Hydrogeochemical Responses of MTMS-Coated Capillary Cover under Heavy Rainfalls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Measurement of Soil Hydrophobicity
2.3. Geotechnical and Hydrogeological Properties
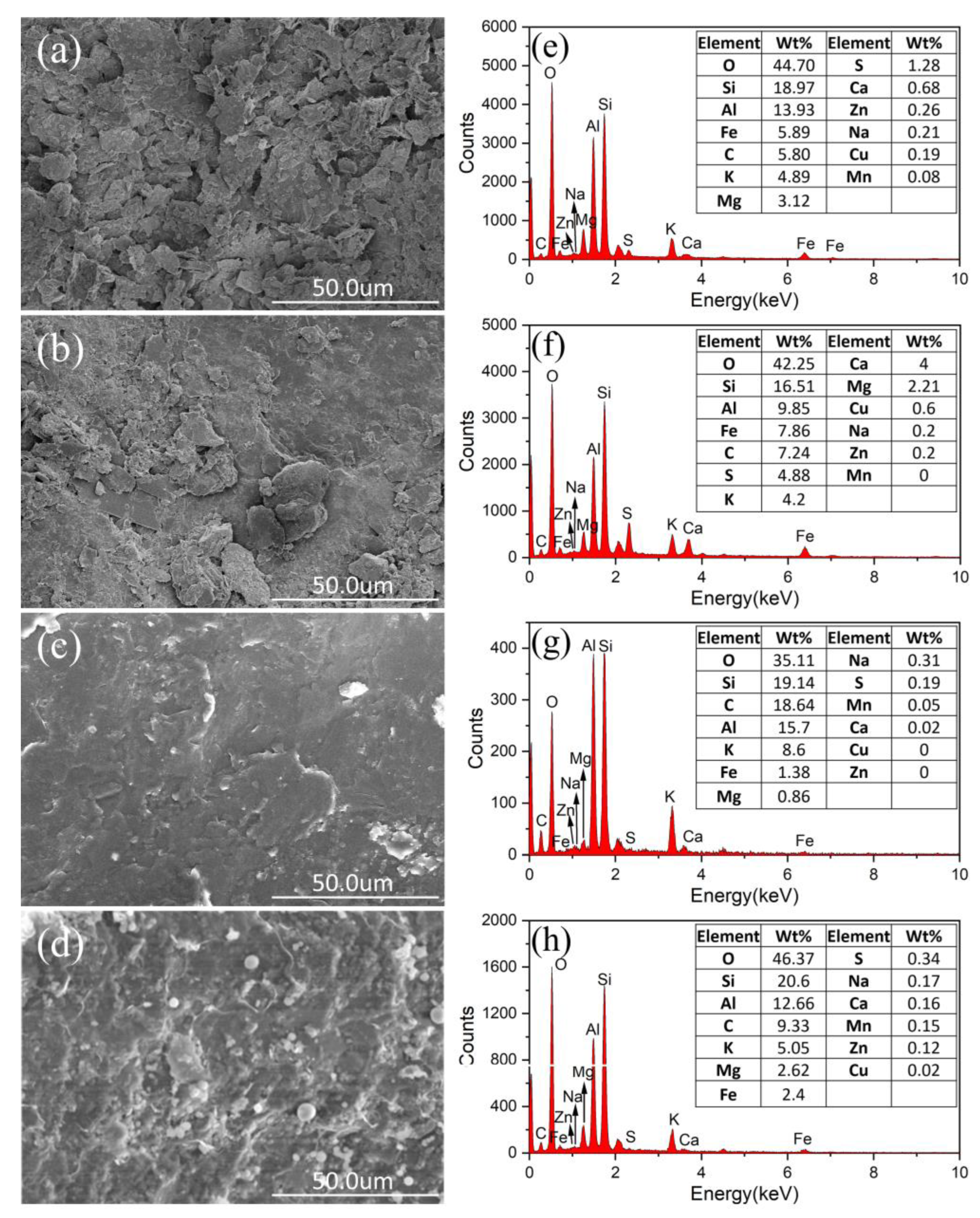
2.4. Mineralogical and Element Composition
2.5. Column Test
2.6. Test Procedures
3. Results
3.1. SWCCs and Permeability Functions
3.2. Selection Criteria of Capillary Cover Materials
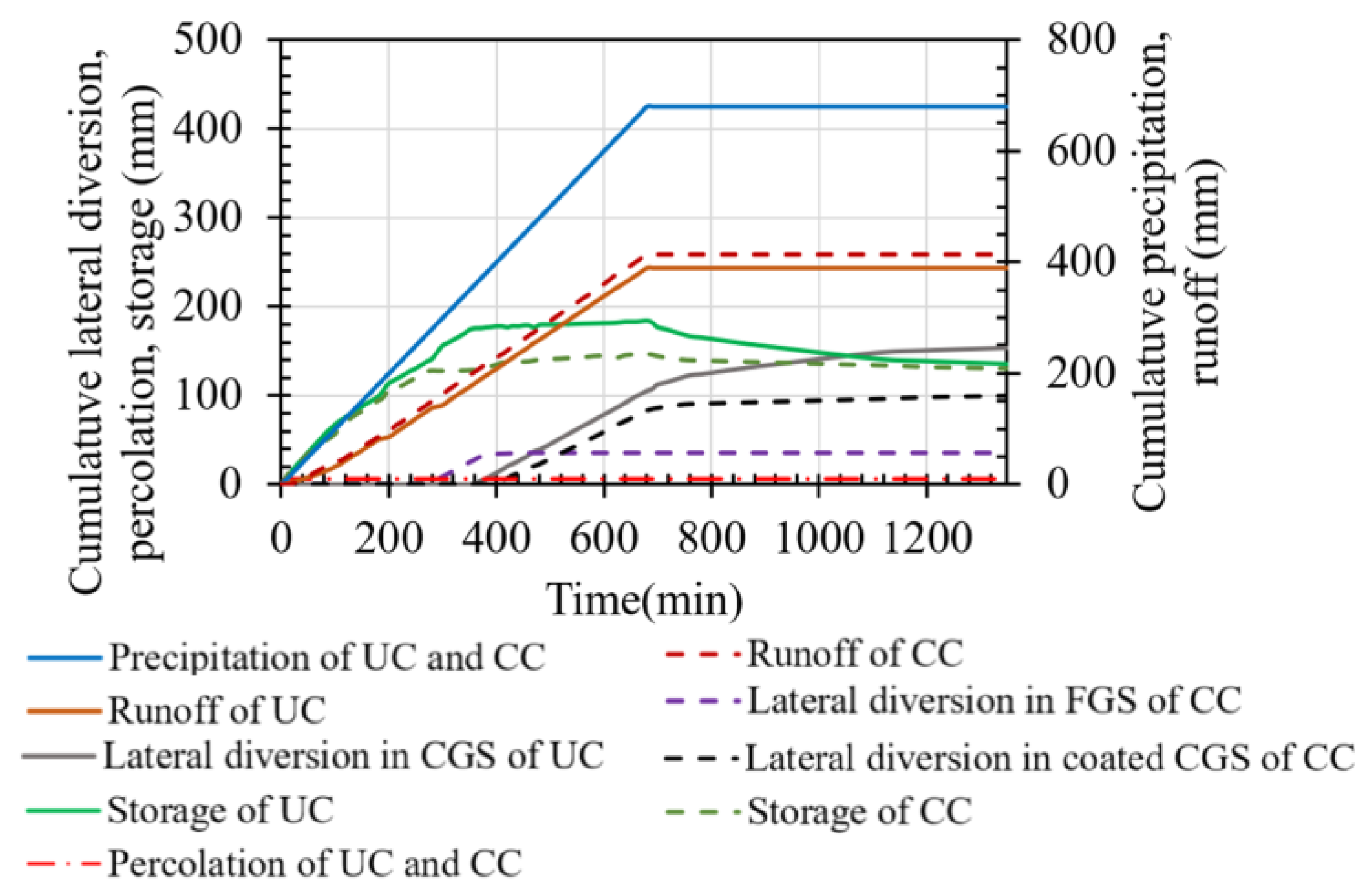
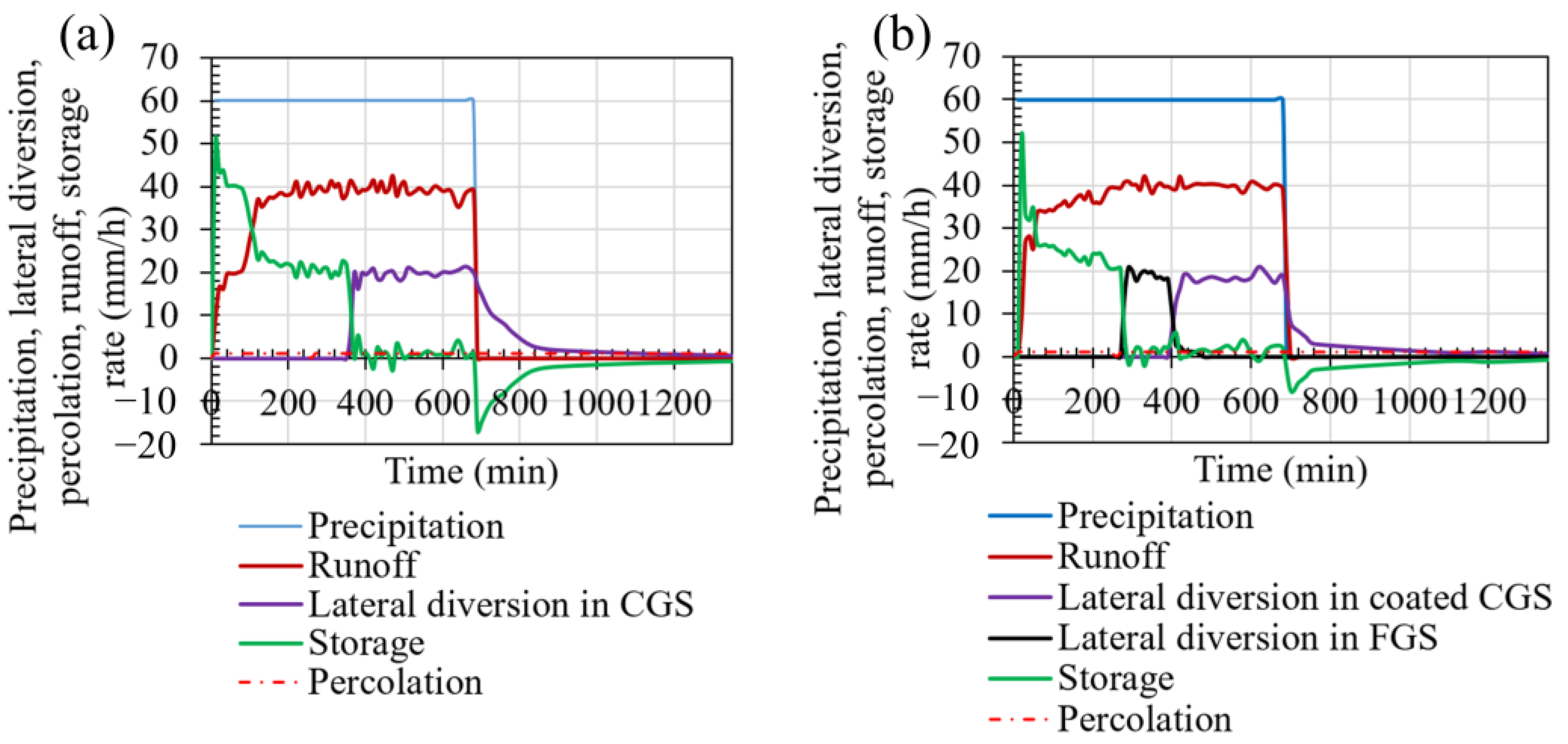
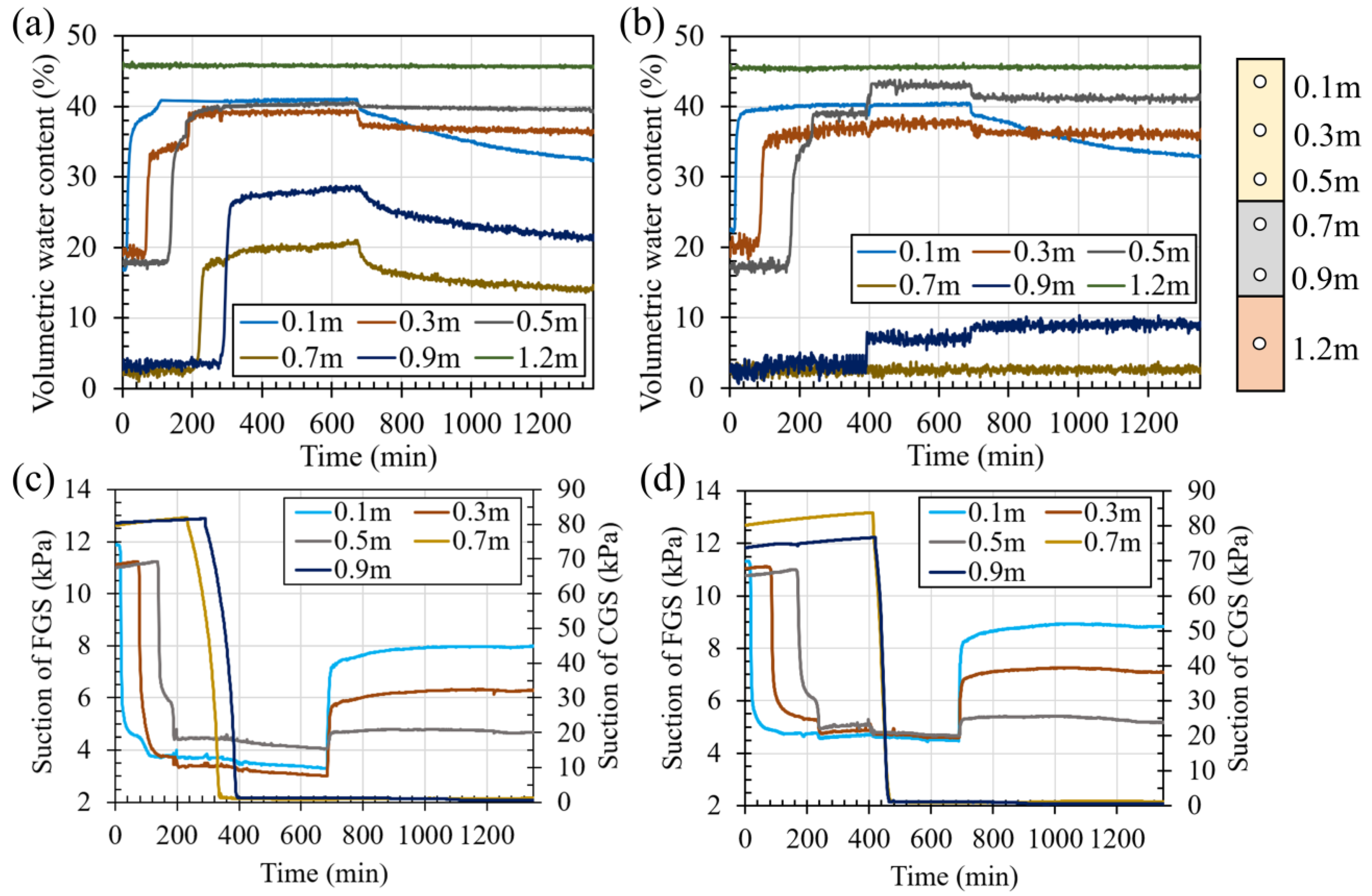
