Abstract
Seaweeds, as biofilters that remediate seawater eutrophication, have been widely applied in integrated cultivations for both ecological and economic benefits. Although Grateloupia turuturu (Rhodophyta) is considered as a qualified species in integrated maricultivation, its growth and biochemical performance under different nitrogen conditions are still unknown. Here, we cultured G. turuturu under two nitrogen sources (nitrate and ammonium) at six concentrations (0, 25, 50, 100, 200, and 400 µM) to investigate its growth and nitrogenous compounds (total and inorganic nitrogen, soluble protein, amino acids, and pigments) as well as the allocation pattern of nitrogen storage pools. Our results showed that G. turuturu was well acclimated to high concentrations of both nitrogen sources, and algal age played an important role in the preference of nitrogen sources. Most of the biochemical compositions in G. turuturu increased significantly with the increased concentrations of nitrogen, except for the protein and nitrate contents. Protein and residual organic nitrogen (RON, mainly amino acids) were found to be the two main nitrogen storage pools in G. turuturu. Our study revealed that G. turuturu can produce more profitable compositions at high nitrogen concentrations, making it a profitably promising biofilter to remediate eutrophication.
1. Introduction
Eutrophication, defined as the excessive richness of nutrients in seawater, results in the dense growth of plants and has become a primary problem in coastal seawater today [1]. Anthropogenic activities such as the release of industrial and residential sewage, fertilizer from land agriculture, and effluents from aquaculture are important drivers of eutrophication [2,3]. The dramatic increase in the biomass of marine vegetation that occurs during eutrophication depletes the oxygen of seawater, thereby leading to the mass mortality of animals [4]. Other adverse effects of eutrophication include increases in the risk of infectious disease, decreases in water transparency and aesthetics, and economic losses [5,6]. Although various strategies have been presented to manage eutrophication, their implementation have been slow and uneven due to practical limitations [7]. One promising method involves the use of macroalgae to remove excess nutrients due to their low cost and high nutrient-uptake efficiency [8]. Various macroalgal species have been studied as biofilters for the bioremediation of aquaculture effluents including Ulva lactuca [9], Undaria pinnatifida [10], Caulerpa lentillifera, and Gracilaria lichenoides [11]. Furthermore, in the aquaculture industry, macroalgae have been investigated in co-cultures with fed species (e.g., fish and shrimp) and other invertebrates in integrated multi-trophic aquaculture systems (IMTA), which effectively reduce eutrophication and thereby increase the sustainability of aquaculture [12]. When choosing the extractive seaweed species for IMTA, apart from the uptake rate, economic value is also an important factor [13]. Hence, it is important to find more seaweed species with both effective nutrient biofiltration capabilities and high added values such as sea vegetables, cosmetics, and bioactivates [14].
Nitrogen is generally considered as the major cause of eutrophication in most coastal ecosystems [15,16]. Both river inputs and mariculture can increase the nitrogen concentration of seawater [17,18]. The Yellow River in China has a dissolved inorganic nitrogen concentration of over 300 µM [17]. The concentrations of nitrate in the Yellow Sea, China, have consistently increased since the 1980s, exceeding levels as high as 23 µM in the Subei Shoal in 2012 [19]. Meanwhile, nitrogen is also a primary essential element for macroalgal growth [20]. Nitrate (NO3−) and ammonium (NH4+) are the two most abundant sources of dissolved inorganic nitrogen in seawater [21] and both are readily absorbed by macroalgae [22]. Several studies have investigated the growth and biochemical responses of algae to nitrate and ammonium, respectively [23,24], and the preference of a nitrogen source has been observed to be species-specific. For example, the green macroalga Ulva fasciata was found to grow faster in ammonium media than that in nitrate media [25], while microalgae Tetraselmis sp. [26] and Isochrysis galbana [27] showed a reversed pattern, with better growth in nitrate media compared to ammonium media. Apart from the nitrogen source, concentration also has different effects on the growth and physio-biochemical characters of macroalgae. Generally, the nitrogen growth kinetics of macroalgae follow typical growth–saturation curves, which means that the macroalgal growth rates increase positively with increases in nitrogen concentration, and then become steady after the nitrogen concentration reaches a saturation point [20]. Different macroalgae have various saturated concentrations, for example, 1.5 µM nitrate or ammonium for Gracilaria foliifera [28], 10 µM nitrate for Saccharina latissima [29], and 30 µM nitrate or ammonium for Cladophora aff. albida [30]. Several studies have shown that the enrichment of nitrogen from low concentrations (6-40 µM) to high concentrations (150–600 µM) can increase the growth rates of macroalgae; however, the differences in RGRs between 300 µM and 600 µM were not found to be significant [31,32,33,34]. Furthermore, the physio-biochemical characteristics (e.g., photosynthesis, pigments, and proteins) of macroalgae have also been investigated with regard to nitrogen concentrations, and their responses patterns have been shown to vary and not always remain consistent with growth performance [31,35,36,37].
Macroalgae first assimilate inorganic nitrogen into small organic molecules such as amino acids, which are then incorporated into macromolecules such as proteins, pigments, and nucleic acids [20]. As the external nitrogen concentrations vary daily and seasonally, macroalgae are able to uptake nitrogen at levels exceeding their growth demand and store the extra nitrogen in cellular storage pools to support subsequent growth in nitrogen-depleted conditions [38]. The important nitrogen storage pools in macroalgae include inorganic nitrogen pools, containing nitrate and ammonium, and organic nitrogen pools, consisting of amino acids, proteins, and pigments [39]. Previous studies have shown that both the nitrogen source and concentration can regulate the accumulation of nitrogen pools in macroalgae [38,40].
Grateloupia turuturu is a red macroalga originally described in Japan [41] that has now spread nearly all over the world as an invasive species [42]. Traditionally, it is consumed as a sea vegetable in Asia [43,44]. In recent decades, it has been proven to be rich in various valuable compounds such as dietary fiber, proteins, pigments, minerals, and lipids, which can be widely used in foods, cosmetics, and clinical and immunological analysis [45,46,47,48]. In addition, Cardoso et al. [49] observed that the bioactive compounds of G. turuturu have antibacterial effects. This echoes the research of Pang et al. [50], who found that G. turuturu can convert the pathogenic Vibrio parahaemolyticus into a non-culturable state and suggested G. turuturu as a potentially ideal candidate to be cultured with other feeding species [51]. However, currently, the growth and biochemical responses of G. turuturu under nitrogen-rich conditions are still unknown, which are also important in the evaluation of whether G. turuturu is an ideal species for integrated mariculture systems from an economic perspective.
In the present study, we investigated the effects of nitrogen source and concentration on the growth and biochemical compositions (total nitrogen, inorganic nitrogen, protein, amino acids, and pigments) of G. turuturu in order to clarify its biomass performances, biochemical variations, and allocation strategies with respect to nitrogen pools under different nitrogen conditions. Our results are expected to provide important basic information for evaluating the eutrophication remediation potential of this species.
