Copper Phytoextraction Using Phyllostachys pubescens
Abstract
1. Introduction
2. Material and Methods
- BCF = Cu contents in all tissues/Cu in soil
- TF = Cu in upper sections/Cu in root and rhizome
Statistical Analyses
3. Results and Discussions
3.1. Cu Soil Removal
3.2. Analysis of Cu Levels in the Plant Organs
| Species and Content of Cu in mg kg−1 | Translocation Factor | Bioconcentration Factor | References |
|---|---|---|---|
| Phyllostachys edulis Content of Cu = 99 | Rhizome 0.60 Stems 0.28 Leaves 0.43 | Root 0.60 Rhizome 0.36 Stems 0.17 Leaves 0.26 | [33] |
| Phyllostachys praecox Content of Cu = 195 | Rhizome 0.91 Stems 0.30 Leaves 1.00 | Root 0.47 Rhizome 0.43 Stems 0.14 Leaves 0.47 | [33] |
| Artemisia vulgaris Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4) | Loam 2 = 0.39 Loam 3 = 0.70 | Loam 2 = 1.45 Loam 3 = 0.87 | [50] |
| Achillea millefolium Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 2 = 0.61 Loam 3 = 0.16 Loam 4 = 0.59 | Loam 2 = 0.61 Loam 3 = 3.94 Loam 4 = 0.37 | [50] |
| Sisymbrium loeselii Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 2 = 0.47 Loam 3 = 0.60 | Loam 2 = 1.44 Loam 3 = 1.21 | [50] |
| Thymus kotschyanus Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 2 = 0.08 Loam 4 = 0.08 | Loam 2 = 4.84 Loam 4 = 1.57 | [50] |
| Rosa canina Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 3 = 0.71 | Loam 3 = 0.73 | [50] |
| Trifolium pratense Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 3 = 1.87 | Loam 3 = 0.65 | [50] |
| Hypericum perforatum Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 3 = 1.27 | Loam 3 = 0.67 | [50] |
| Tussilago farfara Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 3 = 0.84 | Loam = 0.75 | [50] |
| Phleum pratense Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 2 = 0.35 Loam 4 = 0.15 | Loam 2 = 4.64 Loam 4 = 1.11 | [50] |
| Sedum caucasicum Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 4 = 0.31 | Loam 4 = 0.75 | [50] |
| Astrodaucus orientalis Cubioavailable = 184.4 (loam 2), 82.4 (loam 3), 894.4 (loam 4). | Loam 4 = 1.19 | Loam 4 = 0.12 | [50] |
| Rumex acetosella | 1.8–1.9 | [78] | |
| Rubia peregrina Cu conc. = 31 to 251 mg kg−1 | 0.2–0.9 | [78] | |
| Phytolacca americana Cu conc. = 134–75 | 1.8–2.9 | 0.17 | [78] |
| Chenopodium album Cu conc. = 134–75 | 5.9 | [78] | |
| Conyza albida Cu conc. = 134–75 | 1.4–2.3 | [78] | |
