Estimation of Evaporation of Water from a Liquid Desiccant Solar Collector and Regenerator by Using Conservation of Mass and Energy Principles
Abstract
:1. Introduction
2. Materials and Methods
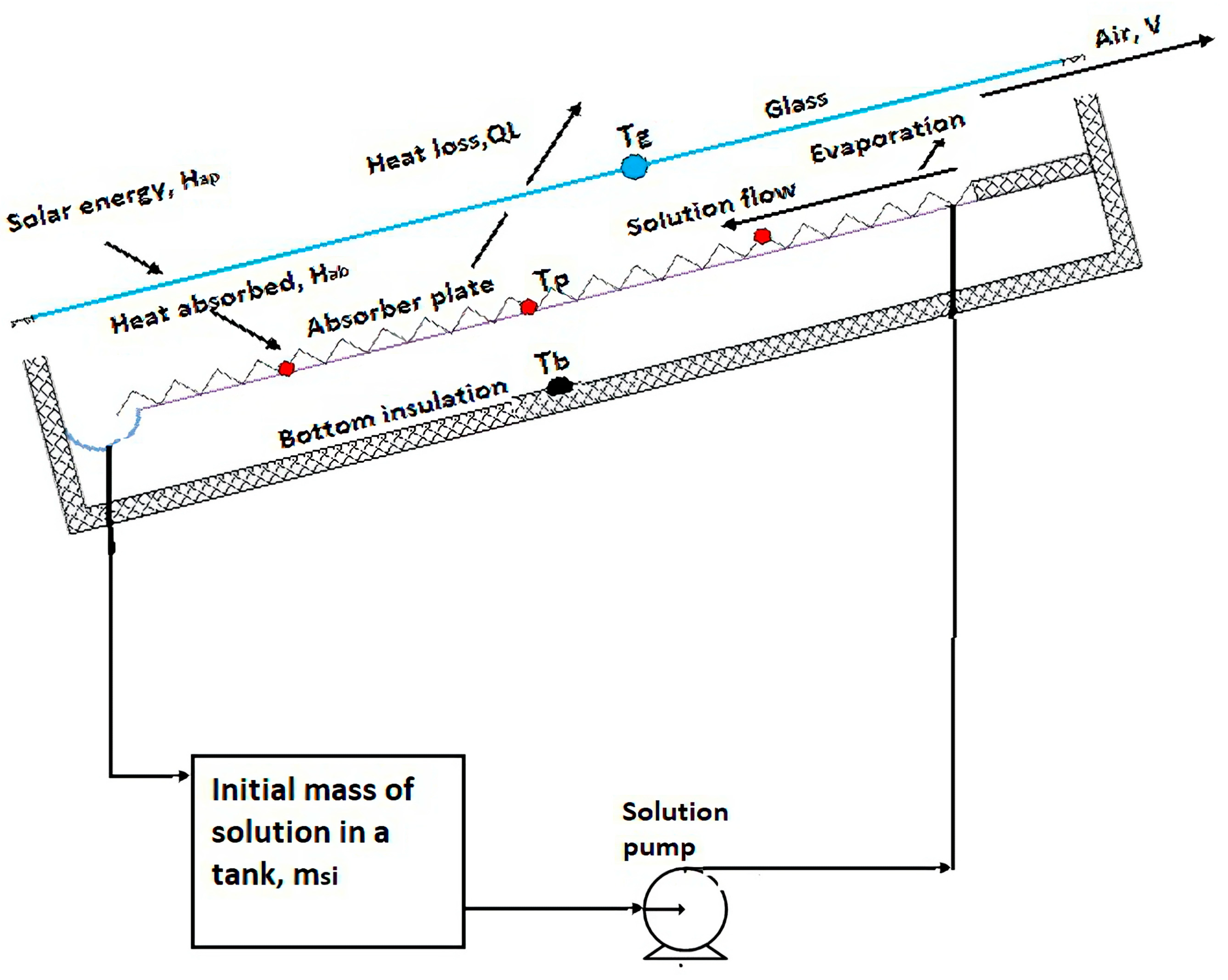
2.1. Experimental Setup and Instruments
2.2. Working Principle of the Solar Regenerator
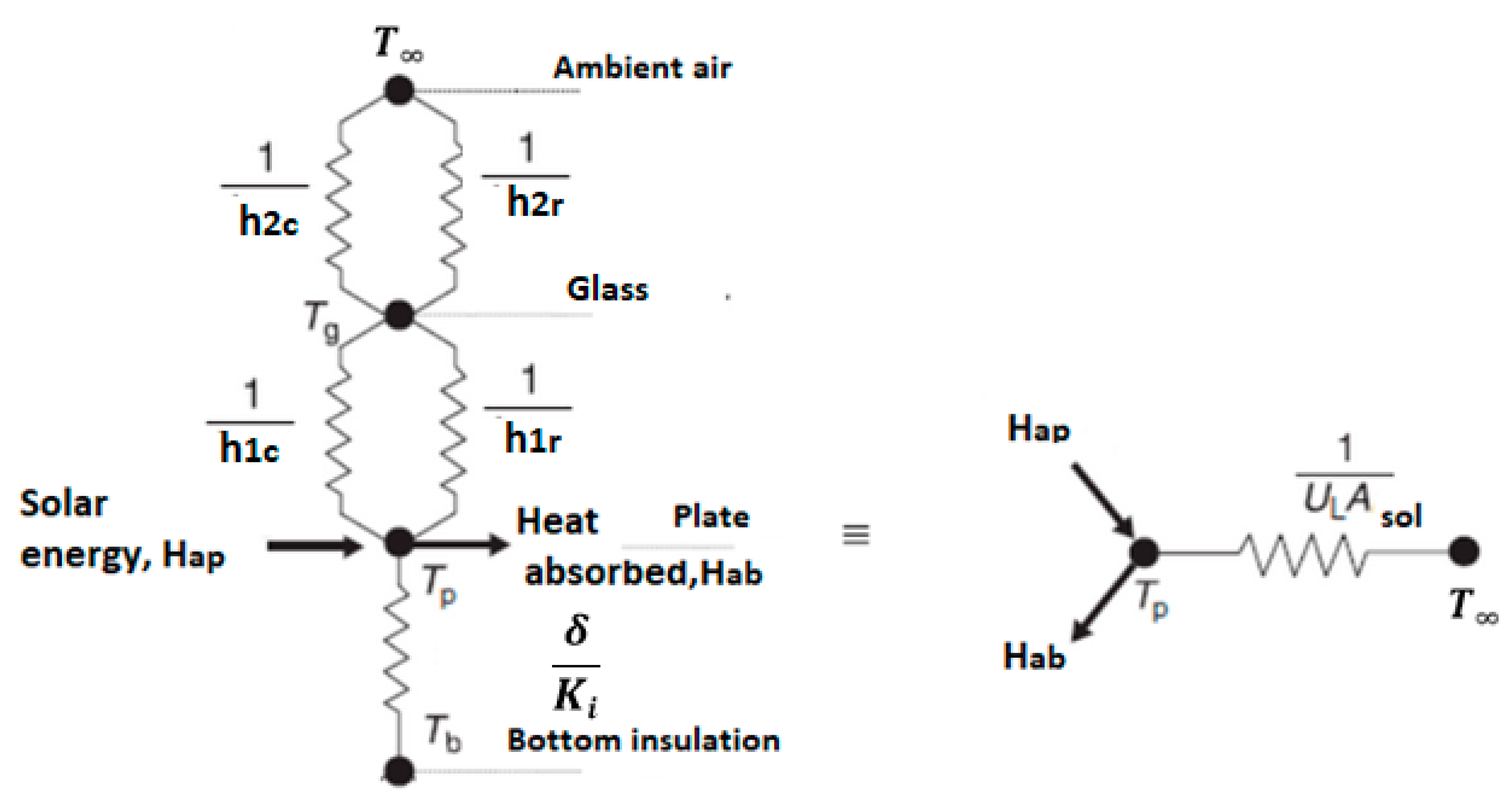
3. Data Reduction
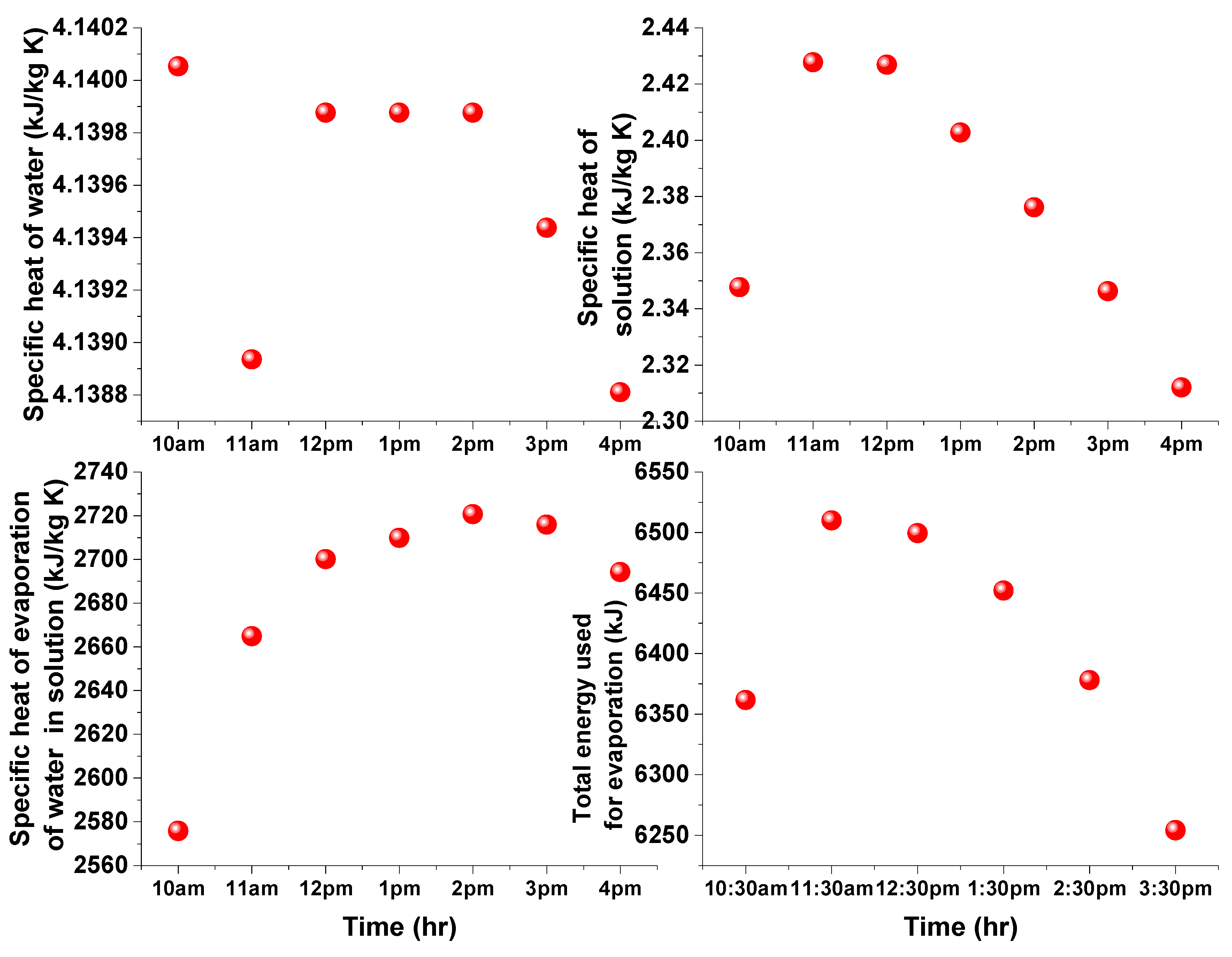
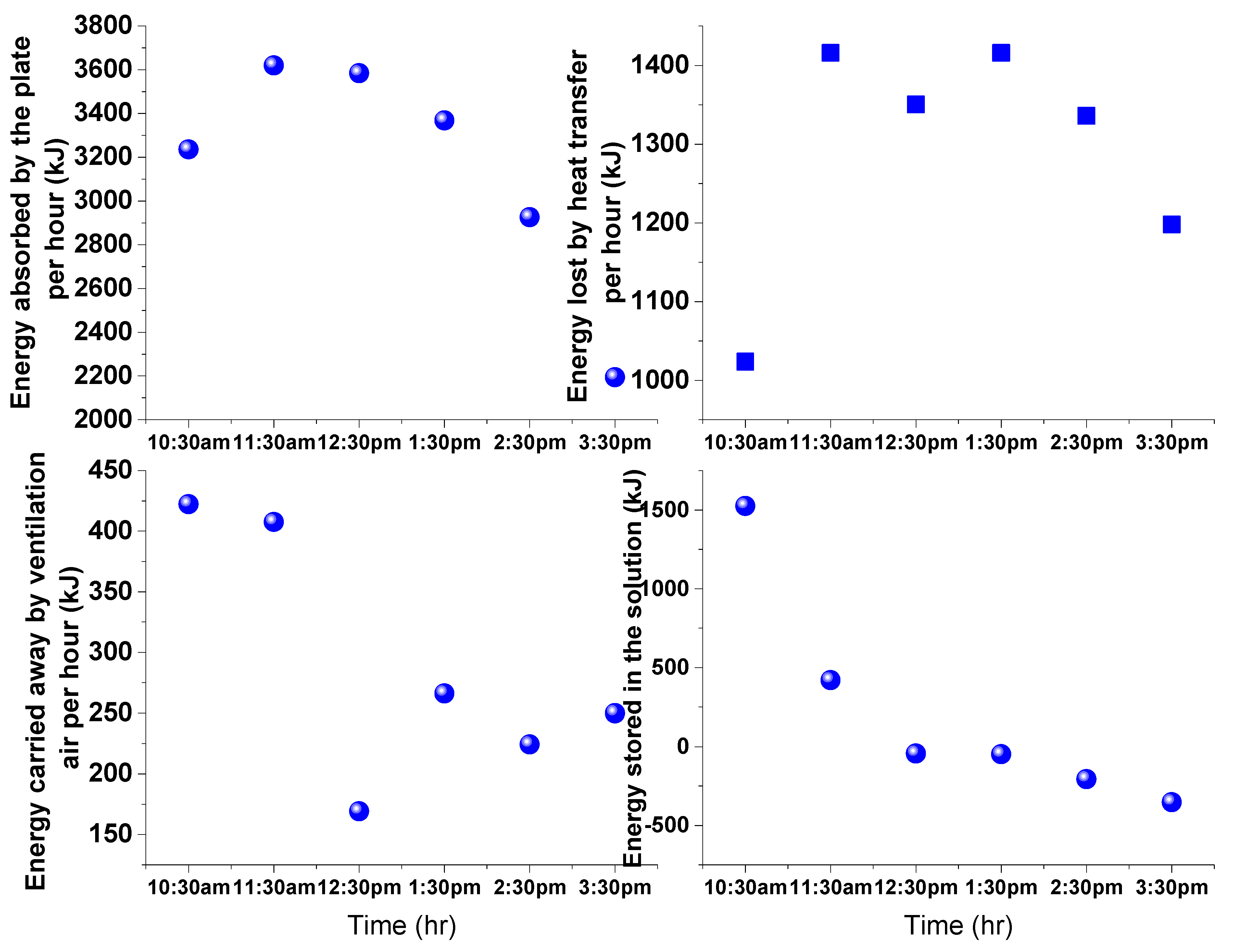
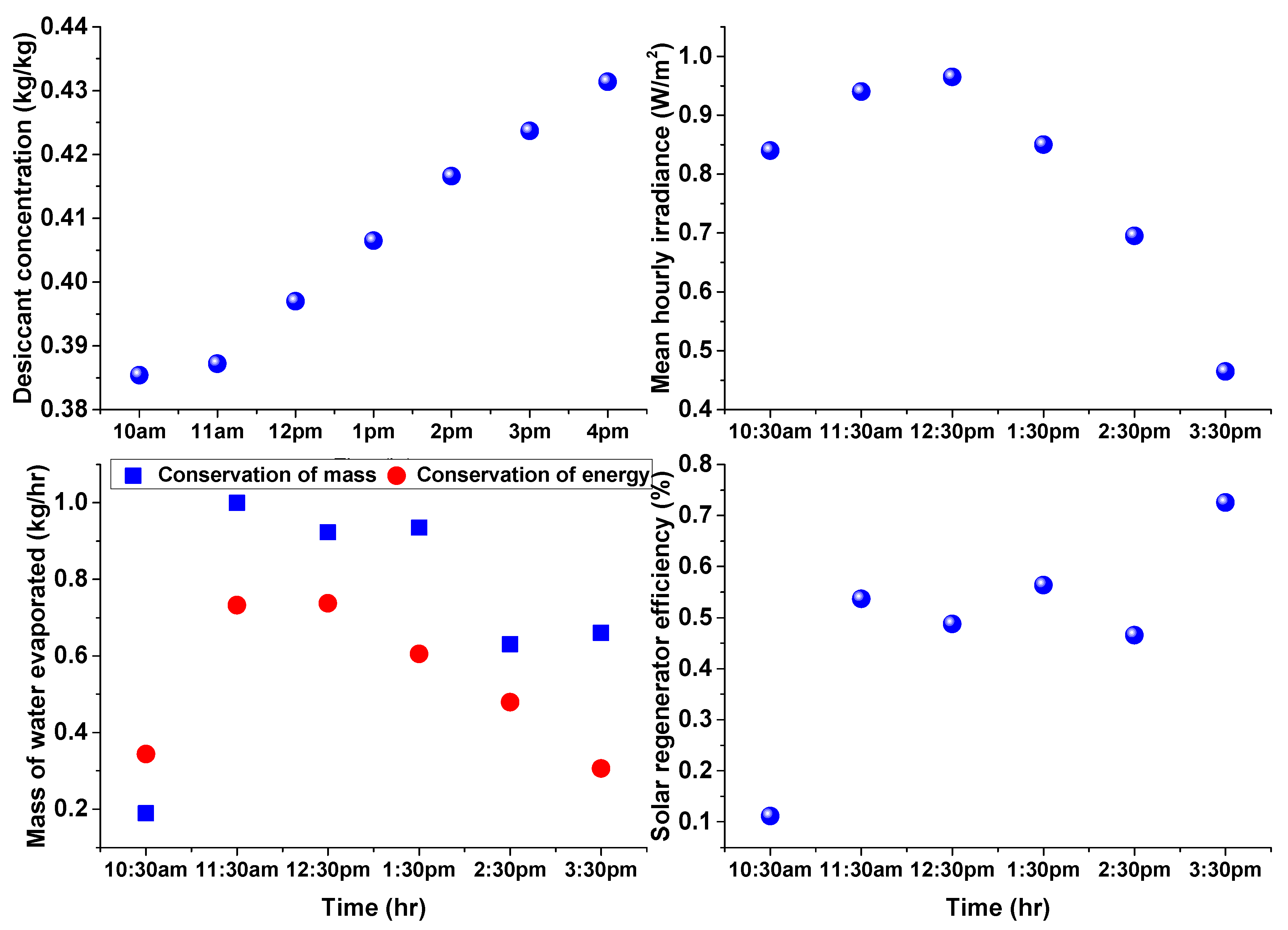
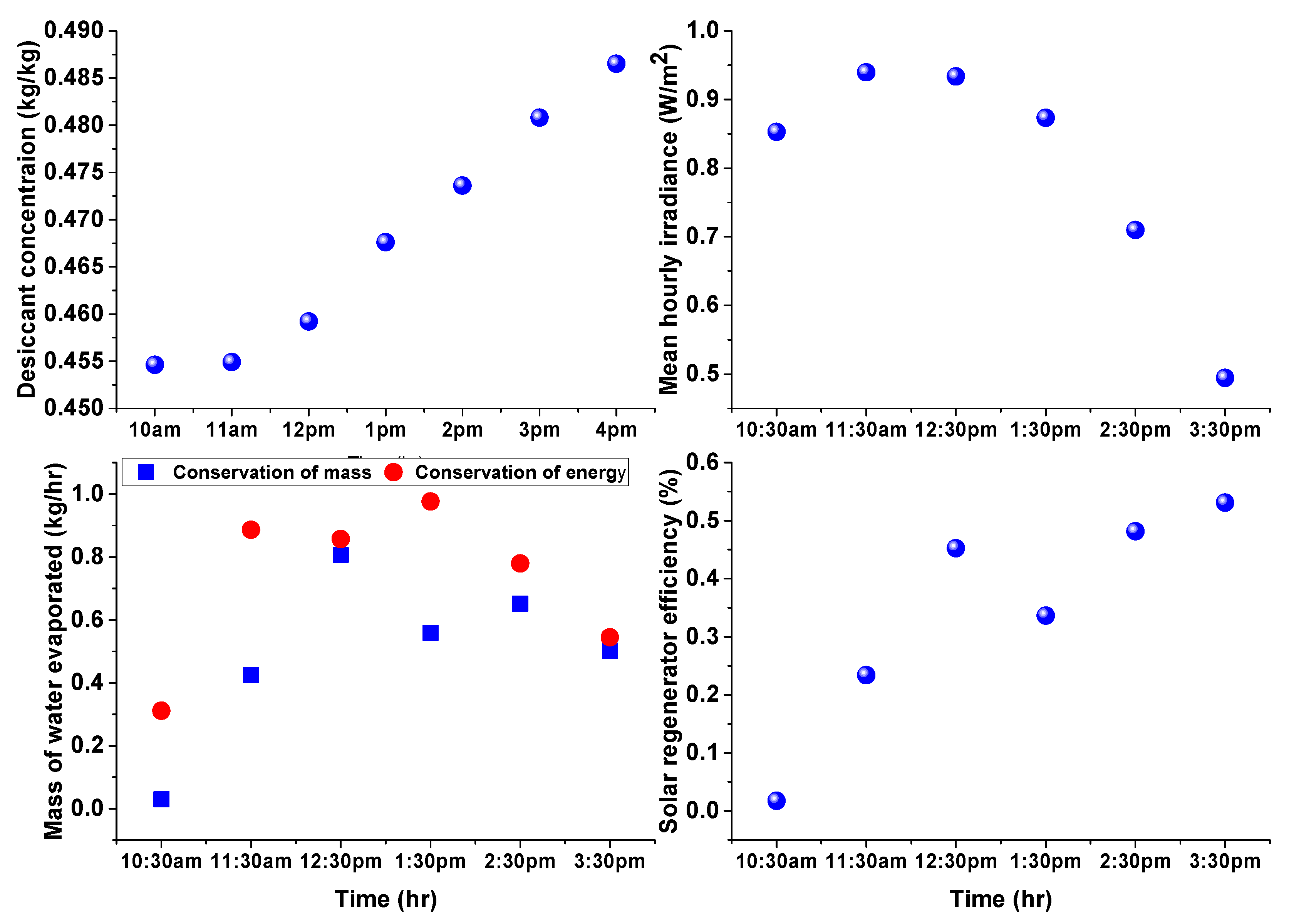
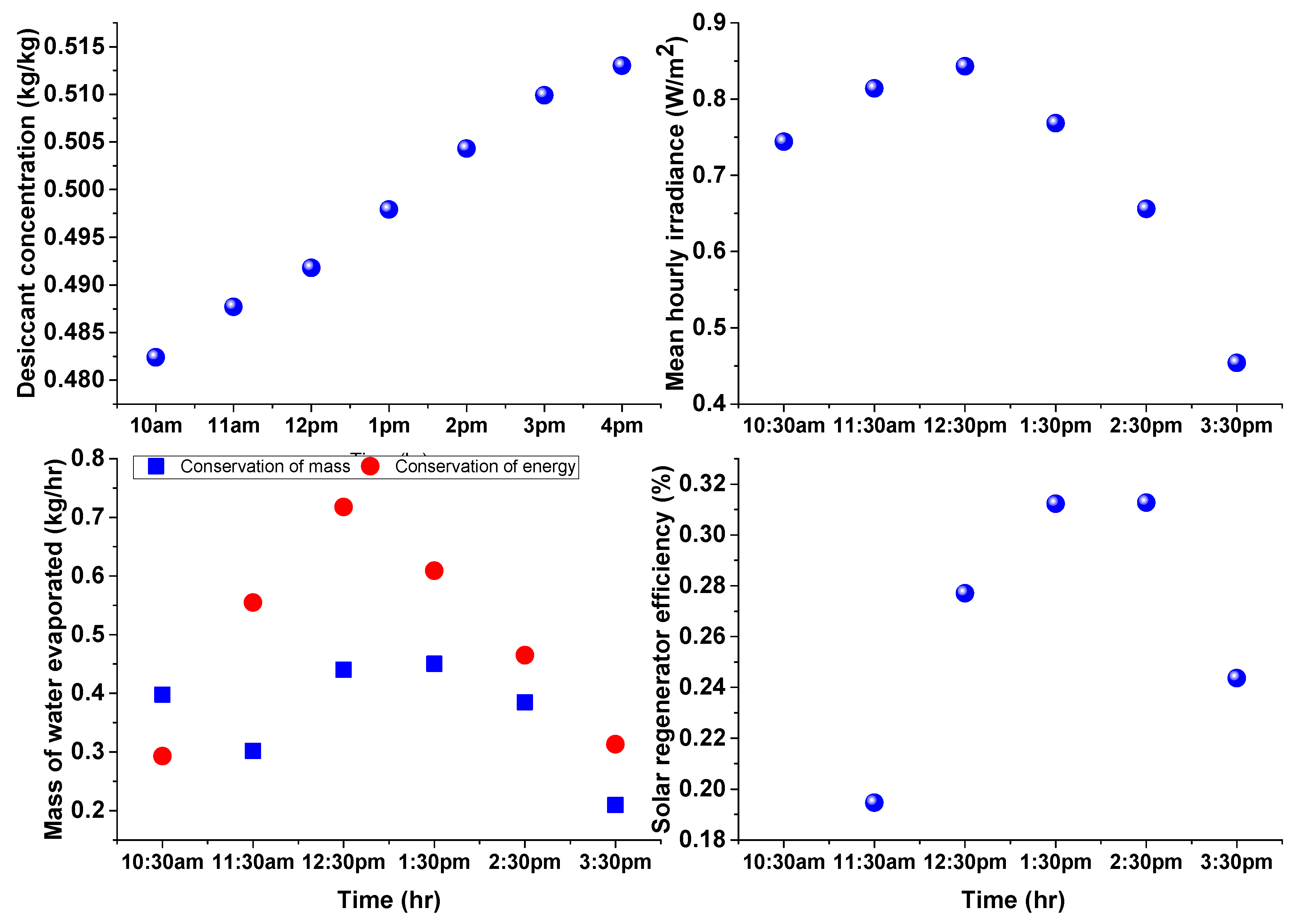
3.1. Estimation of Water Evaporation Using Conservation of Desiccant Mass
Solar Regenerator Efficiency
3.2. Estimation of Water Evaporation Using Conservation of Energy
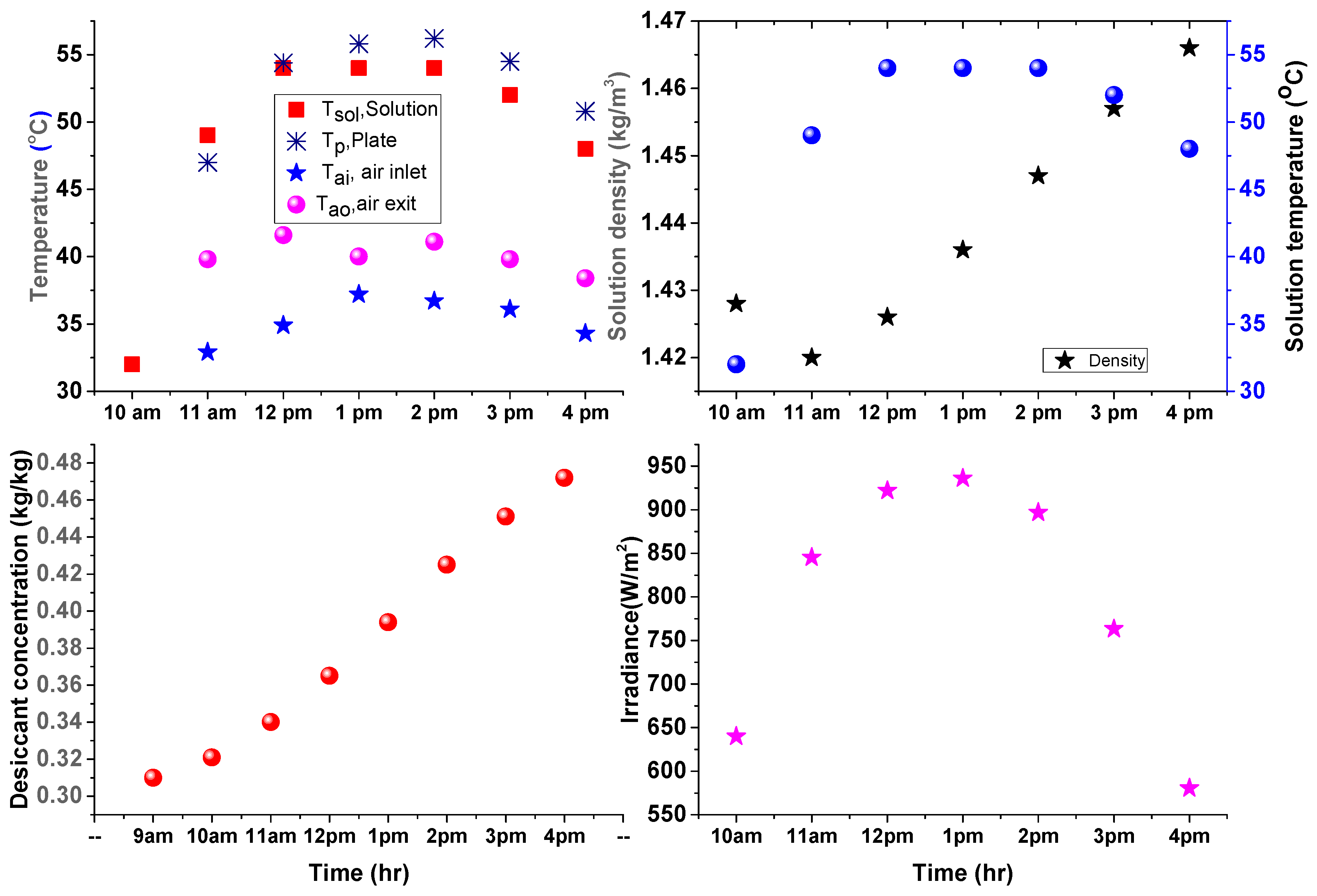
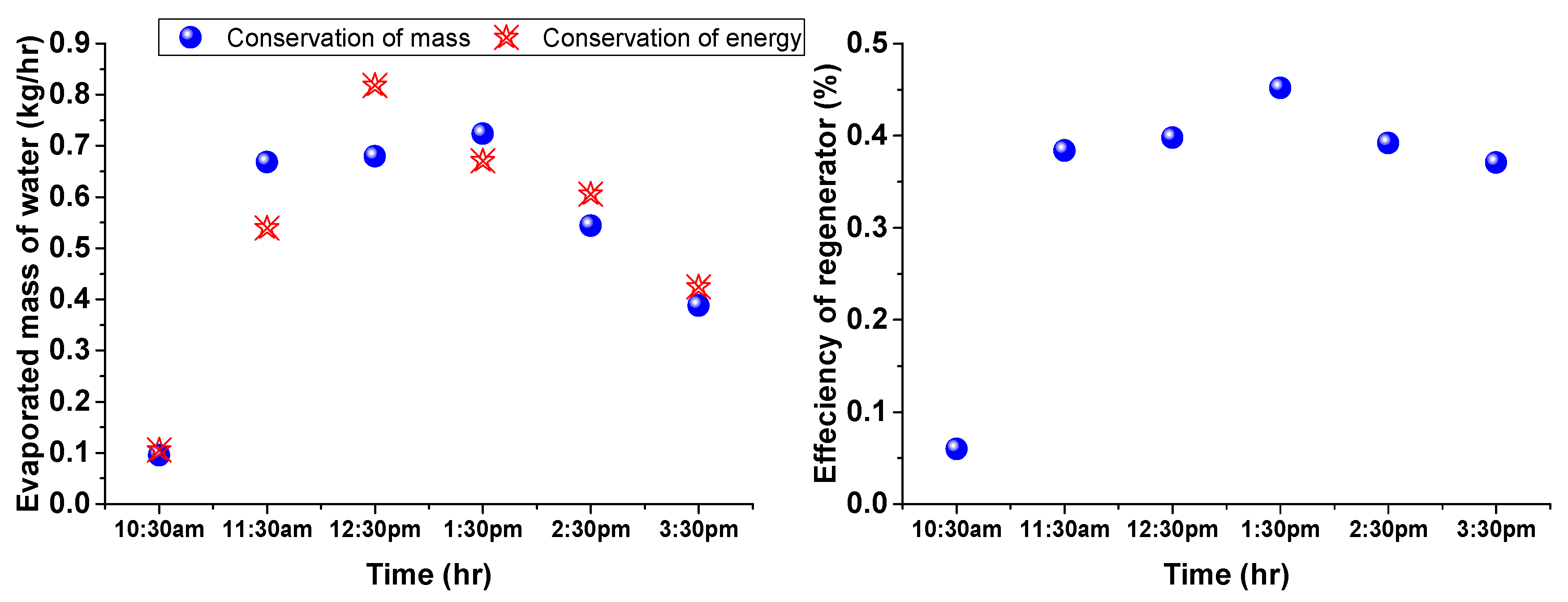
4. Results and Discussion
5. Directions for Further Research
- Simulation of annual regeneration or evaporation performance of different size solar regenerators;
- Optimization of the size of the regenerator solar absorber area compared to the desiccant concentration and storage tank volume;
- Experimental regeneration performance study of different desiccant materials such as potassium formate and lithium chloride solution or hybrid solutions;
- Experimental performance study of the solar regenerator by directly coupling it with a liquid desiccant air conditioning system for a specified latent cooling load in the air dehumidifier;