2. Materials and Methods
2.1. Algal Collection and Laboratory Acclimation
Grateloupia turuturu tetrasporophytes were collected from the intertidal zone of Tuandao (36°02′ N, 122°17′ E), Qingdao, China. These samples were transported immediately to our laboratory in cooler boxes filled with seawater. Healthy tetrasporophytes were selected and fully rinsed with sterilized seawater to remove diatoms and detritus. Next, they were placed in large conical flasks containing 3 L of enriched F/2 medium [52] and incubated with gentle aeration. All chemicals and reagents used in the F/2 medium as well as those mentioned below were of analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The tetrasporophytes were kept at 20 °C with 86 μmol photons m−2 s−1 under a 12:12 h light:dark cycle for pre-cultivation until massive tetraspore release was detected using an Olympus microscope. Then, 5–10 g of one mature terasporopyte was placed in a Petri dish with a diameter of 20 cm filled with 250 mL of enriched F/2 medium. The bottom of the Petri dish was covered with 2 × 2 cm2 glass slides. After 24 h of dark cultivation at 15 °C, the slides were taken out under aseptic conditions and placed in 20 Petri dishes with a diameter of 5 cm filled with 15 mL of enriched F/2 medium. The culture conditions were the same as during pre-cultivation. The culture medium was replaced every three days. After the young red thalli were visible to the naked eye, the slides were cultured in 500 mL of aerated F/2 medium. The experiment was carried out after the young thallus branches grew up to 1–5 cm.
2.2. Culture Experiment
Eight G. turuturu thalli were placed in 400 mL of filtered, autoclaved, and nitrogen-free F/2 medium. NaNO3 or NH4Cl was added to the medium to produce six nitrogen concentrations: 0, 25, 50, 100, 200, and 400 μM. The samples were aerated and cultured at 24 °C with a light intensity of 109–118 μmol photons m−2 s−1 and a photoperiod of 12:12 h light:dark. The salinity of the culture medium was 34 psu. The culture media and flasks were changed twice every week. The experiment was conducted for four weeks, and each treatment had four replicates.
2.3. Measurements of Relative Growth Rate
The fresh weight and length of the G. turuturu thallus were measured at the beginning and end of the experiment. The relative growth rate (RGR; % day−1) was calculated using the following formula (Equation (1)):
where V0 is the initial values of fresh weight or length; Vt is the final values of fresh weight or length; and t is the number of experimental days.
RGR = (ln Vt − ln V0)/t × 100
2.4. Measurements of Total and Inorganic Nitrogen
For the measurement of total nitrogen, samples were dried at 80 °C for 48 h. After measuring the dry weight, the samples were ground to powder using a high-throughput tissue grinder (Scientz-48, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). The total nitrogen content was analyzed using an elemental analyzer (Vario EL III, Elementar, Langenselbold, Germany).
Inorganic nitrogen (NO3− and NH4+) was measured using the ethanol extraction technique described by McGlathery et al. [38] and Hwang et al. [53]. The samples were briefly washed in deionized water and blotted dry. Then, 0.5 g of fresh sample was ground in 15 mL 90% (v/v) ethanol using a mortar and pestle. The mixture was maintained at room temperature for one night. Then, after centrifugation, 3 mL of the supernatant was diluted to 30 mL with deionized water and frozen at −80 °C for the subsequent analysis of NO3− and NH4+. The NO3− and NH4+ contents were determined via the cadmium column reduction method [54] and the hypobromite oxidation method [55], respectively, with a UV–Visible spectrophotometer (U-2900UV/VIS, HITACHI, Tokyo, Japan).
2.5. Measurement of Soluble Protein
Fresh samples (0.1 g) were ground with 0.9 mL phosphate-buffered solution (0.1 M, pH = 7.4) using a mortar and pestle. The extract was centrifuged at 12,000 rpm and 4 °C for 30 min. The absorption of the supernatant was measured at 595 nm using an ultraviolet spectrophotometer. The soluble protein content of the samples was estimated using Coomassie brilliant blue G-250 dye (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and bovine albumin according to the method described by Kochert [56].
2.6. Measurement of Free Amino Acids
Samples were dried at 80 °C for 48 h. After measuring the dry weight, the samples were ground to a powder using a high-throughput tissue grinder. The content of free amino acids was analyzed using an automatic amino acid analyzer (L-8900, Hitachi, Japan). The quantification included 15 free amino acids: aspartic acid, threonine, serine, glutamic acid, proline, glycine, alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, histidine, lysine, and arginine. In addition, the content of flavor amino acids was calculated as the sum of umami flavor amino acids (aspartic and glutamic acids) and sweet flavor amino acids (glycine, alanine, serine, and proline) [57].
2.7. Measurements of Chlorophyll a (Chl a) and Phycoerythrin (PE)
For the measurements of PE, 0.1 g of fresh sample was homogenized with 6 mL of phosphate-buffered solution (0.1 M, pH = 6.5) using a glass homogenizer at 0 °C. Then, the extract was centrifuged at 4000× g rpm and 4 °C for 20 min. The absorption of the supernatant was measured at 565 nm using a UV–Visible spectrophotometer (U-2900UV/VIS, HITACHI, Japan). The content of phycoerythrobilin (mg g−1) was calculated according to the following equation (Equation (2)):
where A565 is the absorption rate of the supernatant at 565 nm; Ve is the volume of the extract solution (mL); I is the optical path of the cuvette (cm); and W is the fresh weight of the samples (g).
PE = 12.4 × A565 × Ve/(I × W × 1000)
For the measurements of Chl a, 6 mL of dimethylformamide (DMF) was added to the centrifuged deposit of samples for the phycoerythrin measurements and maintained at 4 °C in darkness for 1 day. The extracts were centrifuged at 4000 rpm and 4 °C for 10 min. The absorption of the supernatant was measured at 750, 664, 647, and 625 nm using a UV–Visible spectrophotometer (U-2900UV/VIS, HITACHI, Japan). The content of Chl a (mg g−1) was calculated according to the following equation (Equation (3)):
where A625–750 are the absorptions of the extracts at 625, 647, 664, and 750 nm; Ve is the volume of DMF (mL); I is the optical path of the cuvette (cm); and W is the fresh weight of the samples (g).
Chl a = [12.65 × (A664 − A750) − 2.99 × (A647 − A750) − 0.04 × (A625 − A750)] × Ve/(I × W × 1000)
2.8. Data Analysis
All contents of the measured biochemical components were normalized to mg N g−1 DW (N, nitrogen; DW, dry weight). To study the allocation of nitrogen into storage pools, the proportions of the six main nitrogen pools were calculated against the total nitrogen content including proteins, residual organic nitrogen (RON), Chl a, PE, nitrate, and ammonium. The RON pool mainly contains free amino acids and small peptides [58,59]; however, it likely also includes RNA and DNA [60]. This was calculated as the difference between the total nitrogen content and the sum of the measured organic and inorganic pools (i.e., proteins, Chl a, PE, nitrate, and ammonium) [38].
