Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Dry Matter and Oil Content Analysis
2.2. Extraction of Coffee Oil from SCG
2.3. Analysis of Basic Parameters of Coffee Oil after Extraction
2.4. Analysis of Fatty Acids (Esters) Profile
2.5. Major Compounds Analyses and Identification of Unsaponifiable Matter
2.6. Biodiesel Production
3. Results and Discussion
3.1. SCG Drying and Oil Content Analysis
3.2. Analysis of Basic Parameters of Coffee Oil after Extraction
3.3. Analysis of Fatty Acids (Esters) Profile
3.4. Major Compounds Analysis and Identification of Unsaponifiable Matter
3.5. Biodiesel Production
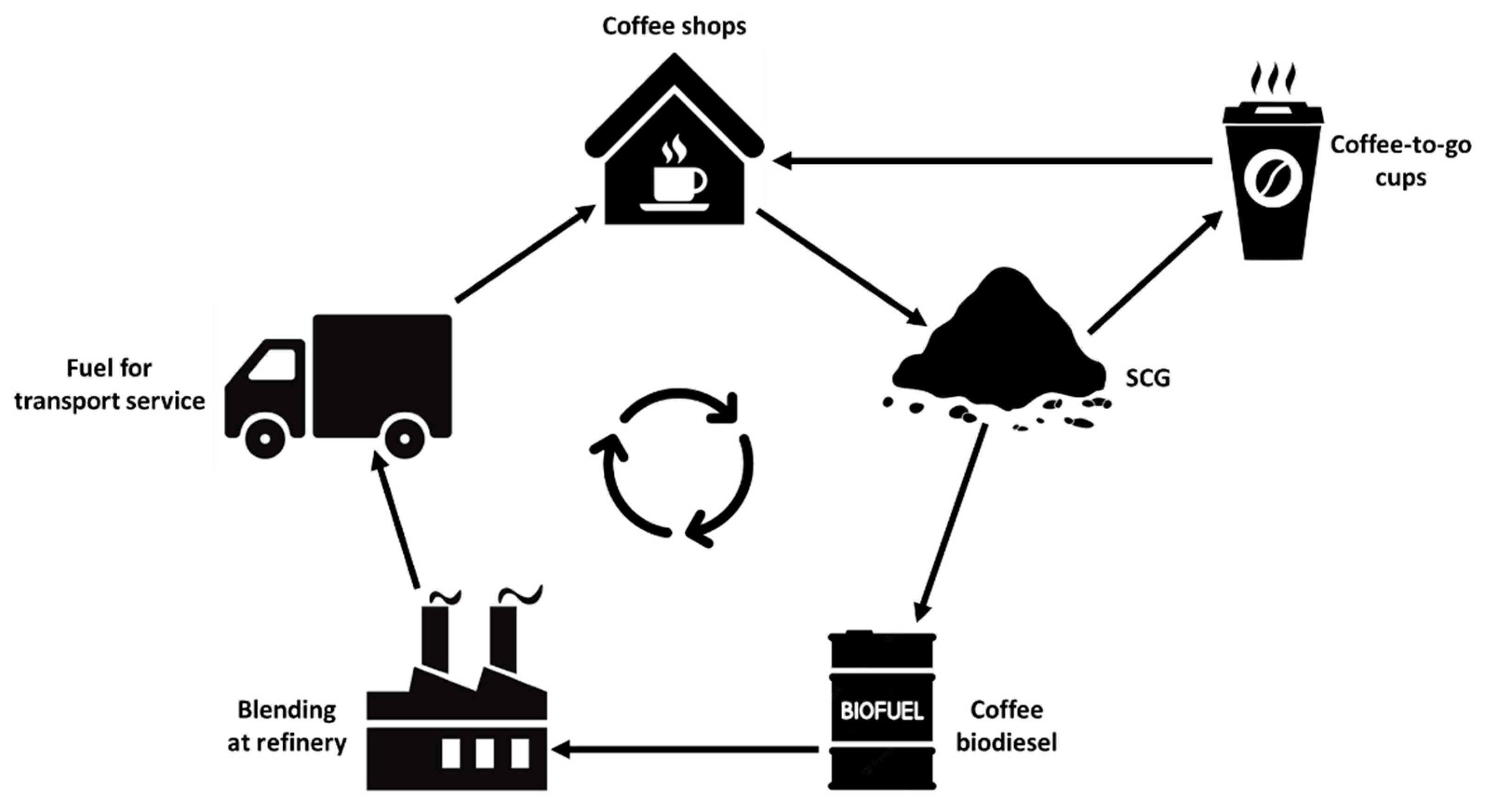
3.6. Coffee Biodiesel Potential Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | acid esterification |
| AV | acid value |
| CFP | cold flow properties |
| CFPP | cold filter plugging point |
| CN | cetane number |
| CO | corn oil |
| DSCG | dried spent coffee grounds |
| FA | fatty acid |
| FFA | free fatty acid |
| IEE | ion-exchange esterification |
| OS | oxidative stability |
| PO | palm oil |
| RI | refractive index |
| RSO | rapeseed oil |
| SBO | soybean oil |
| SCG | spent coffee grounds |
| SCGO | oil extracted from spent coffee grounds |
| SFO | sunflower oil |
| TAG | triacylglyceride/triglyceride |
| TE | transesterification |
| UM | unsaponifiable matter |
| WSCG | wet spent coffee grounds |
References
- Markets and Trades: Coffee. Available online: https://www.fao.org/markets-and-trade/commodities/coffee/en/ (accessed on 10 February 2023).
- Coffee Market Report: July 2022. Available online: https://www.ico.org/documents/cy2021-22/cmr-0922-e.pdf (accessed on 10 February 2023).
- Kafková, V.; Ondrejíčková, P. Assessment of the extracted coffee oil valorisation to biodiesel. Waste Forum 2022, 1, 45–56. [Google Scholar]
- May, G.; Folkerts, J. Breaking new grounds for coffee. Food Sci. Technol. 2021, 35, 28–31. [Google Scholar]
- Perta-Crisan, S.; Ursachi, C.; Munteanu, F.D. Trends in valorisation of spent coffee grounds: A review. Scien. Tech. Bull.-Chem. Food Sci. Eng. 2019, 16, 29–40. [Google Scholar]
- Miladi, M.; Martins, A.A.; Mata, T.M.; Vegara, M.; Pérez-Infantes, M.; Remmani, R.; Ruiz-Canales, A.; Núñez-Gómez, D. Optimization of Ultrasound-Assisted Extraction of Spent Coffee Grounds Oil Using Response Surface Methodology. Processes 2021, 9, 2085. [Google Scholar] [CrossRef]
- Rivera, X.C.S.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life cycle environmental sustainability of valorisation routes for spent coffee grounds: From waste to resources. Resour. Conserv. Recycl. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Unwaste and Reshape. Available online: https://www.kaffeeform.com/en/ (accessed on 10 February 2023).
- More Sustainable Plastics Made a Reality from Coffee Grounds. Available online: https://www.linkedin.com/company/beanused/ (accessed on 10 February 2023).
- Coffee Based Biodegradable Tableware. Available online: https://rekava.com/ (accessed on 10 February 2023).
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- Passadis, K.; Fragoulis, V.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Study of Valorisation Routes of Spent Coffee Grounds. In Proceedings of the Heraklion 2019, Heraklion, Greece, 26–29 June 2019. [Google Scholar]