- Experimental investigation of the thermal and optical characteristics of the solar regenerator parts such as the glass, the paints used for the absorber and the solar absorptance of different desiccant materials.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Area (m2) | |
| Specific heat (kJ/kg K) | |
| Dust coefficient (−); thickness of metal strip welded along the collector left and right length (m) | |
| Energy of evaporation (kJ) | |
| Convective heat transfer coefficient (kJ/kg) | |
| Differential enthalpy of dilution (kJ/kg) | |
| Convective heat transfer coefficient (W/m2K) | |
| Radiation heat transfer coefficient (W/m2K) | |
| Total incident solar energy (kJ) | |
| Total absorbed solar energy (kJ) | |
| Solar radiation (W/m2) | |
| Thermal conductivity (W/m K) | |
| Glazing space (m); perimeter (m) | |
| Nusselt number (-) | |
| Mass (kg) | |
| Initial mass of the desiccant in the tank before starting experiment | |
| Rayleigh number (-) | |
| a result parameter; (varies) | |
| Shade coefficient (-) | |
| Starting time of experiment | |
| Temperature (°C or K) | |
| Heat loss coefficient (W/m2K); uncertainty of an instrument/a calculated variable; (varies) | |
| Wind speed (m/s); Volume (m3) | |
| Greek symbols | |
| Collector absorptivity (-) | |
