Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta)
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Collection and Preparation
2.2. Experimental Setup
2.3. Measurements of Nitrate and Ammonium Uptake Rates
2.4. Measurements of Relative Growth Rates
2.5. Measurements of Total Nitrogen
2.6. Measurement of Soluble Protein
2.7. Measurements of Chlorophyll a (Chl a) and Phycoerythrin (PE)
2.8. Data Analysis
3. Results
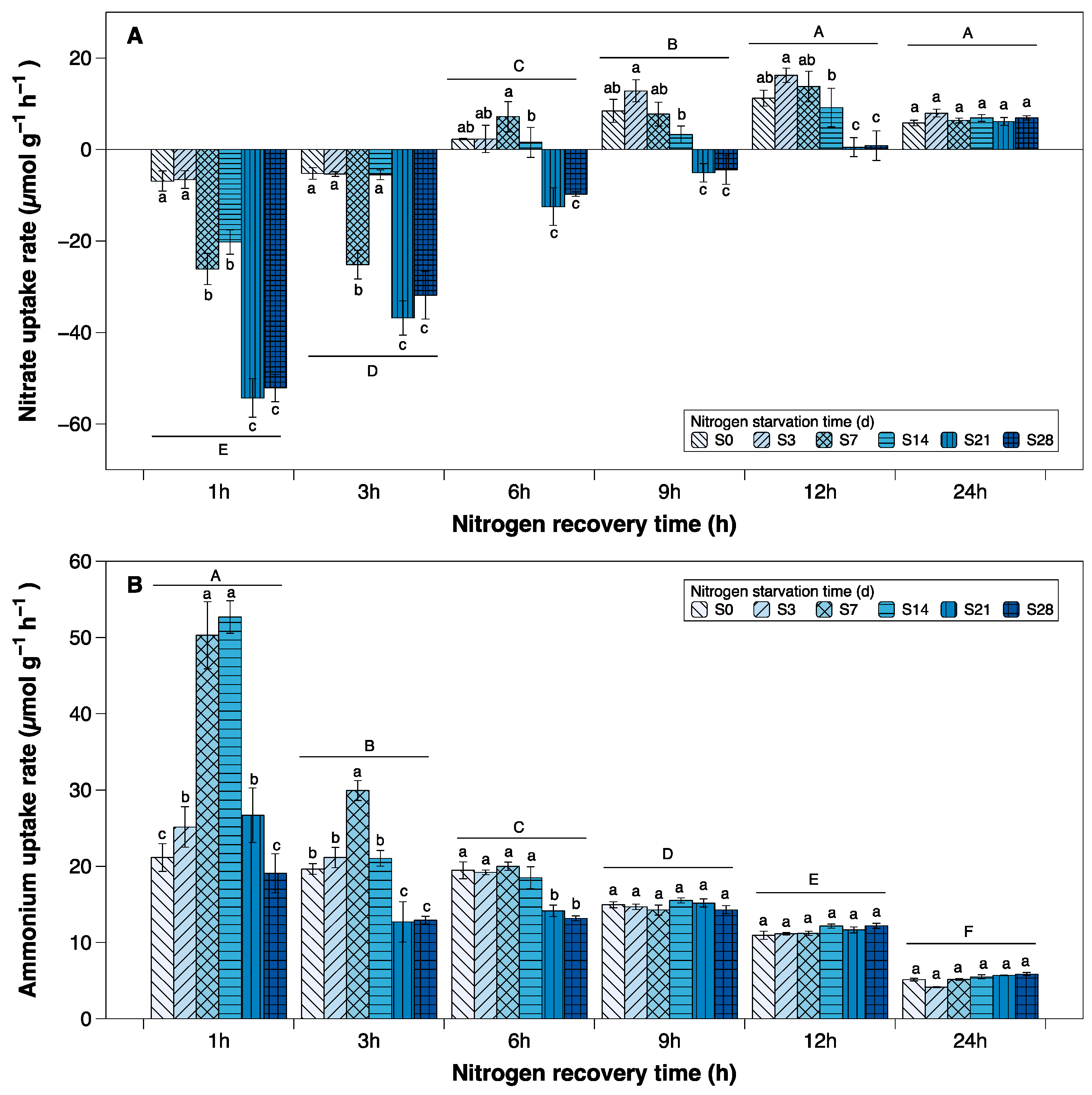
3.1. Uptake Rates of Nitrate and Ammonium
3.2. Relative Growth Rates (RGRs)
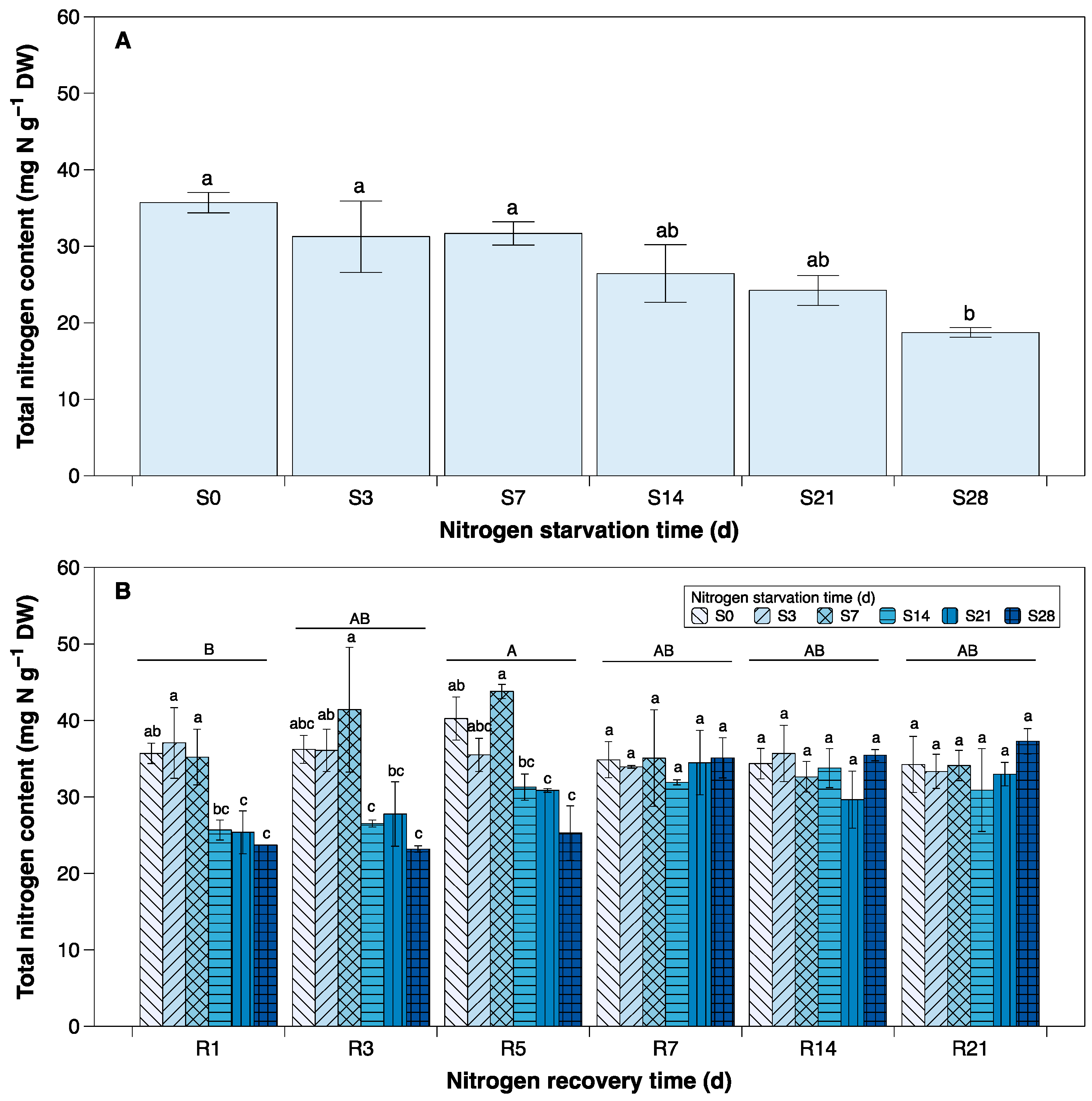
3.3. Total Nitrogen
3.4. Soluble Protein
3.5. Chlorophyll a (Chl a) and Phycoerythrin (PE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; 551p. [Google Scholar]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Young, E.B.; Dring, M.J.; Savidge, G.; Birkett, D.A.; Berges, J.A. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant Cell Environ. 2007, 30, 764–774. [Google Scholar] [CrossRef] [PubMed]
- McGlathery, K.J.; Pedersen, M.F.; Borum, J. Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorphy linum (Chlorophyta). J. Phycol. 1996, 32, 393–401. [Google Scholar] [CrossRef]
- Naldi, M.; Viaroli, P. Nitrate uptake and storage in the seaweed Ulva rigida C. Agardh in relation to nitrate availability and thallus nitrate content in a eutrophic coastal lagoon (Sacca di Goro, Po River Delta, Italy). J. Exp. Mar. Biol. Ecol. 2002, 269, 65–83. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Borum, J. Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. M. Ecol. Prog. Ser. 1996, 142, 261–272. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hori, Y.; Tanida, K.; Imai, I. Population dynamics of the harmful diatom Eucampia zodiacus Ehrenberg causing bleachings of Porphyra thalli in aquaculture in Harima-Nada, the Seto Inland Sea, Japan. Harmful Algae 2007, 6, 763–773. [Google Scholar] [CrossRef]
- Martín, L.A.; Rodríguez, M.C.; Matulewicz, M.C.; Fissore, E.N.; Gerschenson, L.N.; Leonardi, P.I. Seasonal variation in agar composition and properties from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonian coast of Argentina. Phycol. Res. 2013, 61, 163–171. [Google Scholar] [CrossRef]
