 2020, 11, 100073. [Google Scholar] [CrossRef]
- Gul, S.; Gani, K.M.; Govender, I.; Bux, F. Reclaimed Wastewater as an Ally to Global Freshwater Sources: A Pestel Evaluation of the Barriers. J. Water Supply Res. Technol. Aqua 2021, 70, 123–137. [Google Scholar] [CrossRef]
- Crook, J. Innovative Applications in Water Reuse: Ten Case Studies; WateReuse Association: Alexandria, VA, USA, 2015. [Google Scholar]
- Walters, J.; Oelker, G.; Lazarova, V. Producing designer recycled water tailored to customer needs. In Milestones in Water Reuse: The Best Success Stories; IWA Publishing: London, UK, 2013. [Google Scholar] [CrossRef]
- Orange County Sanitation District (OCWD). GWRS—Groundwater Replenishment System. Available online: https://www.ocwd.com/wp-content/uploads/gwrs-technical-brochure-2021.pdf (accessed on 1 March 2022).
- Freeman, S.; Leitner, G.; Crook, J.; Vernon, W. A Clear Advantage—Membrane Filtration Is Gaining Acceptance in The Water Quality Field. Water Environ. Technol. 2002, 14, 16–21. [Google Scholar]
- Wong, J. A Survey of Advanced Membrane Technologies and Their Applications in Water Reuse Projects. In Proceedings of the 76th Annual Technical Exhibition & Conference, Water Environment Federation, Alexandria, VA, USA, 8–12 January 2003. [Google Scholar]
- Florida Department of Environmental Protection. 2021 Reuse Inventory; Division of Water Resource Management Florida Department of Environmental Protection: Tallahassee, FL, USA, 2021. Available online: https://floridadep.gov/sites/default/files/2021%20Reuse%20Inventory.pdf (accessed on 10 December 2021).
- Crook, J. St. Petersburg, Florida, Dual Water System: A Case Study. In Water Conservation, Reuse, and Recycling; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Lim, M.H.; Seah, H. NEWater: A key element of Singapore’s water sustainability. In Milestones in Water Reuse: The Best Success Stories; IWA Publishing: London, UK, 2020. [Google Scholar]
- Lefebvre, O. Beyond Newater: An Insight into Singapore’s Water Reuse Prospects. Curr. Opin. Environ. Sci. Health 2018, 2, 26–31. [Google Scholar]
- Lee, H.; Tan, T.P. Singapore’s experience with Reclaimed Water: NEWater. Int. J. Water Resour. Dev. 2016, 32, 611–621. [Google Scholar] [CrossRef]
- PUB Singapore’s National Water Agency. NEWater; PUB, Singapore’s National Water Agency: Singapore, 2022.
- Christou, A.; Dalias, P.; Neocleous, D. Spatial and Temporal Variations in Evapotranspiration and Net Water Requirements of Typical Mediterranean Crops on the Island of Cyprus. J. Agric. Sci. 2017, 155, 1311–1323. [Google Scholar] [CrossRef]
- SUWANU Europe. The Success Story of Cyprus—Fact Sheet 1—Water Demand and Supply: Facts and Figures. Available online: https://suwanu-europe.eu/wp-content/uploads/2020/01/FS1_FactSheet_Cyprus_Water-demand.pdf (accessed on 5 December 2022).
- SUWANU Europe. The Success Story of Cyprus—Fact Sheet 2—Wastewater Treatment and Reuse. Available online: https://suwanu-europe.eu/wp-content/uploads/2020/01/FS2_FactSheet_Cyprus_Wastewater.pdf (accessed on 5 December 2022).
- Water Development Department (WDD). Recycled Water-Quantities and Use of Recycled Water. Available online: http://www.moa.gov.cy/moa/wdd/Wdd.nsf/index_en/index_en?OpenDocument (accessed on 10 December 2022).
- SUWANU Europe. The Success Story of Cyprus—Fact Sheet 6—Pricing System for Irrigation Water. Available online: https://suwanu-europe.eu/wp-content/uploads/2020/01/FS6_FactSheet_Cyprus_Pricing.pdf (accessed on 5 December 2022).
- Fielding, K.S.; Dolnicar, S.; Schultz, T. Public Acceptance of Recycled Water. Int. J. Water Resour. Dev. 2018, 35, 551–586. [Google Scholar] [CrossRef]
- SUWANU Europe. The Success Story of Cyprus—Fact Sheet 8—Public Acceptance. Available online: https://suwanu-europe.eu/wp-content/uploads/2020/01/FS8_FactSheet_Cyprus_Acceptance.pdf (accessed on 5 December 2022).
- Zhang, Y.; Tang, F.; Li, D.; Li, Y.; Chen, W.; Yang, M. Role of Water Reuse for Tianjin, A Megacity Suffering from Serious Water Shortage. In Milestones in Water Reuse: The Best Success Stories; IWA Publishing: London, UK, 2013. [Google Scholar] [CrossRef]
- Jowett, A.J. China’s Water Crisis: The Case of Tianjin (Tientsin). Geogr. J. 1986, 152, 9. [Google Scholar] [CrossRef]
- Tal, A. Rethinking the Sustainability of Israel’s Irrigation Practices in the Drylands. Water Res. 2016, 90, 387–394. [Google Scholar] [CrossRef]
- Hagin, J.; Oron, G.; Fardous, A.N.; Boulad, A.; Haddad, M.; Khamis, M.; Ben Hur, M. Wastewater treatment and reuse in agricultural production. Final Report Submitted to the USAID-MERC, Project M22-006 and Semi-Annual Reports 2003—2007.
- Hagin, J.; Khamis, M.; Boulad, A.; Al Hadidi, L.; Oron, G. Advanced Wastewater Treatment Technology and Reuse. Semi-Annual Report Submitted to USAID—MERC, Project M28-028 and Semi—Annual Reports 2008/2009.
- Hagin, J.; Khamis, M.; Manassra, A.; Abbadi, J.; Qurie, M.; Bulad, A.; AlHadidi, L.; Semiat, R.; Shaviv, A.; Katz, I.; et al. Treatment and Use of Wastewater for Agricultural Irrigation. In Proceedings International Fertiliser Society; International Fertiliser Society: Leek, UK, 2010. [Google Scholar]
- Tal, A. Seeking sustainability: Israel’s evolving water management strategy. Science 2006, 313, 1081–1084. [Google Scholar] [CrossRef]
- Feitelson, E. The Four Eras of Israeli Water Policies. Glob. Issues Water Policy 2013, 4, 15–32. [Google Scholar] [CrossRef]
- Umwelt Bundesamt (UBA). Recommendations for Deriving EU Minimum Quality Requirements for Water Reuse; Scientific Opinion Paper; Umwelt Bundesamt: Dessau-Rosslau, Germany, 2017. [Google Scholar]
- Hellwig, J.; de Graaf, I.E.M.; Weiler, M.; Stahl, K. Large- Scale Assessment of Delayed Groundwater Responses to Drought. Water Resour. Res. 2020, 56, e2019WR025441. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Gaba, A.; Zimmermann, B.; Jentzsch, T.; Kroemer, K.; Tiemann, Y.; Harsanyi, L.; Buchta, P.; Doelchow, U.; Lipnizki, J.; et al. Reuse of Municipal Wastewater for Different Purposes Based on a Modular Treatment Concept. J. Water Reuse Desalin. 2020, 10, 301–316. [Google Scholar] [CrossRef]
- Meyer, S.; Wehrman, H.A.; Knapp, H.V.; Lin, Y.-F.; Glatfelter, F.E.; Angel, J.R.; Thomason, J.F.; Injerd, D.A. Northeastern Illinois Water Supply Planning Investigations: Opportunities and Challenges of Meeting Water Demand in Northeastern Illinois—Executive Summary. The Illinois State Water Survey. 2012. Available online: https://www.ideals.illinois.edu/items/42621 (accessed on 5 April 2022).
- Illinois State Water Survey (ISWS). Illinois Water Supply Planning. Illinois State Water Survey—Prairie Research Institute. 2022. Available online: https://www.isws.illinois.edu/illinois-water-supply-planning/northeastern-illinois (accessed on 5 April 2022).
- Dziegielewski, B.; Chowdhury, F.J. Scenario-based forecast of Regional Water demands in northeastern Illinois. J. Water Resour. Plan. Manag. 2012, 138, 80–89. [Google Scholar] [CrossRef]
- Starr, J.D. How Chicago manages its distribution system. J. Am. Water Work. Assoc. 1974, 66, 328–331. [Google Scholar] [CrossRef]
- City of Chicago. Water Treatment. Available online: https://www.chicago.gov/city/en/depts/water/supp_info/education/water_treatment.html (accessed on 14 October 2022).
- Metropolitan Water Reclamation District of Greater Chicago (MWRD). Final Effluents. Mwrd.org. Available online: https://mwrd.org/water-reclamation-plants#:~:text=A%20continued%20commitment%20to%20protect%20our%20water%20environmentandtext=The%20boundaries%20of%20the%20MWRD,gallons%20of%20wastewater%20each%20day (accessed on 29 January 2022).
- Public Building Commission of Chicago. Water Reuse Handbook; Public Building Commission of Chicago: Chicago, IL, USA; Available online: https://www.pbcchicago.com/wp-content/uploads/2017/07/PBCWaterReuseHandbook_August2011.pdf (accessed on 29 January 2022).
- Meng, Y. Water Reuse Planning Model for the Greater Chicago Area. Ph.D. Dissertation, Illinois Institute of Technology, Chicago, IL, USA, 2009. Available online: https://proxy.cc.uic.edu/login?url=https://www.proquest.com/dissertations-theses/water-reuse-planning-model-greater-chicago-area/docview/304900610/se-2 (accessed on 1 February 2022).
- Illinois American Water. Reuse of Wastewater Effluent through a Public and Private Partnership. Available online: https://www.faegredrinker.com/webfiles/2016%20Water%20Conference%20-%20Chicago/Wastewater%20Effluent%20-%20Full%20Page.pdf (accessed on 10 February 2022).