The effects of different nitrogen sources and concentrations on RGR and the biochemical compositions were analyzed using a two-way analysis of variance (ANOVA). A Shapiro–Wilk’s test and Levene’s test were used to check the normality of the data and the homogeneity of variance, respectively. Since the homogeneities of total nitrogen (p = 0.001), nitrate (p = 0.001), ammonium (p = 0.007), Chl a (p = 0.004), and PE (p = 0.006) were violated, two-way ANOVA was utilized as ANOVA is reasonably robust to violations of the homogeneity of variance when the group sizes are reasonably similar [61]. Significant differences were further analyzed by pairwise comparisons with a Bonferroni adjustment for multiple comparisons. Differences were considered to be significant at a probability of 5% (p < 0.05). All data are given as the mean ± standard deviation (SD). Data analysis was conducted using SPSS statistical software (version 26.0).
3. Results
3.1. Growth
The RGR of the fresh weight was significantly affected by the nitrogen source and concentration both individually and interactively (nitrogen source effect, F = 424.706, p < 0.001; nitrogen concentration effect, F = 6.016, p < 0.001, interaction effect, F = 2.769, p < 0.05; Figure 1a and Table 1). The RGR of the fresh weight in the NO3−-N-based media was significantly higher than that in the NH4+-N-based media. In the media supplemented with NO3−-N, the RGR of the fresh weight increased significantly with increasing concentrations of NO3−-N. In contrast, the RGR of the fresh weight did not vary significantly in the NH4+-N-based media. The RGR of the length was significantly affected by the nitrogen source (F = 296.339, p < 0.001) and the interaction between the nitrogen source and concentration (F = 3.013, p < 0.05; Figure 1b and Table 1). Similar to the RGR of the fresh weight, the RGR of the length in the NO3−-N-based media was significantly higher than that in the NH4+-N-based media. In the NO3−-N-based media, the highest RGR of the length was at 200 µmol L−1. No differences in the RGRs of the length were found between concentrations in the NH4+-N-based media.
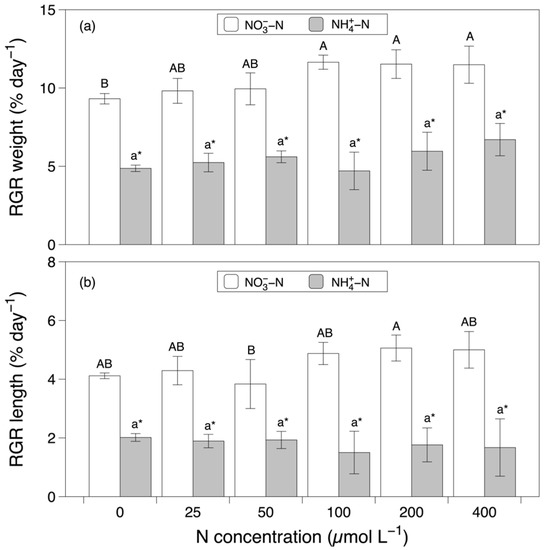
Figure 1.
Relative growth rates (RGRs) of the fresh weight (a) and length (b) of Grateloupia turuturu after 28 days of cultivation under the combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. As the interaction effect was significant, different capital and small letters indicate significant differences among concentrations in the nitrate and ammonium media, respectively. Asterisks indicate significant differences between nitrogen sources at each concentration (p < 0.05).

Table 1.
Results of two-way ANOVA examining the effects of nitrogen source and concentration on the growth and biochemical compositions of Grateloupia turuturu. Statistically significant values are indicated by asterisks (p < 0.05).
3.2. Total and Inorganic Nitrogen
The total nitrogen content was significantly affected by the nitrogen concentration (F = 11.980, p < 0.001; Figure 2 and Table 1). The values of the total nitrogen content increased significantly from concentrations between 0 and 50 µmol L−1 to concentrations between 200 and 400 µmol L−1. With respect to the content of nitrate, the main effect of the nitrogen source (F = 276.252, p < 0.001) and the interaction between the nitrogen source and concentration (F = 2.937, p < 0.05) were significant. After the pairwise comparisons, a significant difference between concentrations could not be detected. The nitrate content in the NO3−-N-based media was significantly lower than that in the NH4+-N-based media (Figure 3a). The content of ammonium was significantly affected by both the nitrogen source and concentration individually (nitrogen source effect, F = 10.341, p < 0.05; nitrogen concentration effect, F = 10.127, p < 0.001). Consistent with the results for nitrate content, the NO3−-N-based media led to significantly lower values of the ammonium content compared to the NH4+-N-based media (Figure 3b). The ammonium content was highest with a concentration of 400 µmol L−1, followed by 100 and 200 µmol L−1, and significantly higher than the values observed with concentrations between 0 and 50 µmol L−1.
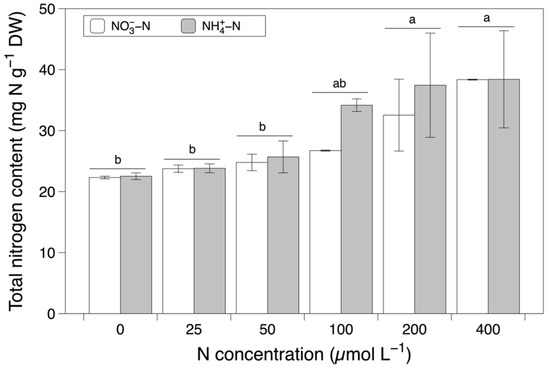
Figure 2.
The total nitrogen content in Grateloupia turuturu after 28 days of cultivation under a combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. Different small letters indicate significant differences between concentrations irrespective of the nitrogen source (p < 0.05).
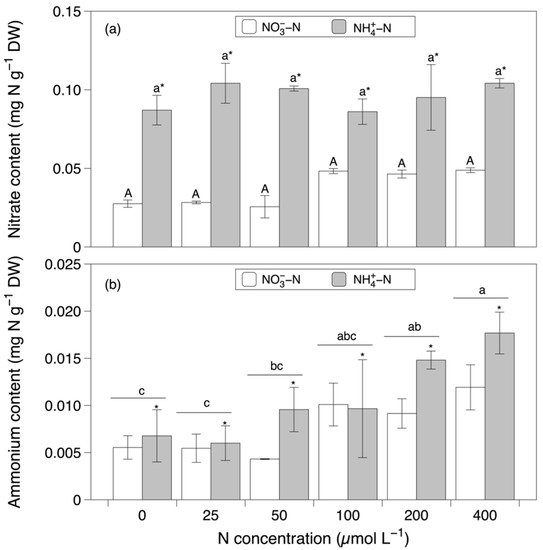
Figure 3.
Nitrate (a) and ammonium (b) content in Grateloupia turuturu after 28 days of cultivation under a combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. As the interaction effect was significant for nitrate, different capital and small letters indicate significant differences among the concentrations in the nitrate and ammonium media, respectively. Asterisks indicate significant differences between nitrogen sources at each concentration (p < 0.05). For ammonium, different small letters indicate significant differences between concentrations, irrespective of the nitrogen source, and asterisks indicate significant differences between nitrogen sources, irrespective of the concentration (p < 0.05).