3.3. Performance of Uncoated Cover
3.4. Performance of the Coated Cover
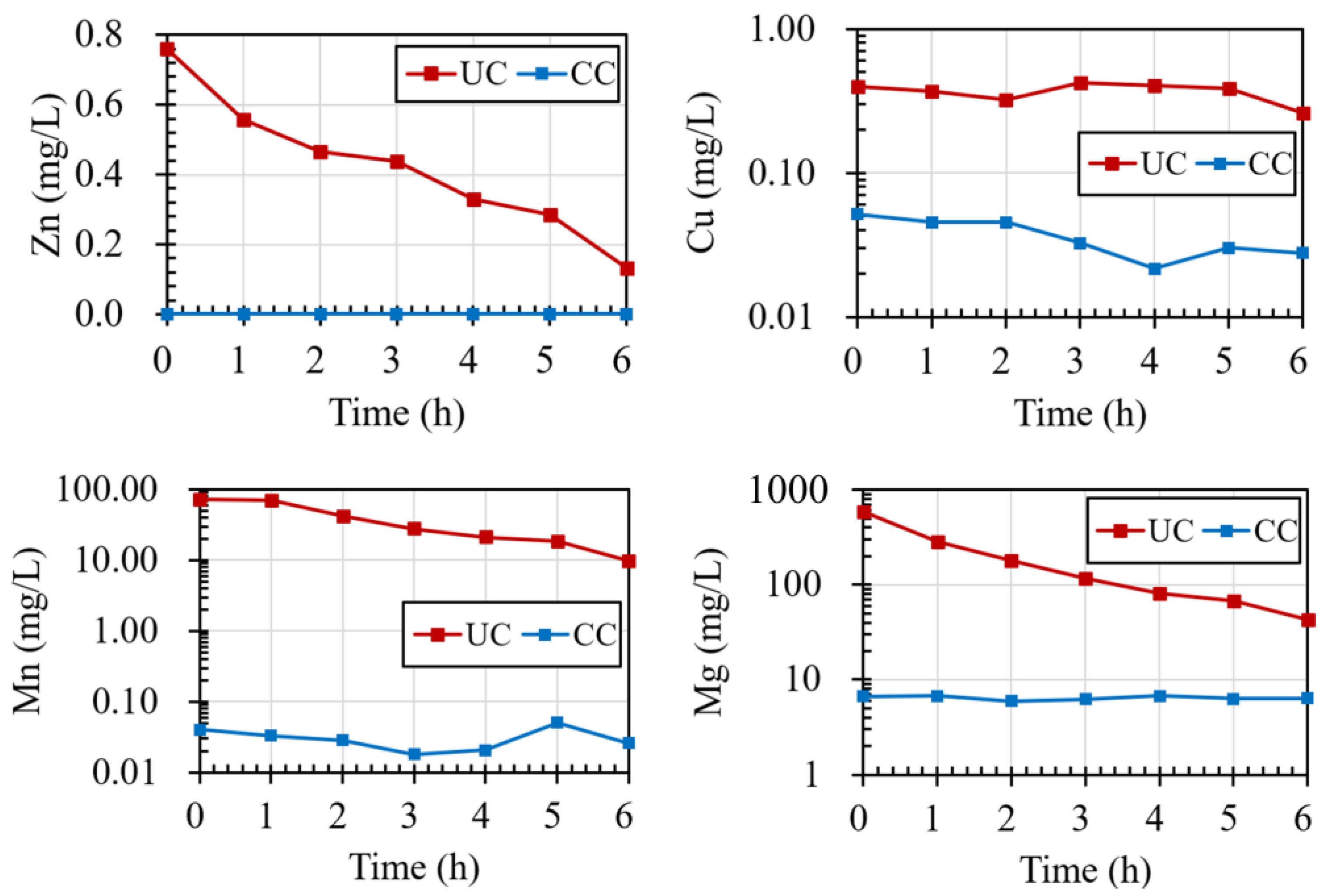
3.5. Chemical Speciation of the Collected Water Samples
4. Discussion
4.1. The Effect of Coated Waste Rock on the Geochemical Behavior of the Covers
4.2. The Effect of Coated Waste Rock on the Hydrogeological Behavior of the Covers
5. Conclusions
- (1)
- Under the condition of heavy rainfall experiment, both uncoated and coated covers exhibited zero leakage.
- (2)