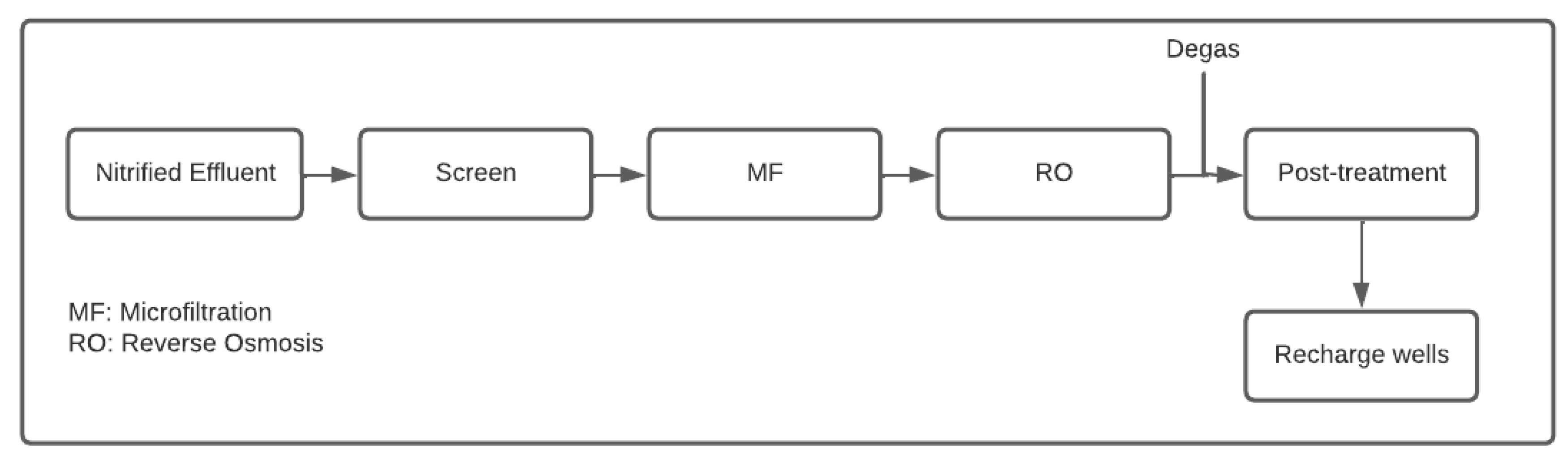
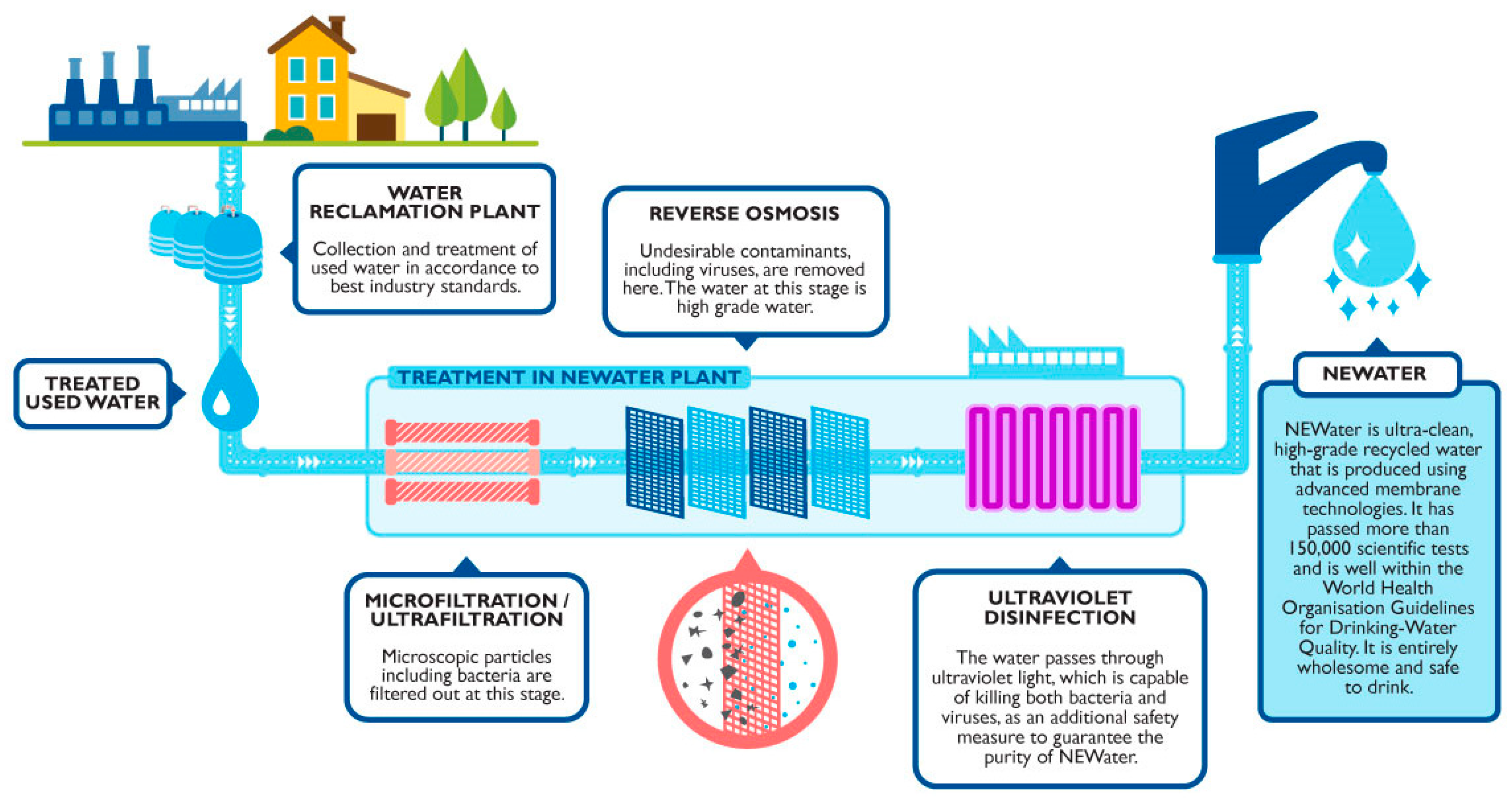
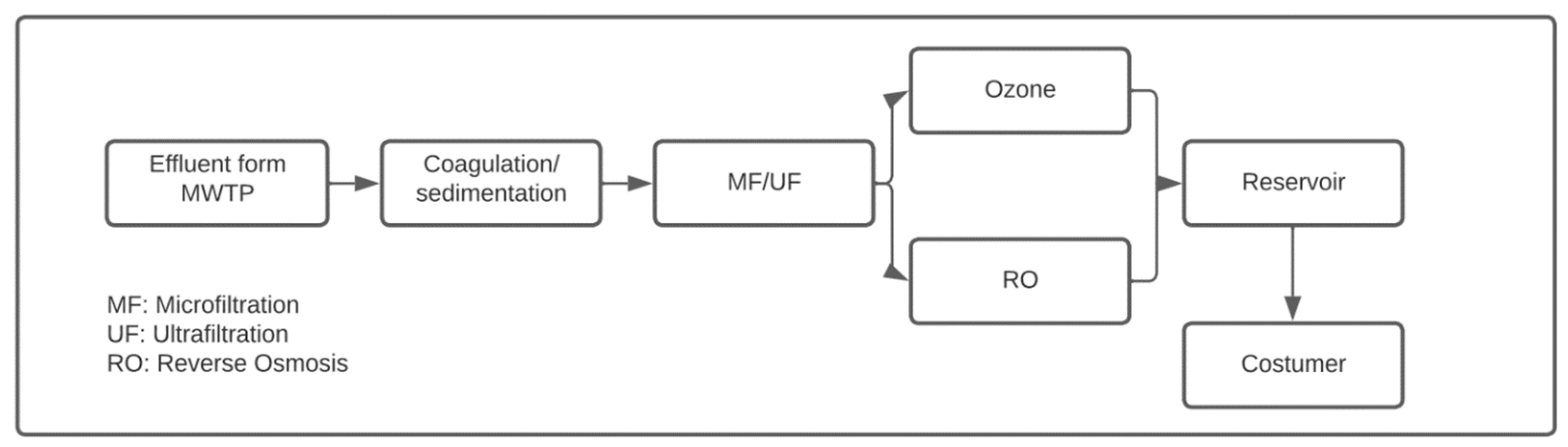

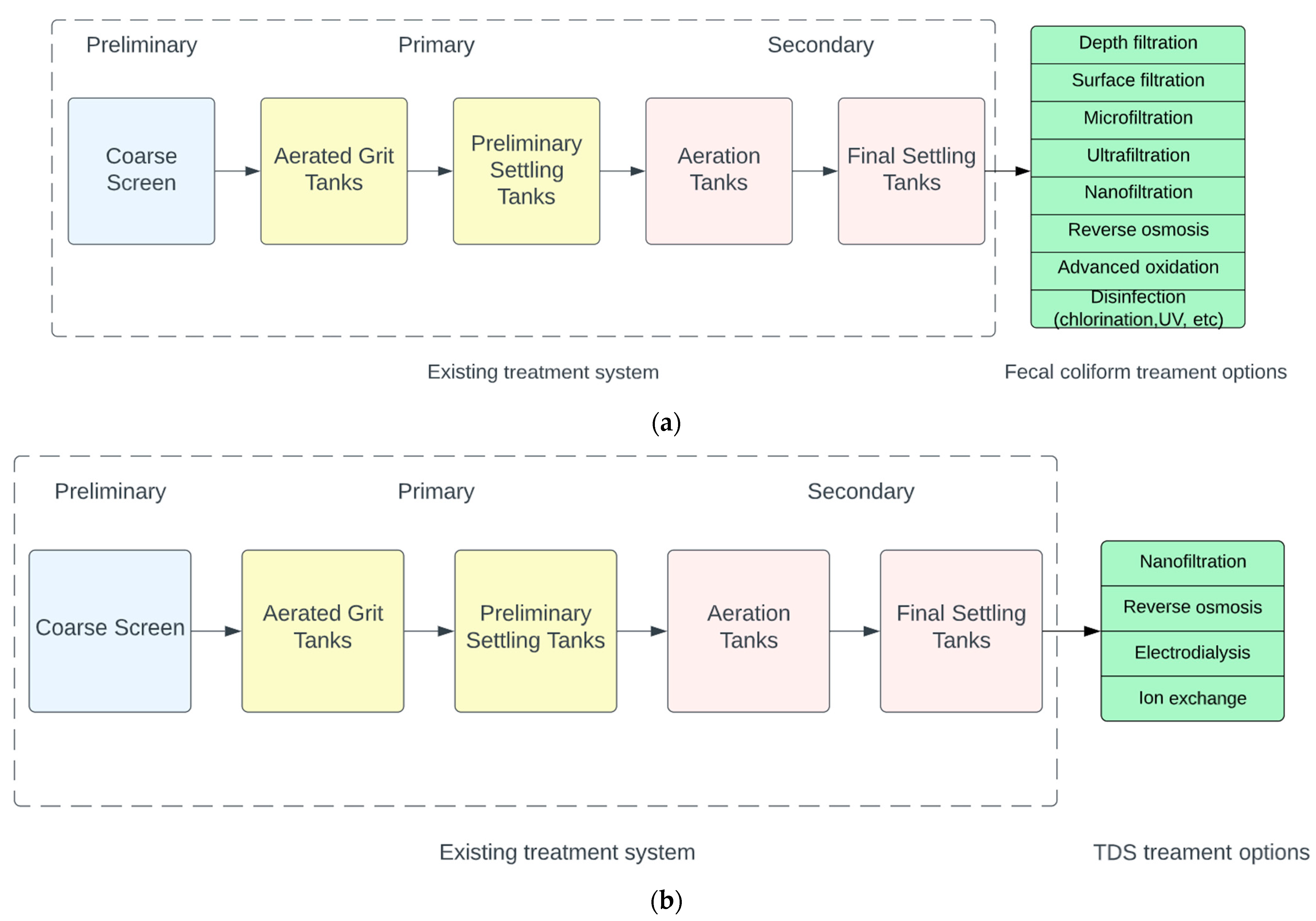



| (a) | ||
| Driver | Importance in Developed countries | Importance in Developing countries |
| Physical characteristics of the environment | ||
| Insufficient water supply | High | High |
| Managing drought and securing a reliable water supply | High | Medium |
| Recycling for meeting agricultural water demand | High | High |
| Inadequate sanitation resulting from the unintentional reuse of wastewater | - | High |
| Water management policies | ||
| Recycling is utilized as a means of mitigating the harmful consequences of discharging treated wastewater and safeguarding the environment, particularly in coastal and tourist regions or in ecologically vulnerable aquatic ecosystems | Medium | - |
| The proper treated wastewater can enhance the ecological conditions in areas with poor water quality | Medium | - |
| Reusing treated wastewater instead of first-use water for drinking water supply | Medium | - |
| The rising recognition among water and wastewater authorities of the financial and ecological advantages of utilizing reused water | Low | - |
| The significant ecological and financial expenses associated with water storage structures, such as dams and reservoirs | Medium | - |
| The increasing successful water recycling initiatives globally | Low | - |
| Social considerations | ||
| Implementing water reuse programs rather than increasing the cost of transporting water from external sources or bearing the expenses associated with advanced wastewater treatment | Medium | - |
| Raising awareness about the ecological consequences connected to excessive consumption of water resources | Low | - |
| To recover substances present in recycled water, such as nitrogen and phosphorus, without incurring any expenses | Low | High |
| The eagerness of the community to embrace the idea of water recycling | Low | - |
| Economic conditions | ||
| As a means of partially covering the expenses of meeting strict standards for wastewater treatment | Medium | - |
| To utilize recycling as a lower-cost method of waste disposal | Medium | Low |
| Safeguarding the environment in tourist destinations | Medium | Medium |
| Physical, social, and economic reasons | ||
| Significant water demands in nearby regions, particularly for urban and industrial purposes | High | High |
| (b) | ||
| Driver | Details | |
| Compliance | The Clean Water Acts defines the degree and type of wastewater treatment needed to fulfill effluent requirements. The CWA prohibits pollution discharges into navigable, fishable, and swimming waters, which requires significant water treatment to increase effluent value and usage. Some municipalities prefer to utilize the highly treated effluents instead of discharging them into waterways. | |
| Viable source substitution | For urban irrigation, air conditioning, and toilet flushing, recycled water may be the most cost-effective and practicable alternative. By reusing recycled water, potable water supply is reduced. | |
| Localized water demand increases | Population growth has increased water demand. Reuse is becoming a new water source alternative in areas where population growth has outpaced the availability of conventional water sources. | |
| Societal pressures | Growing recognition of the importance of water sources has led to the development of regulatory frameworks and organizational structures. | |
| Public acceptance (Yuck Factor) | In any water reuse project, having public support is crucial. However, there is a psychological obstacle, commonly referred to as the “yuck factor”, that exists among the public regarding wastewater reuse projects. To overcome this obstacle, it is essential to inform and involve the public continuously in water reuse projects, thereby gaining their attention and support for such initiatives. |
| Economic viability | Water reuse infrastructure and technology require large capital investments, and while sustainable and cost-effective in the long run, the added treatment and monitoring can be more expensive than other sources. Government subsidies may be necessary, and institutional barriers and differing priorities can make large-scale programs challenging. |
| Policy and regulations | Establishing and implementing guidelines for water reuse is crucial to gain public approval for water recycling. Nevertheless, in certain cases, regulations may impede and create difficulties for the reuse of water. |
| Technical feasibility and energy efficiency | To avoid overtreatment and energy waste, reuse technologies must be designated specifically to the end use. For example, reverse osmosis should be limited to high-end reuse applications, whereas other technologies may be more efficient for non-potable reuses. |
| Innovation | To remove the social, political, and economic constraints to the development of water reuse that is cost competitive. The technology innovation for water reuse should be focus-oriented toward developing of reliability, performance, flexibility and robustness of existing technologies, the development of new cost effective and energy efficient technologies, and other tools for water reuse practices. |
| Reuse Category and Description | Treatment | Reclaimed Water Quality |
|---|---|---|
| Urban Reuse | ||
| Unrestricted | Secondary, filtration, disinfection | pH = 6.0–9.0 ≤10 mg/L BOD ≤2 NTU No detectable fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water in non-potable applications in municipal settings where public access is not restricted. | ||
| Restricted | Secondary, disinfection | pH = 6.0–9.0 ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water in non-potable applications in municipal settings where public access is controlled or restricted by physical or institutional barriers, such as fencing, advisory signage, or temporal access restriction | ||
| Agricultural Reuse | ||
| Food Crops | Secondary, filtration, disinfection | pH = 6.0–9.0 ≤10 mg/L BOD ≤2 NTU No detectable fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water for surface or spray irrigation of food crops which are intended for human consumption, consumed raw. | ||
| Processed Food Crops | Secondary, disinfection | pH = 6.0–9.0 ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coli/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water for surface irrigation of food crops which are intended for human consumption, commercially processed. | ||
| Non-Food Crops | ||
| The use of reclaimed water for irrigation of crops which are not consumed by humans, including fodder, fiber, and seed crops, or to irrigate pastureland, commercial nurseries, and sod farms. | ||
| Impoundments | ||
| Unrestricted | Secondary, filtration, disinfection | pH = 6.0–9.0 ≤10 mg/L BOD ≤2 NTU No detectable fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water in an impoundment in which no limitations are imposed on body-contact. | ||
| Restricted | Secondary, disinfection | ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water in an impoundment where body-contact is restricted. | ||
| Environmental Reuse | ||
| Environmental Reuse | Variable, secondary and disinfection (min) | Variable, but not exceed: ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| The use of reclaimed water to create wetlands, enhance natural wetlands, or sustain stream flows. | ||
| Industrial Reuse | ||
| Once-through Cooling | Secondary | pH = 6.0–9.0 ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| Recirculating Cooling Towers | Secondary, disinfection (chemical coagulation and filtration may be needed) | Variable, depends on recirculation ratio: pH = 6.0–9.0 ≤30 mg/L BOD ≤30 mg/L TSS ≤200 fecal coliform/100 mL 1 mg/L Cl2 residual (min) |
| Groundwater Recharge—Non-potable Reuse | ||
| The use of reclaimed water to recharge aquifers which are not used as a potable drinking water source. | Site-specific and use-dependent, primary (min.) for spreading, secondary (min.) for injection | Site-specific and use dependent |
| Indirect Potable Reuse | ||
| Groundwater Recharge by Spreading into Potable Aquifers | Secondary, filtration, disinfection, soil aquifer treatment | Includes, but not limited to, the following: No detectable total coliform/100 mL 1 mg/L Cl2 residual (min) pH = 6.5–8.5 ≤2 NTU ≤2 mg/L TOC of wastewater origin Meet drinking water standards after percolation through vadose zone |
| Groundwater Recharge by Injection into Potable Aquifers | Secondary, filtration, disinfection, advanced wastewater treatment | Includes, but not limited to, the following: No detectable total coliform/100 mL 1 mg/L Cl2 residual (min) pH = 6.5–8.5 ≤2 NTU ≤2 mg/L TOC of wastewater origin Meet drinking water standards |
| Augmentation of Surface Water Supply Reservoirs | Secondary, filtration, disinfection, advanced wastewater treatment | Includes, but not limited to, the following: No detectable total coliform/100 mL 1 mg/L Cl2 residual (min) pH = 6.5–8.5 ≤2 NTU ≤2 mg/L TOC of wastewater origin Meet drinking water standards |