3.3. Soluble Protein
The soluble protein content was significantly affected by the nitrogen source (F = 40.958, p < 0.001) and the interaction between the nitrogen source and concentration (F = 37.865, p < 0.05; Figure 4 and Table 1). The NH4+-N-based media induced significantly higher contents of soluble protein compared to the NO3−-N-based media at concentrations of 25, 100, 200, and 400 µmol L−1. Various nitrogen concentrations from the same nitrogen source resulted in different trends in the soluble protein contents. In the NO3−-N-based media, no differences in the soluble protein contents were found between the different concentrations. In contrast, a concentration of 100 µmol L−1 resulted in the highest soluble protein content with the NH4+-N based media.
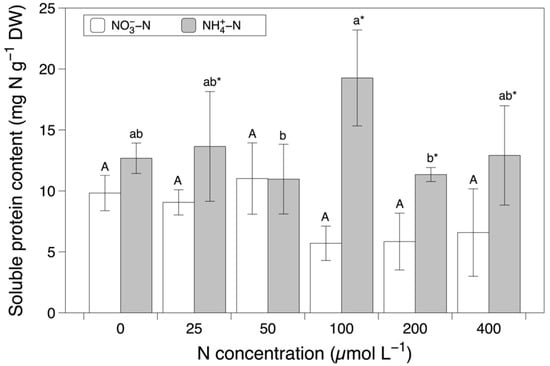
Figure 4.
The soluble protein content in Grateloupia turuturu after 28 days of cultivation under a combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. As the interaction effect was significant, different capital and small letters indicate significant differences among concentrations in the nitrate and ammonium media, respectively. Asterisks indicate significant differences between nitrogen sources at each concentration (p < 0.05).
3.4. Free Amino Acids
The contents of free amino acids and flavor amino acids shared similar response patterns with different treatments. They were significantly affected by the nitrogen concentration and the interaction between the nitrogen source and concentration (p < 0.001, Figure 5 and Table 1). Generally, at concentrations of 100 and 200 µmol L−1, the content of amino acids and flavor amino acids in the NH4+-N-based media were higher than that in the NO3−-N-based media. In contrast, at concentrations between 0 and 50 µmol L−1, the content of amino acids and flavor amino acids in NO3−-N-based media was higher than that in the NH4+-N-based media. In addition, both contents increased along with an increasing concentration. The NH4+-N-based medium with a concentration of 400 µmol L−1 had the highest amino acids and flavor amino acid content at 19.77 ± 1.19 mg N g−1 DW and 9.10 ± 0.48 mg N g−1 DW, respectively.
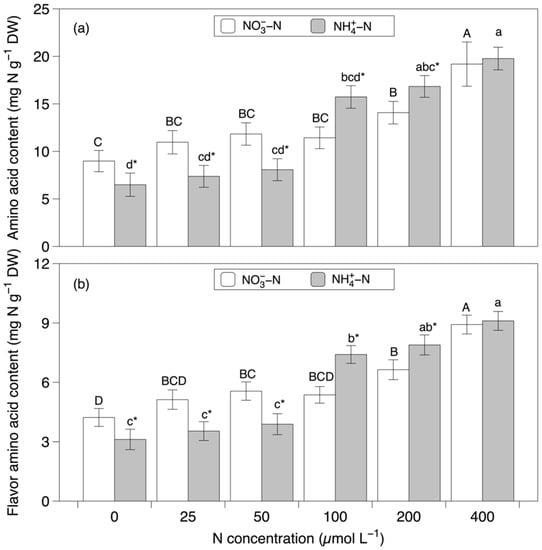
Figure 5.
Amino acid (a) and flavor amino acid (b) contents in Grateloupia turuturu after 28 days of cultivation under a combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. As the interaction effect was significant, different capital and small letters indicate significant differences among the concentrations in nitrate and ammonium media, respectively. Asterisks indicate significant differences between nitrogen sources at each concentration (p < 0.05).
3.5. Chl a and PE
The Chl a content was significantly affected by the nitrogen concentration (F = 15.309, p < 0.001; Figure 6a and Table 1). The Chl a content decreased along with the decreasing concentration. Specifically, the Chl a content was the highest at a concentration of 400 µmol L−1, then significantly decreased at concentrations of 100 and 200 µmol L−1, and remained the lowest at a concentration of 0 µmol L−1. The PE content was significantly affected by the nitrogen source (F = 18.528, p < 0.001), concentration (F = 22.527, p < 0.001), and the interaction between the nitrogen source and concentration (F = 8.812, p < 0.001; Figure 6b and Table 1). At concentrations of 100–400 µmol L−1, the PE content was significantly higher in the NH4+-N-based media than in the NO3−-N based media. For the other concentrations, no significant differences between nitrogen sources were detected. Generally, the PE content increased with the increasing concentration. The pairwise comparisons showed that the highest PE content occurred in the 400 µmol L−1 NH4+-N-based media.
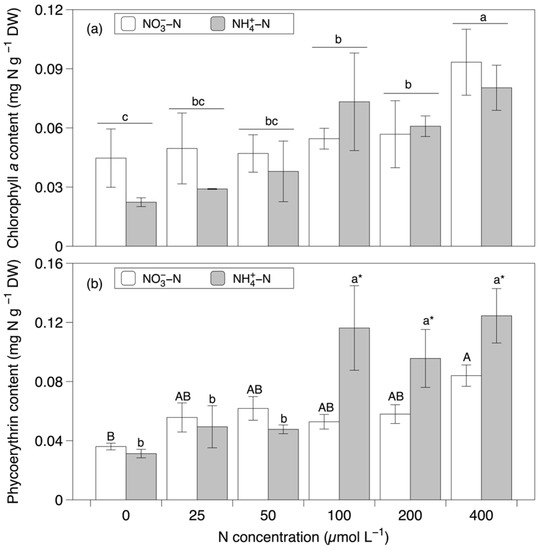
Figure 6.
The chlorophyll a (a) and phycoerythrin (b) contents in Grateloupia turuturu after 28 days of cultivation under a combination of two nitrogen sources at six concentrations. Data represent the mean ± SD. As the interaction effect was significant for phycoerythrin, different capital and small letters indicate significant differences among concentrations in the nitrate and ammonium media, respectively. Asterisks indicate significant differences between nitrogen sources at each concentration (p < 0.05). For chlorophyll a, different small letters indicate significant differences between concentrations, irrespective of the nitrogen source (p < 0.05).