- Zhao, L.S.; Su, H.N.; Li, K.; Xie, B.B.; Liu, L.N.; Zhang, X.Y.; Chen, X.L.; Huang, F.; Zhou, B.C.; Zhang, Y.Z. Supramolecular architecture of photosynthetic membrane in red algae in response to nitrogen starvation. Biochim. Biophys. Acta 2016, 1857, 1751–1758. [Google Scholar] [CrossRef]
- Zhao, L.S.; Li, K.; Wang, Q.M.; Song, X.Y.; Su, H.N.; Xie, B.B.; Zhang, X.Y.; Huang, F.; Chen, X.L.; Zhou, B.C.; et al. Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci. Rep. 2017, 7, 8542. [Google Scholar] [CrossRef]
- Liu, X.; Wen, J.; Chen, W.; Du, H. Physiological effects of nitrogen deficiency and recovery on the macroalga Gracilariopsis lemaneiformis (Rhodophyta). J. Phycol. 2019, 55, 830–839. [Google Scholar] [CrossRef]
- Liu, X.; Wen, J.; Zheng, C.; Jia, H.; Chen, W.; Du, H. The impact of nitrogen deficiency and subsequent recovery on the photosynthetic performance of the red macroalga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2699–2707. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Yuan, C.; Li, T.; Li, A. Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply. J. Appl. Phycol. 2019, 31, 2797–2809. [Google Scholar] [CrossRef]
- Liefer, J.D.; Garg, A.; Campbell, D.A.; Irwin, A.J.; Finkel, Z.V. Nitrogen starvation induces distinct photosynthetic responses and recovery dynamics in diatoms and prasinophytes. PLoS ONE 2018, 13, e0195705. [Google Scholar] [CrossRef]
- Cullen, J.J.; Yang, X.; MacIntyre, H.L. Nutrient limitation of marine photosynthesis. In Primary Productivity and Biogeochemical Cycles in the Sea; Falkowski, P.G., Woodhead, A.D., Vivirto, K., Eds.; Springer: Boston, MA, USA, 1992; Volume 43, pp. 69–88. [Google Scholar]
- Li, C.; Ariga, I.; Mikami, K. Difference in nitrogen starvation-inducible expression patterns among phylogenetically diverse ammonium transporter genes in the red seaweed Pyropia yezoensis. Am. J. Plant Sci. 2019, 10, 1325–1349. [Google Scholar] [CrossRef]
- Schmollinger, S.; Muhlhaus, T.; Boyle, N.R.; Blaby, I.K.; Casero, D.; Mettler, T.; Moseley, J.L.; Kropat, J.; Sommer, F.; Strenkert, D.; et al. Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 2014, 26, 1410–1435. [Google Scholar] [CrossRef]