| Collector inclination (°) | |
| Desiccant concentration in solution (kg/kg) | |
| Density (kg/m3) | |
| Glass transmissivity | |
| Stefan Boltzmann constant (Wm-2K-4) | |
| Reduced temperature of water (-) | |
| Change in time (s) | |
| Collection cum regeneration efficiency (%) | |
| Kinematic viscosity of air (m2 s−2) | |
| Insulator thickness (m) | |
| Subscripts | |
| Air | |
| ab | Absorbed; absorber |
| Air inlet | |
| ap | Aperture |
| Air outlet | |
| Bottom | |
| Convection | |
| Dust coefficient; | |
| f | Final |
| f1,f2 | Auxiliary concentration and temperature functions |
| i | Initial; insulator |
| Glass | |
| Loss; Length | |
| Optical | |
| Plate | |
| Radiation | |
| Regeneration | |
| Side | |
| Initial solution; solution inlet | |
| Final solution | |
| Solution outlet | |
| Solution; solar | |
| Solar regenerator air inlet area | |
| Top; total | |
| v | Vapor |
References
- Cheng, Q.; Zhang, X. Review of solar regeneration methods for liquid desiccant air-conditioning system. Energy Build. 2013, 67, 426–433. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, T. Exergy investigation of three ideal regeneration methods for liquid desiccant: Thermal air, mechanical vapor recompression and electrodialysis regeneration. Energy Build. 2021, 249, 111258. [Google Scholar] [CrossRef]