3.6. Allocation of Nitrogen Pools
Table 2 and Table 3 show the allocation ratios of the nitrogen pools under different concentrations of NO3−-N and NH4+-N. The protein and RON nitrogen pools were the largest, accounting for nearly 99%. The percentage of the protein nitrogen pool gradually decreased with an increase in nitrogen concentration, while the RON pool gradually increased and became the largest storage pool. The proportion of pigment pools and inorganic nitrogen pools was relatively low. For the pigment pools, the PE nitrogen pool was generally larger than the Chl a nitrogen pool. The PE nitrogen pool reached a maximum of 0.34% at an NH4+-N concentration of 100 μmol L−1. The Chl a nitrogen pool reached a maximum of 0.24% at an NO3−-N concentration of 400 μmol L−1. The NH4+-N pool was lower than the NO3−-N pool, accounting for the smallest proportion of the total nitrogen pool.

Table 2.
The effects of the NO3−-N concentration on the allocation of nitrogen pools in Grateloupia turuturu. RON—residual organic nitrogen.

Table 3.
Effects of the NH4+-N concentration on the allocation of nitrogen pools in Grateloupia turuturu. RON—residual organic nitrogen.
4. Discussion
Our study showed that the growth rate of Grateloupia turuturu in nitrate media was higher than that in ammonium media, which is in accordance with several observations in microalgae such as Tetraselmis sp. [26] and Isochrysis galbana [27]. In contrast, the green macroalga Ulva fasciatia was previously found to grow faster in ammonium media than in nitrate media [25], highlighting the species-specific responses of algal growth to nitrogen sources. In addition, the growth differences between the nitrogen sources were observed at a concentration of 0 μmol L−1. This could be because the samples used in the nitrate media (averaged initial fresh weight of thalli: 0.0051 g) were younger than the samples used in the ammonium media (averaged initial fresh weight of thalli: 0.0188 g), leading to a faster growth in the nitrate media, implying that algal age plays an important role in algal performances under different nitrogen sources. Therefore, the effects of nitrogen sources on the growth of G. turuturu should be carefully evaluated. Our study showed that an increased nitrate concentration enhanced the growth rates of the fresh weight. Similarly, other macroalgae species have also been found to have higher growth rates in enriched nitrate conditions including Ulva rigida [31], Pyropia haitanensis [33], and Saccharina japonica [34]. In contrast, an increase in ammonium concentration did not change the growth rates of G. turuturu in our study. Such invariable growth rates may suggest that G. turuturu is unable to incorporate additional ammonium into growth once the ammonium concentration reaches its saturation levels. This steady status was also observed in RGRs at nitrate concentrations between 100 and 400 µmol L−1; these results are in accordance with the unchanged growth rates of Pyropia haitanesis under 300 and 600 µM nitrate media [33]. In addition, our results show that G. turuturu was well acclimated to high concentrations (400 µM) of both nitrogen sources. In contrast, the growth of the dinoflagellate Alexandrium minutum was previously found to decrease once the ammonium concentration exceeded 50 µM [62]. Such inhibition might be due to the fact that high concentrations of ammonium are toxic for algae [63]. Furthermore, the repression of growth by excessive nitrate and ammonium concentrations is more often observed in microalgae, rather than in macroalgae [64,65,66]. This discrepancy could be attributed to the different physiological characteristics of macro- and microalgae as well as the fact that the nitrogen concentration used for microalgae (mM level) was much higher than that used for macroalgae (µM level) [33,65].
The total nitrogen content of macroalgae is an effective bioindicator to evaluate the relative magnitude of nitrogen enrichment in seawater [22] as it correlates well with the nitrogen concentration [67,68]. Such correlations were also verified in our study, reflected by the gradually increased total nitrogen content along with the improved concentrations of nitrate and ammonium. Interestingly, the patterns of the total nitrogen contents did not parallel the growth rate patterns, which remained unchanged at different concentrations of nitrogen, except for the fresh weights in nitrate media. This may be due to the fact that the nitrogen uptake was temporally decoupled with growth; therefore, the total nitrogen content may reflect the nutrient loading of seaweeds more directly [22,69,70].
The inorganic nitrogen pool is the most direct nitrogen reservoir in algae after they absorb external nitrogen. As discussed above, inorganic nitrogen can accumulate in algae when the nitrogen assimilation and incorporation rates are lower than the nitrogen uptake rate. Our study illustrated that the internal nitrate contents were far higher than the internal ammonium contents, which have been observed in other macroalgae species such as Fucus versiculosus, Fucus serratus, and Laminaria digitata [71,72]. A possible explanation for this result may be that nitrate needs to be reduced before assimilation and incorporation. In contrast, ammonium can be directly utilized by algae and thereby has a higher rate of conversion than nitrate. In addition, exceedingly high contents of ammonium have toxic effects on algae [73,74]. Our study indicated that although G. turuturu was surrounded in the media with high contents of ammonium, it has evolved to keep ammonium at a low and safe level in its cells.
Our results showed that the ammonium media stimulated greater protein synthesis compared to the nitrate media. The preference of a nitrogen source for synthesizing protein is species-specific. For instance, Ulva fenestrata and Gracilaria pacifica were found to produce more proteins in ammonium-enriched media than in nitrate-enriched media [40], which is in accordance with our study. In contrast, the microalga Tetraselmis sp. showed the opposite patterns to our findings, synthesizing more proteins in NaNO3 than in the NH4Cl media [26]. In addition, the increased nitrogen concentration did not significantly change the production of proteins, except the 100 µmol L−1 ammonium medium. Such patterns differ from most previous studies, which have shown protein contents increasing with higher nitrogen levels [31,75]. Meanwhile, situations where higher nitrate concentrations decreased the protein contents have also been reported, for example, in the microalga Spirulina sp. [76].
The contents of the amino acids and flavor amino acids showed a positive correlation with the concentration of nitrogen in our study. Likewise, an increase in amino acid content upon the addition of nitrogen has also been observed in Ulva intestinalis [23] and Pyropia yezoensis [32]. Such patterns may be explained by the fact that amino acids are the initial products of nitrogen assimilation, especially glutamine and glutamate [27], and may respond to nitrogen concentration more rapidly compared to growth and other biochemical parameters. Previous studies have shown that glutamine can increase rapidly and substantially after the addition of inorganic nitrogen [77]. One interesting finding is that at low concentrations of nitrogen (0–50 μmol L−1), the amino acid content in ammonium media was lower than that in nitrate media, whereas at high concentrations of nitrogen (100–200 μmol L−1), the amino acid content in the ammonium media was higher than that in the nitrate media. These reverse patterns may imply that G. turuturu is able to regulate the concentration of cellular ammonium by synthesizing amino acids for self-protection. That is, G. turuturu converts the extra ammonium into amino acids rapidly to avoid the toxicity of ammonium over-accumulation.
Chl a and phycobiliproteins, as the main antenna pigments in red algae, are considered as important nitrogen reserves, and their contents are generally assumed to be strongly related to environmental nitrogen concentrations, reflected by the increase in pigments upon the enrichment of nutrients [78,79]. Our results for the pigment contents support the above assumption. Furthermore, the contents of Chl a did not differ significantly between the two nitrogen sources, demonstrating that G. turuturu prefers both nitrate and ammonium to synthesize Chl a. In contrast, the PE content was significantly higher in the ammonium media than in the nitrate media when the concentrations of nitrogen were high (100–400 μmol L−1). This illustrates that G. turuturu prefers to store extra ammonium in phycobiliproteins rather than Chl a, suggesting that phycobiliproteins play an important role in regulating the concentration of cellular ammonium.