| Type of Surface Water | Fecal Coli./100 mL | Note |
|---|---|---|
| Seasonally Protected Water | 200 | Any waters that support primary contact from May through October. |
| Year-Round Protected Waters | 2000 | Applicable to any public and food processing water intake. |
| Unprotected Waters | Not subjected to Fecal Coli. Standards | When meeting certain characteristic of unprotected waters.
|
| Water Reclamation Plant | Highest Fecal Coliform Concentration (CFU/100 mL) | Average Fecal Coliform Concentration (CFU/100 mL) | ||
|---|---|---|---|---|
| May–October | November–April | May–October | November–April | |
| Stickney | 48,000 | 76,000 | 15,758 | 12,042 |
| Calumet | 150 | 57,000 | 45 | 6827 |
| O’Brien | 3500 | 52,000 | 113 | 1817 |
| Egan | 200 | 7500 | 27 | 2213 |
| Kirie * | 90 | 10 | 11 | 10 |
| Hanover Park * | 40 | 10 | 13 | 10 |
| Lemont | 220,000 | 210,000 | 33,827 | 20,086 |
| (a) | ||||||||||||||
| Constituent Class | Secondary Treatment | Secondary with nutrient removal | Depth filtration | Surface filtration | Microfiltration | Ultrafiltration | Dissolved Air Flotation | Nano filtration | Reverse Osmosis | Electro dialysis | Carbon adsorption | Ion exchange | Advanced Oxidation | Disinfection |
| Suspended Solids | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | - | - | - | - |
| Colloidal Solids | - | - | - | - | ✓ | ✓ | ✓ | - | - | ✓ | - | - | - | - |
| Particulate Organic Matter | - | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | - | ✓ | - |
| Dissolved Organic Matter | ✓ | ✓ | - | - | - | - | - | ✓ | ✓ | - | ✓ | - | ✓ | ✓ |
| Nitrogen | - | ✓ | - | - | - | - | - | - | ✓ | - | - | ✓ | - | - |
| Phosphorous | - | ✓ | - | - | - | - | - | - | ✓ | - | - | - | - | - |
| Trace Constituents | - | - | - | - | - | - | - | ✓ | ✓ | - | ✓ | ✓ | ✓ | - |
| Total Dissolved Solids | - | - | - | - | - | - | - | ✓ | ✓ | ✓ | - | ✓ | - | - |
| Bacteria | - | - | ✓ | ✓ | ✓ | ✓ | - | ✓ | ✓ | - | - | - | ✓ | ✓ |
| Protozoan Cysts and Oocysts | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ |
| Viruses | - | - | - | - | - | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ |
| (b) | ||||||||||||||
| Overall Treatment Objective | Unit Processes | TOC | TSS | TDS | Trace Chemical Constituents | Pathogens 3 | ||||||||
| Removal of Suspended Solids | Media Filtration, Microfiltration and ultrafiltration | Partial removal | High removal | None | None | High removal 3 | ||||||||
| Reducing the Concentration of Dissolved Chemicals | NF/RO | 90% removal | High removal | High removal | High removal 1 | High removal | ||||||||
| ED/EDR | None | None | High removal | None | None | |||||||||
| PAC | High removal | None | None | Partial removal | None | |||||||||
| GAC | 40–60% removal | High removal | None | 40–60% removal | Partial removal | |||||||||
| Ion exchange | None | None | High removal | Partial removal | None | |||||||||
| Biofiltration | High removal, High degradation 2 | High removal | None | High degradation 2 | Partial removal | |||||||||
| Ozone | None | None | None | High degradation * | High degradation | |||||||||
| Disinfection and Removal of Trace Organic Compounds | UV | None | None | None | Partial degradation * | High degradation | ||||||||
| Free Chlorine | None | None | None | Partial degradation * | High degradation | |||||||||
| Chloramines 4 | None | None | None | None | Partial degradation * | |||||||||
| PAA 5 | None | None | None | Partial degradation * | High degradation | |||||||||
| Pasteurization 5 | None | None | None | Partial degradation | High degradation | |||||||||
| Ozone | None | None | None | High degradation * | High degradation | |||||||||
| Chlorine dioxide | None | None | None | Partial degradation * | High degradation | |||||||||
| Advanced oxidation processes (UV/H2O2, O3/H2O2, UV/Cl2) | None | None | None | High degradation * | High degradation | |||||||||
| Category | Tertiary Treatment Process | Reuse Purposes | Location | |
|---|---|---|---|---|
| Pre-Treatment-Filtration | Disinfection | |||
| Urban Reuse | Flocculation Media Filtration | Chlorination | Non-potable irrigation (residential, commercial, industrial) | El Segundo, CA, SUA |
| Agriculture | Flocculation Multi-media Filters | Chlorination | Raw-eaten vegetables and fruits | Monterey One, CA, USA |
| None (Membrane Bioreactor effluent) | Ultraviolet | Vineyards | American Canyon, CA, USA | |
| Coagulation Flocculation Cloth Media Filter | Ultraviolet | Raw-eaten fruits | Pajaro Valley, CA, USA | |
| Industrial | Microfiltration | Reverse Osmosis (Single Pass) Decarbonation | Industrial—Boiler Feed (BF) water | El Segundo, CA, USA |
| Microfiltration | Reverse Osmosis (Single Pass) Ozone Decarbonation | Industrial—Low-Pressured Boiler Feed | El Segundo, CA, USA | |
| Microfiltration | Reverse Osmosis (Double Pass) Ozone Decarbonation | High-Pressure Boiler Feed | El Segundo, CA, USA | |
| Sand Filter | Addition of corrosion inhibitors, sodium hypochlorite, acid, and antifoaming agents (at power plant) | Cooling towers | Denver, CO, USA | |
| Media Filtration | Oxidized Coagulation Disinfected (UV or Chlorine) | Pulp and paper (newspaper) | Los Angeles, CA, USA | |
| Gravity Filter | Chlorination | Textile (carpet dyeing) | Santa Fe, CA, USA | |
| Granular Coal | Ultraviolet | Geyser recharge for electricity | Santa Rosa, CA, USA | |
| Lime Softening Filtration | Chlorination | Cooling towers | Baltimore, MD, USA | |
| Environmental | Automatic Backwash with Sand Media | Chlorination | Wetlands | Orlando, FL, USA |
| Indirect Potable Reuse | Microfiltration | Reverse Osmosis UV with Hydrogen Peroxide Lime Treatment | Groundwater recharge | Orange County, CA, USA |
| Lime Clarification Media Filtration | Granulated Activated Carbon Ion Exchange Chlorination | Fairfax, VA, USA | ||
| Media Filtration | Reverse Osmosis Ultraviolet with Advanced Oxidation Process Chlorination | Groundwater recharge via riverbank filtration | Arapahoe County, CO, USA | |
| Potable Reuse | Flocculation Biologically Active Carbon Filtration Microfiltration Ozonation Granular Activated Carbon | Ultraviolet Chlorination | Drinking Water (preliminary approval) | Castle Rock, CO, USA |
| Granular Activated Carbon Filtration | Reverse Osmosis Ultraviolet with Advanced Oxidation Process | Drinking Water (Undergoing regulatory approval) | El Paso, TX, USA | |
| Combination | Granular Coal | Ultraviolet | Farmlands Vineyards Public urban landscaping | Santa Rosa, CA, USA |
| None | UV | Agricultural Irrigation (Vineyards) Landscape Irrigation (excludes golf courses) Industrial use Other—Construction site dust control Other—In-plant use at City WRF | American Canyon, CA, USA | |
| Microfiltration | Chlorine/Dechlorination Reverse Osmosis Ultraviolet | Irrigation Industrial Streamflow Augmentation (future direction) Groundwater Recharge (future direction) | Santa Clara, CA, USA | |
| Process | Advantages | Disadvantages |
| Advanced oxidation processes (AOP) Photolysis Heterogeneous and homogeneous photocatalytic reactions non-catalytic wet air oxidation (WAO) Catalytic wet air oxidation (CWAO) Supercritical water gasification |
|
|
| Adsorption/filtration Commercial activated carbons (CAC) Commercial activated alumina (CAA) Sand Mixed materials Silica gel |
|
|
| Biological methods Bioreactors Biological activated sludge (BAS) Microbiological treatments Enzymatic decomposition Lagoon |
|
|
| Coagulation/flocculation |
|
|
| Dialysis Electrodialysis (ED) Electro-electrodialysis (EED) Emulsion liquid membranes (ELM) Supported liquid membranes Membrane filtration Microfiltration (MF) Ultrafiltration (UF) Nanofiltration (NF) Reverse osmosis |
|
|
| Ion exchange Chelating resins Selective resins Microporous resins Polymeric adsorbents Polymer-based hybrid adsorbents |
|
|
| (a) | |||||||
| Capacity (million gallons per day, MGD) | Treatment Technologies | Total Capital Cost (USD /kgal per year) | Annualized Capital Cost (USD /kgal) | Capital Cost (USD /kgal) | Annual Capital Cost + O&M Cost (USD /kgal) | End Uses | Facility |
| 5 | Secondary treated water–Filtration–UV | 5.73 | 0.5 | 0.35 | 0.85 | Landscape irrigation | Desert Breeze, NV, USA |
| 10 | Secondary treated water–Filtration–UV | 4.23 | 0.37 | 0.68 | 1.05 | Landscape irrigation | Durango Hills, NV, USA |
| 16.4 | Advanced Activated Sludge Treatment | 1.14 | 0.1 | 0.05 | 0.15 | Landscape irrigation, amenity reservoir | Trinity River Authority, TX, USA |
| 30 | Biologically aerated filters–Flocculation–Sedimentation–Filtration–Disinfection | 13.57 | 1.18 | 1.06 | 2.24 | Landscape irrigation, Industrial cooling, zoo | Denver Water, CO, USA |
| 40 | Biological Nutrient Removal (BNR) secondary treated water–Filtration–Chlorine Disinfection | 18.75 | 1.63 | 1.02 | 2.65 | Irrigation, industrial cooling, laundry, paper processing | West Basin, CA, USA |