- Carlucci, R.; Jäger, S.N.; Labadie, G.R. Steryl glucosides recovered from biodiesel tank deposits are an excellent source of phytosterols. Ind. Crops Prod. 2022, 187, 115307. [Google Scholar] [CrossRef]
- Gómez-de la Cruz, F.J.; Cruz-Peragón, F.; Casanova-Peláez, P.J.; Palomar-Carnicero, J.M. A vital stage in the large-scale production of bio-fuels from spent coffee grounds: The drying kinetics. Fuel Process. Technol. 2015, 130, 188–196. [Google Scholar] [CrossRef]
- Tun, M.M.; Raclavská, H.; Juchelková, D.; Růžičková, J.; Šafář, M.; Štrbová, K.; Gikas, P. Spent coffee ground as renewable energy source: Evaluation of the drying processes. J. Environ. Manag. 2020, 275, 111204. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vergara, H.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Solomakou, N.; Tsafrakidou, P.; Goula, A.M. Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent. Sustainability 2022, 14, 9358. [Google Scholar] [CrossRef]
- Blinová, L.; Bartosova, A.; Sirotiak, M. Biodiesel Production from Spent Coffee Grounds. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 113–121. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2017, 72, 240–254. [Google Scholar] [CrossRef]
- Caetano, N.; Silva, V.; Mata, T. Valorization of Coffee Grounds for Biodiesel Production. In Proceedings of the 5th International Conference on Safety and Environment in the Process, Milan, Italy, 3–6 June 2012. [Google Scholar]
- Kourmentza, C.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Spent coffee grounds make much more than waste: Exploring recent advances and future exploitation strategies for the valorization of an emerging food waste stream. J. Clean. Prod. 2018, 172, 980–992. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Araujo, M.N.; Santos, K.C.; Carmo Diniz, N.; Carvalho, J.C.; Corazza, M.L. A biorefinery approach for spent coffee grounds valorization using pressurized fluid extraction to produce oil and bioproducts: A systematic review. Bioresour Technol. Rep. 2022, 18, 101013. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Wang, F.M.; Juan, H.Y.; Su, C.H. Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Bioresour Technol. 2020, 296, 122334. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crops Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Lee, Y.G.; Song, Y.; Nguyen, D.-T.; Bae, H.-J. An integrated process for conversion of spent coffee grounds into value-added materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar] [CrossRef]
- Sugebo, B. A review on enhanced biofuel production from coffee by-products using different enhancement techniques. Mater. Renew. Sustain. Energy 2022, 11, 91–103. [Google Scholar] [CrossRef]
- Haile, M. Integrated volarization of spent coffee grounds to biofuels. Biofuel Res. J. 2014, 2, 65–69. [Google Scholar] [CrossRef]
- Bijla, L.; Aissa, R.; Bouzid, H.A.; Sakar, E.H.; Ibourki, M.; Laknifli, A.; Gharby, A. Spent Coffee Ground Oil as a Potential Alternative for Vegetable Oil Production: Evidence from Oil Content, Lipid Profiling, and Physicochemical Characterization. Biointerface Res. Appl. Chem. 2022, 12, 6308–6320. [Google Scholar] [CrossRef]