- Msanne, J.; Xu, D.; Konda, A.R.; Casas-Mollano, J.A.; Awada, T.; Cahoon, E.B.; Cerutti, H. Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 2012, 75, 50–59. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Y.; Li, L.; Yi, N.; Liu, Y.; Qaseem, M.F.; Li, H.; Wu, A.M. Physiological and transcriptomic responses to nitrogen deficiency in Neolamarckia cadamba. Front. Plant Sci. 2021, 12, 747121. [Google Scholar] [CrossRef]
- Jian, J.; Zeng, D.; Wei, W.; Lin, H.; Li, P.; Liu, W. The combination of RNA and protein profiling reveals the response to nitrogen depletion in Thalassiosira pseudonana. Sci. Rep. 2017, 7, 8989. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.; Yuan, C.; Zhang, Y.; Xu, J.; Zheng, H.; Xiang, W.; Li, A. Linking lipid accumulation and photosynthetic efficiency in Nannochloropsis sp. under nutrient limitation and replenishment. J. Appl. Phycol. 2020, 32, 1619–1630. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, P.; Feng, J.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. The roles of starch and lipid in Chlorella sp. during cell recovery from nitrogen starvation. Bioresour. Technol. 2018, 247, 58–65. [Google Scholar] [CrossRef]
- Valledor, L.; Furuhashi, T.; Recuenco-Munoz, L.; Wienkoop, S.; Weckwerth, W. System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol. Biofuels 2014, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Williams, E.; Wang, D.Z.; Xie, Z.X.; Hsia, R.C.; Jenck, A.; Halden, R.; Li, J.; Chen, F.; Place, A.R. Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol. 2013, 162, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Lin, Z.-X. NH4+-N over-compensatory uptake of Gracilaria lemaneiformis under the stress of nutrients deficiency. Oceanol. Limnol. Sin. 2005, 36, 307–312, (In Chinese with English Abstract). [Google Scholar]
- Li, D.-P.; Ma, Z.-L.; Li, H.; Ding, G.; Xin, M.-L.; Wu, H.-Y.; Guo, W. NH4+-N over-compensatory uptake of Sargassum horneri under the stress of nutrients deficiency. Oceanol. Limnol. Sin. 2018, 49, 904–909, (In Chinese with English Abstract). [Google Scholar]
- Xia, B.M. Flora Algarum Marinarum Sinicarum, Tomus II Rhodophyta No. III Gelidiales, Cryptonemiales, Hildenbrandiales; Science Press: Beijing, China, 2004; 203p. (In Chinese) [Google Scholar]
- Yoshida, T.; Suzuki, M.; Yoshinaga, K. Check list of marine algae of Japan (Revised in 2015). Jpn. J. Phycol. 2015, 63, 129–189. [Google Scholar]