| 12.5 | Microfiltration-Reverse Osmosis (RO)–Advanced Oxidation | 30.72 | 2.68 | 2.38 | 5.6 | Indirect Potable Reuse | West Basin, CA, USA |
| 10 | Activated Sludge Secondary Treatment with Denitrification–Anaerobic Digestion–Lime Treatment–Sand Filtration-Ozonation-Biologically Active Granular Activated Carbon Filtration–Final Disinfection | 23.46 | 2.05 | 0.33 | 2.38 | Indirect Potable Reuse | El Paso Water, TX, USA |
| 20 | Biological Nutrient Removal (BNR) secondary treated water–Filtration–Chlorine Disinfection–Soil Aquifer Treatment | 11.26 | 0.98 | 1.18 | 2.16 | Indirect Potable Reuse | Inland Empire, CA, USA |
| 24 | Biological Nutrient Removal (BNR) secondary treated water–Sodium Hypochlorite Disinfection–Treatment Wetlands | 3.92 | 0.34 | 0.35 | 0.69 | Indirect Potable Reuse | Casey WRF/Huie Wetlands Clayton Co., GA, USA |
| 70 | Enhanced Primary Treatment–Activated Sludge and Trickling Filter Secondary Treatment–Microfiltration (MF)–Reverse Osmosis (RO)–Advanced Oxidation (ultraviolet light and hydrogen peroxide) | 20.0 | 1.74 | 1.16 | 2.90 | Indirect Potable Reuse | Orange Co. GWRS, CA, USA |
| (b) | |||||||
| Community | Potable Water Rates (First Tiers Only) | Reclaimed Water Rates | |||||
| Rate per 1000 gal | Use | Rater per 1000 gal | Use | ||||
| Tucson, AZ, USA | 2.19 | 1–15 ccf | 2.45 | Variable on all use | |||
| 7.82 | 16–30 ccf | ||||||
| Dublin San Ramon Services District, CA, USA | 3.28 | Tier 1 Volume charge, first 22,440 gallons | 3.19 | Flat rate volume charge | |||
| 3.48 | Tier 2 Volume Charge over 22,440 gallons | ||||||
| Eastern Municipal Water District, CA, USA | 2.07 | Tier 1 Indoor use | 0.8 | R-452 Non-Ag, Secondary, Disinfected-2009 | |||
| 3.79 | Tier 2 Outdoor use | 0.88 | R-452 Non-Ag, Tertiary, Disinfected, Filtered-2009 | ||||
| Glendale Water and Power, CA, USA | 3.18 | Commercial Rate | 2.39 | Non-potable purposes | |||
| Irvine Ranch Water District, CA 1, USA | 1.62 | Residential Detached Base Rate 5–9 ccf | 1.44 | Landscape Irrigation Base Index 41–100% ET | |||
| 3.34 | Residential Detached Inefficient Rate 10–14 ccf | 3.01 | Landscape Irrigation Inefficient Index 101–110% ET | ||||
| 5.78 | Residential Detached Excessive Rate 15–19 ccf | 5.2 | Landscape Irrigation Excessive Index 111–120% ET | ||||
| Orange Country, FL, USA | 1.04 | 0–3000 gal | 0.74 | Variable on >4000 gal/month | |||
| 1.39 | 4000–10,000 gal | ||||||
| St. Petersburg, FL, USA | 3.45 | 0–5600 gal | 17.63 | Unmetered–First acre | |||
| 10.1 | Unmetered > 1 acre | ||||||
| 0.5 | Metered | ||||||
| El Paso, TX, USA | 1.94 | Over 4 ccf | 1.24 | Variable on all use | |||
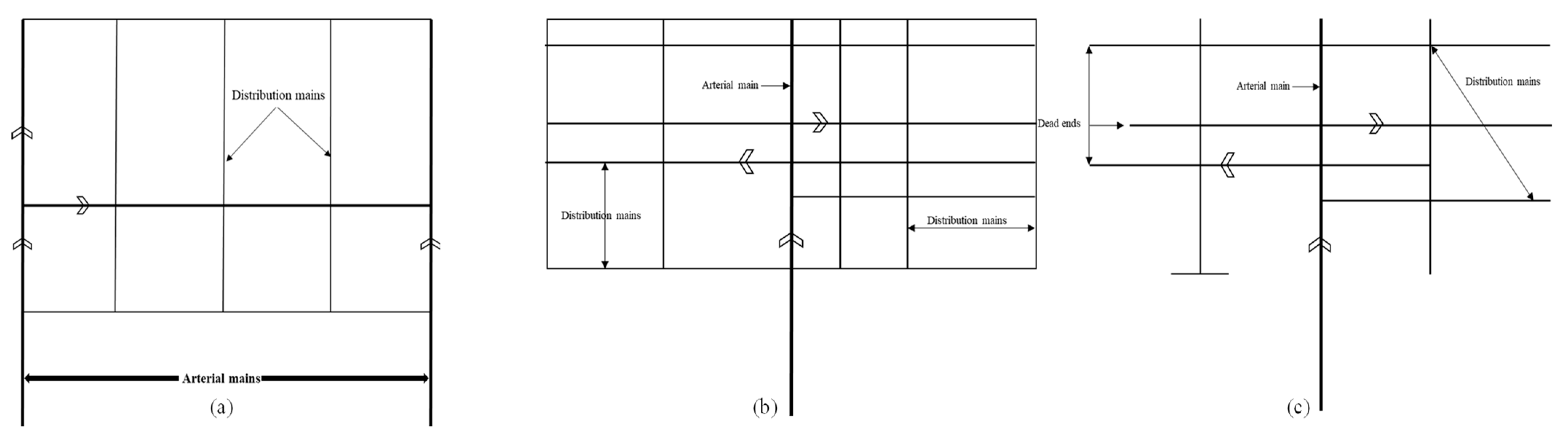
| System Type | Description | Notes |
|---|---|---|
| Loop | The areas that are going to be served are surrounded by large feeder mains, and smaller cross feed lines are connected to the main loop. | Reclaimed water is distributed from two directions to the main reuse area. Looped systems have less head loss than tree system. |
| Grid | The piping is set out in a checkerboard arrangement, and the size of the pipe typically decreases as the distance from the source increases. | Pipe size reduction will reduce material costs and has similar advantages as the loop system. |
| Tree | It utilizes a single main that decreases in size the further away it is from the source. | Usually used for systems that do not need the higher level of reliability that loop and grid systems offer. The accumulation of build-up in dead ends can be avoided with regular line flushing. |
| Treatment Technology | Description |
|---|---|
| Scaleban | Scaleban is an innovation that enables industries to accomplish water saving and zero liquid discharge (ZLD). Scaleban solves the following typical issues connected with the use of wastewater treatment in cooling towers: hard water scaling, total suspended solids removal, corrosion, and biofouling. Cooling towers can be operated at greater TDS levels with the Scaleban system. |
| Forward Osmosis | FO is driven by differential osmotic pressure, and water diffusion occurs from lower (the feed side) to higher concentration (the draw side). |
| Activated Glass Media Filter | The Activated Glass Media Filter is a filter product produced from an aluminosilicate filter medium, which is subjected to a distinctive three-stage physiochemical activation process to attain an optimal particle size, shape, and charge. This process increases the filter’s surface area by up to 300 times, resulting in improved mechanical and electrostatic filtering for enhanced effectiveness. |
| Vacuum Distillation | Vacuum distillation is a method used to purify substances that cannot be easily distilled under normal atmospheric pressure. This process separates impurities according to their varying boiling points. |
| Volute | Volute is a sludge dewatering device that continuously removes water and moisture from sludge. |
| Solar Detoxification | Solar detoxification is a process that utilizes ultraviolet (UV) light and a catalyst to eliminate harmful organic compounds and toxic substances from wastewater. |
| Sustainable Wastewater Treatment | Effluent is classified into three streams according to their TDS and COD concentration level. Recommendation of sustainable treatment then provided for each stream. |
| Technical and Infrastructure Challenge | Frequency of Occurrence in Previous Studies | Preventive Strategy | Corrective Strategy |
|---|---|---|---|
| Aging infrastructure | 51 | Geographic Information System (GIS) | Capital Investment |
| Improper maintenance of water infrastructure | 47 | Implementing appropriate policies and measures | - |
| Traditional wastewater treatment methods | 39 | Protection, accommodation, and retreatment of infrastructure | |
| The interdisciplinary nature of infrastructure systems | 32 | GIS | - |
| Awareness of infrastructure resilience and role of media | |||
| Loss of disinfectant residuals | 26 | The Environmental Protection Agency Network (EPANET) | - |
| Escalating physical threats | 21 | GIS | - |
| The Water Network Tool for Resilience (WNTR) | |||
| Redundancy in the water distribution systems | 16 | - | Examining decisions on management techniques |
| Interdependencies of water and wastewater infrastructure to electric power | 14 | Intervention’s framework | - |
| Storage capacity in the wastewater collection system | 14 | Increasing the storage capacity of wastewater collection system | - |
| Implementing appropriate policies and measures | |||
| Backup power and structural stability of drinking and wastewater treatment and pumping facilities | 7 | - | - |
| Inefficient pond sand filters | 4 | Efficient pond sand filters | - |
| Unauthorized structures | 3 | Implementing appropriate policies and measures | - |
| Emerging Contaminants | Initial Powdered Activated Carbon (PAC) Concentration | Removal Efficiency Using PAC (%) | Initial Granular Activated Carbon (GAC) Concentration | Removal Efficiency Using GAC (%) |
|---|---|---|---|---|
| Bezafibrate | 1300 | 90 | - | - |
| 17-Alphaethylestradiol | 0.24 ± 0.07 | 83.3 | <20 | 50 |
| 17-Beta estradiol | 4.68 ± 0.89 | 99.9 | 2 | >43 |
| Diclofenac | NR | 96–98 | NR | >98 |
| Propranolol | NR | 91–94 | - | - |
| Sulfamethoxazole | NR | 58 | NR | 90 |
| Clarithromycin | NR | 88 | - | - |
| Carbamazepine | NR | 92 | 66 | 23 |
| Iopromide | NR | 70 | NR | >80 |
| Mecoprop | NR | 65 | - | - |
| Bisphenol A | 12.60 ± 2.02 | 53 | NR | 66 |
| Erythromycin | - | - | 300 ± 200 | 99.9 |
| Ciproflaxacin | - | - | 130 | 82.3 |
| Carbamazepine | - | - | NR | 23 |
| Nonylphenol | - | - | NR | 84 |
| Triclosan | - | - | NR | 95 |
| Galaxolide | - | - | NR | 79 |
| Project/Location | Plant Capacity | Type of Use | Benefits | Cost/Revenue/Funding |
|---|---|---|---|---|
| Hampton Roads Sanitation | 15 MGD | Industrial | Reduce costs to the nearby refinery for process, provide more secure water supply in drought condition, conserve potable water resources, and reduce the nutrient load released to the river | Capital cost: USD 2.6 million O&M cost: USD 135,000–USD 150,000 (fiscal year 2003) |
| Irvine Ranch Water District | 15 MGD and 5.5 MGD | Landscape and agricultural irrigation | Maximize drinking water supply, conserve potable water by switching recycled water for non-potable uses, and minimize the mount of treated wastewater that must be sent to regional wastewater agency for disposal through an ocean outfall | O&M cost for treatment and distribution: USD 6.6 million |
| Monterey County Water Recycling Project | 30 MGD | Agricultural | Conserve potable water for agricultural, reduce sea water intrusion by 40 to 50% | Capital cost: USD 78 million Total cost to treat and deliver to agricultural areas: USD 225/ac-ft Revenue: USD 6 million annually |
| San Antonio Water System | 116 MGD (total from 4 plants) | Industrial and commercial | 51% cost saving from potable water rates, reduce dependency on the existing aquifers supply, reduce cost for fertilizer due to nutrients recovered from wastewater | Capital cost: USD 124 million |