| Rumex induratus Cu conc. = 134–75 | 2 | [78] | |
| Phyllostachys pubescens Cu conc. = 50, 100, 300, 600 | Steam = 0.23–0.18–0.22–0.18 Leaves = 0.15–0.09–0.10–0.07 | Roots = 0.78–1.41–0.63–0.54 Steam = 0.18–0.26–0.14–0.04 Leaves = 0.12–0.13–0.06–0.04 | [21] |
| Lemna minor Cu conc. = 0.25–0.50–0.75–1.0 | 0.360 0.191 0.335 0.432 | [12] | |
| Azolla filiculoides Cu conc. = 0.25–0.50–0.75–1.0 | 1.600 0.903 1.038 0.954 | [12] | |
| Daucus carota Cu conc. = Inceptisol Entisol, 198.6–91.3 | 0.6–3.2 | [79] | |
| Phyllostachys pubescens Cu conc. = 100 mg kg−1 | 0.18 = Stem 0.09 = Leaves | 1.41 = Root 0.26 = Stem 0.13 = Leaves | [20] |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef]
- Go, J.L.; Madrazo, C.F.; Orbecido, A.H.; de Castro, M.E.; Deocaris, C.C.; Belo, L.P. Analysis of the copper removal kinetics of the Philippine giant bamboo (Dendrocalamus asper) in hydroponics. Heliyon 2021, 7, e06208. [Google Scholar] [CrossRef] [PubMed]
- Karczewska, A.; Mocek, A.; Goliński, P.; Mleczek, M. Phytoremediation of copper-contaminated soil. In Phytoremediation: Management of Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2015; Volume 2, pp. 143–170. [Google Scholar]
- Fernandes, J.C.; Henriques, F.S. Biochemical, Physiological, and Structural Effects of Excess Copper in Plants. Bot. Rev. 1991, 57, 246–273. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace metals in soils-a current issue in Poland. Acta Univ. Wratislav. Pr. Bot. 2001, 79, 13–20. [Google Scholar]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chem, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Ranieri, E. Chromium and nickel control in full- and small-scale subsuperficial flow constructed wetlands. Soil Sediment Contam. 2012, 21, 802–814. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Ranieri, E.; Torretta, V. Process enhancement for maximization of methane production in codigestion biogas plants. Manag. Environ. Qual. Int. J. 2016, 27, 289–298. [Google Scholar] [CrossRef]
- Budiawan, B.; Auerkari, E.L. Effects of Chromium on Human Body. Res. Gate. 2017, 13, 317403540. [Google Scholar]
- Das, B.K.; Das, P.K.; Das, B.P.; Dash, P. Green Technolgy. to limit the effects of hexavalent chromium contaminated water bodies on public health and vegetation at industrial sites. J. Appl. Bio. Biotech. 2021, 9, 28–35. [Google Scholar]
