Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Purification of the Pathogen
2.3. Fungus Characterization
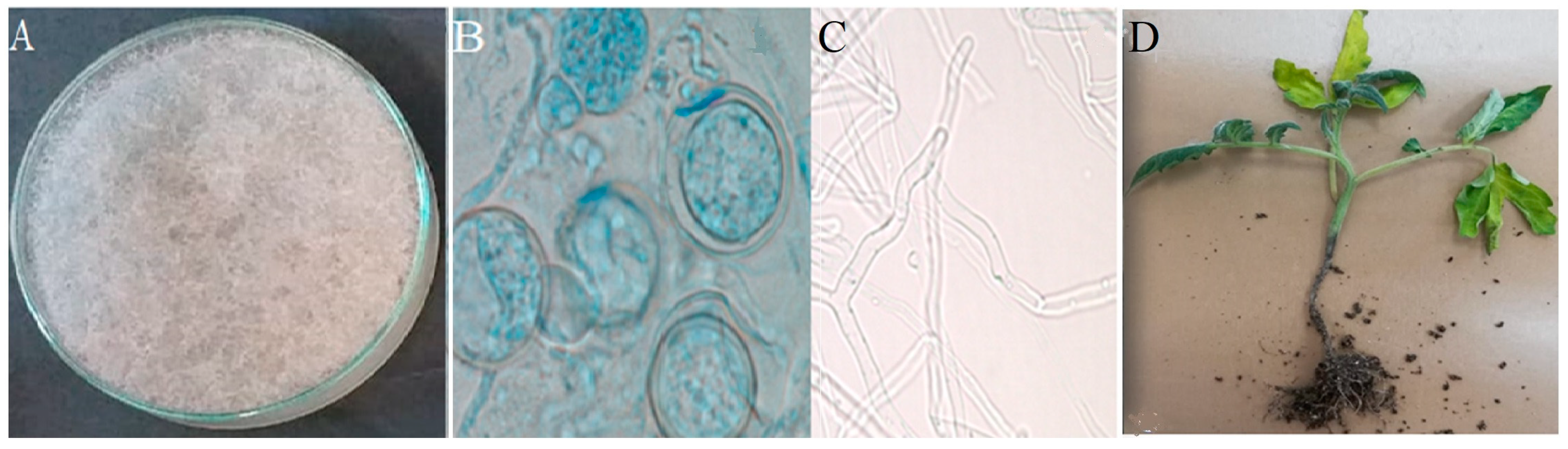
2.3.1. Morphological Identification
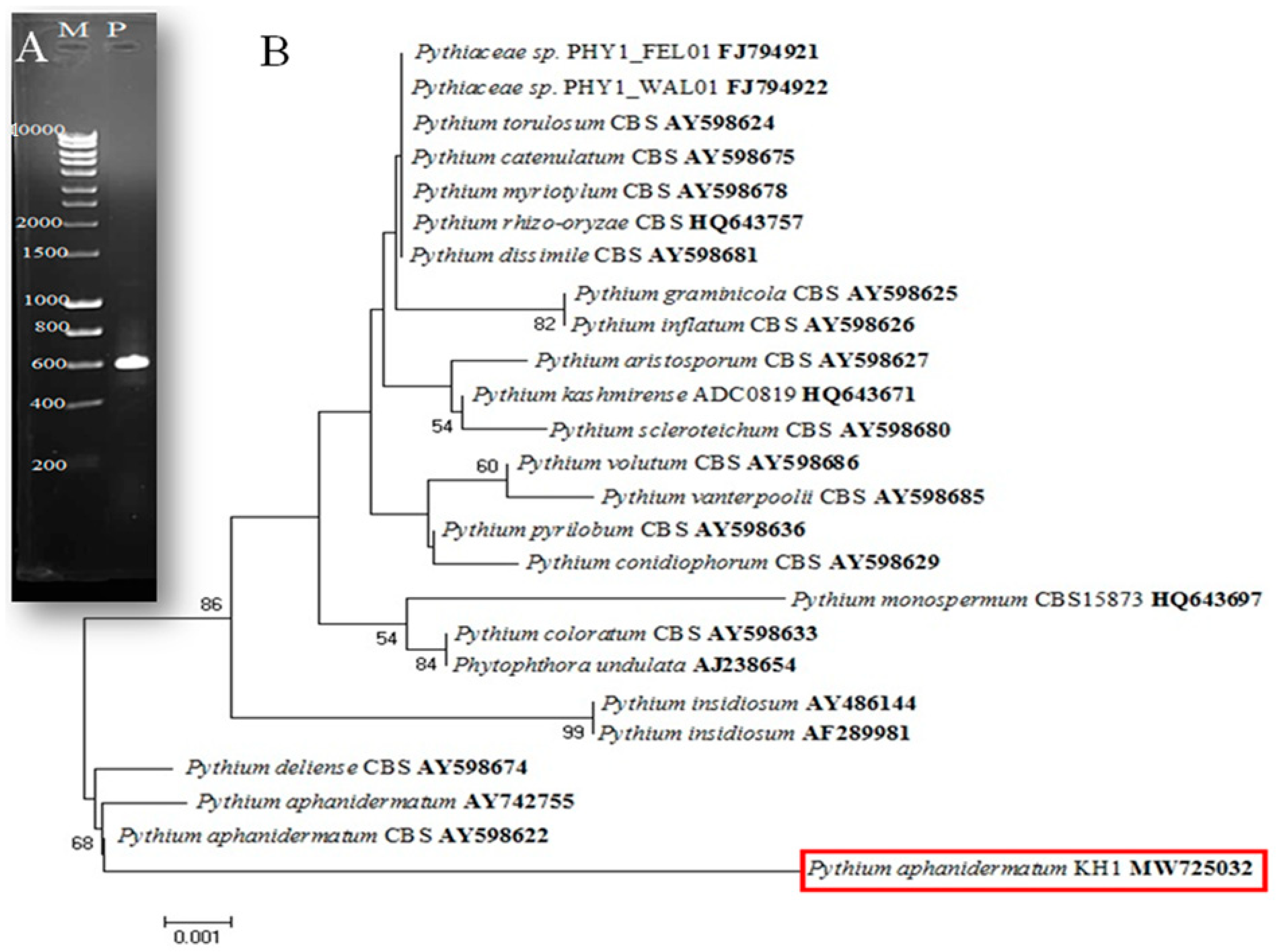
2.3.2. Molecular Identification of Fungi Using PCR
DNA Isolation of Fungal Cells
Agarose Gel Electrophoresis
Detection of the Fungus by ITS-PCR
PCR Product Purification
DNA Sequencing and Phylogenetic Construction
2.4. Greenhouse Experiment
2.5. Sample Collection and RNA Isolation
2.6. Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.7. Real-Time PCR
2.8. HPLC Analysis
2.9. Statistical Analysis
| Gene | Abbreviation | Sequence 5′-----3′ | Function | References |
|---|---|---|---|---|
| Beta-actin | β-actin | Frw-ATGCCATTCTCCGTCTTGACTTG Rev-GAACCTAAGCCACGATACCA | Housekeeping gene used for normalizing gene expression levels in many different types of cells. | [31] |
| Pathogenesis-related protein-1 | PR-1 | Frw-TTCTTCCCTCGAAAGCTCAA Rev-CGCTACCCCAGGCTAAGTTT | Plays a role in the plant’s defense against pathogens. PR-1 can bind to fungal cell walls and inhibit fungal growth; considered as a biomarker for plant stress response. | [32] |
| β-1,3-glucanases | PR-2 | Frw-TCACCAAACTATTGGATTTCAA Rev-GACTCAATTTTTGACTTCTTAATCC | Encodes the production of β-1,3-glucanase enzyme, which plays a role in defense against fungal pathogens. | [33] |
| Chitinase | PR-3 | Frw-ACTGGAGGATGGGCTTCAGCA Rev-TGGATGGGGCCTCGTCCGAA | Encoding the production of chitinase, the enzyme that breaks down chitin, found in the cell walls of fungi (cell wall degrading enzyme). | |
| Chitin binding protein | PR-4 | Frw-GACAACAATGCGGTCGTCAAGG Rev-AGCATGTTTCTGGAATCAGGCTG | Encodes a protein called chitinase-binding protein in plants, which binds to chitin and prevents fungal growth. | [34] |
| Thaumatin-like protein | PR-5 | Frw-ATGGGGTAAACCACCAAACA Rev-GTTAGTTGGGCCGAAAGACA | Thaumatin-like proteins (TLPs) are part of the family of PR proteins. Plants make more of these proteins when they are stressed by both biotic and abiotic factors. The PR-5 gene encodes for a type of TLP in plants, and it may act by disrupting the integrity of the cell walls of pathogens. | [32] |