- Compared with the uncovered cover layer, the MTMS coating increases the contact angle and WEV of the waste rock, delays rainwater entering the CGS layer for 180 min, and increases the water storage capacity of the FGS layer by 15 mm.
- (3)
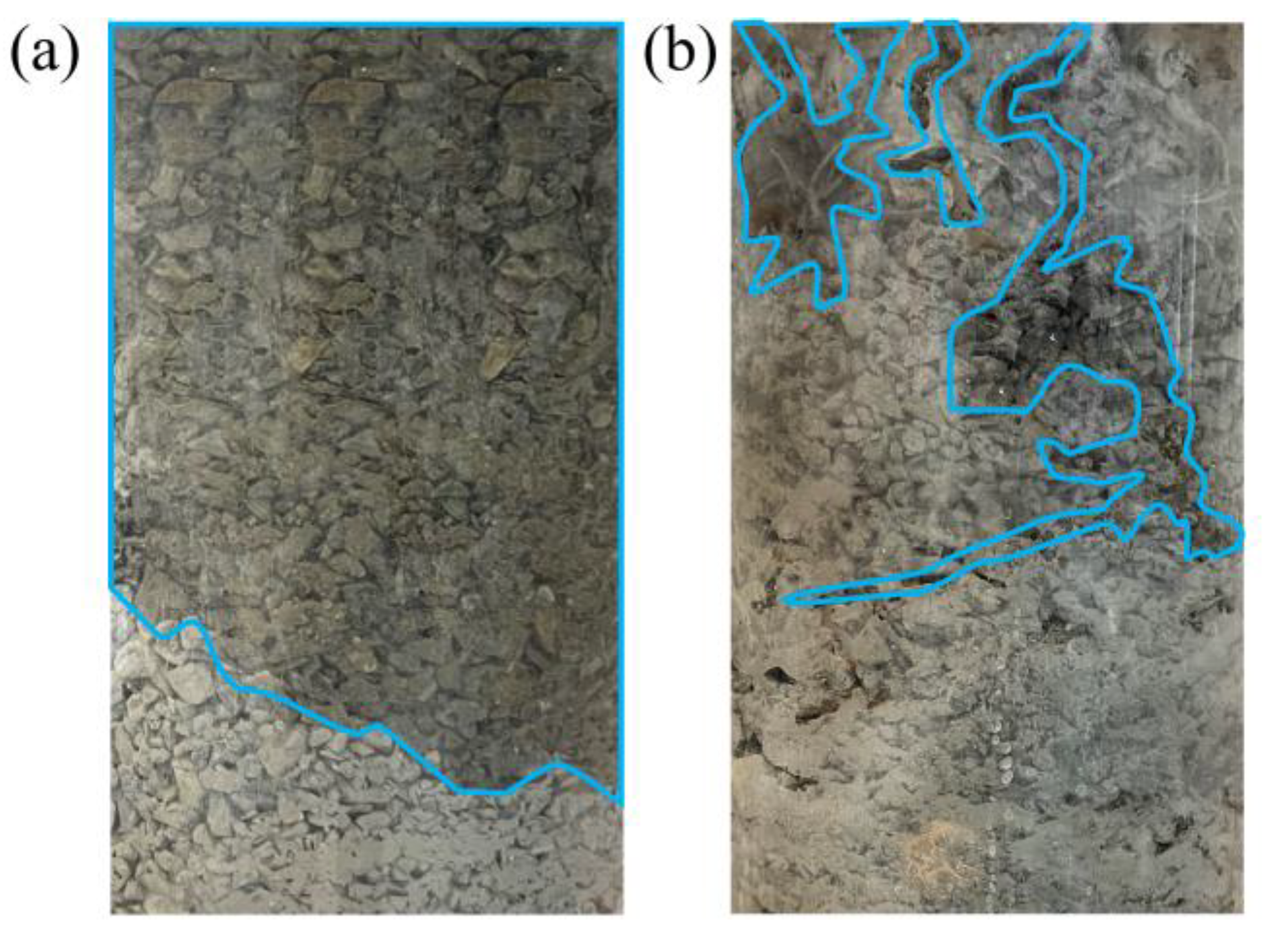
- Through SEM-EDX testing of the surface morphology and elemental content of the waste rock before and after passivation, the surface of the coated waste rock is smoother and the Fe and S content is reduced, which reduces the potential for acid generation of the waste rock. The passivation film adheres well to the surface of the waste rock.