- Al-Baldawi, I.A.; Yasin, S.R.; Jasim, S.S.; Abdullah, S.R.S.; Almansoory, A.F.; ’Izzati Ismail, N. Removal of copper by Azolla filiculoides and Lemna minor: Phytoremediation potential, adsorption kinetics and isotherms. Heliyon 2022, 8, e11456. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Maiti, S.K. Risk assessment of potentially toxic elements in soils and vegetables around coal-fired thermal power plant: A case study of Dhanbad, India. Environ. Monit. Assess. 2020, 192, 699. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Taverac, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Wong, C.S.; Li, X.; Thornton, I. Urban environmental geochemistry of trace metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Ranieri, E.; Świetlik, J. DBPs control in European drinking water treatment plants using chlorine dioxide: Two case studies. J. Environ. Eng. Landsc. Manag. 2010, 18, 85–91. [Google Scholar] [CrossRef]
- Van Lienden, C.; Shan, L.; Rao, S.; Ranieri, E.; Young, T.M. Metals removal from stormwater by commercial and non-commercial granular activated carbons. Water Environ. Res. 2010, 82, 351–356. [Google Scholar] [CrossRef]
- Ciudin, R.; Isarie, C.; Cioca, L.; Petrescu, V.; Nederita, V.; Ranieri, E. Vacuum waste collection system for an historical city centre. Sci. Bull. 2014, 76, 215–222. [Google Scholar]
- Ragazzi, M.; Rada, E.C.; Ranieri, E.; Masi, S.; Montanaro, C. Critical analysis of the integration of residual municipal solid waste incineration and selective collection in two Italian tourist areas. Waste Manag. Res. 2014, 32, 551–555. [Google Scholar]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of MB (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef]
- Chen, J.R.; Peng, D.L.; Shafi, M.; Li, S.; Wu, J.S.; Ye, Z.Q.; Wang, Y.; Yan, W.B.; Liu, D. Phytoremediation potential of moso bamboo (Phyllostachys pubescens) for zinc and ultrastructure changes under zinc toxicity. Russ. J. Ecol. 2015, 46, 444–449. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II) and Ni(II) from single- and multimetal solutions by recycled waste porous glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- D’Onghia, G.; Ranieri, E.; Ranieri, F.; Petrella, A.; Spagnolo, V.; Ranieri, A.C. Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study. Toxics 2021, 9, 312. [Google Scholar]