| Water Conserv II | 42 MGD | Agricultural, commercial, and rapid infiltration basins (RIB) to recharge aquifer | Elimination of discharge to environmentally sensitive surface waters, demand reduction on aquifer, and enhanced aquifer storage | Capital cost: USD 277 million O&M and distribution cost: USD 4.8 million USEPA funding: USD 100 million |
| Pinellas County’s Reclaimed Water Program | 9 MGD and 33 MGD | Irrigation of public access areas | Reduce cost for potable water purchases, and additional potable water savings | Cost to upgrade WTP: USD 150 million Capital cost for water transmission and distribution: USD 140 million Annual O&M cost: USD 1.2 million Revenue: USD 87 million Grants from SWFWMD: USD 28 million |
| End Users | Treatment Technologies |
|---|---|
| Non-potable irrigation (residential, commercial, industrial)—Title 22 | HRC, tertiary media filter, Cl disinfection |
| Groundwater injection for West Coast Basin Seawater Barrier | Ozone, MF, RO, UV-AOP, decarbonation, Cl disinfection |
| Low Pressure Boiler Feed (LPBF) for Chevron Refinery—Industrial | Ozone, MF, RO (Single Pass), decarbonation |
| High Pressure Boiler Feed (HPBF) for Chevron Refinery—Industrial | Ozone, MF, RO (Double Pass), decarbonation |
| Ammonia-free water for cooling towers at Chevron Refinery—Industrial | BAF |
| Ammonia-free water for cooling towers at Torrance Refinery—Industrial | BAF |
| BF water for Torrance Refinery—Industrial | MF, RO (Single Pass), decarbonation |
| Ammonia-free water for cooling towers at Marathon Refinery—Industrial | BAF |
| BF water for Marathon Refinery—Industrial | MF, RO (Single Pass), decarbonation |
| WWTP | Capacity (m3/day) | Capacity (Person Equivalent (PE)) | Biological Treatment Process Applied | Tertiary Treatment Process Applied |
|---|---|---|---|---|
| Anthoupoli | 13,000 | 130,000 | Membrane Bioreactor (UF) | - |
| Vathia Gonia (NSB) | 22,000 | 202,000 | Membrane Bioreactor (MF) | UV disinfection |
| Larnaca | 18,000 | 100,000 | Membrane Bioreactor | Sand filtration—Chlorination |
| Moni Limassol | 40,000 | 272,000 | Conventional Activated Sludge | Sand filtration—Chlorination |
| Paphos | 19,500 | 160,000 | Conventional Activated Sludge | Sand filtration—Chlorination |
| Paralimni-Ayia Napa | 21,000 | 125,000 | Conventional Activated Sludge | Chlorination |
| Total | 133,500 | UF: Ultrafiltration; MF: Microfiltration | ||
| Irrigation Water Quality | Jordan (after 2 Seasons) | Israel (after 6 Years) | |||
|---|---|---|---|---|---|
| EC (dS/m) | EC(dS/m) | Sodium Adsorption Ratio (SAR) | |||
| Depth (cm) | 0–20 | 20–40 | - | - | |
| Effluent from Secondary Treatment | 3.24 | 3.01 | 16 | 25 | |
| UF Permeate | 2.83 | 2.71 | 9 | 20 | |
| RO Permeate | - | - | 2 | 3 | |
| UF-RO mix | 30–70 | - | - | 6 | 16 |
| 50–50 | 1.14 | 0.99 | - | - | |
| 70–30 | - | - | 5 | 12 | |
| Parameter | Unit | Municipal Water | Treated Water | ||
|---|---|---|---|---|---|
| South Water Purification Plant | Jardine Water Purification Plant (Central) | Jardine Water Purification Plant (North) | Stickney | ||
| Temperature | °C | 14 | 13.7 | 13.7 | 17.3 |
| Turbidity | N.T.U. | 0.167 | 0.3 | 0.1 | - |
| pH | 7.87 | 7.9 | 7.9 | 7.1 | |
| Dissolved Oxygen | mg/L | - | - | - | 8.3 |
| BOD5 | mg/L | - | - | - | <6 |
| Total Solids | mg/L | 189.3 | 184.0 | 187.3 | 697 |
| Total Dissolved Solids | mg/L | 157.3 | 156.0 | 156.3 | 692 |
| Hardness | mg as CaCO3/L | 138.3 | 138.3 | 139.0 | 246 |
| Total Alkalinity | mg as CaCO3/L | 101.8 | 102.2 | 101.1 | - |
| Calcium | mg/L | 35.0 | 35.1 | 35.3 | 63.6 |
| Magnesium | mg/L | 12.2 | 12.3 | 12.5 | 21.1 |
| Sodium | mg/L | 8.8 | 8.8 | 8.8 | - |
| Potassium | mg/L | 1.4 | 1.4 | 1.4 | - |
| Ammonia | N mg/L | <0.1 | <0.1 | <0.1 | <0.7 |
| Nitrite | N mg/L | <0.25 | <0.25 | <0.25 | - |
| Nitrate | N mg/L | 0.316 | 0.309 | 0.307 | - |
| Total Phosphate | mg/L | 1.2 | 1.2 | 1.2 | 0.76 |
| Chloride | mg/L | 16.0 | 15.9 | 16.0 | 450 |
| Fluoride | mg/L | 0.7 | 0.7 | 0.7 | 0.5 |
| (a) | ||||||||
| Parameter | Symbol | Unit | Stickney (South West) | Stickney (West) | ||||
| Min | Avg | Max | Min | Avg | Max | |||
| Flow | MGD | 136 | 302 | 794 | 64 | 302 | 582 | |
| Acidity | pH | Not Reported | Not Reported | |||||
| Biological Oxygen Demand | BOD5 | mg/L | 83 | 373 | 2691 | 51 | 183 | 912 |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | Data Not Available | Data Not Available | ||||
| Fat, Oil, and Grease | FOG | mg/L | <5 | <12 | 40 | 6 | 18 | 38 |
| Suspended Solids | SS | mg/L | 68 | 655 | 8320 | 45 | 256 | 1480 |
| Volatile SS | VSS | mg/L | 64 | 458 | 6280 | 39 | 194 | 1060 |
| Total Solids | TS | mg/L | 544 | 1220 | 5360 | 636 | 880 | 1280 |
| Total Volatile Solids | VTS | mg/L | 136 | 506 | 2390 | 140 | 344 | 608 |
| Total Kjeldahl Nitrogen | TKN | mg/L | 13 | 46 | 213 | 11 | 37 | 85 |
| Ammonia | NH3-N | mg/L | 3.5 | 20.0 | 60.2 | 7.6 | 22.8 | 46.8 |
| Nitrite Nitrate | NO2-N + NO3-N | mg/L | <0.25 | <0.20 | 1.25 | <0.25 | <0.28 | 2.73 |
| Phosphorous | P-TOT | mg/L | 2.72 | 11.49 | 56.39 | 2.23 | 7.16 | 18.38 |
| Phosphorous | P-SOL | mg/L | Data Not Available | Data Not Available | ||||
| Cyanides | CN | mg/L | <0.005 | <0.012 | 0.031 | <0.005 | <0.008 | 0.018 |
| Amenable, Cyanide | CN AM | mg/L | <0.005 | <0.004 | 0.007 | <0.005 | <0.004 | 0.009 |
| Phenol | Phenol | ug/L | <0.005 | <0.012 | 0.031 | <5 | <26 | 138 |
| Fluoride | F | mg/L | 0.3 | 0.6 | 0.8 | 0.4 | 0.7 | 0.8 |
| Total Organic Carbon | TOC | mg/L | Data Not Available | Data Not Available | ||||
| Arsenic | As | mg/L | 0.002 | 0.005 | 0.020 | <0.002 | <0.003 | 0.007 |
| Barium | Ba | mg/L | 0.044 | 0.117 | 0.632 | 0.040 | 0.067 | 0.121 |
| Cadmium | Cd | mg/L | <0.002 | <0.002 | 0.009 | <0.002 | <0.002 | 0.003 |
| Chromium | Cr | mg/L | <0.003 | <0.022 | 0.184 | <0.003 | <0.016 | 0.036 |
| Copper | Cu | mg/L | 0.033 | 0.125 | 1.053 | 0.027 | 0.072 | 0.158 |
| Iron | Fe | mg/L | 1.14 | 4.07 | 24.89 | 0.75 | 1.78 | 4.03 |
| Soluble Iron | Sol Fe | mg/L | 0.06 | 0.23 | 1.87 | 0.03 | 0.11 | 0.16 |
| Lead | Pb | mg/L | 0.004 | 0.021 | 0.136 | 0.003 | 0.010 | 0.024 |
| Manganese | Mn | mg/L | 0.089 | 0.203 | 1.089 | 0.049 | 0.094 | 0.213 |
| Mercury | Hg | ug/L | <0.5 | <0.4 | 0.6 | <0.5 | <0.4 | <0.5 |
| Nickel | Ni | mg/L | 0.006 | 0.030 | 0.292 | 0.005 | 0.013 | 0.024 |
| Selenium | Se | mg/L | <0.004 | <0.003 | 0.009 | <0.004 | <0.003 | 0.004 |
| Silver | Ag | mg/L | <0.004 | <0.003 | 0.014 | <0.004 | <0.003 | <0.004 |
| Zinc | Zn | mg/l | 0.081 | 0.285 | 1.573 | 0.065 | 0.156 | 0.337 |
| Antimony | Sb | mg/L | <0.002 | <0.002 | 0.005 | <0.002 | <0.002 | 0.003 |
| Beryllium | Be | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Thallium | Tl | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium(6+) | Cr6 | ug/L | <3 | <2 | <3 | <3 | <2 | 8 |
| Sulphate | SO4 | mg/L | Data Not Available | 42 | 61 | 86 | ||
| Chloride | Cl | mg/L | Data Not Available | Data Not Available | ||||
| (b) | ||||||||
| Parameter | Symbol | Unit | Calumet | O’Brien | ||||
| Min | Avg | Max | Min | Avg | Max | |||
| Flow | MGD | Not Reported | Not Reported | |||||
| Acidity | pH | 6.9 | 7.4 | 8.0 | Not Reported | |||
| Biological Oxygen Demand | BOD5 | mg/L | 41 | 189 | 2084 | 30 | 108 | 289 |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | 27 | 139 | 2662 | 13 | 73 | 135 |
| Fat, Oil, and Grease | FOG | mg/L | 8 | 23 | 66 | 8 | 26 | 54 |
| Suspended Solids | SS | mg/L | 18 | 267 | 2388 | 37 | 126 | 417 |
| Volatile SS | VSS | mg/L | 12 | 211 | 2200 | Data Not Available | ||
| Total Solids | TS | mg/L | 592 | 1119 | 2959 | 478 | 687 | 982 |
| Total Volatile Solids | VTS | mg/L | 138 | 358 | 1232 | Data Not Available | ||
| Total Kjeldahl Nitrogen | TKN | mg/L | 11 | 26 | 77 | 6 | 24 | 36 |
| Ammonia | NH3-N | mg/L | 3.6 | 14.0 | 26.9 | 5.1 | 16.0 | 23.7 |
| Nitrite Nitrate | NO2-N + NO3-N | mg/L | <0.25 | <0.26 | 2.39 | <0.25 | <0.30 | 2.69 |
| Phosphorous | P-TOT | mg/L | 1.74 | 7.18 | 21.50 | 0.99 | 3.71 | 7.58 |
| Phosphorous | P-SOL | mg/L | 0.70 | 3.95 | 17.00 | 0.84 | 2.00 | 2.72 |
| Cyanides | CN | mg/L | <0.005 | <0.010 | 0.051 | <0.005 | <0.007 | 0.020 |
| Amenable, Cyanide | CN AM | mg/L | <0.005 | <0.004 | <0.010 | <0.005 | <0.004 | 0.008 |
| Phenol | Phenol | ug/L | <5 | <22 | 105 | <5 | <21 | 52 |
| Fluoride | F | mg/L | 0.4 | 0.5 | 0.7 | 0.4 | 0.6 | 0.9 |
| Total Organic Carbon | TOC | mg/L | 34 | 103 | 771 | 25 | 73 | 113 |
| Arsenic | As | mg/L | <0.002 | <0.004 | 0.009 | <0.002 | <0.002 | 0.003 |
| Barium | Ba | mg/L | 0.036 | 0.095 | 0.244 | 0.033 | 0.048 | 0.076 |
| Cadmium | Cd | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium | Cr | mg/L | <0.004 | <0.006 | 0.025 | <0.004 | <0.003 | 0.004 |
| Copper | Cu | mg/L | 0.017 | 0.061 | 0.229 | 0.025 | 0.042 | 0.106 |
| Iron | Fe | mg/L | 0.86 | 3.32 | 12.01 | 0.43 | 0.88 | 3.01 |
| Soluble Iron | Sol Fe | mg/L | 0.06 | 0.48 | 3.57 | 0.09 | 0.15 | 0.19 |