- Dang, C.-H.; Nguyen, T.-D. Physicochemical Characterization of Robusta Spent Coffee Ground Oil for Biodiesel Manufacturing. Waste Biomass Valorization 2019, 10, 2703–2712. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete utilization of wet spent coffee grounds waste as a novel feedstock for antioxidant, biodiesel, and bio-char production. Ind. Crops Prod. 2019, 138, 111484. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization dor Standardization: Geneva, Switzerland, 2017.
- EN 14103:2020; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic Acid Methyl Ester Contents. European Committee for Standardization: Bruxelles, Belgium, 2020.
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Transesterification of vegetable oil to biodiesel fuel using alkaline catalyst. Fuel 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Kocsisová, T.; Cvengroš, J.; Lutišan, J. High-temperature esterification of fatty acids with methanol at ambient pressure. Eur. J. Lipid Sci. Technol. 2005, 107, 87–92. [Google Scholar] [CrossRef]
- Özbay, N.; Oktar, N.; Tapan, N.A. Esterification of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel 2008, 87, 1789–1798. [Google Scholar] [CrossRef]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental impacts of biodiesel production from waste spent coffee grounds and its implementation in a compression ignition engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, A.K.; Singh, P. Effect of Moisture Content on the Mechanical Oil Extraction of Canola Seeds. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 965–977. [Google Scholar] [CrossRef]
- Sharma, A.; Ray, A.; Singhal, R.S. A biorefinery approach towards valorization of spent coffee ground: Extraction of the oil by supercritical carbon dioxide and utilizing the defatted spent in formulating functional cookies. Future Foods 2021, 4, 100090. [Google Scholar] [CrossRef]
- Kang, B.-J.; Jeon, J.-M.; Bhatia, S.K.; Kim, D.-H.; Yang, Y.-H.; Jung, S.; Yoon, J.-J. Two-Stage Bio-Hydrogen and Polyhydroxyalkanoate Production: Upcycling of Spent Coffee Grounds. Polymers 2023, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Harahap, F.A.U.; Heru, H.; Darni, Y.; Ginting, S.B. Extraction and Characterization of Coffee Oil From Instant-Coffee Waste. JBAT 2019, 8, 59–64. [Google Scholar] [CrossRef]
- Patra, C.J.; Kumaran, P.; Praveen, R.; Senthil Kumar, A. Production of biodiesel from spent coffee grounds by transesterification and its byproducts as fuel additives. Int. J. Chem. Sci. 2019, 14, 590–596. [Google Scholar]
- Obruca, S.; Benešová, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates. New Biotechnol. 2015, 31, 569–574. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of Spent Coffee Grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- The Determination of Phosphorus, Sulfur, Sodium, Potassium, Calcium, and Magnesium in Biodiesel. Available online: https://www.spectroscopyonline.com/view/determination-phosphorus-sulfur-sodium-potassium-calcium-and-magnesium-biodiesel (accessed on 14 March 2023).