3. Results and Discussion
3.1. Isolation and Identification of the Fungus Isolate
3.2. Greenhouse Trail and Pathogenicity Test
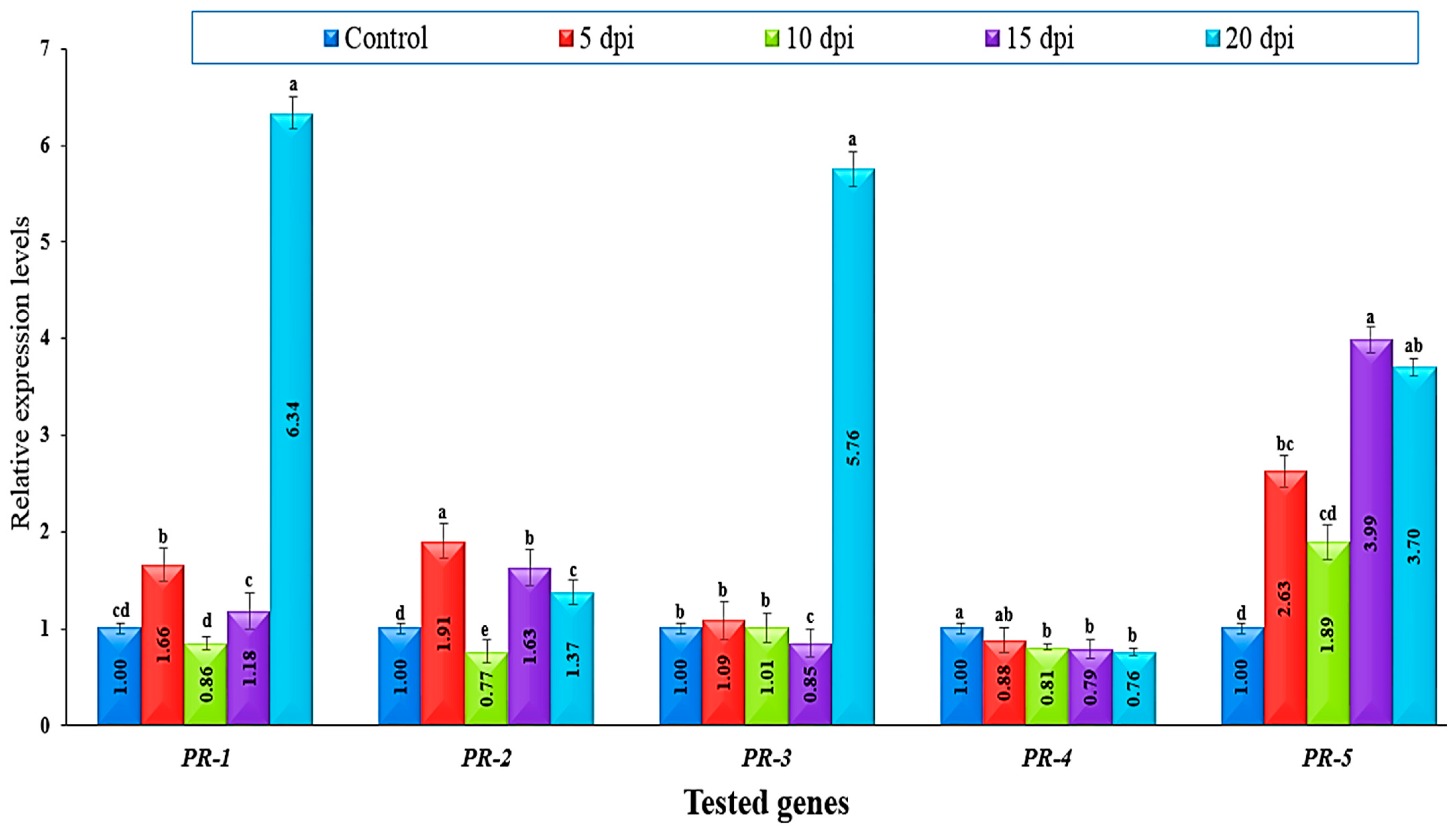
3.3. The Expression Levels of PR-Related Genes
3.4. HPLC Profiling of Tomato Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chellemi, D.O.; Gamliel, A.; Katan, J.; Subbarao, K.V. Development and deployment of systems-based approaches for the management of soilborne plant pathogens. Phytopathology 2016, 106, 216–225. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Schweiger, W.; Steiner, B. Breeding for resistance to head blight caused by Fusarium spp. in wheat. CABI Rev. 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Elshahawy, I.; Abouelnasr, H.M.; Lashin, S.M.; Darwesh, O.M. First report of Pythium aphanidermatum infecting tomato in Egypt and its control using biogenic silver nanoparticles. J. Plant Prot. Res. 2018, 58, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Deacon, J. Cellulose Decomposition by Pythium and Its Relevance to Substrate-Groups of Fungi. Trans. Br. Mycol. Soc. 1979, 72, 469–477. [Google Scholar] [CrossRef]
- Kipngeno, P.; Losenge, T.; Maina, N.; Kahangi, E.; Juma, P. Efficacy of Bacillus subtilis and Trichoderma asperellum against Pythium aphanidermatum in tomatoes. Biol. Control 2015, 90, 92–95. [Google Scholar] [CrossRef]
- Gupta, S.; Nawaz, K.; Parween, S.; Roy, R.; Sahu, K.; Kumar Pole, A.; Khandal, H.; Srivastava, R.; Kumar Parida, S.; Chattopadhyay, D. Draft genome sequence of Cicer reticulatum L., the wild progenitor of chickpea provides a resource for agronomic trait improvement. DNA Res. 2017, 24, 1–10. [Google Scholar] [PubMed]
- Siam, G.; Abdelhakim, T. Analysis of the Tomato Value Chain in Egypt and Establishment of an Action Plan to Increase Its Efficiency. Ph.D. Thesis, CIHEAM-IAMM, Montpellier, France, 2018; p. 118. [Google Scholar]