| Lead | Pb | mg/L | <0.002 | <0.008 | 0.040 | <0.002 | <0.004 | 0.016 |
| Manganese | Mn | mg/L | 0.082 | 0.185 | 0.536 | 0.037 | 0.057 | 0.126 |
| Mercury | Hg | ug/L | <0.5 | <0.4 | <0.5 | <0.5 | <0.4 | <0.5 |
| Nickel | Ni | mg/L | 0.004 | 0.009 | 0.030 | 0.004 | 0.010 | 0.087 |
| Selenium | Se | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 |
| Silver | Ag | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 |
| Zinc | Zn | mg/l | 0.052 | 0.197 | 0.917 | 0.047 | 0.091 | 0.189 |
| Antimony | Sb | mg/L | <0.002 | <0.002 | 0.004 | <0.002 | <0.002 | 0.002 |
| Beryllium | Be | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Thallium | Tl | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium(6+) | Cr6 | ug/L | <3 | <2 | <3 | <3 | <2 | <3 |
| Sulphate | SO4 | mg/L | Data Not Available | Data Not Available | ||||
| Chloride | Cl | mg/L | Data Not Available | 100.98 | 277.24 | 2019.7 | ||
| (c) | ||||||||
| Parameter | Symbol | Unit | Egan | Kirie | ||||
| Min | Avg | Max | Min | Avg | Max | |||
| Flow | MGD | Data Not Available | Data Not Available | |||||
| Acidity | pH | 6.6 | 7.4 | 7.7 | Data Not Available | |||
| Biological Oxygen Demand | BOD5 | mg/L | 86 | 176 | 441 | 26 | 138 | 260 |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | 69 | 119 | 252 | Data Not Available | ||
| Fat, Oil, and Grease | FOG | mg/L | 9 | 35 | 70 | <5 | <21 | 65 |
| Suspended Solids | SS | mg/L | 80 | 186 | 721 | 474 | 922 | 2070 |
| Volatile SS | VSS | mg/L | Data Not Available | Data Not Available | ||||
| Total Solids | TS | mg/L | 672 | 932 | 1894 | 22 | 157 | 1180 |
| Total Volatile Solids | VTS | mg/L | Data Not Available | Data Not Available | ||||
| Total Kjeldahl Nitrogen | TKN | mg/L | 15 | 30 | 56 | 4 | 28 | 93 |
| Ammonia | NH3-N | mg/L | 6.0 | 17.1 | 27.4 | 2.5 | 17.4 | 32.7 |
| Nitrite Nitrate | NO2-N + NO3-N | mg/L | <0.25 | <2.19 | 5.08 | <0.25 | <0.87 | 2.75 |
| Phosphorous | P-TOT | mg/L | 2.43 | 6.37 | 11.20 | 0.93 | 4.35 | 19.15 |
| Phosphorous | P-SOL | mg/L | 1.12 | 3.98 | 5.17 | 0.37 | 2.11 | 3.88 |
| Cyanides | CN | mg/L | <0.005 | <0.006 | 0.017 | <0.005 | <0.005 | 0.014 |
| Amenable, Cyanide | CN AM | mg/L | Data Not Available | <0.001 | <0.001 | 0.001 | ||
| Phenol | Phenol | ug/L | 0.4 | 0.6 | 0.7 | <5 | <26 | 67 |
| Fluoride | F | mg/L | 6 | 26 | 46 | 0.3 | 0.6 | 0.8 |
| Total Organic Carbon | TOC | mg/L | 43 | 95 | 180 | 67 | 97 | 126 |
| Arsenic | As | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | 0.002 |
| Barium | Ba | mg/L | 0.037 | 0.053 | 0.135 | 0.039 | 0.061 | 0.104 |
| Cadmium | Cd | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.002 | 0.008 |
| Chromium | Cr | mg/L | <0.004 | <0.004 | 0.015 | <0.004 | <0.004 | 0.011 |
| Copper | Cu | mg/L | 0.027 | 0.067 | 0.288 | 0.016 | 0.068 | 0.116 |
| Iron | Fe | mg/L | 0.40 | 0.93 | 4.17 | 0.36 | 1.02 | 3.32 |
| Soluble Iron | Sol Fe | mg/L | 0.08 | 0.17 | 0.31 | 0.09 | 0.19 | 0.29 |
| Lead | Pb | mg/L | <0.002 | <0.002 | 0.010 | <0.002 | <0.003 | 0.010 |
| Manganese | Mn | mg/L | 0.050 | 0.073 | 0.113 | 0.041 | 0.069 | 0.126 |
| Mercury | Hg | ug/L | <0.5 | <0.4 | <0.5 | <0.5 | <0.4 | <0.5 |
| Nickel | Ni | mg/L | 0.004 | 0.009 | 0.022 | 0.004 | 0.014 | 0.038 |
| Selenium | Se | mg/L | <0.004 | <0.003 | 0.004 | <0.004 | <0.003 | 0.004 |
| Silver | Ag | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | 0.006 |
| Zinc | Zn | mg/L | 0.043 | 0.109 | 0.436 | 0.031 | 0.112 | 0.251 |
| Antimony | Sb | mg/L | <0.002 | <0.002 | 0.002 | <0.002 | <0.002 | 0.002 |
| Beryllium | Be | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Thallium | Tl | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium(6+) | Cr6 | ug/L | <3 | <2 | <3 | <3 | <2 | <3 |
| Sulphate | SO4 | mg/L | Data Not Available | 35 | 78 | 93 | ||
| Chloride | Cl | mg/L | 128.28 | 209.12 | 476.71 | 106.24 | 236.11 | 1084.30 |
| (d) | ||||||||
| Parameter | Symbol | Unit | Hanover Park | Lemont | ||||
| Min | Avg | Max | Min | Avg | Max | |||
| Flow | MGD | Data Not Available | Data Not Available | |||||
| Acidity | pH | 6.3 | 7.0 | 7.7 | 6.9 | 7.4 | 7.8 | |
| Biological Oxygen Demand | BOD5 | mg/L | 27 | 187 | 916 | 41 | 176 | 379 |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | Not Reported | 22 | 103 | 242 | ||
| Fat, Oil, and Grease | FOG | mg/L | 14 | 42 | 71 | <5 | <15 | 34 |
| Suspended Solids | SS | mg/L | 30 | 145 | 729 | 35 | 298 | 9300 |
| Volatile SS | VSS | mg/L | Data Not Available | 25 | 220 | 1832 | ||
| Total Solids | TS | mg/L | 598 | 796 | 1474 | 674 | 1423 | 9678 |
| Total Volatile Solids | VTS | mg/L | Data Not Available | 136 | 401 | 7722 | ||
| Total Kjeldahl Nitrogen | TKN | mg/L | 12 | 40 | 83 | 11 | 36 | 66 |
| Ammonia | NH3-N | mg/L | 7.2 | 27.0 | 36.9 | 5.7 | 18.5 | 31.7 |
| Nitrite Nitrate | NO2-N + NO3-N | mg/L | <0.25 | <0.27 | 4.74 | <0.25 | <0.63 | 4.14 |
| Phosphorous | P-TOT | mg/L | 1.85 | 5.85 | 13.24 | 1.45 | 5.53 | 11.91 |
| Phosphorous | P-SOL | mg/L | 2.07 | 3.73 | 4.79 | Data Not Available | ||
| Cyanides | CN | mg/L | <0.005 | <0.006 | 0.015 | <0.005 | <0.010 | 0.031 |
| Amenable, Cyanide | CN AM | mg/L | <0.001 | <0.001 | 0.001 | Data Not Available | ||
| Phenol | Phenol | ug/L | 7 | 37 | 64 | <5 | <11 | 19 |
| Fluoride | F | mg/L | 0.3 | 0.6 | 0.7 | 0.5 | 0.9 | 1.0 |
| Total Organic Carbon | TOC | mg/L | 32 | 128 | 314 | Data Not Available | ||
| Arsenic | As | mg/L | <0.002 | <0.001 | 0.002 | <0.002 | <0.001 | 0.003 |
| Barium | Ba | mg/L | 0.035 | 0.049 | 0.084 | 0.042 | 0.064 | 0.138 |
| Cadmium | Cd | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium | Cr | mg/L | <0.004 | <0.003 | 0.004 | <0.004 | <0.003 | 0.009 |
| Copper | Cu | mg/L | 0.018 | 0.049 | 0.122 | 0.023 | 0.084 | 0.310 |
| Iron | Fe | mg/L | 0.32 | 0.66 | 1.99 | 0.27 | 1.09 | 3.52 |
| Soluble Iron | Sol Fe | mg/L | 0.09 | 0.16 | 0.22 | 0.05 | 0.13 | 0.18 |
| Lead | Pb | mg/L | <0.002 | <0.002 | 0.005 | <0.002 | <0.003 | 0.009 |
| Manganese | Mn | mg/L | 0.040 | 0.071 | 0.128 | 0.020 | 0.066 | 0.148 |
| Mercury | Hg | ug/L | <0.5 | <0.4 | <0.5 | <0.5 | <0.4 | 0.7 |
| Nickel | Ni | mg/L | 0.002 | 0.003 | 0.005 | 0.002 | 0.003 | 0.009 |
| Selenium | Se | mg/L | <0.004 | <0.003 | 0.004 | <0.004 | <0.003 | <0.004 |
| Silver | Ag | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 |
| Zinc | Zn | mg/l | 0.035 | 0.090 | 0.235 | 0.031 | 0.148 | 0.558 |
| Antimony | Sb | mg/L | <0.002 | <0.002 | 0.003 | <0.002 | <0.002 | 0.002 |
| Beryllium | Be | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Thallium | Tl | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 |
| Chromium(6+) | Cr6 | ug/L | <3 | <2 | <3 | <3 | <2 | <3 |
| Sulphate | SO4 | mg/L | Data Not Available | Data Not Available | ||||
| Chloride | Cl | mg/L | 90.93 | 169.40 | 474.69 | Data Not Available | ||
| Water Reclamation Plant | Primary | Secondary | Filtration | Disinfection |
|---|---|---|---|---|
| Stickney | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | ✕ | ✕ |
| O‘Brien | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | ✕ | UV Disinfection |
| Calumet | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | ✕ | Chlorination/ Dechlorination |
| Egan | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | Sand and Anthracite | Chlorination/ Dechlorination |
| Kirie | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | Sand and Anthracite | Chlorination/ Dechlorination |
| Hanover Park | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | Sand and Anthracite | Chlorination/ Dechlorination |
| Lemont | Coarse Screen Aerated Grit Tanks Preliminary Settling Tanks | Activated Sludge Aeration Tanks Final Settling Tanks | ✕ | ✕ |
| (a) | |||||||||
| Parameter | Symbol | Unit | Stickney | ||||||
| Min | Mean | Max | |||||||
| Flow | MGD | 260 | 595 | 1267 | |||||
| Acidity | pH | 6.7 | 7.1 | 7.4 | |||||
| Dissolved Oxygen | DO | mg/L | 5.3 | 8.3 | 10.5 | ||||
| Temperature | Temp. | °C | 8.5 | 17.3 | 25.3 | ||||
| Biological Oxygen Demand | BOD5 | mg/L | <2 | <6 | 16 | ||||
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | <2 | <2 | 6 | ||||
| Total Solids | TS | mg/L | 480 | 697 | 1031 | ||||
| Total Volatile Solids | TVS | mg/L | 28 | 189 | 540 | ||||
| Suspended Solids | SS | mg/L | <4 | <5 | 15 | ||||
| Total Kjeldahl Nitrogen | TKN | mg/L | 1 | 2 | 6 | ||||
| Ammonia | NH3-N | mg/L | <0.3 | <0.7 | 4.5 | ||||
| Unionized Ammonia | Un-ionNH3-N | mg/L | 0 | 0 | 0 | ||||
| Nitrite Nitrate | NO2 + NO3-N | mg/L | 2.21 | 7.64 | 13.48 | ||||
| Phosphorous | P-Tot | mg/L | 0.16 | 0.76 | 4.53 | ||||
| Phosphorous | P-Sol | mg/L | Data Not Available | ||||||
| Chloride | Cl | mg/L | 112.41 | 450.21 | 1967.00 | ||||
| Fluoride | F | mg/L | 0.3 | 0.5 | 0.6 | ||||
| Fats, Oils, and Greases | FOG | mg/L | <5 | <4 | <5 | ||||
| Phenol | Phenol | ug/L | <5 | <4 | 12 | ||||
| Fecal Coliform | Fecal col. | cfu/100 mL | 1100 | GM = 7790 | 37,000 | ||||
| Sulphate | SO4 | mg/L | 48 | 89 | 113 | ||||
| Cyanides | CN | mg/L | <0.005 | <0.006 | 0.019 | ||||
| Arsenic | As-Tot | mg/L | <0.002 | <0.002 | 0.002 | ||||