- (4)
- The water quality of the lateral drainage from the cover system constructed using raw materials from the mine met the discharge requirements. In addition, the passivation treatment technology reduced the concentration of heavy-metal ions in the lateral drainage by over 90%, which holds promises as regards environmental protection.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Albright, W.H.; Benson, C.H.; Gee, G.W.; Roesler, A.C.; Abichou, T.; Apiwantragoon, P.; Lyles, B.F.; Rock, S.A. Field water balance of landfill final covers. J. Environ. Qual. 2004, 33, 2317–2332. [Google Scholar] [CrossRef] [PubMed]
- Bussière, B.; Benzaazoua, M.; Aubertin, M.; Mbonimpa, M. A laboratory study of covers made of low-sulphide tailings to prevent acid mine drainage. Environ. Geol. 2004, 45, 609–622. [Google Scholar] [CrossRef]
- Aubertin, M.; Cifuentes, E.; Apithy, S.A.; Bussiere, B.; Molson, J.; Chapuis, R.P. Analyses of water diversion along inclined covers with capillary barrier effects. Can. Geotech. J. 2009, 46, 1146–1164. [Google Scholar] [CrossRef]
- Sinnathamby, G.; Phillips, D.H.; Sivakumar, V.; Paksy, A. Landfill cap models under simulated climate change precipitation: Impacts of cracks and root growth. Géotechnique 2014, 64, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Jian-yong, S.; Xue-de, Q.; Yue-bing, Z. Experimental methods for interface behaviors of geosynthetics in landfills. Chin. J. Geotech. Eng. 2010, 32, 688–692. [Google Scholar]
- Albright, W.H.; Benson, C.H.; Gee, G.W.; Abichou, T.; McDonald, E.V.; Tyler, S.W.; Rock, S.A. Field performance of a compacted clay landfill final cover at a humid site. J. Geotech. Geoenviron. Eng. 2006, 132, 1393–1403. [Google Scholar] [CrossRef]
- Ng, C.W.; Chen, R.; Coo, J.L.; Liu, J.; Ni, J.; Chen, Y.M.; Zhan, L.-T.; Guo, H.W.; Lu, B.W. A novel vegetated three-layer landfill cover system using recycled construction wastes without geomembrane. Can. Geotech. J. 2019, 56, 1863–1875. [Google Scholar] [CrossRef]
- O’kane, M.; Wilson, G.; Barbour, S. Instrumentation and monitoring of an engineered soil cover system for mine waste rock. Can. Geotech. J. 1998, 35, 828–846. [Google Scholar] [CrossRef]
- Yanful, E.K.; Simms, P.H.; Payant, S.C. Soil covers for controlling acid generation in mine tailings: A laboratory evaluation of the physics and geochemistry. Water Air Soil Pollut. 1999, 114, 347–375. [Google Scholar] [CrossRef]
- Morris, C.E.; Stormont, J.C. Parametric study of unsaturated drainage layers in a capillary barrier. J. Geotech. Geoenvironmental Eng. 1999, 125, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Argunhan-Atalay, C.; Yazicigil, H. Modeling and performance assessment of alternative cover systems on a waste rock storage area. Mine Water Environ. 2018, 37, 106–118. [Google Scholar] [CrossRef]
- Apiwantragoon, P.; Benson, C.H.; Albright, W.H. Field Hydrology of Water Balance Covers for Waste Containment. J. Geotech. Geoenviron. Eng. 2015, 141, 04014101. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.W.; Liu, J.; Chen, R.; Xu, J. Physical and numerical modeling of an inclined three-layer (silt/gravelly sand/clay) capillary barrier cover system under extreme rainfall. Waste Manag. 2015, 38, 210–221. [Google Scholar] [CrossRef]
- Chen, R.; Huang, J.; Leung, A.K.; Chen, Z.; Chen, Z. Experimental investigation on water release and gas emission of evapotranspirative capillary barrier landfill covers. Soil Sci. Soc. Am. J. 2022, 86, 311–323. [Google Scholar] [CrossRef]
- Zheng, S.; Xing, X.; Lourenço, S.D.; Cleall, P.J. Cover systems with synthetic water-repellent soils. Vadose Zone J. 2021, 20, e20093. [Google Scholar] [CrossRef]
- Lourenço, S.; Jones, N.; Morley, C.; Doerr, S.; Bryant, R. Hysteresis in the soil water retention of a sand–clay mixture with contact angles lower than ninety degrees. Vadose Zone J. 2015, 14, vzj2014.07.0088. [Google Scholar] [CrossRef]
- Wijewardana, N.S.; Kawamoto, K.; Moldrup, P.; Komatsu, T.; Kurukulasuriya, L.C.; Priyankara, N.H. Characterization of water repellency for hydrophobized grains with different geometries and sizes. Environ. Earth Sci. 2015, 74, 5525–5539. [Google Scholar] [CrossRef]
- Subedi, S.; Kawamoto, K.; Jayarathna, L.; Vithanage, M.; Moldrup, P.; de Jonge, L.W.; Komatsu, T. Characterizing time-dependent contact angles for sands hydrophobized with oleic and stearic acids. Vadose Zone J. 2012, 11, vzj2011-0055. [Google Scholar] [CrossRef]
- Bardet, J.-P.; Jesmani, M.; Jabbari, N. Permeability and compressibility of wax-coated sands. Géotechnique 2014, 64, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.S.H.; Lourenço, S.D.N. Comparison of three silane compounds to impart water repellency in an industrial sand. Géotechnique Lett. 2016, 6, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; Lourenço, S. Conditions to induce water repellency in soils with dimethyldichlorosilane. Géotechnique 2016, 66, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Lamparter, A.; Bachmann, J.; Goebel, M.-O.; Woche, S. Carbon mineralization in soil: Impact of wetting–drying, aggregation and water repellency. Geoderma 2009, 150, 324–333. [Google Scholar] [CrossRef]
- Bachmann, J.; Woche, S.; Goebel, M.O.; Kirkham, M.; Horton, R. Extended methodology for determining wetting properties of porous media. Water Resour. Res. 2003, 39. [Google Scholar] [CrossRef]
- Dell’Avanzi, E.; Guizelini, A.; Da Silva, W.; Nocko, L.; Buzzi, O. Potential Use of Induced Soil-Water Repellency Techniques to Improve the Performance of Landfill’s Alternative Final Cover Systems; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rahardjo, H.; Santoso, V.; Leong, E.; Ng, Y.; Tam, C.; Satyanaga, A. Use of recycled crushed concrete and Secudrain in capillary barriers for slope stabilization. Can. Geotech. J. 2013, 50, 662–673. [Google Scholar] [CrossRef]
- Boulanger-Martel, V.; Bussière, B.; Côté, J.; Mbonimpa, M. Influence of freeze–thaw cycles on the performance of covers with capillary barrier effects made of crushed rock–bentonite mixtures to control oxygen migration. Can. Geotech. J. 2016, 53, 753–764. [Google Scholar] [CrossRef]
- Nason, P.; Jia, Y.; Maurice, C.; Alakangas, L.; Öhlander, B. Biodegradation of biosolids under aerobic conditions: Implications for cover materials for sulfide mine tailings remediation. Mine Water Environ. 2016, 35, 273–282. [Google Scholar] [CrossRef]
- Hey, C.; Simms, P. Preliminary assessment of biosolids in covers with capillary barrier effects. Eng. Geol. 2021, 280, 105973. [Google Scholar] [CrossRef]