- Hafez, E.E.; Abdelkhalek, A.A.; Abd El-Wahab, A.S.E.-D.; Galal, F.H. Altered gene expression: Induction/suppression in leek elicited by Iris Yellow Spot Virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol. Equip. 2013, 27, 4061–4068. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Behiry, S.I.; Ashmawy, N.A.; Abdelkhalek, A.A.; Younes, H.A.; Khaled, A.E.; Hafez, E.E. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J. Plant Dis. Prot. 2018, 125, 197–204. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Barzegar, P.E.F.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Barzegar, K.E.F. The current natural/chemical materials and innovative technologies in periodontal diseases therapy and regeneration: A narrative review. Mater. Today Commun. 2022, 32, 104099. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Hafez, E. Differential induction and suppression of the potato innate immune system in response to Alfalfa mosaic virus infection. Physiol. Mol. Plant Pathol. 2020, 110, 101485. [Google Scholar] [CrossRef]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) following Cucumber mosaic virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Gillings, M.R.; Tesoriero, L.A.; Gunn, L.A. Detection of double-stranded RNA and virus-like particles in Australian isolates of Pythium irregular. Plant Pathol. 1993, 1, 6–15. [Google Scholar] [CrossRef]
- Sneh, B.; Burpee, L.; Qgoshi, A. Identification of Rhizocto-Nia Species; APS Press: Saint Paul, MN, USA, 1991; p. 133. [Google Scholar]
- Van der Plaats-Niterink, A.J. Monogrpah of the Genus Pythium. In Studies Mycology; No. 21; Centra Albareau vor Schimmel Cultures: Baarh, The Netherlands, 1981. [Google Scholar]
- Leck, A. Lactophenol Cotton Blue Slide Mounts. Community Eye Health 1999, 12, 24. [Google Scholar]