According to our experimental results, proteins and RON (mainly amino acids) are the two most important nitrogen storage pools, accounting for over 99% of the total nitrogen. Similar findings were recorded in other macroalgae species such as U. fenestrata and G. pacifica [40] and Chaetomorpha linum [38]. Furthermore, the RON pool was generally larger than the protein pool, and its proportion increased along with the enrichment of nitrogen. Amino acids are the junction of inorganic nitrogen assimilation and macromolecule synthesis [23]. The increased accumulation of amino acids illustrates that the assimilation rate of inorganic nitrogen into amino acids exceeded the incorporation rate of amino acids into proteins, indicating that free amino acids play a more important role in nitrogen storage than proteins. The low percentage of inorganic nitrogen in the nitrogen pool indicates a rapid turnover rate and has little importance as a nitrogen repository. Ammonium is generally observed to have low proportions of nitrogen pools in macroalgae [38,40,58]. In contrast, nitrate storage has been found to vary in different macroalgal species [72], from less than 1% of total nitrogen in U. fenestrata [40] to up to one-third of total nitrogen in Laminaria longicruri [80]. Chl a and PE also accounted for less than 1% of total nitrogen in G. turuturu, regardless of the nitrogen source and concentration. For Chl a, our findings are in agreement with previous studies suggesting it is not a major nitrogen storage pool [40,59,81]. In contrast, the portion of PE in G. turuturu was lower than that of the previously reported portions (4–11% of total nitrogen) in other red macroalgae such as G. pacifica [40], Gracilaria gracilis [82], and Gracilaria tikvahiae [83]. In summary, the differences in the proportions of the main nitrogen pools between our study and others highlight that the proportions of nitrogen storage pools are species-specific; further investigations are needed to fully understand the allocation strategies of nitrogen pools in macroalgae.
Understanding the performance of G. turuturu in different nitrogen concentrations can help us evaluate its application in the bioremediation of ocean eutrophication. In our study, although the nitrogen removal rates were not investigated, G. turuturu showed an increasing growth trend upon the enrichment of nitrogen, without inhibition in high nitrogen concentrations (up to 400 µmol L−1). This suggests that G. turuturu is well acclimated to high concentrations of external nitrogen, enabling it to be a potentially feasible alternative for bioremediation. Further studies such as those measuring the nitrogen uptake rates of G. turuturu are necessary. In addition, the high growth rate in nitrate media compared to ammonium indicates that G. turuturu prefers nitrate, which is relevant for remediation in aquaculture effluents, as more nitrate is found in aquaculture effluents than ammonium [8]. Furthermore, G. turuturu increased profitable compositions (e.g., amino acids and PE) in high concentrations of nitrogen, which benefits value addition and is an added argument for the use of G. turuturu in integrated mariculture systems [12].
5. Conclusions
In conclusion, our results show that G. turuturu is well acclimated to high nitrogen concentrations, and nitrate especially stimulates an increase in biomass. The variation in nitrogen pools highlights that the RON pool, which mainly includes amino acids, is the largest nitrogen storage pool under high nitrogen concentrations. Our detailed analysis of the nitrogen-related composition in G. turuturu helps us to understand its allocation strategies for nitrogen in eutrophication conditions. In addition, by comparing our results with those of other studies, it was highlighted that growth and biochemical performance under various nitrogen concentrations are species-specific. Therefore, involving more macroalgal species under the same experimental design is desirable to further understand the differences between species-specific responses as well as the range of responses of individuals within the macroalgal species. Our findings such as the increased high-value compositions under high nitrogen levels aid in providing recommendations for the application of G. turuturu as a biofilter in eutrophication management and IMTA.
Author Contributions
Conceptualization, methodology, investigation, formal analysis, writing–original draft, Q.W.; Investigation, visualization, data curation, L.L.; Conceptualization, formal analysis, writing–original draft and review, H.L.; Validation, resources, Q.G.; Conceptualization, methodology, writing–review and check, project administration, funding acquisition, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Fund for the Central Universities (No. 202262002) and the Young Talent Program of Ocean University of China (No. 202212015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Yan Liu of Ocean University of China for the experimental assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Moal, M.; Gascuel-Odoux, C.; Menesguen, A.; Souchon, Y.; Etrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Selman, M.; Greenhalgh, S. Eutrophication: Sources and drivers of nutrient pollution. Renew. Resour. J. 2010, 26, 19–26. [Google Scholar]
- Huang, C.; Wang, X.; Yang, H.; Li, Y.; Wang, Y.; Chen, X.; Xu, L. Satellite data regarding the eutrophication response to human activities in the plateau lake Dianchi in China from 1974 to 2009. Sci. Total Environ. 2014, 485–486, 1–11. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Bruno, J.F.; Petes, L.E.; Harvell, C.D.; Hettinger, A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003, 6, 1056–1061. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Malone, T.C.; Newton, A. The globalization of cultural eutrophication in the coastal ocean: Causes and consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Ben-Ari, T.; Neori, A.; Ben-Ezra, D.; Shauli, L.; Odintsov, V.; Shpigel, M. Management of Ulva lactuca as a biofilter of mariculture effluents in IMTA system. Aquaculture 2014, 434, 493–498. [Google Scholar] [CrossRef]
- Cahill, P.L.; Hurd, C.L.; Lokman, M. Keeping the water clean—Seaweed biofiltration outperforms traditional bacterial biofilms in recirculating aquaculture. Aquaculture 2010, 306, 153–159. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Q.; Dong, S.; Tian, X. A comparative study of the nutrient uptake and growth capacities of seaweeds Caulerpa lentillifera and Gracilaria lichenoides. J. Appl. Phycol. 2016, 28, 3083–3089. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Chopin, T.; Robinson, S.M.C.; Troell, M.; Neori, A.; Buschmann, A.H.; Fang, J. Multitrophic integration for sustainable marine aquaculture. In The Encyclopedia of Ecology, Ecological Engineering; Jørgensen, S.E., Fath., B.D., Eds.; Elsevier: Oxford, UK, 2008; Volume 3, pp. 2463–2475. [Google Scholar]