- Lee, Y.P.; Kang, S.Y. A Catalogue of the Seaweeds in Korea; Jeju National University Press: Jeju, Republic of Korea, 2001; 662p. [Google Scholar]
- Perestenko, L.P. Red Algae of the Far-Eastern Seas of Russia; Russian Academy of Sciences: St. Petersburg, Russia, 1996; 330p. [Google Scholar]
- Capistrant-Fossa, K.; Brawley, S.H. Unexpected reproductive traits of Grateloupia turuturu revealed by its resistance to bleach-based biosecurity protocols. Botanica Marina 2019, 62, 83–96. [Google Scholar] [CrossRef]
- Mathieson, A.C.; Dawes, C.J.; Pederson, J.; Gladych, R.A.; Carlton, J.T. The Asian red seaweed Grateloupia turuturu (Rhodophyta) invades the Gulf of Maine. Biol. Invasions 2007, 10, 985–988. [Google Scholar] [CrossRef]
- Nyberg, C.D.; Wallentinus, I. Can species traits be used to predict marine macroalgal introductions? Biol. Invasions 2005, 7, 265–279. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. In Eleventh International Seaweed Symposium. Developments in Hydrobiogy; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 22, pp. 513–516. [Google Scholar]
- Bangmei, X.; Abbott, I.A. Edible seaweeds of China and their place in the Chinese diet. Econ. Bot. 1987, 41, 341–353. [Google Scholar] [CrossRef]
- Denis, C.; Massé, A.; Fleurence, J.; Jaouen, P. Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae Grateloupia turuturu: Process definition and up-scaling. Sep. Purif. Technol. 2009, 69, 37–42. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia turuturu. J. Appl. Phycol. 2012, 25, 425–432. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the biochemical composition of the edible seaweed Grateloupia turuturu Yamada harvested from two sampling sites on the Brittany coast (France): The influence of storage method on the extraction of the seaweed pigment R-phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and analysis of compounds with antibacterial potential from the red alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodriguez-Alcala, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Pang, S.J.; Xiao, T.; Bao, Y. Dynamic changes of total bacteria and Vibrio in an integrated seaweed–abalone culture system. Aquaculture 2006, 252, 289–297. [Google Scholar] [CrossRef]