- He, B.B.; Van Gerpen, J.H.; Thompson, J.C. Sulfur content in selected oils and fats and their corresponding methyl esters. Appl. Eng. Agric. 2009, 25, 223–226. [Google Scholar] [CrossRef]
- O’Brien, R.D. Soybean Oil Purification. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 377–408. [Google Scholar]
- Marx, S.; Venter, R.; Karmee, S.K.; Louw, J.; Truter, C. Biofuels from spent coffee grounds: Comparison of processing routes. Biofuels 2022, 13, 537–543. [Google Scholar] [CrossRef]
- Supang, W.; Ngamprasertsith, S.; Sakdasri, W.; Sawangkeaw, R. Ethyl acetate as extracting solvent and reactant for producing biodiesel from spent coffee grounds: A catalyst- and glycerol-free process. J. Supercrit. Fluids 2022, 186, 105586. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Stageman, N.E.; Fortune, C.M.; Chuck, C.J. Effect of the type of bean, processing, and geographicallocation on the biodiesel produced from waste coffee grounds. Energy Fuels 2014, 28, 1166–1174. [Google Scholar] [CrossRef]
- EN 14214; Liquid Petroleum Products—Fatty acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization: Bruxelles, Belgium, 2019.
- Kumbhar, V.; Pandey, A.; Sonawane, C.R.; El-Shafay, A.S.; Panchal, H.; Chamkha, A.J. Statistical analysis on prediction of biodiesel properties from its fatty acid composition. Case Stud. Therm. Eng. 2022, 30, 101775. [Google Scholar] [CrossRef]
- Chuah, L.F.; Yusup, S.; Aziz, A.R.A.; Klemeš, J.J.; Bokhari, A.; Abdullah, M.Z. Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Technol. Environ. Policy 2016, 18, 473–482. [Google Scholar] [CrossRef]
- Chemat, A.; Ravi, H.K.; Hostequin, A.C.; Burney, H.; Tomao, V.; Fabiano-Tixier, A.-S. Valorization of spent coffee grounds by 2-methyloxolane as bio-based solvent extraction. Viable pathway towards bioeconomy for lipids and biomaterials. OCL 2022, 29, 7. [Google Scholar] [CrossRef]
- Massaya, J.; Pereira, A.P.; Mills-Lamptey, B.; Benjamin, J.; Chuck, C.J. Conceptualization of a spent coffee grounds biorefinery: A review of existing valorisation approaches. Food Bioprod. Process. 2019, 118, 149–166. [Google Scholar] [CrossRef]
- Akgün, N.A.; Bulut, H.; Kikic, I.; Solinas, D. Extraction behavior of lipids obtained from spent coffee grounds usingsupercritical carbon dioxide. Chem. Eng. Technol. 2014, 37, 1975–1981. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D. Towards the sustainable and circular bioeconomy: Insights on spent coffee grounds valorization. Sci. Total Environ. 2022, 833, 155113. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.N.; Do, H.Q.; Giang Duong, H.T.; Perng, Y.S.; Dam, V.N.; Nguyen, V.T.; Bui, H.M. Taguchi optimization and life cycle assessment of biodiesel production from spent ground coffee. Environ. Dev. Sustain. 2021, 24, 12900–12916. [Google Scholar] [CrossRef]
- Statistical Office of the Slovak Republic. Available online: http://datacube.statistics.sk/#!/view/sk/VBD_SLOVSTAT/ps2040rs/v_ps2040rs_00_00_00_sk (accessed on 10 February 2023).