- Chopin, T.; Neori, A.; Buschmann, A.; Pang, S.; Sawhney, M. Diversification of the aquaculture sector: Seaweed cultivation, integrated multi-trophic aquaculture, integrated sequential biorefineries. Glob. Aquacult. Advocate 2011, 14, 58–60. [Google Scholar]
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Paerl, H.W. Why does N-limitation persist in the world’s marine waters? Mar. Chem. 2018, 206, 1–6. [Google Scholar] [CrossRef]
- Wang, B.; Xin, M.; Wei, Q.; Xie, L. A historical overview of coastal eutrophication in the China Seas. Mar. Pollut. Bull. 2018, 136, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.G.; Glenn, E.P.; Conn, J.; Moore, D.; Walsh, T.; Akutagawa, M. Cultivation of Gracilaria parvispora (Rhodophyta) in shrimp-farm effluent ditches and floating cages in Hawaii: A two-phase polyculture system. Aquaculture 2001, 193, 239–248. [Google Scholar] [CrossRef]
- Li, H.-M.; Zhang, C.-S.; Han, X.-R.; Shi, X.-Y. Changes in concentrations of oxygen, dissolved nitrogen, phosphate, and silicate in the southern Yellow Sea, 1980–2012: Sources and seaward gradients. Estuar. Coast. Shelf Sci. 2015, 163, 44–55. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; 551p. [Google Scholar]
- Gruber, N. Chapter 1—The marine nitrogen cycle: Overview and challenges. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1–50. [Google Scholar]
- Fong, P. Chapter 20—Macroalgal-dominated ecosystems. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 917–947. [Google Scholar]
- Taylor, M.W.; Barr, N.G.; Grant, C.M.; Rees, T.A.V. Changes in amino acid composition of Ulva intestinalis (Chlorophyceae) following addition of ammonium or nitrate. Phycologia 2006, 45, 270–276. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Hurd, C.L.; Beardall, J.; Hepburn, C.D. Restricted use of nitrate and a strong preference for ammonium reflects the nitrogen ecophysiology of a light-limited red alga. J. Phycol. 2015, 51, 277–287. [Google Scholar] [CrossRef]
- Shahar, B.; Shpigel, M.; Barkan, R.; Masasa, M.; Neori, A.; Chernov, H.; Salomon, E.; Kiflawi, M.; Guttman, L. Changes in metabolism, growth and nutrient uptake of Ulva fasciata (Chlorophyta) in response to nitrogen source. Algal Res. 2020, 46, 101781. [Google Scholar] [CrossRef]
- Kim, G.; Mujtaba, G.; Lee, K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Roopnarain, A.; Sym, S.; Gray, V.M. Effect of nitrogenous resource on growth, biochemical composition and ultrastructure of Isochrysis galbana (Isochrysidales, Haptophyta). Phycol. Res. 2015, 63, 43–50. [Google Scholar] [CrossRef]
- DeBoer, J.A.; Guigli, H.J.; Israel, T.L.; D’Elia, C.F. Nutritional studies of two red algae. I. Growth rate as a function of nitrogen source and concentration. J. Phycol. 1978, 14, 261–266. [Google Scholar] [CrossRef]
- Chapman, A.R.O.; Markham, J.W.; Lüning, K. Effects of nitrate concentration on the growth and physiology of Laminaria saccharia (Phaeophyta) in culture. J. Phycol. 1978, 14, 195–198. [Google Scholar] [CrossRef]
- Gordon, D.M.; Birch, P.B.; McComb, A.J. Effects of inorganic phosphorus and nitrogen on the growth of an estuarine Cladophora in Culture. Bot. Mar. 1981, 24, 93–106. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef]
- Gao, G.; Gao, Q.; Bao, M.; Xu, J.; Li, X. Nitrogen availability modulates the effects of ocean acidification on biomass yield and food quality of a marine crop Pyropia yezoensis. Food Chem. 2019, 271, 623–629. [Google Scholar] [CrossRef]
- Chen, B.; Zou, D.; Ma, J. Interactive effects of elevated CO2 and nitrogen–phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). J. Appl. Phycol. 2015, 28, 1235–1243. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Li, J.; Gong, Q. Effects of elevated pCO2 and nutrient enrichment on the growth, photosynthesis, and biochemical compositions of the brown alga Saccharina japonica (Laminariaceae, Phaeophyta). PeerJ 2019, 7, e8040. [Google Scholar] [CrossRef]
- Cabello-Pasini, A.; Figueroa, F.L. Effect of nitrate concentration on the relationship between photosynthetic oxygen evolution and electron transport rate in Ulva rigida (Chlorophyta). J. Phycol. 2005, 41, 1169–1177. [Google Scholar] [CrossRef]
- Nederlof, M.A.J.; Neori, A.; Verdegem, M.C.J.; Smaal, A.C.; Jansen, H.M. Ulva spp. performance and biomitigation potential under high nutrient concentrations: Implications for recirculating IMTA systems. J. Appl. Phycol. 2022, 34, 2157–2171. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Li, J.; Wang, Q.; Gong, Q. Nutrient enrichment regulates the growth and physiological responses of Saccharina japonica to ocean acidification. J. Ocean Univ. China 2020, 19, 895–901. [Google Scholar] [CrossRef]
- McGlathery, K.J.; Pedersen, M.F.; Borum, J. Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorphy linum (Chlorophyta). J. Phycol. 1996, 32, 393–401. [Google Scholar] [CrossRef]
- Liu, J.-W.; Dong, S.-L. Comparative studies on utilizing nitrogen capacity between two macroalgae Gracilaria tenuistipitata var. liui (Rhodophyta) and Ulva pertusa (Chlorophyta) I. Nitrogen storage under nitrogen enrichment and starvation. J. Environ. Sci. 2001, 13, 318–322. [Google Scholar]
- Naldi, M.; Wheeler, P.A. Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. J. Phycol. 1999, 35, 70–77. [Google Scholar] [CrossRef]
- Yamada, Y. Note on some Japanese algae VIII. Sci. Pap. Inst. Algol. Res. Fac. Sci. Hokkaido Imp. Univ. 1941, 2, 195–215. [Google Scholar]
- Capistrant-Fossa, K.; Brawley, S.H. Unexpected reproductive traits of Grateloupia turuturu revealed by its resistance to bleach-based biosecurity protocols. Bot. Mar. 2019, 62, 83–96. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. In Eleventh International Seaweed Symposium; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 513–516. [Google Scholar]
- Bangmei, X.; Abbott, I.A. Edible seaweeds of China and their place in the Chinese diet. Econ. Bot. 1987, 41, 341–353. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodriguez-Alcala, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia Turuturu. J. Appl. Phycol. 2012, 25, 425–432. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the biochemical composition of the edible seaweed Grateloupia turuturu Yamada harvested from two sampling sites on the Brittany coast (France): The influence of storage method on the extraction of the seaweed pigment R-phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and analysis of compounds with antibacterial potential from the red alga Grateloupia Turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef]