- Tiwari, K.L.; Jadhav, S.K.; Gupta, S. Modified CTAB technique for isolation of DNA from some medicinal plants. Res. J. Med. Plant 2012, 6, 65–73. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; University of Texas South Western Medical Center: Dallas, TX, USA, 1989. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984; ISBN 0471870927. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2009; Volume 2. [Google Scholar]
- Pasumarthy, K.K.; Mukherjee, S.K.; Choudhury, N.R. The presence of tomato leaf curl Kerala virus AC3 protein enhances viral DNA replication and modulates virus induced gene-silencing mechanism in tomato plants. Virol. J. 2011, 8, 178. [Google Scholar] [CrossRef]
- Wang, X.; El Hadrami, A.; Adam, L.R.; Daayf, F. Differential activation and suppression of potato defence responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol. 2008, 57, 1026–1037. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M. Contrasting regulation of NO and ROS in potato defense-associated metabolism in response to pathogens of different lifestyles. PLoS ONE 2016, 11, e0163546. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Lev, S.; Gepstein, S.; Horwitz, B.A. A compatible interaction of Alternaria brassicicola with Arabidopsis thaliana ecotype DiG: Evidence for a specific transcriptional signature. BMC Plant Biol. 2009, 9, 31. [Google Scholar] [CrossRef]
- Badotti, F.; de Oliveira, F.S.; Garcia, C.F.; Vaz, A.B.M.; Fonseca, P.L.C.; Nahum, L.A.; Oliveira, G.; Góes-Neto, A. Effectiveness of ITS and sub-regions as DNA barcode markers for the identification of Basidiomycota (Fungi). BMC Microbiol. 2017, 17, 42. [Google Scholar] [CrossRef]
- Matsumoto, C.; Kageyama, K.; Suga, H.; Hyakumachi, M. Phylogenetic relationships of Pythium species based on ITS and 5.8 S sequences of the ribosomal DNA. Mycoscience 1999, 40, 321–331. [Google Scholar] [CrossRef]
- Barboza, E.A.; Cabral, C.S.; Rossato, M.; Martins, F.; Reis, A. Pythium and Phytopythium species associated with weeds collected in vegetable production fields in Brazil. Lett. Appl. Microbiol. 2022, 74, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Eswaran, A.; Sangeetha, G. Occurrence, virulence and pathogenicity of species of Pythium inciting damping-off disease in chilli. J. Mycol. Plant Pathol. 2010, 40, 67. [Google Scholar]