- Kabambi, A.K.; Bussiere, B.; Demers, I. Hydrogeological Behaviour of Covers with Capillary Barrier Effects Made of Mining Materials. Geotech. Geol. Eng. 2017, 35, 1199–1220. [Google Scholar] [CrossRef]
- Larochelle, C.G.; Bussière, B.; Pabst, T. Acid-generating waste rocks as capillary break layers in covers with capillary barrier effects for mine site reclamation. Water Air Soil Pollut. 2019, 230, 57. [Google Scholar] [CrossRef]
- Duchesne, J.; Doye, I. Effectiveness of covers and liners made of red mud bauxite and/or cement kiln dust for limiting acid mine drainage. J. Environ. Eng. 2005, 131, 1230–1235. [Google Scholar] [CrossRef]
- Mollamahmutoğlu, M.; Yilmaz, Y. Potential use of fly ash and bentonite mixture as liner or cover at waste disposal areas. Environ. Geol. 2001, 40, 1316–1324. [Google Scholar] [CrossRef]
- Soares, A.B.; Ubaldo, M.d.O.; de Souza, V.P.; Moreira Soares, P.S.; Barbosa, M.C.; Mendonça, R.M.G. Design of a Dry Cover Pilot Test for Acid Mine Drainage Abatement in Southern Brazil. I: Materials Characterization and Numerical Modeling. Mine Water Environ. 2009, 28. [Google Scholar] [CrossRef]
- Dublet-Adli, G.; Pabst, T.; Okkenhaug, G.; Sætre, C.; Vårheim, A.; Tvedten, M.; Gelena, S.; Smebye, A.; Kvennås, M.; Breedveld, G. Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site. Minerals 2021, 11, 987. [Google Scholar] [CrossRef]
- Hosseini, M.; Karapanagiotis, I. Materials with Extreme Wetting Properties: Methods and Emerging Industrial Applications; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Saulick, Y.; Lourenço, S.D.; Baudet, B.A. Optimising the hydrophobicity of sands by silanisation and powder coating. Géotechnique 2021, 71, 250–259. [Google Scholar] [CrossRef]
- Dong, Y.; Zeng, W.; Lin, H.; He, Y. Preparation of a novel water-soluble organosilane coating and its performance for inhibition of pyrite oxidation to control acid mine drainage at the source. Appl. Surf. Sci. 2020, 531, 147328. [Google Scholar] [CrossRef]
- Chau, H.W.; Biswas, A.; Vujanovic, V.; Si, B.C. Relationship between the severity, persistence of soil water repellency and the critical soil water content in water repellent soils. Geoderma 2014, 221, 113–120. [Google Scholar] [CrossRef]
- Hewelke, E.; Gozdowski, D.; Korc, M.; Małuszyńska, I.; Górska, E.B.; Sas, W.; Mielnik, L. Influence of soil moisture on hydrophobicity and water sorptivity of sandy soil no longer under agricultural use. Catena 2022, 208, 105780. [Google Scholar] [CrossRef]
- Zheng, S.; Lourenço, S.D.; Cleall, P.J.; Chui, T.F.M.; Ng, A.K.; Millis, S.W. Hydrologic behavior of model slopes with synthetic water repellent soils. J. Hydrol. 2017, 554, 582–599. [Google Scholar] [CrossRef]
- Doerr, S.H. On standardizing the ‘water drop penetration time’and the ‘molarity of an ethanol droplet’techniques to classify soil hydrophobicity: A case study using medium textured soils. Earth Surf. Process. Landf. J. Br. Geomorphol. Group 1998, 23, 663–668. [Google Scholar] [CrossRef]
- ASTM D422; Standard Test Method for Particle-Size Analysis of Soils. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D4318; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D698; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D5084-16a; Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D2434-22; Standard Test Method for Permeability of Granular Soils (Constant Head). ASTM International: West Conshohocken, PA, USA, 2022.
- Bate, B.; Nie, S.; Chen, Z.; Zhang, F.; Chen, Y. Construction of soil–water characteristic curve of granular materials with toroidal model and artificially generated packings. Acta Geotech. 2021, 16, 1949–1960. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Wu, Q. Water-entry value as an alternative indicator of soil water-repellency and wettability. J. Hydrol. 2000, 231, 76–83. [Google Scholar] [CrossRef]
- Rahardjo, H.; Tami, D.; Leong, E. Effectiveness of sloping capillary barriers under high precipitation rates. In Proceedings of the 2nd International Conference on Problematic Soils, Petaling Jaya, Malaysia, 3–6 December 2006; pp. 3–5. [Google Scholar]
- Ecole Polytechnique (Montréal, Québec). Centre de développement technologique, Aubertin, M. Evaluation en Laboratoire des Barrières Sèches Construites à Partir de Résidus Miniers: Rapport Final; École polytechnique de Montréal: Montreal, QC, Canada, 1995. [Google Scholar]
- Smesrud, J.K.; Selker, J.S. Effect of soil-particle size contrast on capillary barrier performance. J. Geotech. Geoenviron. Eng. 2001, 127, 885–888. [Google Scholar] [CrossRef]
- Parent, S.-É.; Cabral, A. Design of inclined covers with capillary barrier effect. Geotech. Geol. Eng. 2006, 24, 689–710. [Google Scholar] [CrossRef]
- Shaikh, J.; Bordoloi, S.; Yamsani, S.K.; Sekharan, S.; Rakesh, R.R.; Sarmah, A.K. Long-term hydraulic performance of landfill cover system in extreme humid region: Field monitoring and numerical approach. Sci. Total Environ. 2019, 688, 409–423. [Google Scholar] [CrossRef]
- Hopp, L.; McDonnell, J.J.; Condon, P. Lateral subsurface flow in a soil cover over waste rock in a humid temperate environment. Vadose Zone J. 2011, 10, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Zhan, L.-T.; Li, G.-Y.; Jiao, W.-G.; Lan, J.-W.; Chen, Y.-M.; Shi, W. Performance of a compacted loess/gravel cover as a capillary barrier and landfill gas emissions controller in Northwest China. Sci. Total Environ. 2020, 718, 137195. [Google Scholar] [CrossRef]
- Bussière, B.; Aubertin, M.; Chapuis, R.P. The behavior of inclined covers used as oxygen barriers. Can. Geotech. J. 2003, 40, 512–535. [Google Scholar] [CrossRef]
- Khire, M.V.; Benson, C.H.; Bosscher, P.J. Capillary barriers: Design variables and water balance. J. Geotech. Geoenviron. Eng. 2000, 126, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Huminicki, D.M.; Rimstidt, J.D. Iron oxyhydroxide coating of pyrite for acid mine drainage control. Appl. Geochem. 2009, 24, 1626–1634. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 2. Rate control by oxide coatings. Geochim. Et Cosmochim. Acta 1990, 54, 395–402. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Dagenais, A.-M.; Archambault, M. Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ. Geol. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- GB 25467; Emission Standard of Pollutants for Copper, Nickel and Cobalt Industry. China Environmental Press: Beijing, China, 2010.