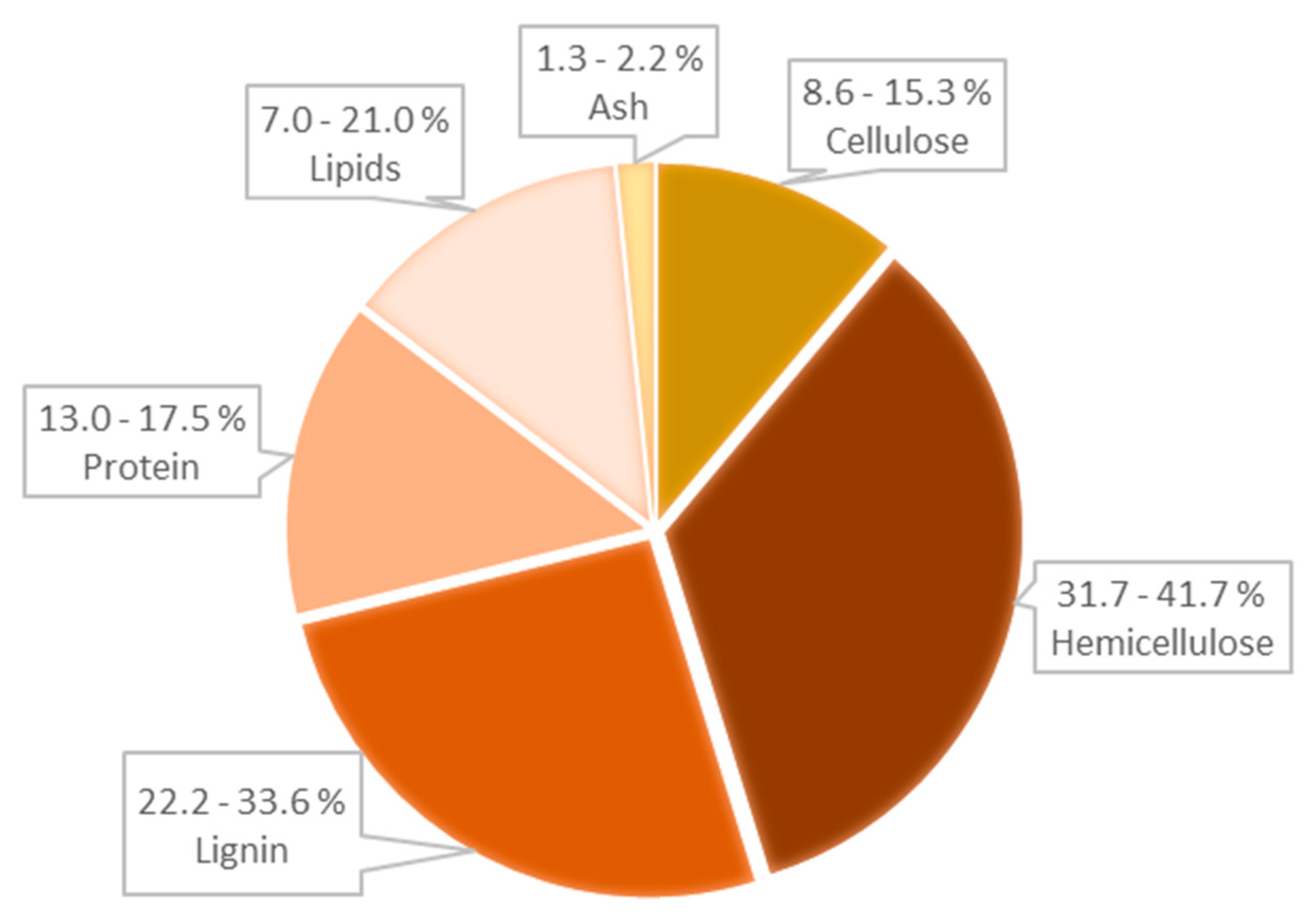
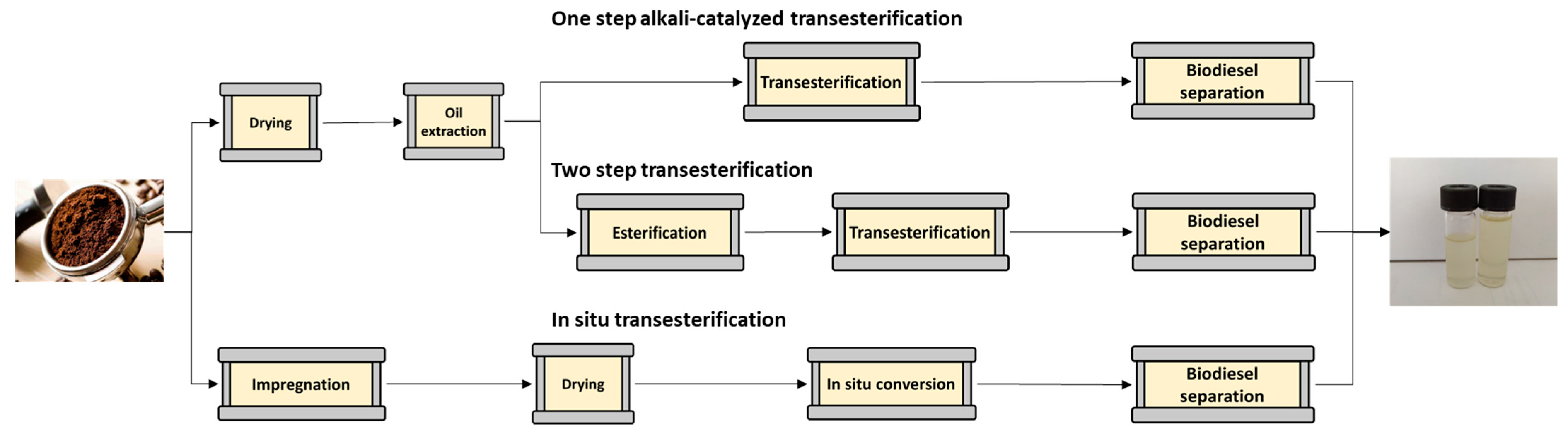

| Roasted Coffee Beans | WSCG | DSCG | |
|---|---|---|---|
| Sample weight (g) | 50.45 | 90.50 | 48.43 |
| Conversion to kg | 1 | 1.79 | 0.96 |
| Dry matter (wt.%) | 98.5 | 50.9 | 95.1 |
| Dry matter (kg) | 0.985 | 0.913 | 0.913 |
| Oil content (wt.%) | 12.78 | 5.98–6.26 | 8.12–9.55 |
| Sample | SCGO | Rapeseed Oil * | Corn Oil * |
|---|---|---|---|
| AV (mg KOH/g) | 9.5 | 1.6 | 22.3 |
| P (mg/kg) | 24.5 | 406.1 | 12.7 |
| Ca (mg/kg) | 5.1 | 73.1 | 0.2 |
| Mg (mg/kg) | 7.3 | 28.9 | 1.3 |
| Na (mg/kg) | 1.4 | 0.7 | 2.9 |
| K (mg/kg) | 4.9 | 111.7 | 9.3 |
| S (mg/kg) | 35.6 | 20.7 | 14.9 |
| Water content (wt.%) | 0.01 | 0.06 | 0.4 |
| FA (% hm.) | SCGO Analysis 1 | SCGO Analysis 2 | SCGO (lit.) [18,32] | RSO * | CO * | SFO | SBO | PO |
|---|---|---|---|---|---|---|---|---|
| C16:0 | 32.97 | 33.14 | 27.5–43.6 | 4.6 | 12.8 | 4.7–8.0 | 9.7–13.3 | 39.1–45.5 |
| C18:0 | 7.16 | 7.34 | 5.3–19.6 | 1.7 | 2.1 | 2.8–4.1 | 3.3–4.9 | 3.3–5.2 |
| C18:1 | 9.36 | 9.22 | 5.5–24.0 | 64.5 | 28.4 | 15.3–28.0 | 21.5–25.5 | 38.2–43.6 |
| C18:2 | 45.45 | 41.94 | 25.8–49.9 | 18.7 | 53.9 | 61.2–73.9 | 50.8–55.9 | 8.3–11.9 |
| C18:3 | 1.44 | 1.26 | 0.8–4.1 | 7.7 | 1.3 | 0.0–0.4 | 4.7–8.9 | 0.1–0.5 |
| C20:0 | 2.51 | 2.68 | 0.0–6.9 | 0.6 | 0.4 | 0.1–0.4 | 0.1–0.6 | 0.2–0.5 |