- Garibaldi, A.; Gilardi, G.; Ortu, G.; Gullino, M.L. First report of damping-off caused by Pythium aphanidermatum on leaf beet (Beta vulgaris subsp. vulgaris) in Italy. Plant Dis. 2013, 97, 292. [Google Scholar] [CrossRef]
- Dai, L.; Wang, D.; Xie, X.; Zhang, C.; Wang, X.; Xu, Y.; Wang, Y.; Zhang, J. The novel gene VpPR4-1 from Vitis pseudoreticulata increases powdery mildew resistance in transgenic Vitis vinifera L. Front. Plant Sci. 2016, 7, 695. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Graham, J.H.; Cubero, J.; Ravnskov, S. Biocontrol traits of plant growth suppressive arbuscular mycorrhizal fungi against root rot in tomato caused by Pythium aphanidermatum. Eur. J. Plant Pathol. 2012, 133, 361–369. [Google Scholar] [CrossRef]
- Moghaddam, G.A.; Rezayatmanda, Z.; Esfahani, M.N.; Khozaei, M. Genetic defense analysis of tomatoes in response to early blight disease, Alternaria alternata. Plant Physiol. Biochem. 2019, 142, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Gamir, J.; Darwiche, R.; Van’t Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of pathogenesis-related protein 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Al Daoude, A.; Shoaib, A.; Al-Shehadah, E.; Jawhar, M.; Arabi MI, E. Pathogenesis—Related genes responses in barley plants challenged with pathogenic fungi with different lifestyles. Cereal Res. Commun. 2020, 48, 341–346. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, B.K. Isolation of a basic 34 kiloDalton β-1, 3-glucanase with inhibitory activity against Phytophthora capsicifrom pepper stems. Physiol. Mol. Plant Pathol. 1997, 50, 103–115. [Google Scholar] [CrossRef]
- Sierra-Gomez, Y.; Annia Rodrıguez-Hernandez, P.C.-S.; Homero Gomez-Velasco, A.H.-S.; Siliqi, D.; Rodrıguez-Romero, A. A biophysical and structural study of two chitinases from Agave tequilana and their potential role as defense proteins. FEBS J. 2019, 286, 4778–4796. [Google Scholar] [CrossRef]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21. [Google Scholar]