- Pang, S.J.; Xiao, T.; Shan, T.F.; Wang, Z.F.; Gao, S.Q. Evidences of the intertidal red alga Grateloupia turuturu in turning Vibrio parahaemolyticus into non-culturable state in the presence of light. Aquaculture 2006, 260, 369–374. [Google Scholar] [CrossRef]
- Charrier, B.; Abreu, M.H.; Araujo, R.; Bruhn, A.; Coates, J.C.; De Clerck, O.; Katsaros, C.; Robaina, R.R.; Wichard, T. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol. 2017, 216, 967–975. [Google Scholar] [CrossRef]
- Mahadevan, K. Chapter 13—Seaweeds: A sustainable food source. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 347–364. [Google Scholar]
- Marquez, G.P.B.; Santiañez, W.J.E.; Trono, G.C.; de la Rama, S.R.B.; Takeuchi, H.; Hasegawa, T. Chapter 16—Seaweeds: A sustainable fuel source. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 421–458. [Google Scholar]
- Jagtap, A.S.; Meena, S.N. Chapter 23—Seaweed farming: A perspective of sustainable agriculture and socio-economic development. In Natural Resources Conservation and Advances for Sustainability; Jhariya, M.K., Meena, R.S., Banerjee, A., Meena, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 493–501. [Google Scholar]
- Xiao, X.; Agusti, S.; Yu, Y.; Huang, Y.; Chen, W.; Hu, J.; Li, C.; Li, K.; Wei, F.; Lu, Y.; et al. Seaweed farms provide refugia from ocean acidification. Sci. Total Environ. 2021, 776, 145192. [Google Scholar] [CrossRef]
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef]
- Zhao, S. Marine Algae and Algae Culture Science; National Defense Industry Press: Beijing, China, 2012; 407p. (In Chinese) [Google Scholar]
- Niwa, K.; Harada, K. Physiological responses to nitrogen deficiency and resupply in different blade portions of Pyropia yezoensis f. narawaensis (Bangiales, Rhodophyta). J. Exp. Mar. Biol. Ecol. 2013, 439, 113–118. [Google Scholar] [CrossRef]
- Roesijadi, G.; Copping, A.E.E.; Huesemann, M.H.H.; Forster, J.; Benemann, J.R.; Thom, R.M. Techno-Economic Feasibility Analysis of Offshore Seaweed Farming for Bioenergy and Biobased Products. 2008. Available online: https://arpa-e.energy.gov/sites/default/files/Techno-Economic%20Feasibility%20Analysis%20of%20Offshore%20Seaweed%20Farming%20for%20Bioenergy%20and%20Biobased%20Products-2008.pdf (accessed on 28 March 2023).