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar]
- Khummalai, N.; Boonamnuayvitaya, V. Suppression of arsenopyrite surface oxidation by sol-gel coatings. J. Biosci. Bioeng. 2005, 99, 277–284. [Google Scholar] [CrossRef]
- Stormont, J.C.; Morris, C.E. Method to estimate water storage capacity of capillary barriers. J. Geotech. Geoenviron. Eng. 1998, 124, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Movasat, M.; Tomac, I. Assessment of Physical Properties of Water-Repellent Soils. J. Geotech. Geoenviron. Eng. 2021, 147, 06021010. [Google Scholar] [CrossRef]

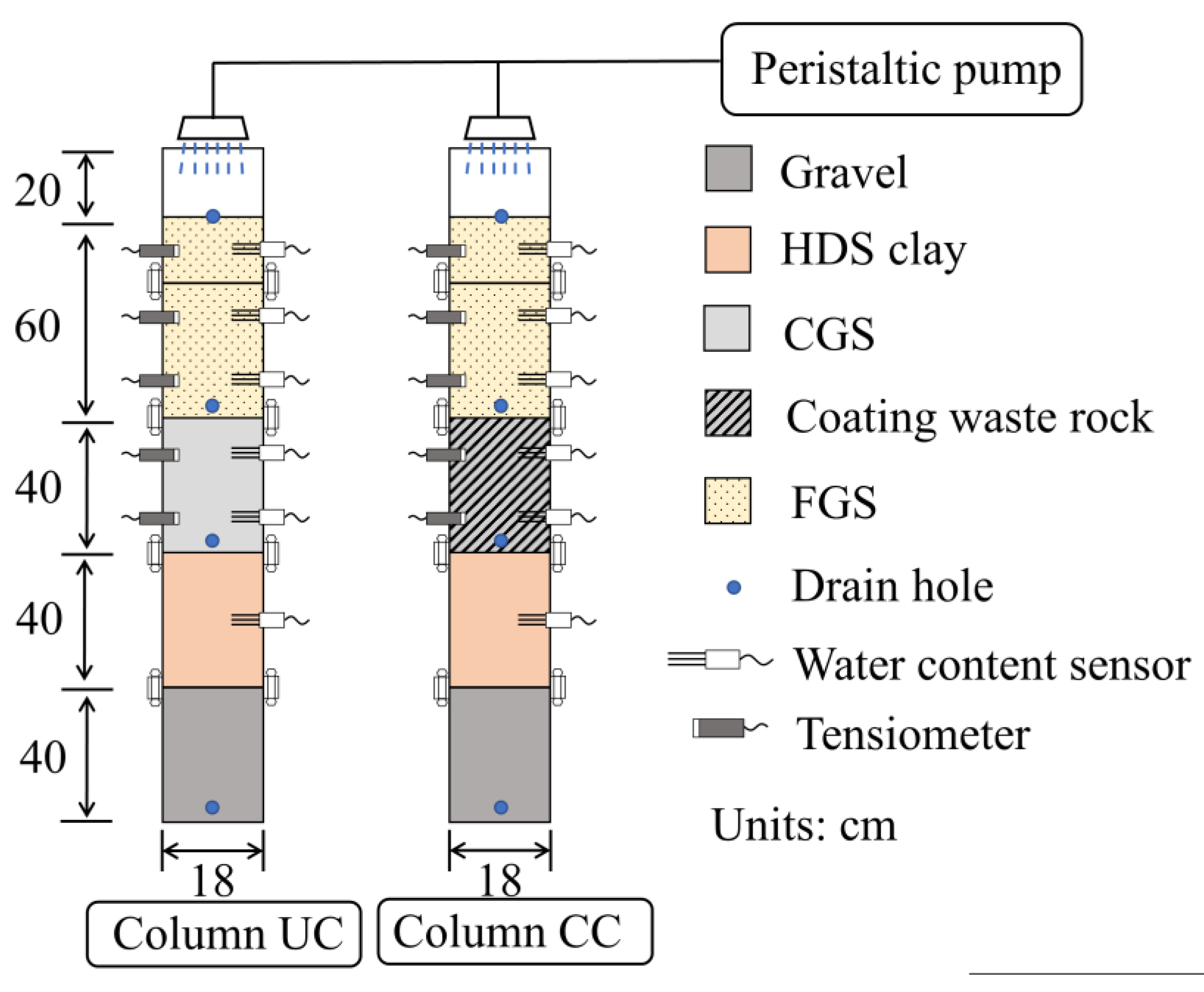
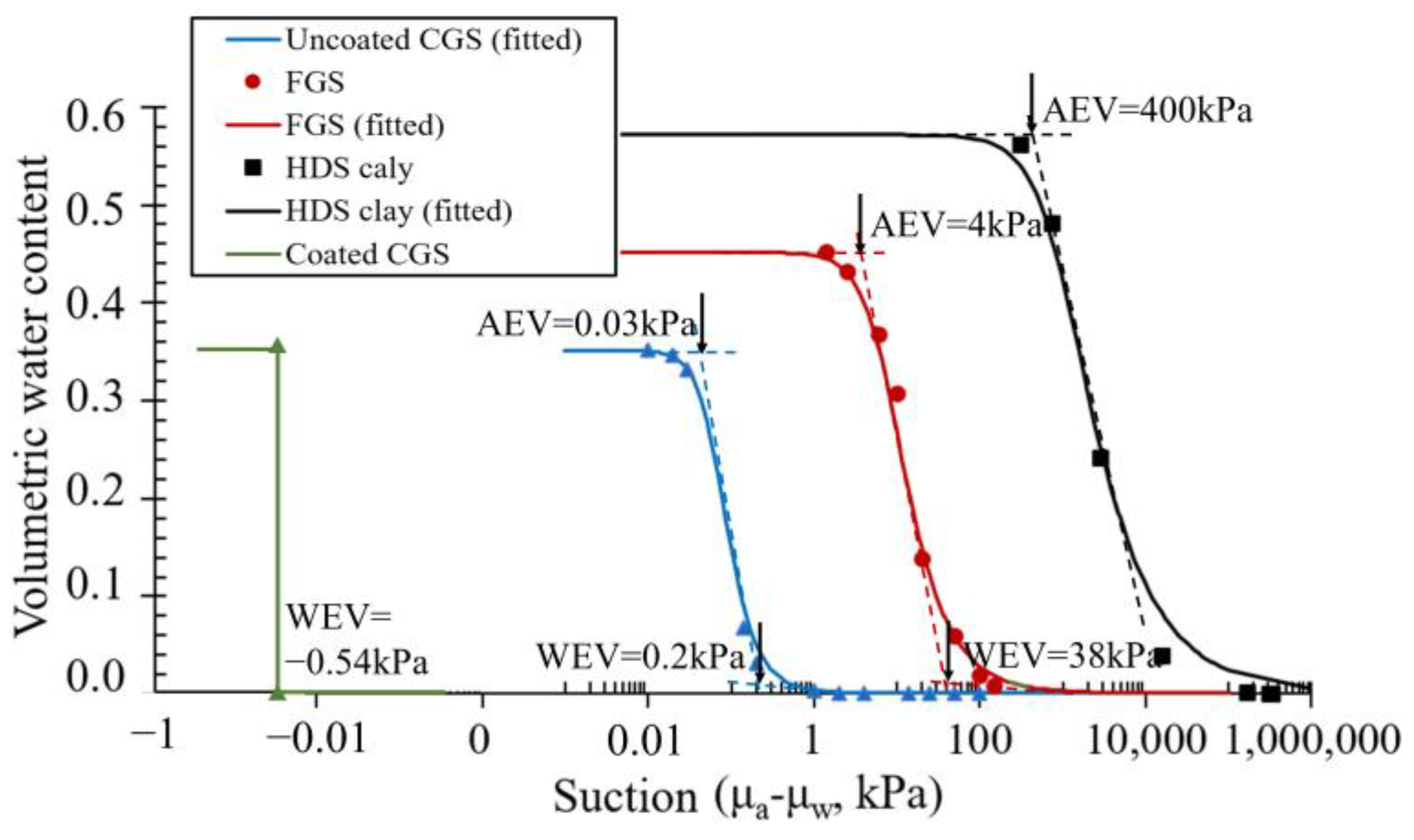
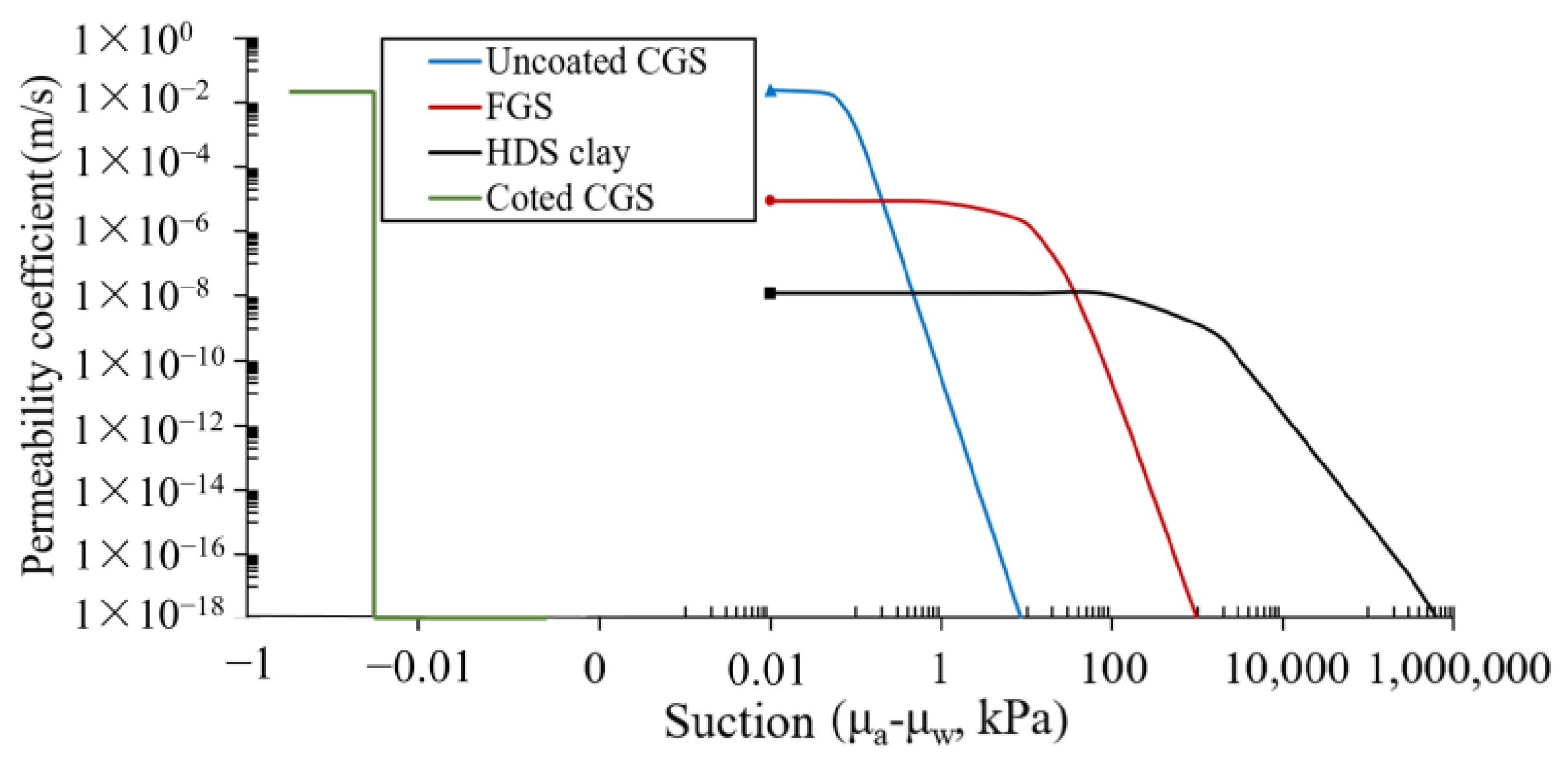
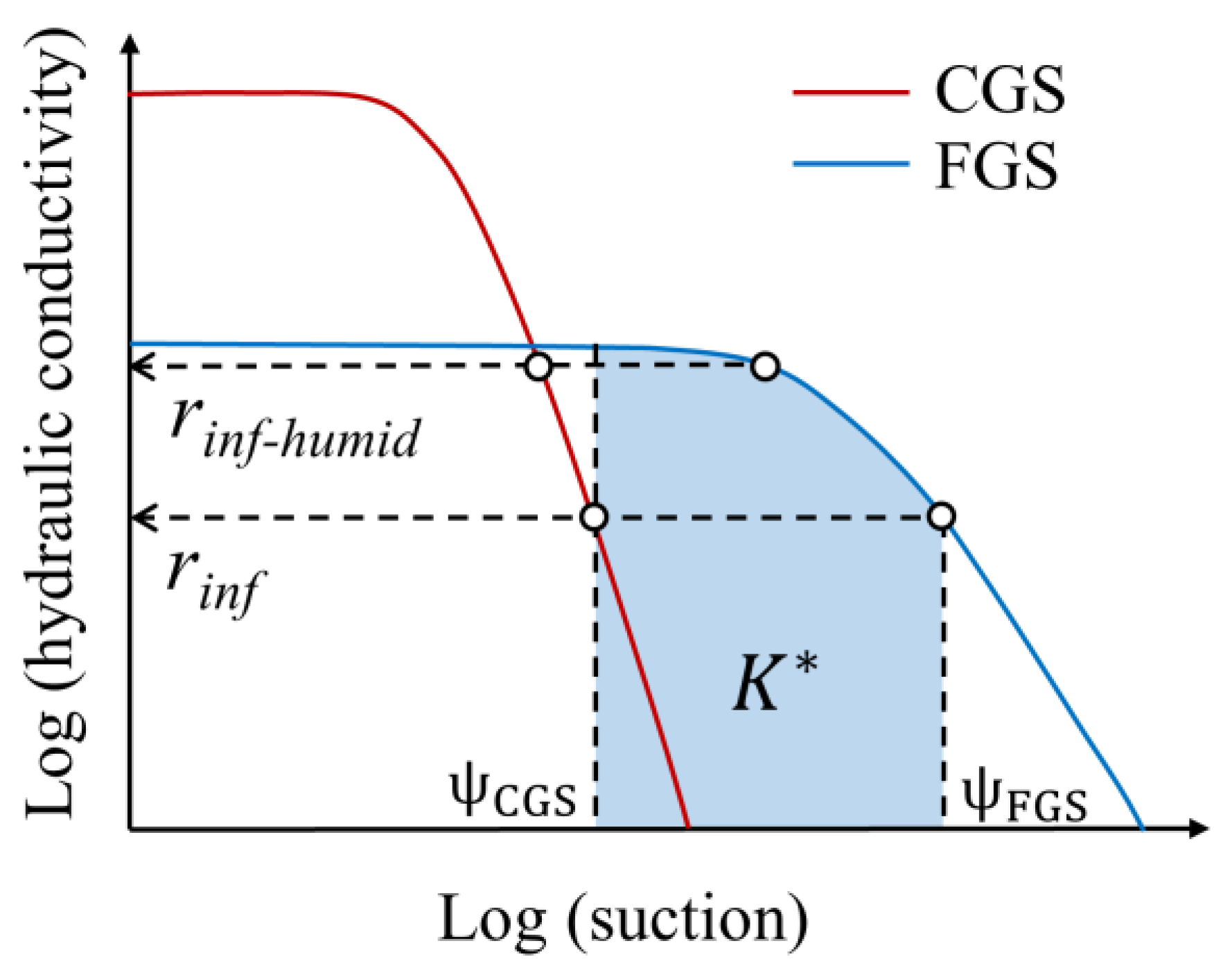
| Parameter | FGS | CGS | HDS Clay |
|---|---|---|---|
| Specific gravity, | 2.73 | - | 2.37 |
| Atterberg limits | |||
| Liquid limit | 20.3 | - | 61.4 |
| Plastic limit | 1.9 | - | 40.3 |
| Plasticity index | 18.3 | - | 21.1 |
| Grain-size diameter | |||
| 0.015 | 0.43 | 0.0013 | |
| 0.085 | 2.5 | 0.0038 | |
| 0.18 | 6.0 | 0.0095 | |
| Coefficient of uniformity, | 12 | 2.4 | 7.3 |
| Coefficient of curvature, | 2.68 | 2.42 | 1.17 |
| Maximum dry densities (g/cm3) | 1.77 | 1.842 | 1.15 |
| Optimum water content (%) | 12.2 | - | 47.3 |
| ksat (m/s) | 8.12 × 10−6 | 2.37 × 10−2 | 1.12 × 10−8 |
| Air entry value (kPa) | 4 | 0.03 | 400 |
| (kPa−1) | 0.13 | 14.5 | 1 × 10−3 |
| n | 2.1 | 2.8 | 1.7 |
| m | 0.52 | 0.64 | 0.41 |
| Tailing | HDS Clay | Waste Rock | Coated Waste Rock | |
|---|---|---|---|---|