- Singh, R.; Tiwari, J.K.; Sharma, V.; Rawat, S. Role of Pathogen related protein families in defence mechanism with potential role in applied biotechnology. Int. J. Adv. Res. 2014, 2, 210–226. [Google Scholar]
- El-Hadary, M.H.; Tayel, A.A. Differential expression of tomato chitinases upon Alternaria solani inoculation with special reference to a modified purification zymogram. Egypt. J. Exp. Biol. (Bot.) 2013, 9, 9–17. [Google Scholar]
- Jongedijk, E.; Tigelaar, H.; van Roekel, J.S.C.; Bres-Vloemans, S.A.; Dekker, I.; van den Elzen, P.J.M.; Cornelissen, B.J.C.; Melchers, L.S. Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica Int. J. Plant Breed. 1995, 85, 173–180. [Google Scholar] [CrossRef]
- Wubben, J.P.; Lawrence, C.B.; De Wit, P.J.G.M. Differential induction of chitinase and 1,3-β-glucanase gene expression in tomato by Cladosporium fulvum and its race-specific elicitors. Physiol. Mol. Plant Pathol. 1996, 48, 105–116. [Google Scholar] [CrossRef]
- Zhu, Q.; Maher, E.A.; Masoud, S.; Dixon, R.A.; Lamb, C.J. Enhanced protection against fungal attack by constitutive co--expression of chitinase and glucanase genes in transgenic tobacco. Bio/Technology 1994, 12, 807–812. [Google Scholar] [CrossRef]
- Jach, G.; Görnhardt, B.; Mundy, J.; Logemann, J.; Pinsdorf, E.; Leah, R.; Schell, J.; Maas, C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.; Hashem, M.; Balbaa, M.M.; Ma, E.; Ahmed, S.A. Induction of New Defensin Genes in Tomato Plants via Pathogens-Biocontrol Plant Pathology & Microbiology Induction of New Defensin Genes in Tomato Plants via Pathogens-Biocontrol Agent Interaction. Plant Pathol. Microbiol. 2013, 4, 167. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G. Plant thaumatin-like proteins: Function, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, H.; Kato, H.; Nakatsu, T.; Oda, J.; Yamada, Y.; Sato, F. Purification and characterization of tobacco pathogenesis-related protein PR-5d, an antifungal thaumatin-like protein. Plant Cell Physiol. 