| Barium | Ba-Tot | mg/L | 0.009 | 0.020 | 0.046 | ||||
| Cadmium | Cd-Tot | mg/L | <0.002 | <0.001 | <0.002 | ||||
| Chromium | Cr-Tot | mg/L | <0.004 | <0.003 | <0.004 | ||||
| Copper | Cu-Tot | mg/L | <0.002 | <0.003 | 0.022 | ||||
| Iron | Fe-Tot | mg/L | 0.04 | 0.07 | 0.13 | ||||
| Lead | Pb-Tot | mg/L | <0.002 | <0.001 | <0.002 | ||||
| Manganese | Mn-Tot | mg/L | 0.004 | 0.015 | 0.032 | ||||
| Iron | Fe-Sol | mg/L | 0.02 | 0.03 | 0.04 | ||||
| Mercury | Hg | mg/L | <0.5 | <1.1 | 3.0 | ||||
| Mercury LL | HgLL | mg/L | Data Not Available | ||||||
| Nickel | Ni-Tot | mg/L | 0.003 | 0.008 | 0.019 | ||||
| Selenium | Se-Tot | mg/L | <0.004 | <0.003 | <0.004 | ||||
| Silver | Ag-Tot | mg/L | <0.004 | <0.003 | <0.004 | ||||
| Zinc | Zn-Tot | mg/L | <0.010 | <0.021 | 0.050 | ||||
| Antimony | Sb-Tot | mg/L | <0.002 | <0.001 | 0.002 | ||||
| Beryllium | Be-Tot | mg/L | <0.002 | <0.001 | <0.002 | ||||
| Thallium | Tl-Tot | mg/L | <0.002 | <0.001 | <0.002 | ||||
| Chromium(6+) | Cr+6-Tot | ug/L | <3 | <2 | <3 | ||||
| Calcium | Ca-Tot | mg/L | 38.01 | 63.63 | 101.60 | ||||
| Magnesium | Mg-Tot | mg/L | 11.59 | 21.11 | 33.73 | ||||
| Hardness | Hardness | mg/L | 143 | 246 | 387 | ||||
| Amenable, Cyanide | AMENCN | mg/L | <0.005 | <0.004 | <0.005 | ||||
| Total Organic Carbon | TOC | mg/L | Data Not Available | ||||||
| Total Nitrogen | Tot_N | mg/L | 4 | 9 | 14 | ||||
| (b) | |||||||||
| Parameter | Symbol | Unit | Calumet | O’Brien | |||||
| Min | Mean | Max | Min | Mean | Max | ||||
| Flow | MGD | 105 | 239 | 452 | 98 | 199 | 427 | ||
| Acidity | pH | 6.8 | 7.2 | 7.5 | 6.8 | 7.1 | 7.2 | ||
| Dissolved Oxygen | DO | mg/L | 5.5 | 7.8 | 9.9 | 7.5 | 9.2 | 11.5 | |
| Temperature | Temp. | °C | 8 | 15 | 22 | Data Not Available | |||
| Biological Oxygen Demand | BOD5 | mg/L | <2 | <7 | 21 | <2 | <6 | 29 | |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | <2 | <3 | 7 | <2 | <2 | 13 | |
| Total Solids | TS | mg/L | 520 | 864 | 2512 | Data Not Available | |||
| Total Volatile Solids | TVS | mg/L | 46 | 148 | 414 | Data Not Available | |||
| Suspended Solids | SS | mg/L | <4 | <5 | 11 | 2 | 4 | 13 | |
| Total Kjeldahl Nitrogen | TKN | mg/L | <1 | <1 | 4 | <1 | <1 | <4 | |
| Ammonia | NH3-N | mg/L | <0.3 | <0.4 | 2.8 | <0.3 | <0.5 | 2.3 | |
| Unionized Ammonia | Un-ionNH3-N | mg/L | Data Not Available | Data Not Available | |||||
| Nitrite Nitrate | NO2 + NO3-N | mg/L | 3.91 | 8.14 | 17.06 | 4.72 | 9.44 | 14.15 | |
| Phosphorous | P-Tot | mg/L | 1.16 | 3.64 | 7.39 | 0.49 | 1.80 | 2.90 | |
| Phosphorous | P-Sol | mg/L | 1.05 | 3.52 | 7.14 | 1.01 | 1.97 | 2.55 | |
| Chloride | Cl | mg/L | 115.18 | 352.50 | 1273.03 | 100.95 | 346.85 | 2014.83 | |
| Fluoride | F | mg/L | 0.4 | 0.5 | 0.6 | 0.3 | 0.5 | 0.6 | |
| Fats, Oils, and Greases | FOG | mg/L | <5 | <4 | <5 | <5 | <4 | 5 | |
| Phenol | Phenol | ug/L | <5 | <4 | 9 | <5 | <4 | 5 | |
| Fecal Coliform | Fecalcol. | cfu/100 mL | <10 | GM 15 | 57,000 | <10 | GM ≤ 66 | 52,000 | |
| Sulphate | SO4 | mg/L | Data Not Available | Data Not Available | |||||
| Cyanides | CN | mg/L | <0.005 | <0.006 | 0.023 | <0.005 | <0.005 | 0.025 | |
| Arsenic | As-Tot | mg/L | 0.002 | 0.003 | 0.004 | <0.002 | <0.001 | <0.002 | |
| Barium | Ba-Tot | mg/L | 0.011 | 0.021 | 0.040 | 0.018 | 0.027 | 0.052 | |
| Cadmium | Cd-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium | Cr-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Copper | Cu-Tot | mg/L | 0.002 | 0.003 | 0.008 | 0.003 | 0.005 | 0.008 | |
| Iron | Fe-Tot | mg/L | 0.06 | 0.10 | 0.13 | 0.04 | 0.05 | 0.09 | |
| Lead | Pb-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Manganese | Mn-Tot | mg/L | 0.010 | 0.031 | 0.091 | 0.005 | 0.010 | 0.044 | |
| Iron | Fe-Sol | mg/L | 0.03 | 0.04 | 0.08 | 0.02 | 0.03 | 0.04 | |
| Mercury | Hg | mg/L | Data Not Available | Data Not Available | |||||
| Mercury LL | HgLL | mg/L | <0.5 | <4.4 | 20.8 | <0.5 | <21.7 | 624.3 | |
| Nickel | Ni-Tot | mg/L | 0.002 | 0.004 | 0.009 | 0.002 | 0.009 | 0.144 | |
| Selenium | Se-Tot | mg/L | <0.004 | <0.003 | 0.004 | <0.004 | <0.003 | 0.004 | |
| Silver | Ag-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Zinc | Zn-Tot | mg/L | 0.011 | 0.018 | 0.027 | 0.017 | 0.028 | 0.047 | |
| Antimony | Sb-Tot | mg/L | <0.002 | <0.001 | 0.002 | <0.002 | <0.001 | 0.002 | |
| Beryllium | Be-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Thallium | Tl-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium(6+) | Cr+6-Tot | ug/L | <3 | <2 | <3 | <3 | <2 | <3 | |
| Calcium | Ca-Tot | mg/L | 45.72 | 73.22 | 106.22 | 38.32 | 59.78 | 99.34 | |
| Magnesium | Mg-Tot | mg/L | 14.90 | 25.24 | 40.73 | 12.48 | 20.87 | 33.37 | |
| Hardness | Hardness | mg/L | 179 | 287 | 411 | 147 | 235 | 381 | |
| Amenable, Cyanide | AMENCN | mg/L | <0.005 | <0.004 | <0.005 | <0.005 | <0.004 | <0.005 | |
| Total Organic Carbon | TOC | mg/L | 6 | 9 | 12 | 5 | 7 | 24 | |
| Total Nitrogen | Tot_N | mg/L | 5 | 9 | 23 | <8 | <11 | <17 | |
| (c) | |||||||||
| Parameter | Symbol | Unit | Egan | Kirie | |||||
| Min | Mean | Max | Min | Mean | Max | ||||
| Flow | MGD | 16.3 | 21.8 | 50.7 | 14.32 | 32.35 | 116.93 | ||
| Acidity | pH | 7.0 | 7.2 | 7.4 | 7.0 | 7.4 | 7.8 | ||
| Dissolved Oxygen | DO | mg/L | 6.3 | 6.9 | 8.5 | 6.6 | 8.0 | 9.1 | |
| Temperature | Temp. | °C | 46 | 61 | 73 | ||||
| Biological Oxygen Demand | BOD5 | mg/L | 4 | 5 | 12 | <2 | <3 | 9 | |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | <2 | <2 | 6 | <2 | <2 | 4 | |
| Total Solids | TS | mg/L | Data Not Available | Data Not Available | |||||
| Total Volatile Solids | TVS | mg/L | Data Not Available | Data Not Available | |||||
| Suspended Solids | SS | mg/L | 2 | 3 | 6 | <2 | <2 | 24 | |
| Total Kjeldahl Nitrogen | TKN | mg/L | <1 | <2 | <5 | <1 | <1 | 4 | |
| Ammonia | NH3-N | mg/L | <0.3 | <0.3 | 1.9 | <0.3 | <0.3 | 3.4 | |
| Unionized Ammonia | Un-ionNH3-N | mg/L | Data Not Available | Data Not Available | |||||
| Nitrite Nitrate | NO2 + NO3-N | mg/L | 5.86 | 17.37 | 22.76 | 3.50 | 8.13 | 12.20 | |
| Phosphorous | P-Tot | mg/L | 0.42 | 3.42 | 5.10 | 0.16 | 0.67 | 2.13 | |
| Phosphorous | P-Sol | mg/L | 0.60 | 3.17 | 4.34 | <0.15 | <0.72 | 2.09 | |
| Chloride | Cl | mg/L | 133.59 | 207.08 | 478.00 | 107.28 | 248.75 | 695.32 | |
| Fluoride | F | mg/L | 0.3 | 0.5 | 0.6 | 0.3 | 0.5 | 0.8 | |
| Fats, Oils, and Greases | FOG | mg/L | <5 | <4 | <5 | <5 | <4 | <5 | |
| Phenol | Phenol | ug/L | <5 | <4 | 7 | <5 | <4 | 23 | |
| Fecal Coliform | Fecal col. | cfu/100 mL | <10 | GM 15 | 7500 | <10 | GM 7 | 90 | |
| Sulphate | SO4 | mg/L | Data Not Available | 42 | 77 | 92 | |||
| Cyanides | CN | mg/L | <0.005 | <0.005 | 0.011 | <0.005 | <0.004 | 0.012 | |
| Arsenic | As-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Barium | Ba-Tot | mg/L | 0.018 | 0.024 | 0.048 | 0.015 | 0.027 | 0.053 | |
| Cadmium | Cd-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium | Cr-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Copper | Cu-Tot | mg/L | 0.004 | 0.008 | 0.013 | 0.002 | 0.004 | 0.008 | |
| Iron | Fe-Tot | mg/L | 0.05 | 0.07 | 0.09 | 0.04 | 0.05 | 0.08 | |
| Lead | Pb-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Manganese | Mn-Tot | mg/L | 0.007 | 0.015 | 0.049 | 0.010 | 0.015 | 0.038 | |
| Iron | Fe-Sol | mg/L | 0.04 | 0.06 | 0.10 | 0.03 | 0.04 | 0.05 | |
| Mercury | Hg | mg/L | 0.6 | 1.1 | 5.3 | Data Not Available | |||
| Mercury | HgLL | mg/L | Data Not Available | <0.5 | <8.6 | 170.2 | |||
| Nickel | Ni-Tot | mg/L | 0.002 | 0.004 | 0.008 | 0.003 | 0.006 | 0.012 | |
| Selenium | Se-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | 0.004 | |
| Silver | Ag-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Zinc | Zn-Tot | mg/L | 0.016 | 0.025 | 0.031 | 0.011 | 0.029 | 0.058 | |
| Antimony | Sb-Tot | mg/L | <0.002 | <0.001 | 0.002 | <0.002 | <0.001 | 0.002 | |
| Beryllium | Be-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Thallium | Tl-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium(6+) | Cr+6-Tot | ug/L | <3 | <2 | <3 | <3 | <2 | <3 | |
| Calcium | Ca-Tot | mg/L | 55.00 | 67.85 | 102.80 | 38.53 | 74.97 | 107.17 | |
| Magnesium | Mg-Tot | mg/L | 20.72 | 25.76 | 37.09 | 13.12 | 27.70 | 39.42 | |
| Hardness | Hardness | mg/L | 223 | 275 | 409 | 150 | 301 | 430 | |
| Amenable, Cyanide | AMEN CN | mg/L | Data Not Available | Data Not Available | |||||
| Total Organic Carbon | TOC | mg/L | 7 | 10 | 19 | 6 | 7 | 10 | |
| Total Nitrogen | Tot_N | mg/L | Data Not Available | Data Not Available | |||||
| (d) | |||||||||
| Parameter | Symbol | Unit | Hanover Park | Lemont | |||||
| Min | Mean | Max | Min | Mean | Max | ||||
| Flow | MGD | 3.59 | 6.44 | 13.64 | 1.33 | 2.43 | 4.21 | ||
| Acidity | pH | 6.5 | 6.8 | 7.2 | 7 | 7.3 | 7.6 | ||
| Dissolved Oxygen | DO | mg/L | 7.4 | 9.7 | 12.3 | 2 | 5.2 | 8.5 | |
| Temperature | Temp. | °C | 11 | 45 | 74 | ||||
| Biological Oxygen Demand | BOD5 | mg/L | <2 | <6 | 20 | <2 | <9 | 57 | |
| Five-day Carbonaceous Biochemical Oxygen Demand | CBOD5 | mg/L | <2 | <3 | 7 | <2 | <4 | 16 | |