- Sgobba, F.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Menduni, G.; Ranieri, A.C.; Hoelzl, C.; Rossmadl, H.; Brehm, C.; Mackowiak, V.; et al. Compact and portable quartz-enhanced photoacoustic spectroscopy sensor for carbon monoxide environmental monitoring in urban areas. Photoacoustics 2022, 25, 100318. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Peer, W.A.; Baxter, I.R.; Richards, E.L.; Freeman, J.L.; Murphy, A.S. Phytoremediation and hyperaccumulator plants. In Molecular Biology of Metal Homeostasis and Detoxification; Tamás, M.J., Martinoia, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 299–340. [Google Scholar]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Quadro, M.S.; Camargo, F.A.; Andreazza, R. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. J. Environ. Manag. 2020, 256, 109953. [Google Scholar] [CrossRef]
- Zayed, M.A.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulations of Pb, Cu and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Muraje, H. Mass Propagation of Bamboo and its Adaptability to Wastewater Gardens in Horticulture. Ph.D Thesis, Jomo Kenyatta University of Agriculture and Thechology, Nairobi, Kenya, 2009. [Google Scholar]
- Bosire, G.O. Rehabilitation and Phytoremediation of Heavy Metal Polluted Riverine Wetlands using Bamboo for Phytoextraction in Kibera. Ph.D Thesis, Kenyatta University, Nairobi, Kenya, 2014. [Google Scholar]
- Favas, P.J.C.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of Soils Contaminated with Metals and Metalloids at Mining Areas: Potential of Native Flora. Environ. Risk Assessm. Soil Contam. 2014, 3, 485–516. [Google Scholar]
- Were, F.H.; Wafula, G.A.; Wairungu, S. Phytoremediation using bamboo to reduce the risk of chromium exposure from a contaminated tannery site in Kenya. J. Health Pollut. 2017, 7, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, R.; Mahimairaja, S.; Bharani, A.; Gayathri, P. Enhanced phytoremediation technology. for chromium contaminated soils using biological amendments. Int. J. Sci. Tech. 2014, 3, 153–162. [Google Scholar]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of heavy metals: An eco-friendly and sustainable approach. In Bioremediation and BioTechnology; Springer: Cham, Switzerland, 2020; pp. 215–231. [Google Scholar]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and its mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 78. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, S.M.; Page, S.E.; Balzter, H.; Ullah, A.; Ali, S.; Mukhamezhanova, A.S. Environmental sustainability and resilience in a polluted ecosystem via phytoremediation of heavy metals and plant physiological adaptations. J. Clean. Prod. 2023, 385, 135733. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Salam, M.M.A.; Ma, C.; Chen, G. Impacts of bamboo biochar on the phytoremediation potential of Salix psammophila grown in multi-metals contaminated soil. Int. J. Phytoremediation 2021, 23, 387–399. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes. Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.; Lahori, A.; Wang, Q.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Al-Duaij, O. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1356. [Google Scholar] [CrossRef]
- Song, U.; Park, H. Importance of biomass management acts and policies after phytoremediation. J. Ecol. Environ. 2017, 41, 13. [Google Scholar] [CrossRef]
- Cozma, P.; Hlihor, R.M.; Roșca, M.; Minuț, M.; Diaconu, M.; Gavrilescu, M. Coupling Phytoremediation with Plant Biomass Valorisation and Metal Recovery: An Overview. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; IEEE: Piscataway, NJ, USA; pp. 1–4. [Google Scholar]
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable valorization of biomass: From assisted phytoremediation to green energy production. In Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 29–51. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders -strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Brooks, R.R.; Chambers, M.F.; Nicks, L.J.; Robinson, B.H. Phytomining. Trends Plant Sci. 1998, 3, 359–362. [Google Scholar] [CrossRef]
- Rotkittikhun, P.; Kruatrachue, M.; Chaiyarat, R.; Ngernsansaruay, C.; Pokethitiyook, P.; Paijitprapaporn, A.; Baker, A.J. Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ. Pollut. 2006, 144, 681–688. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Mercury remediation potential of Brassica juncea (L.) Czern. for clean-up of flyash contaminated sites. Chemosphere 2020, 248, 125857. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper phytoremediation potential of wild plant species growing in the mine polluted areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Song, X.Z.; Peng, C.H.; Zhou, G.M.; Jiang, H.; Wang, W.G.; Xiang, W.H. Climate warming-induced upward shift of MB population on Tianmu Mountain, China. J. Mt. Sci. 2013, 10, 363–369. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Wang, Y.; Wu, J.; Ye, Z.; Liu, C.; Zhong, B.; Guo, H.; He, L.; Liu, D. Organic acid compounds in root exudation of Moso Bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 20977–20984. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, C.; Jiang, P.; Xu, Q. Review of carbon fixation in bamboo forests in China. Bot. Rev. 2011, 77, 262–270. [Google Scholar] [CrossRef]
- Yen, T. Comparing aboveground structure and aboveground carbon storage of an age series of moso bamboo forests subjected to different management strategies. J. For. Res. 2015, 20, 1–8. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C. Phytoremediation potential of moso bamboo (Phyllostachys pubescens) intercropped with Sedum plumbizincicola in metal-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 27244–27253. [Google Scholar] [CrossRef] [PubMed]
- Kalimeris, A.; Ranieri, E.; Founda, D.; Norrant, D. Variability modes of precipitation along a Central Mediterranean area and their relations with ENSO, NAO, and other climatic patterns. Atm. Res. 2017, 198, 56–80. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998; Volume 21, p. 1378. [Google Scholar]
- Xiao, F.; Gu, Z.; Sarkissian, A.; Ji, Y.; Yang, Y.; Rang, L.; Zeng, Q.; Huang, P.; Chen, H. Phytoremediation of potentially toxic elements in a polluted industrial soil using Poinsettia. Physiol. Mol. Biol. Plants 2021, 27, 675–686. [Google Scholar] [CrossRef]
- Michaud, A.; Chappellaz, C.; Hinsinger, P. Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant Soil 2008, 310, 151–165. [Google Scholar] [CrossRef]
- Collin, B.; Doelsch, E.; Keller, C.; Panfili, F.; Meunier, J.D. Effects of silicon and copper on bamboo grown hydroponically. Environ. Sci. Pollut. Res. 2013, 20, 6482–6495. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Islam, E. Lead accumulation and tolerance of MB (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ. Sci. B. 2015, 16, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mulama, J.K.; Fundi, S.I.; Gwaya, O.; Chelule, K. Effectiveness of Bamboo (Phyllostachys pubescens) and Papyrus (Papyrus cyperus) in uptake of Heavy Metals from Soil Contaminated with Petroleum Sludge. Am. J. Environ. Eng. 2020, 10, 51–58. [Google Scholar]