- Mehta, J.; Rane, M. Liquid desiccant based solar air conditioning system with novel evacuated tube collector as regenerator. Procedia Eng. 2013, 51, 688–693. [Google Scholar] [CrossRef]
- Mullick, S.C.; Gupta, M.C. Solar Desorption of Absorbent Solution. Sol. Energy 1973, 16, 19–24. [Google Scholar] [CrossRef]
- Singh, R.P.; Das, R.K. Progressive Development and Challenges Faced by Solar Rotary Desiccant-Based Air-Conditioning Systems: A Review. Processes 2021, 9, 1785. [Google Scholar] [CrossRef]
- Jani, D.B.; Mishra, M.; Sahoo, P.K. Solid desiccant air conditioner—A state of the art review. Renew. Sustain. Energy Rev. 2016, 60, 1451–1469. [Google Scholar] [CrossRef]
- Alizadeh, S.; Haghgou, H.R. Development of a Pilot Plant Solar Liquid Desiccant Air Conditioner for the Northern Region of Iran. J. Renew. Energy Environ. 2022, 3, 63–70. [Google Scholar]
- Kumar, K.; Singh, A.; Shaik, S.; Saleel, C.A.; Aabid, A.; Baig, M. Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach. Sustainability 2022, 14, 3441. [Google Scholar] [CrossRef]
- Naik, B.; Joshi, M.; Muthukumar, P.; Sultan, M.; Miyazaki, T.; Shamshiri, R.; Ashraf, H. Investigating Solid and Liquid Desiccant Dehumidification Options for Room Air-Conditioning and Drying Applications. Sustainability 2020, 12, 10582. [Google Scholar] [CrossRef]