| C20:1 | 0.34 | 0.39 | 0.3–3.2 | 12.0 | 0.3 | 0.0–0.2 | 0.0–0.4 | 0.1–0.2 |
| C22:0 | 0.53 | 0.62 | 0.4–1.2 | 0.3 | 0.2 | 0.4–0.9 | 0.1–0.5 | 0.0–0.1 |
| C24:0 | 0.24 | 0.25 | 0.1–0.3 | 0.1 | 0.2 | 0.2–0.4 | 0.0–0.3 | 0.0–0.1 |
| Compound | Area (%) |
|---|---|
| Free fatty acid | 22.49 |
| Monoglycerides | 1.14 |
| Sterols | 1.89 |
| Diglycerides | 9.06 |
| Triglycerides | 65.42 |
| RT (min) | Area (Ab ∗ s) | Compound | Quality | Mol Weight (amu) | CAS Number |
|---|---|---|---|---|---|
| 15.237 | 355,763,565 | Caffeine | 97 | 194.08 | 000058-08-2 |
| 20.197 | 121,181,206 | (Z)-9-Octadecenamide | 99 | 281.272 | 000301-02-0 |
| 27.303 | 24,486,743 | Stigmasterol | 99 | 412.371 | 000083-48-7 |
| 27.929 | 21,656,564 | γ-Sitosterol | 99 | 414.386 | 000083-47-6 |
| 25.202 | 20,499,232 | β-Tocopherol | 99 | 416.365 | 000148-03-8 |
| 18.452 | 15,666,889 | n-Hexadecanoic acid | 93 | 256.24 | 000057-10-3 |
| 25.971 | 7,061,250 | Vitamin E | 99 | 430.381 | 000059-02-9 |
| 28.123 | 6,068,755 | (3-β,24Z)-Stigmasta-5,24(28)-dien-3-ol | 95 | 412.371 | 000481-14-1 |
| 23.65 | 2,930,511 | Squalene | 99 | 410.391 | 000111-02-4 |
| FFA (wt.%) | SCGO | TE | AE + TE | IEE + TE |
|---|---|---|---|---|
| Refractive index (20 °C) | 1.4725 | 1.4545 | 1.4550 | 1.4490 |
| Ester content (wt.%) | - | 84.98 | 89.62 | 65.44 |
| C16:0 | 33.14 | 28.15 | 31.29 | 19.12 |