| Quartz | 52.8 | 34.2 | 17.5 | 9.7 |
| Muscovite | 31.7 | 16.9 | 47.5 | 17.2 |
| Clinochlore | 5.7 | 12.4 | 15.7 | 7.9 |
| Calcite | - | 10.5 | 4.2 | 1.1 |
| Microcline | 7.3 | 5.9 | - | - |
| Kaolinite | 2.4 | - | - | - |
| Albite | - | 20.2 | - | - |
| Pyrite | - | 4.7 | 2.2 | |
| Rutile | - | - | 1 | 0.4 |
| Gypsum | - | - | 7.3 | 2.8 |
| Cronstedtite | - | - | - | 0.4 |
| Nacrite | - | - | 1.3 | - |
| Calcium Sulfate | - | - | 1 | - |
| MTMS | - | - | - | 58.3 ** |
| Element | Units | Tailing | HDS Clay | Waste Rock | Coated Waste Rock |
|---|---|---|---|---|---|
| Fe | wt% | 3.66 | 3.96 | 6.87 | 5.31 |
| Al | wt% | 1.77 | 0.17 | 2.28 | 1.80 |
| K | wt% | 2.38 | 0.07 | 2.37 | 2.34 |
| Na | wt% | 0.50 | 0.86 | 1.91 | 0.51 |
| Ca | mg/kg | 255.6 | 5794.4 | 910.2 | 159.8 |
| Cu | mg/kg | 1320.1 | 390.6 | 1852.4 | 1630.5 |
| Zn | mg/kg | 50.8 | 118.9 | 802.6 | 28.4 |
| As | mg/kg | 102.2 | 90.9 | 219.0 | 99.6 |
| Sr | mg/kg | 9.2 | 24.3 | 16.7 | 1.1 |
| Mo | mg/kg | 90.4 | 4.0 | 104.2 | 51.1 |
| Cd | mg/kg | 1.68 | 1.90 | 2.47 | 0.83 |
| Ba | mg/kg | 94.8 | 11.6 | 586.9 | 27.7 |
| Hg | mg/kg | 0.28 | 0.33 | 0.22 | 0.31 |
| Pb | mg/kg | 10.1 | 5.6 | 16.8 | 8.6 |
| Mg | mg/kg | 124.9 | 1068.6 | 436.2 | 142.6 |
| Cr | mg/kg | 63.0 | 11.3 | 76.1 | 79.3 |
| Mn | mg/kg | 267.1 | 949.8 | 225.0 | 192.8 |
| Co | mg/kg | 48.1 | 71.4 | 91.8 | 149.5 |
| Ni | mg/kg | 38.3 | 86.6 | 55.4 | 71.9 |
| Reference | Climate | WEVCGS | AEVFGS | ksat, FGS | |||
|---|---|---|---|---|---|---|---|
| [8] | Humid | 0.4 | 2 | 200 | 3 | 5.7 × 10−5 | 68.2 |