1997, 38, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochemistry 2002, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krebitz, M.; Wagner, B.; Ferreira, F.; Peterbauer, C.; Campillo, N.; Witty, M.; Kolarich, D.; Steinkellner, H.; Scheiner, O.; Breiteneder, H. Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein. J. Mol. Biol. 2003, 329, 721–730. [Google Scholar] [CrossRef]
- Khatri, R.K.; Shastry, R.P.; Reddy, P.N.; Nema, K.G. Metabolic changes in rice leaves infected by Entyloma oryzae. Indian Phytopathol. 1985, 38, 769–771. [Google Scholar]
- Naik, M.K.; Hiremath, P.C.; Hiremath, S.V. Post Infectional Changes in the Betelvine Leaves Infected with Colletotrichum gloeosporioides. Indian Phytopathol. 1988, 41, 370–372. [Google Scholar]
- Nema, A.G. Sugar and phenol contents of betelvine leaves after inoculation with leaf spot bacterium. Indian Phytopathol. 1989, 42, 31–37. [Google Scholar]
- Ejike, C.E.C.C.; Gong, M.; Udenigwe, C.C. Phytoalexins from the Poaceae: Biosynthesis, function and prospects in food preservation. Food Res. Int. 2013, 52, 167–177. [Google Scholar] [CrossRef]
- Behiry, S.; Soliman, S.A.; Massoud, M.A.; Abdelbary, M.; Kordy, A.M.; Abdelkhalek, A.; Heflish, A. Trichoderma pubescens Elicit Induced Systemic Resistance in Tomato Challenged by Rhizoctonia solani. J. Fungi 2023, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Na, H.; Kwack, Y.; Chun, C. Secondary metabolite profiling in various parts of tomato plants. Hortic. Sci. Technol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwack, Y.; Lee, J.H.; Chun, C. Antimicrobial activity of various parts of tomato plants varied with different solvent extracts. plant Pathol. J. 2019, 35, 149. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef]