- Elmer, T.; Worall, M.; Wu, S.; Riffat, S. Experimental evaluation of a liquid desiccant air conditioning system for tri-generation/waste-heat-driven applications. Int. J. Low-Carbon Technol. 2017, 12, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Collier, R.K. The analysis and simulation of an open cycle absorption refrigeration system. Sol. Energy 1979, 23, 357–366. [Google Scholar] [CrossRef]
- Gandhidasan, P. A simple analysis of an open regeneration system. Sol. Energy 1983, 31, 343–345. [Google Scholar] [CrossRef]
- Peng, C.P.; Howell, J.R. Analysis of open inclined surface solar regenerators for absorption cooling applications-comparison between numerical and analytical models. Sol. Energy 1982, 28, 265–268. [Google Scholar] [CrossRef]
- Haim, I.; Grossman, G.; Shavit, A. Simulation and analysis of open cycle absorption systems for solar cooling. Sol. Energy 1992, 49, 515–534. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.L. Experimental study of a forced convection solar collector/regenerator for open cycle absorption cooling. J. Sol. Energy Eng. 1994, 116, 194–199. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.L. Optimum glazing height a glazed solar collector/regenerator for open cycle absorption cooling. Energy 1994, 19, 925–931. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.L. The effect of heat recovery on experimental study of a forced convection solar collector/regenerator for open cycle absorption cooling. J. Sol. Energy Eng. 1995, 54, 19–24. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.L. A simulation study of performance evaluation of single glazed and double glazed solar collectors/regenerators for open cycle absorption solar cooling system. Sol. Energy 2001, 71, 263–268. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Novak, K.S.; Wood, B.D. Unglazed collector/regenerator performance for a solar assisted open cycle absorption cooling system. Sol. Energy 1993, 50, 59–73. [Google Scholar] [CrossRef]
- Alizadeh, S.; Saman, W.Y. Modeling and performance of a forced flow solar collector/regenerator using liquid desiccant. Sol. Energy 2002, 72, 143–154. [Google Scholar] [CrossRef]
- Alizadeh, S.; Saman, W.Y. An experimental study of forced flow solar collector/regenerator using liquid desiccant. Sol. Energy 2002, 73, 345–362. [Google Scholar] [CrossRef]
- Kabeel, A.E. Augmentation of the performance of solar regenerator of open absorption cooling system. Renew. Energy 2005, 30, 327–338. [Google Scholar] [CrossRef]
- Elsarrag, E. Evaporation rate of a novel tilted solar liquid desiccant regeneration system. Sol. Energy 2008, 82, 663–668. [Google Scholar] [CrossRef]
- Yutong, L.; Yang, H. Experimental study of an open-cycle solar collector/regenerator using liquid desiccant for air conditioning. Int. J. Green Energy 2010, 7, 273–288. [Google Scholar] [CrossRef]