- Sanchez, P.G.; Fernandez, L.P.; Trejo, L.T.; Alcantar, G.G.; Cruz, J.D.; Papadopoulos, A.P. Heavy metal accumulation in beans and its impact on growth and yield under soilless culture. Acta Hortic. 1999, 481, 617. [Google Scholar] [CrossRef]
- Stingu, A.; Volf, I.; Popa, V.I. Study of copper and cadmium accumulation by bean. Environ. Eng. Manag. J. 2009, 8, 1247–1252. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Li, C.; Zhang, X.; Gu, L.; Huang, Z.; Huang, Z. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J. Hazard. Mater. 2021, 416, 125898. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef]
- Shahid, M.; Rafiq, S.S.M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Landi, C.; Liberatori, G.; Cotugno, P.; Sturba, L.; Vannuccini, M.L.; Massari, F.; Miniero, D.V.; Tursi, A.; Shaba, E.; Behnisch, P.A.; et al. First Attempt to Couple Proteomics with the AhR Reporter Gene Bioassay in Soil Pollution Monitoring and Assessment. Toxics 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Kovács, G.P.; Gyuricza, C.; Neményi, A. Potential use of bamboo in the phytoremediation in of heavy metals: A review. Acta Agrar. Debr. 2022, 1, 91–97. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Gautam, M.; Pandey, D.; Agrawal, M. Phytoremediation of metals using lemongrass (Cymbopogon citratus (DC) Stapf.) grown under different levels of red mud in soil amended with biowastes. Int. J. Phytoremediat. 2017, 19, 555–562. [Google Scholar] [CrossRef]
- Ullah, S.; Mahmood, T.; Iqbal, Z.; Naeem, A.; Ali, R.; Mahmood, S. Phytoremediative potential of salt-tolerant grass species for cadmium and lead under contaminated nutrient solution. Int. J. Phytoremediat. 2019, 21, 1012–1018. [Google Scholar] [CrossRef]
- Sajad, M.A.; Khan, M.S.; Bahadur, S.; Naeem, A.; Ali, H.; Batool, F.; Shuaib, M.; Batool, S. Evaluation of chromium phytoremediation potential of some plant species of Dir Lower. Acta Ecol. Sin. 2020, 40, 158–165. [Google Scholar]
- Ullah, S.; Mahmood, S.; Ali, R.; Khan, M.R.; Akhtar, K.; Depar, N. Comparing chromium phyto-assessment in Brachiaria mutica and Leptochloa fusca growing on chromium polluted soil. Chemosphere 2021, 269, 128728. [Google Scholar] [CrossRef]