- Ortega-García, J.G.; Montes-Belmont, R.; Rodríguez-Monroy, M.; Ramírez-Trujillo, J.A.; Suárez-Rodríguez, R.; Sepúlveda-Jiménez, G. Effect of Trichoderma asperellum applications and mineral fertilization on growth promotion and the content of phenolic compounds and flavonoids in onions. Sci. Hortic. 2015, 195, 8–16. [Google Scholar] [CrossRef]
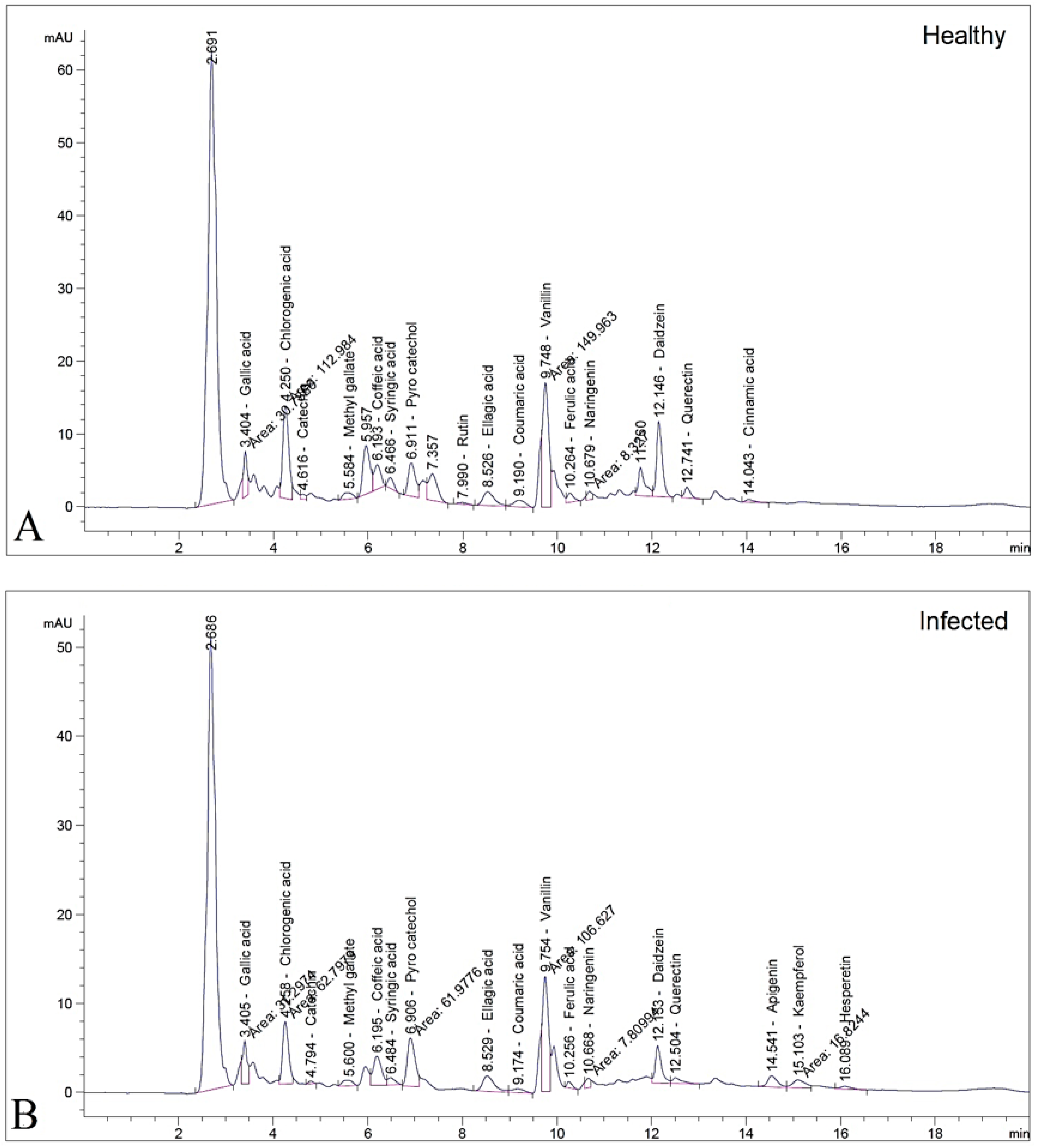
| Polyphenolic Compounds | Concentration (µg/g) | |
|---|---|---|
| Control | Infected | |
| Gallic acid | 192.4 | 195.6 |
| Chlorogenic acid | 1110.4 | 617.2 |
| Catechin | 72.4 | 30.7 |
| Methyl gallate | 45.8 | 35.8 |
| Caffeic acid | 182.1 | 219.2 |
| Syringic acid | 62.8 | 45.0 |
| Pyro catechol | 476.7 | 603.3 |
| Rutin | 30.6 | 0.0 |
| Ellagic acid | 609.8 | 578.9 |
| Coumaric acid | 28.6 | 13.7 |
| Vanillin | 421.0 | 299.3 |
| Ferulic acid | 54.5 | 23.3 |
| Naringenin | 57.9 | 54.3 |
| Daidzein | 403.5 | 177.6 |
| Querectin | 102.3 | 67.9 |
| Cinnamic acid | 7.2 | 0.0 |
| Apigenin | 0.0 | 86.8 |
| Kaempferol | 0.0 | 134.1 |
| Hesperetin | 0.0 | 19.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.A.; Al-Askar, A.A.; Sobhy, S.; Samy, M.A.; Hamdy, E.; Sharaf, O.A.; Su, Y.; Behiry, S.I.; Abdelkhalek, A. Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction. Sustainability 2023, 15, 6551. https://doi.org/10.3390/su15086551
Soliman SA, Al-Askar AA, Sobhy S, Samy MA, Hamdy E, Sharaf OA, Su Y, Behiry SI, Abdelkhalek A. Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction. Sustainability. 2023; 15(8):6551. https://doi.org/10.3390/su15086551
Chicago/Turabian StyleSoliman, Seham A., Abdulaziz A. Al-Askar, Sherien Sobhy, Marwa A. Samy, Esraa Hamdy, Omaima A. Sharaf, Yiming Su, Said I. Behiry, and Ahmed Abdelkhalek. 2023. "Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction" Sustainability 15, no. 8: 6551. https://doi.org/10.3390/su15086551
APA StyleSoliman, S. A., Al-Askar, A. A., Sobhy, S., Samy, M. A., Hamdy, E., Sharaf, O. A., Su, Y., Behiry, S. I., & Abdelkhalek, A. (2023). Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction. Sustainability, 15(8), 6551. https://doi.org/10.3390/su15086551