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings —1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Sun, X.Y.; Hong, L.C.; Ye, H.M. Experiment determining nitrate nitrogen in water samples by on-line cadmium column reduc- tion-flow injection method. Water Resour. Prot. 2010, 26, 75–77, (In Chinese with English Abstract). [Google Scholar]
- Wu, Z.-Z. Improved method of NH4+-N determined by hypobromite oxidation in water. Mar. Environ. Sci. 2007, 26, 85–87, (In Chinese with English abstract). [Google Scholar]
- Kochert, G. Protein determination by dye binding. In Handbook of Phycological Methods: Physiological and Biochemical Method; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: Cambridge, UK, 1978; pp. 92–93. [Google Scholar]
- Pallant, J. SPSS Ssurvival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 7th ed.; Open University Press: London, UK, 2010; 378p. [Google Scholar]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Differential growth response of Ulva lactuca to ammonium and nitrate assimilation. J. Appl. Phycol. 2010, 23, 345–351. [Google Scholar] [CrossRef]
- Bracken, M.E.S.; Stachowicz, J.J. Seaweed diversity enhances nitrogen uptake via complementary use of nitrate and ammonium. Ecology 2006, 87, 2397–2403. [Google Scholar] [CrossRef]
- Vonk, J.A.; Middelburg, J.J.; Stapel, J.; Bouma, T.J. Dissolved organic nitrogen uptake by seagrasses. Limnol. Oceanogr. 2008, 53, 542–548. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Buschmann, A.H.; Sousa-Pinto, I.; Yarish, C. Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. J. Exp. Mar. Biol. Ecol. 2011, 407, 190–199. [Google Scholar] [CrossRef]
- Rees, T.A.V.; Dobson, B.C.; Bijl, M.; Morelissen, B. Kinetics of nitrate uptake by New Zealand marine macroalgae and evidence for two nitrate transporters in Ulva intestinalis L. Hydrobiologia 2007, 586, 135–141. [Google Scholar] [CrossRef]
- Fan, X.; Xu, D.; Wang, Y.; Zhang, X.; Cao, S.; Mou, S.; Ye, N. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: Implications for the explosion in green tides. J. Appl. Phycol. 2013, 26, 537–544. [Google Scholar] [CrossRef]
- Naldi, M.; Wheeler, P.A. Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. J. Phycol. 1999, 35, 70–77. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, L.; Li, H.; Gong, Q.; Gao, X. Effects of nitrogen source and concentration on the growth and biochemical composition of the red seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta). Sustainability 2023, 15, 4210. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Liu, X.; Zhong, M.; Chen, W.; Wang, F.; Du, H. Response of Gracilaria lemaneiformis to nitrogen deprivation. Algal Res. 2018, 34, 82–96. [Google Scholar] [CrossRef]
- Cole, A.J.; Angell, A.R.; de Nys, R.; Paul, N.A. Cyclical changes in biomass productivity and amino acid content of freshwater macroalgae following nitrogen manipulation. Algal Res. 2015, 12, 477–486. [Google Scholar] [CrossRef]