- Brun, L.A.; Maillet, J.; Richarte, J.; Herrmann, P.; Remy, J.C. Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils. Environ. Pollut. 1998, 102, 151–161. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Fernández-Calviño, D.; Pérez-Rodríguez, P.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Copper and zinc in rhizospheric soil of wild plants growing in long-term acid vineyard soils. Insights on availability and metal remediation. Sci. Total Environ. 2019, 672, 389–399. [Google Scholar] [CrossRef]
- Melo, G.W.; Furini, G.; Brunetto, G.; Comin, J.J.; Simão, D.G.; Marques, A.C.R.; Marchezan, C.; Silva, I.C.B.; Souza, M.; Soares, C.R.; et al. Identification and phytoremediation potential of spontaneous species in vineyard soils contaminated with copper. Int. J. Phytoremediat. 2021, 24, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]

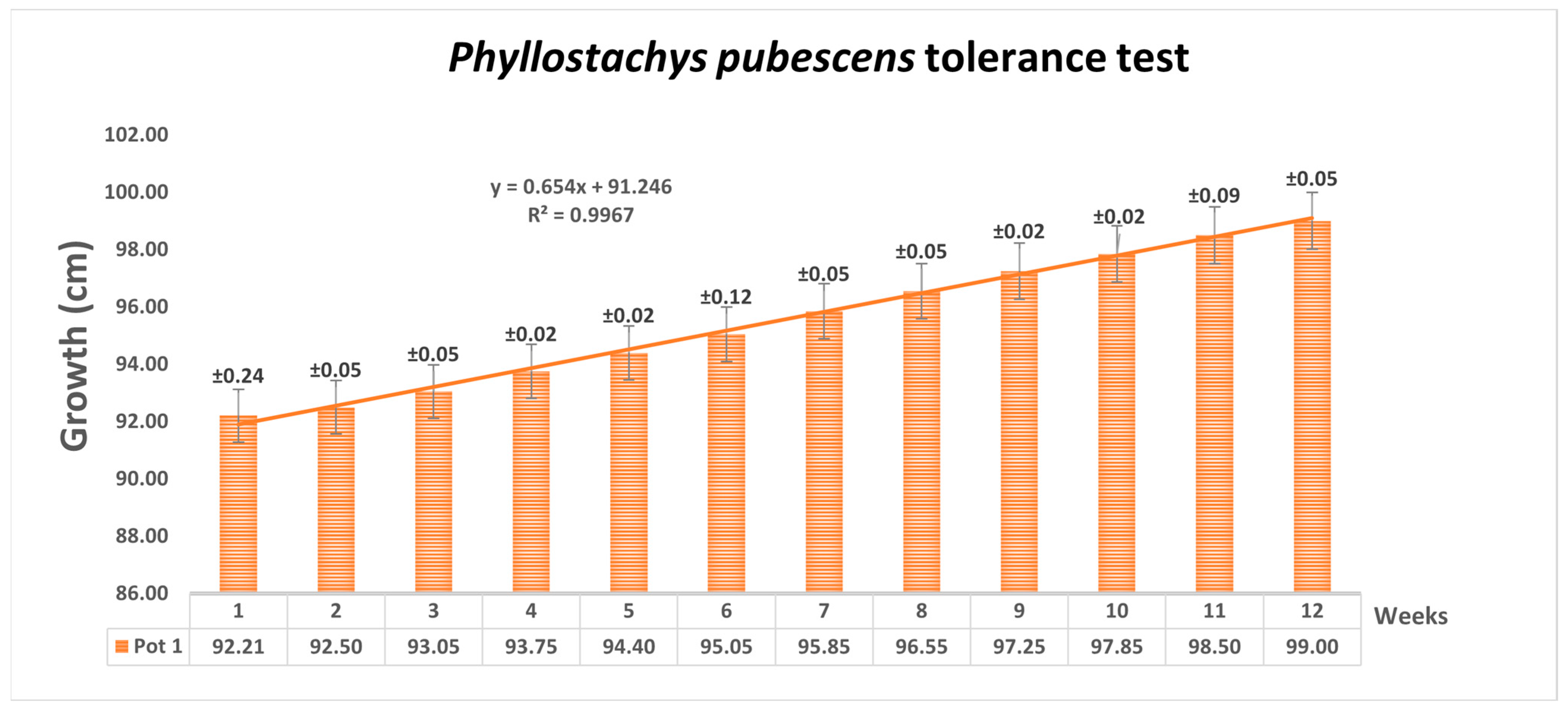

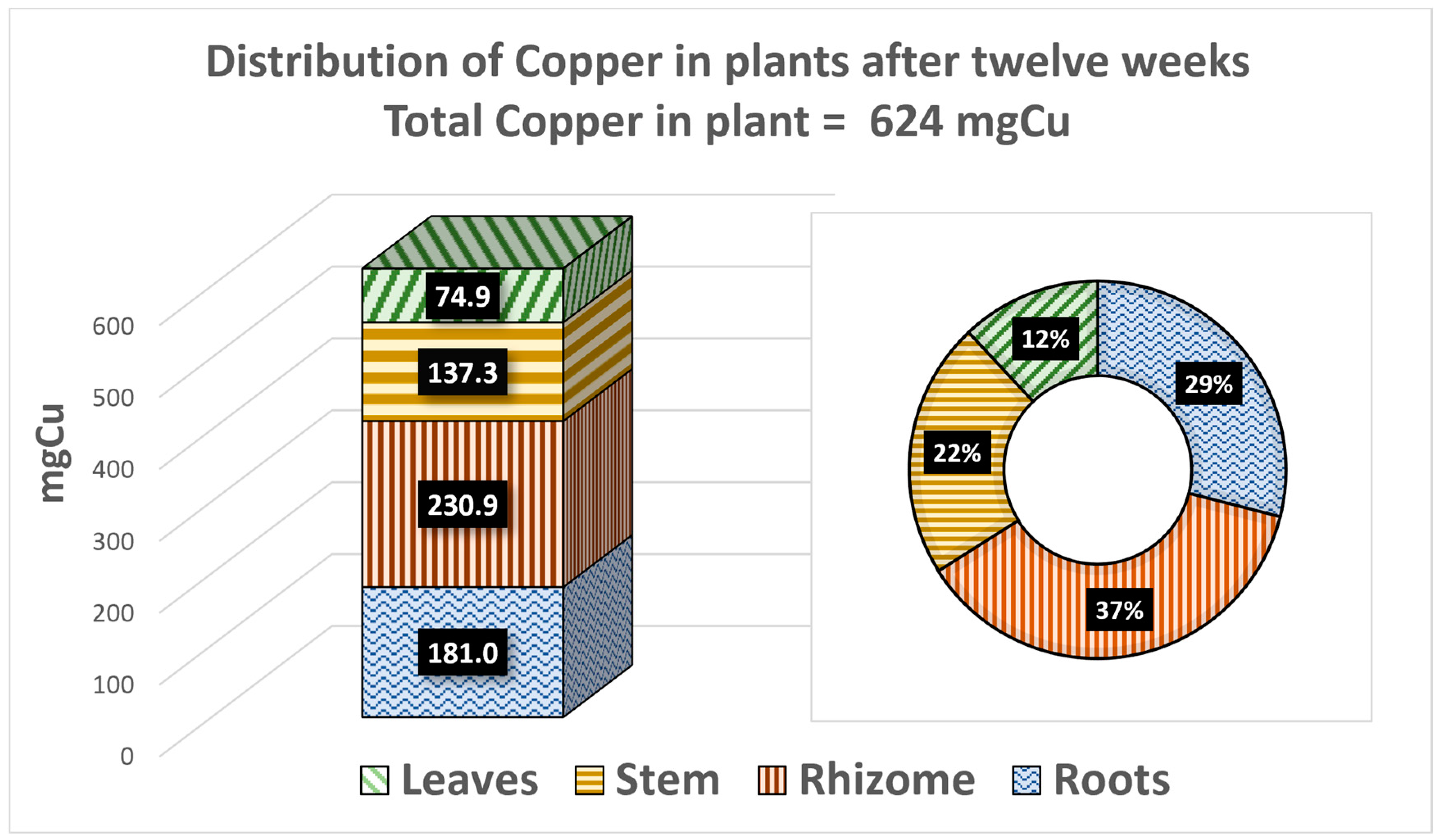
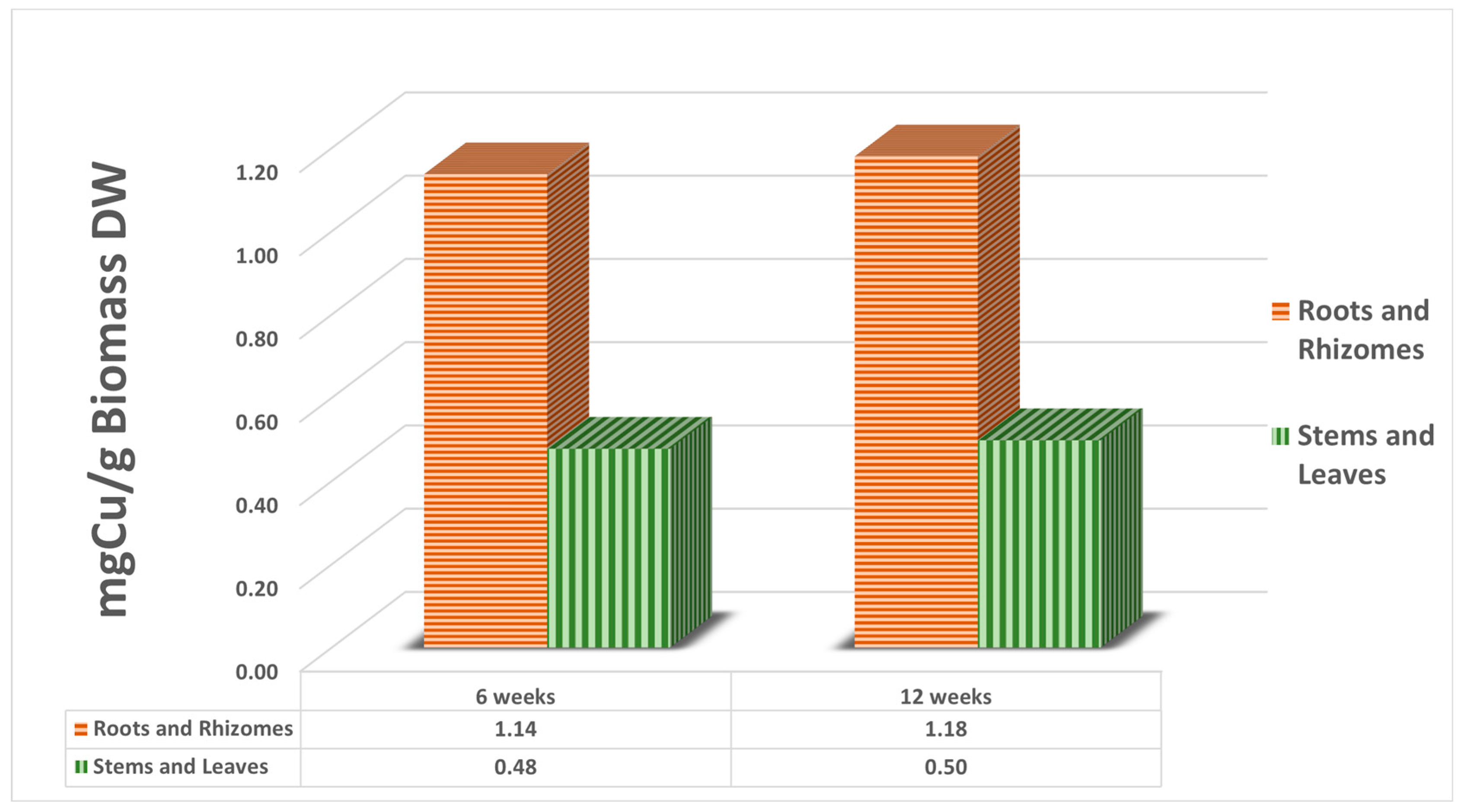
| Organs | mg Cu (Six w.) | mg Cu (Twelve w.) | g Biomass (Six w.) | g Biomass (Twelve w.) | mg Cu/g (Six w.) | mg Cu/g (Twelve w.) |
|---|---|---|---|---|---|---|
| Roots | 26.39 | 59.5 | 125.8 | 163.1 | 0.58 | 0.56 |
| Rhizomes | 54.72 | 67.0 | 170.2 | 185.8 | 0.56 | 0.62 |
| Stems | 41.51 | 50.8 | 332.5 | 388.4 | 0.06 | 0.07 |
| Leaves | 13.46 | 16.5 | 30.5 | 37.6 | 0.42 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranieri, E.; D’Onghia, G.; Ranieri, F.; Melian Herrera, J.A.; Lopopolo, L.; Spagnolo, V.L.; Ranieri, A.C. Copper Phytoextraction Using Phyllostachys pubescens. Sustainability 2023, 15, 5238. https://doi.org/10.3390/su15065238
Ranieri E, D’Onghia G, Ranieri F, Melian Herrera JA, Lopopolo L, Spagnolo VL, Ranieri AC. Copper Phytoextraction Using Phyllostachys pubescens. Sustainability. 2023; 15(6):5238. https://doi.org/10.3390/su15065238
Chicago/Turabian StyleRanieri, Ezio, Gianfranco D’Onghia, Francesca Ranieri, Jose Alberto Melian Herrera, Luigi Lopopolo, Vincenzo Luigi Spagnolo, and Ada Cristina Ranieri. 2023. "Copper Phytoextraction Using Phyllostachys pubescens" Sustainability 15, no. 6: 5238. https://doi.org/10.3390/su15065238
APA StyleRanieri, E., D’Onghia, G., Ranieri, F., Melian Herrera, J. A., Lopopolo, L., Spagnolo, V. L., & Ranieri, A. C. (2023). Copper Phytoextraction Using Phyllostachys pubescens. Sustainability, 15(6), 5238. https://doi.org/10.3390/su15065238