| [55] | Humid | 270 | 720 | >1000 | 3 | 2.9 × 10−8 | 5.5 |
| [56] | Humid | 0.042 | 1.2 | >1000 | 2 | 2.3 × 10−5 | - |
| [57] | Semi-humid | 0.31 | 7.3 | 2194 | 5 | 1.8 × 10−7 | - |
| [12] | Semi-arid | 280 | 24 | - | 2 | 1.0 × 10−7 | - |
| [58] | Semi-arid | 1 | 3.5 | 7 | 3 | 5.1 × 10−5 | 8.4 |
| [59] | Arid and semi-arid | 92 | 74 | 97.8 | 1 | 2.7 × 10−4 | - |
| This study | Humid | 0.2 | 4 | 190 | 4 | 8.1 × 10−6 | 107 |
| Sample Name | Fe (mg/L) | Cu (mg/L) | Zn (mg/L) | Mn (mg/L) | Mg (mg/L) |
|---|---|---|---|---|---|
| Drainage of FGS | 0 | 0.059 | 0.038 | 0.046 | 8.516 |
| Drainage of coated CGS | 0 | 0.045 | 0 | 0.033 | 5.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Chen, J.; Yang, Y.; Zhao, H.; Zhan, L.; Bate, B. Hydrogeochemical Responses of MTMS-Coated Capillary Cover under Heavy Rainfalls. Sustainability 2023, 15, 6667. https://doi.org/10.3390/su15086667
Xia L, Chen J, Yang Y, Zhao H, Zhan L, Bate B. Hydrogeochemical Responses of MTMS-Coated Capillary Cover under Heavy Rainfalls. Sustainability. 2023; 15(8):6667. https://doi.org/10.3390/su15086667
Chicago/Turabian StyleXia, Liangxiong, Jiakai Chen, Yixin Yang, Hongfen Zhao, Liangtong Zhan, and Bate Bate. 2023. "Hydrogeochemical Responses of MTMS-Coated Capillary Cover under Heavy Rainfalls" Sustainability 15, no. 8: 6667. https://doi.org/10.3390/su15086667
APA StyleXia, L., Chen, J., Yang, Y., Zhao, H., Zhan, L., & Bate, B. (2023). Hydrogeochemical Responses of MTMS-Coated Capillary Cover under Heavy Rainfalls. Sustainability, 15(8), 6667. https://doi.org/10.3390/su15086667