- Pang, S.J.; Xiao, T.; Shan, T.F.; Wang, Z.F.; Gao, S.Q. Evidences of the intertidal red alga Grateloupia turuturu in turning Vibrio parahaemolyticus into non-culturable state in the presence of light. Aquaculture 2006, 260, 369–374. [Google Scholar] [CrossRef]
- Pang, S.J.; Xiao, T.; Bao, Y. Dynamic changes of total bacteria and Vibrio in an integrated seaweed–abalone culture system. Aquaculture 2006, 252, 289–297. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Hwang, S.-P.L.; Williams, S.L.; Brinkhuis, B.H. Changes in internal dissolved nitrogen pools as related to nitrate uptake and assimilation in Gracilaria tikvahiae McLachlan (Rhodophyta). Bot. Mar. 1987, 30, 11–20. [Google Scholar] [CrossRef]
- Sun, X.Y.; Hong, L.C.; Ye, H.M. Experiment determining nitrate nitrogen in water samples by on-line cadmium column reduc- tion-flow injection method. Water Resour. Prot. 2010, 26, 75–77, (In Chinese with English Abstract). [Google Scholar]
- Wu, Z.-Z. Improved method of NH4+-N determined by hypobromite oxidation in water. Mar. Environ. Sci. 2007, 26, 85–87, (In Chinese with English Abstract). [Google Scholar]
- Kochert, G. Protein determination by dye binding. In Handbook of Phycological Methods: Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: Cambridge, UK, 1978; pp. 92–93. [Google Scholar]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Fujita, R.M.; Wheeler, P.A.; Edwards, R.L. Metabolic regulation of ammonium uptake by Ulva rigida (Chlorophyta): A compartmental analysis of the rate-limiting step for uptake. J. Phycol. 1988, 24, 560–566. [Google Scholar] [CrossRef]
- Rosenberg, C.; Ramus, J. Ecological growth strategies in the seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): Soluble nitrogen and reserve carbohydrates. Mar. Biol. 1982, 66, 251–259. [Google Scholar] [CrossRef]
- Dortch, Q.; Clayton, J.R.; Thoresen, S.S.; Ahmed, S.I. Species differences in accumulation of nitrogen pools in phytoplankton. Mar. Biol. 1984, 81, 237–250. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 7th ed.; Open University Press: London, UK, 2020; 378p. [Google Scholar]
- Chang, F.H.; McClean, M. Growth responses of Alexandrium minutum (Dinophyceae) as a function of three different nitrogen sources and irradiance. N. Z. J. Mar. Freshw. Res. 1997, 31, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front. Microbiol. 2018, 9, 3250. [Google Scholar] [CrossRef]
- Chen, L.; Xing, R.; Jiang, A.; Yao, Y.; Zhou, G. Effects of nitrogen source and N/P on growth and photosynthesis in the invasive marine macroalga Chaetomorpha valida. Environ. Sci. Pollut. Res. Int. 2020, 27, 24272–24283. [Google Scholar]
- Mousavi, M.; Mehrzad, J.; Najafi, M.F.; Zhiani, R.; Shamsian, S.A.A. Nitrate and ammonia: Two key nitrogen sources for biomass and phycocyanin production by Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2022, 34, 2271–2281. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Mancini-Filho, J.; Schinke, K.P.; Aidar, E. Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: An evaluation for aquaculture. Phycologia 2002, 41, 158–168. [Google Scholar] [CrossRef]
- Fong, P.; Kamer, K.; Boyer, K.E.; Boyle, K.A. Nutrient content of macroalgae with differing morphologies may indicate sources of nutrients for tropical marine systems. Mar. Ecol. Prog. Ser. 2001, 220, 137–152. [Google Scholar] [CrossRef]
- Costanzo, S.D.; O’Donohue, M.J.; Dennison, W.C. Gracilaria edulis (Rhodophyta) as a biological indicator of pulsed nutrients in oligotrophic waters. J. Phycol. 2000, 36, 680–685. [Google Scholar] [CrossRef]
- Martins, I.; Pardal, M.Â.; Lillebø, A.I.; Flindt, M.R.; Marques, J.C. Hydrodynamics as a major factor controlling the occurrence of green macroalgal blooms in a eutrophic estuary: A case study on the influence of precipitation and river management. Estuar. Coast. Shelf Sci. 2001, 52, 165–177. [Google Scholar] [CrossRef]
- Uya, M.; Maggi, E.; Mori, G.; Nuccio, C.; Gribben, P.E.; Bulleri, F. Carry over effects of nutrient addition on the recovery of an invasive seaweed from the winter die-back. Mar. Environ. Res. 2017, 126, 37–44. [Google Scholar] [CrossRef]
- Young, E.B.; Berges, J.A.; Dring, M.J. Physiological responses of intertidal marine brown algae to nitrogen deprivation and resupply of nitrate and ammonium. Physiol. Plant 2009, 135, 400–411. [Google Scholar] [CrossRef]
- Young, E.B.; Dring, M.J.; Savidge, G.; Birkett, D.A.; Berges, J.A. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant Cell Environ. 2007, 30, 764–774. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour. Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef]
- Gutierrez, J.; Kwan, T.A.; Zimmerman, J.B.; Peccia, J. Ammonia inhibition in oleaginous microalgae. Algal Res. 2016, 19, 123–127. [Google Scholar] [CrossRef]
- Msuya, F.E.; Neori, A. Effect of water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J. Appl. Phycol. 2008, 20, 1021–1031. [Google Scholar] [CrossRef]
- Moraes, L.; da Rosa, G.M.; de Souza, M.d.R.A.Z.; Costa, J.A.V. Carbon dioxide biofixation and production of Spirulina sp. LEB 18 biomass with different concentrations of NaNO3 and NaCl. Braz. Arch. Biol. Technol. 2018, 61, e18150711. [Google Scholar] [CrossRef]
- Barr, N.G.; Rees, T.A.V. Nitrogen status and metabolism in the green seaweed Enteromorpha intestinalis: An examination of three natural population. Mar. Ecol. Prog. Ser. 2003, 249, 133–144. [Google Scholar] [CrossRef]
- Andria, J.; Vergara, J.; Perez-Llorens, J.L. Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cádiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur. J. Phycol. 1999, 34, 497–504. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Neefus, C.D.; Chung, I.K.; Yarish, C. Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J. Appl. Phycol. 2007, 19, 431–440. [Google Scholar] [CrossRef]
- Chapman, A.R.O.; Craigie, J.S. Seasonal growth in Laminaria longicruris: Relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar. Biol. 1977, 40, 197–205. [Google Scholar] [CrossRef]
- Duke, C.S.; Litaker, R.W.; Ramus, J. Seasonal variation in RuBPCase activity and N allocation in the chlorophyte seaweeds Ulva curvata (Kutz.) De Toni and Codium decorticatum (Woodw.) Howe. J. Exp. Mar. Biol. Ecol. 1987, 112, 145–164. [Google Scholar] [CrossRef]
- Smit, A.J.; Robertson, B.L.; du Preez, D.R. Influence of ammonium-N pulse concentrations and frequency, tank condition and nitrogen starvation on growth rate and biochemical composition of Gracilaria Gracilis. J. Appl. Phycol. 1996, 8, 473–481. [Google Scholar] [CrossRef]
- Bird, K.T.; Habig, C.; DeBusk, T. Nitrogen allocation and storage patterns in Gracilaria tikvahiae (Rhodophyta). J. Phycol. 1982, 18, 344–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).