- Conde, M.R. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- ASHRAE. ASHRAE Handbook Fundamentals; ASHRAE: Atlanta, GA, USA, 2009. [Google Scholar]
- Gezahegn, H.T.; Mullick, S.C.; Jain, S. Transient Performance of a Liquid Desiccant Solar Regenerator. In Proceedings of the 2014 Purdue Conferences, International Refrigeration and Air Conditioning Conference, Paper 1486, West Lafayette, IN, USA, 14–17 July 2014. [Google Scholar]
- Foster, R.; Ghassemi, M.; Cota, A. Solar Energy: Renewable Energy and the Environment; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; p. 300. [Google Scholar]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 1980; pp. 1–918. [Google Scholar]
- Dunn, P. Measurement and Data Analysis for Engineering and Science; McGraw Hill: Boca Raton, FL, USA, 2005. [Google Scholar]


| S. No. | Instrument | Uncertainty |
|---|---|---|
| 1 | Densimeter | |
| 2 | Pyranometer | of pyranometer reading |
| 3 | Thermometer | |
| 4 | Thermocouple wire | |
| 5 | RTD | |
| 6 | Tape rule/Scale |
| S. No. | Parameter | Values |
|---|---|---|
| 1 | Initial solution mass | 34.8 kg |
| 2 | Initial concentration () | 43.6 kg/kg |
| 3 | Solution tank size | 57 cm × 57 cm × 33 cm |
| 4 | Collector areas (apearure and absorber) | 1.92 m × 0.9 m and 1.84 m × 0.82 m |
| 5 | Collector inclination | 14° |
| 6 | Wind speed, | 0.4 m/s |
| 7 | Glass transmisivity | 0.85 |
| 8 | Absorber plate absoptance | 0.9 |
| 9 | Shade coefcient | 0.02 |