| Total Solids | TS | mg/L | Data Not Available | 672 | 1179 | 2584 | |||
| Total Volatile Solids | TVS | mg/L | Data Not Available | 86 | 198 | 428 | |||
| Suspended Solids | SS | mg/L | <2 | <4 | 18 | <4 | <6 | 38 | |
| Total Kjeldahl Nitrogen | TKN | mg/L | <1 | <2 | <5 | <1 | <2 | 6 | |
| Ammonia | NH3-N | mg/L | <0.3 | <0.3 | 1.6 | <1.5 | <0.6 | 3.7 | |
| Unionized Ammonia | Un-ionNH3-N | mg/L | Data Not Available | Data Not Available | |||||
| Nitrite Nitrate | NO2 + NO3-N | mg/L | 7.4 | 18.89 | 25.5 | 5.12 | 16.75 | 25.07 | |
| Phosphorous | P-Tot | mg/L | 1.08 | 3.58 | 4.9 | <0.15 | <3.01 | 4.3 | |
| Phosphorous | P-Sol | mg/L | 1.01 | 3.53 | 4.72 | ||||
| Chloride | Cl | mg/L | 96.06 | 165.4 | 393.84 | 156.09 | 409.62 | 1263.34 | |
| Fluoride | F | mg/L | 0.3 | 0.5 | 0.6 | 0.4 | 0.8 | 1 | |
| Fats, Oils, and Greases | FOG | mg/L | <5 | <4 | 5 | <5 | <4 | <5 | |
| Phenol | Phenol | ug/L | <5 | <4 | 7 | <5 | <4 | 12 | |
| Fecal Coliform | Fecal col. | cfu/100 mL | <10 | GM 9 | 40 | 910 | GM 11,805 | 220,000 | |
| Sulphate | SO4 | mg/L | Data Not Available | Data Not Available | |||||
| Cyanides | CN | mg/L | <0.005 | <0.005 | 0.01 | <0.005 | <0.006 | 0.016 | |
| Arsenic | As-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Barium | Ba-Tot | mg/L | 0.022 | 0.03 | 0.056 | 0.03 | 0.039 | 0.059 | |
| Cadmium | Cd-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium | Cr-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Copper | Cu-Tot | mg/L | 0.004 | 0.007 | 0.011 | 0.004 | 0.009 | 0.026 | |
| Iron | Fe-Tot | mg/L | 0.04 | 0.05 | 0.12 | 0.05 | 0.08 | 0.28 | |
| Lead | Pb-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Manganese | Mn-Tot | mg/L | 0.005 | 0.018 | 0.053 | 0.005 | 0.015 | 0.056 | |
| Iron | Fe-Sol | mg/L | 0.03 | 0.04 | 0.05 | 0.04 | 0.05 | 0.06 | |
| Mercury | Hg | mg/L | Data Not Available | Data Not Available | |||||
| Mercury | HgLL | mg/L | <0.5 | <1.0 | 2.4 | <0.5 | <1.0 | 2.5 | |
| Nickel | Ni-Tot | mg/L | <0.002 | <0.002 | 0.004 | <0.002 | <0.001 | 0.002 | |
| Selenium | Se-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | 0.005 | |
| Silver | Ag-Tot | mg/L | <0.004 | <0.003 | <0.004 | <0.004 | <0.003 | <0.004 | |
| Zinc | Zn-Tot | mg/L | 0.026 | 0.044 | 0.061 | 0.017 | 0.033 | 0.066 | |
| Antimony | Sb-Tot | mg/L | <0.002 | <0.001 | 0.002 | <0.002 | <0.001 | <0.002 | |
| Beryllium | Be-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Thallium | Tl-Tot | mg/L | <0.002 | <0.001 | <0.002 | <0.002 | <0.001 | <0.002 | |
| Chromium(6+) | Cr+6-Tot | ug/L | <3 | <2 | <3 | <3 | <2 | <3 | |
| Calcium | Ca-Tot | mg/L | 49.99 | 64.66 | 102.82 | 65.65 | 91.57 | 120.32 | |
| Magnesium | Mg-Tot | mg/L | 17.83 | 24.46 | 38.17 | 25.15 | 32.49 | 38.38 | |
| Hardness | Hardness | mg/L | 199 | 262 | 412 | 267 | 362 | 458 | |
| Amenable, Cyanide | AMEN CN | mg/L | Data Not Available | Data Not Available | |||||
| Total Organic Carbon | TOC | mg/L | 9 | 12 | 17 | Data Not Available | |||
| Total Nitrogen | Tot_N | mg/L | Data Not Available | Data Not Available | |||||
| Water Parameter | Limit | Stickney | Calumet | O’Brien | Egan | Kirie | Hanover Park | Lemont |
|---|---|---|---|---|---|---|---|---|
| Urban Reuse (Unrestricted): The use of reclaimed water for non-potable applications in municipal settings where public access is not restricted (toilet flushing, air conditioning, irrigation of parks, residential landscaping, school yards, etc.) | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤10 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| NTU | ≤2 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fecal Coli (cfu/100 mL) | None | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Urban Reuse (Restricted): The use of reclaimed water where public exposure is controlled. Irrigation of areas such as highway median, and subsurface irrigation | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Agricultural Reuse (Food Crops): The use of reclaimed water to irrigate food crops that are intended to be eaten raw | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤10 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| NTU | ≤2 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fecal Coli (cfu/100 mL) | None | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Agricultural Reuse (Processed Food Crops and Non-Food Crops): The use of reclaimed water to irrigate crops that are processed before consumed, and irrigation of non-food crops (seed crops, industrial crops, processed food crops, fodder crops, orchard crops, etc. | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Impoundments (Unrestricted): The use of reclaimed water in an impoundment in which no limitations are imposed on body-contact water recreation activities (some states categorize snowmaking) | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤10 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| NTU | ≤2 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fecal Coli (cfu/100 mL) | None | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Impoundments (Restricted): The use of reclaimed water in an impoundment where body contact is restricted (some states include fishing and boating) | ||||||||
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Environmental Reuse: The use of reclaimed water to create, enhance, sustain, or augment water bodies, including wetlands, aquatic habitats, or stream flow | ||||||||
| Variable but not exceed: | ||||||||
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Industrial Reuse: The use of reclaimed water in industrial applications and facilities, power production, and extraction of fossil fuels | ||||||||
| Industrial Reuse (Once-through Cooling) | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Industrial Reuse (Recirculating Cooling Towers) | ||||||||
| pH | 6.0–9.0 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| BOD (mg/L) | ≤30 | <6 | <7 | <6 | 5 | <3 | <6 | <9 |
| TSS (mg/L) | ≤30 | <5 | <5 | 4.0 | 3.0 | <2 | <4 | <6 |
| Fecal Coli (cfu/100 mL) | ≤200 | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Groundwater Recharge (Non-potable Reuse): | ||||||||
| Site Specific | ||||||||
| Groundwater Recharge (Indirect Potable Reuse) | ||||||||
| pH | 6.5–8.5 | 7.1 | 7.2 | 7.1 | 7.2 | 7.4 | 6.8 | 7.3 |
| TOC (mg/L) | ≤2 | N/A | 9 | 7 | 10 | 7 | 12 | N/A |
| NTU | ≤2 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fecal Coli (cfu/100 mL) | None | GM = 7790 | GM 15 | GM 66 | GM 15 | GM 7 | GM 9 | GM 11,805 |
| Chlorine (mg/L) | 1 (min) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Meet Drinking Water Standards | ||||||||
| Reuse Category | Stickney | Calumet | O’Brien | Egan | Kirie | Hanover Park | Lemont |
|---|---|---|---|---|---|---|---|
| Urban Reuse (Unrestricted) | |||||||
| Urban Reuse (Restricted) | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Agricultural Reuse (Food Crops) | |||||||
| Agricultural Reuse (Processed Food Crops and Non-Food Crops) | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Impoundments (Unrestricted) | |||||||
| Impoundments (Restricted) | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Environmental Reuse | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Industrial Reuse (Once-through Cooling) | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Industrial Reuse (Recirculating Cooling Towers) | ♦ | ♦ | ♦ | ♦ | ♦ | ||
| Groundwater Recharge (Non-potable Reuse) | Site Specific | ||||||
| Groundwater Recharge (Potable Reuse) | Meet Drinking Water Standards | ||||||
| Facility | Unit | Min | Avg. | Max |
|---|---|---|---|---|
| Stickney WRP | mg/L | 476 | 692 | 1016 |
| Calumet WRP | 440 | 793 | 2501 | |
| Lemont WRP | 624 | 1147 | 2546 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, K.R.; Kandou, V.; Havrelock, R.; El-Khattabi, A.R.; Cordova, T.; Wilson, M.D.; Nelson, B.; Trujillo, C. Reuse of Treated Wastewater: Drivers, Regulations, Technologies, Case Studies, and Greater Chicago Area Experiences. Sustainability 2023, 15, 7495. https://doi.org/10.3390/su15097495
Reddy KR, Kandou V, Havrelock R, El-Khattabi AR, Cordova T, Wilson MD, Nelson B, Trujillo C. Reuse of Treated Wastewater: Drivers, Regulations, Technologies, Case Studies, and Greater Chicago Area Experiences. Sustainability. 2023; 15(9):7495. https://doi.org/10.3390/su15097495
Chicago/Turabian StyleReddy, Krishna R., Valeria Kandou, Rachel Havrelock, Ahmed Rachid El-Khattabi, Teresa Cordova, Matthew D. Wilson, Braeden Nelson, and Citlalli Trujillo. 2023. "Reuse of Treated Wastewater: Drivers, Regulations, Technologies, Case Studies, and Greater Chicago Area Experiences" Sustainability 15, no. 9: 7495. https://doi.org/10.3390/su15097495
APA StyleReddy, K. R., Kandou, V., Havrelock, R., El-Khattabi, A. R., Cordova, T., Wilson, M. D., Nelson, B., & Trujillo, C. (2023). Reuse of Treated Wastewater: Drivers, Regulations, Technologies, Case Studies, and Greater Chicago Area Experiences. Sustainability, 15(9), 7495. https://doi.org/10.3390/su15097495