| C18:0 | 7.34 | 6.26 | 7.09 | 4.14 |
| C18:1 | 9.22 | 8.15 | 9.47 | 6.94 |
| C18:2 | 41.94 | 37.36 | 36.04 | 31.82 |
| C18:3 | 1.26 | 1.20 | 1.01 | 0.98 |
| C20:0 | 2.68 | 2.25 | 2.63 | 1.35 |
| Unidentified matter (wt.%) | - | 1.26 | 0.18 | 1.65 |
| Year | 2021 | 2020 | 2019 | 2018 | 2017 |
|---|---|---|---|---|---|
| Consumption (tons) | 15,450 | 13,900 | 15,609 | 17,010 | 13,680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafková, V.; Kubinec, R.; Mikulec, J.; Variny, M.; Ondrejíčková, P.; Ház, A.; Brisudová, A. Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery. Sustainability 2023, 15, 5612. https://doi.org/10.3390/su15075612
Kafková V, Kubinec R, Mikulec J, Variny M, Ondrejíčková P, Ház A, Brisudová A. Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery. Sustainability. 2023; 15(7):5612. https://doi.org/10.3390/su15075612
Chicago/Turabian StyleKafková, Valentína, Róbert Kubinec, Jozef Mikulec, Miroslav Variny, Petra Ondrejíčková, Aleš Ház, and Adriana Brisudová. 2023. "Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery" Sustainability 15, no. 7: 5612. https://doi.org/10.3390/su15075612
APA StyleKafková, V., Kubinec, R., Mikulec, J., Variny, M., Ondrejíčková, P., Ház, A., & Brisudová, A. (2023). Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery. Sustainability, 15(7), 5612. https://doi.org/10.3390/su15075612