| 10 | Collector heat loss coefcient | 13.7 W/m2 K |
| 11 | Volume flow rate of solution | 10 lpm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tafesse, G.H.; Ahmed, G.M.S.; Badruddin, I.A.; Kamangar, S.; Hussien, M. Estimation of Evaporation of Water from a Liquid Desiccant Solar Collector and Regenerator by Using Conservation of Mass and Energy Principles. Sustainability 2023, 15, 6520. https://doi.org/10.3390/su15086520
Tafesse GH, Ahmed GMS, Badruddin IA, Kamangar S, Hussien M. Estimation of Evaporation of Water from a Liquid Desiccant Solar Collector and Regenerator by Using Conservation of Mass and Energy Principles. Sustainability. 2023; 15(8):6520. https://doi.org/10.3390/su15086520
Chicago/Turabian StyleTafesse, Gezahegn Habtamu, Gulam Mohammed Sayeed Ahmed, Irfan Anjum Badruddin, Sarfaraz Kamangar, and Mohamed Hussien. 2023. "Estimation of Evaporation of Water from a Liquid Desiccant Solar Collector and Regenerator by Using Conservation of Mass and Energy Principles" Sustainability 15, no. 8: 6520. https://doi.org/10.3390/su15086520
APA StyleTafesse, G. H., Ahmed, G. M. S., Badruddin, I. A., Kamangar, S., & Hussien, M. (2023). Estimation of Evaporation of Water from a Liquid Desiccant Solar Collector and Regenerator by Using Conservation of Mass and Energy Principles. Sustainability, 15(8), 6520. https://doi.org/10.3390/su15086520