- Liu, J.-W.; Dong, S.-L. Comparative studies on utilizing nitrogen capacity between two macroalgae Gracilaria tenuistipitata var. liui (Rhodophyta) and Ulva pertusa (Chlorophyta) I. Nitrogen storage under nitrogen enrichment and starvation. J. Environ. Sci. 2001, 13, 318–322. [Google Scholar]
- Yang, J.; Yin, Y.; Yu, D.; He, L.; Shen, S. Activation of MAPK signaling in response to nitrogen deficiency in Ulva prolifera (Chlorophyta). Algal Res. 2021, 53, 102153. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Different behaviour between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Int. J. Plant Biol. 2020, 11, 8567. [Google Scholar] [CrossRef]
- Young, E.B.; Berges, J.A.; Dring, M.J. Physiological responses of intertidal marine brown algae to nitrogen deprivation and resupply of nitrate and ammonium. Physiol. Plant. 2009, 135, 400–411. [Google Scholar] [CrossRef]




| Factors | df | F | p |
|---|---|---|---|
| Nitrate uptake rates | |||
| Starvation time | 5 | 696.190 | <0.001 * |
| Recovery time | 5 | 273.300 | <0.001 * |
| Interaction | 25 | 34.384 | <0.001 * |
| Ammonium uptake rates | |||
| Starvation time | 5 | 973.315 | <0.001 * |
| Recovery time | 5 | 153.071 | <0.001 * |
| Interaction | 25 | 75.990 | <0.001 * |
| Parameter | Factor | df | F | p |
|---|---|---|---|---|
| RGRs | Starvation time | 5 | 9.720 | 0.008 * |
| Total nitrogen | Starvation time | 5 | 8.659 | 0.003 * |
| Protein | Starvation time | 5 | 7.869 | <0.001 * |
| Chl a | Starvation time | 5 | 52.869 | <0.001 * |
| PE | Starvation time | 5 | 31.921 | <0.001 * |
| Factors | df | F | p |
|---|---|---|---|
| RGRs | |||
| Starvation time | 5 | 13.216 | <0.001 * |
| Recovery time | 5 | 6.880 | <0.001 * |
| Interaction | 25 | 1.238 | 0.274 |
| Total nitrogen | |||
| Starvation time | 5 | 14.325 | <0.001 * |
| Recovery time | 5 | 3.149 | 0.015 * |
| Interaction | 25 | 3.309 | <0.001 * |
| Protein | |||
| Starvation time | 5 | 26.845 | <0.001 * |
| Recovery time | 5 | 2.439 | 0.039 * |
| Interaction | 25 | 2.202 | 0.003 * |
| Chl a | |||
| Starvation time | 5 | 82.955 | <0.001 * |
| Recovery time | 5 | 14.247 | <0.001 * |
| Interaction | 25 | 3.537 | <0.001 * |
| PE | |||
| Starvation time | 5 | 77.971 | <0.001 * |
| Recovery time | 5 | 5.655 | <0.001 * |
| Interaction | 25 | 1.752 | 0.027 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lan, L.; Zhang, J.; Wang, Q.; Liu, Y.; Li, H.; Gong, Q.; Gao, X. Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta). Sustainability 2023, 15, 7032. https://doi.org/10.3390/su15097032
Chen Y, Lan L, Zhang J, Wang Q, Liu Y, Li H, Gong Q, Gao X. Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta). Sustainability. 2023; 15(9):7032. https://doi.org/10.3390/su15097032
Chicago/Turabian StyleChen, Yining, Lan Lan, Jing Zhang, Qiaohan Wang, Yan Liu, Huiru Li, Qingli Gong, and Xu Gao. 2023. "Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta)" Sustainability 15, no. 9: 7032. https://doi.org/10.3390/su15097032
APA StyleChen, Y., Lan, L., Zhang, J., Wang, Q., Liu, Y., Li, H., Gong, Q., & Gao, X. (2023). Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia turuturu (Halymeniaceae, Rhodophyta). Sustainability, 15(9), 7032. https://doi.org/10.3390/su15097032






