Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Pyrolysis Conditions
2.2. Biochar Characterisation
2.3. Statistical Analysis
3. Results
3.1. Characterisation of Grapevine-Pruning Residues
3.2. Biochar Characterisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSA | Specific Surface Area |
| EC | Electrical conductivity |
| FTIR | Fourier transform infrared spectroscopy |
| TC | Total carbon |
| ICP-OES | Inductively coupled plasma—optical emission spectrometry |
| BET | Brunauer–Emmett–Teller method |
| SEM | Scanning Electron Microscope |
| ANOVA | Analysis of variance |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| Ca | Calcium |
| S | Sulfur |
| Mg | Magnesium |
| Na | Sodium |
| As | Arsenic |
| Fe | Iron |
| Ni | Nickel |
| Zn | Zinc |
| Cu | Copper |
| Mo | Molybdenum |
| Pb | Lead |
| Se | Selenium |
| Mn | Manganese |
References
- Maletić, E.; Karoglan Kontić, J.; Pejić, I. Vinova Loza : Ampelografija, Ekologija, Oplemenjivanje; Školska Knjiga: Zagreb, Croatia, 2008; ISBN 9789530311480. [Google Scholar]
- OIV (International Organization for Vine and Wine). World Vitiviniculture Situtation; OIV: Dijon, France, 2015. [Google Scholar]
- Hapih.hr Annual Report for 2019. Available online: https://www.hapih.hr/wp-content/uploads/2020/11/CSH-Godisnje-izvjesce-za-2019.pdf (accessed on 10 January 2023).
- Ministarstvo poljoprivrede Republike Hrvatske. SPECIFIKACIJA ZOI Hrvatska Istra Sukladno Uredbi 1308/2013, Članak 94. Za Zaštitu Oznake Izvornosti Sukladno Članku 93; Ministarstvo poljoprivrede Republike Hrvatske: Zagreb, Croatia, 2013.
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An Overview of Climate Change Impacts on European Viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Santos, J.A. Modelling Climate Change Impacts on Viticultural Yield, Phenology and Stress Conditions in Europe. Glob. Chang. Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Schultz, H.R. Climate Change and Viticulture: Research Needs for Facing the Future. J. Wine Res. 2010, 21, 113–116. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, S.K.; Krishna, H. The Effect of Certain Rootstocks on the Grape Cultivar “Pusa Urvashi” (Vitis Vinifera L.). Int. J. Fruit Sci. 2010, 10, 16–28. [Google Scholar] [CrossRef]
- Köse, B.; Karabulut, B.; Ceylan, K. Effect of Rootstock on Grafted Grapevine Quality. Eur. J. Hortic. Sci. 2014, 79, 197–202. [Google Scholar]
- Jogaiah, S.; Oulkar, D.P.; Banerjee, K.; Sharma, J.; Patil, A.G.; Maske, S.R.; Somkuwar, R.G. Biochemically Induced Variations during Some Phenological Stages in Thompson Seedless Grapevines Grafted on Different Rootstocks. S. Afr. J. Enol. Vitic. 2013, 34, 36–45. [Google Scholar] [CrossRef]
- Salomé, C.; Coll, P.; Lardo, E.; Metay, A.; Villenave, C.; Marsden, C.; Blanchart, E.; Hinsinger, P.; Le Cadre, E. The Soil Quality Concept as a Framework to Assess Management Practices in Vulnerable Agroecosystems: A Case Study in Mediterranean Vineyards. Ecol. Indic. 2016, 61, 456–465. [Google Scholar] [CrossRef]
- Parlavecchia, M.; D’Orazio, V.; Loffredo, E. Wood Biochars and Vermicomposts from Digestate Modulate the Extent of Adsorption-Desorption of the Fungicide Metalaxyl-m in a Silty Soil. Environ. Sci. Pollut. Res. 2019, 26, 35924–35934. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.M.; Cantos-Villar, E. Grapevine Cane’s Waste Is a Source of Bioactive Stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, G. Viticultural Wood Waste as a Source of Polyphenols of Interest: Opportunities and Perspectives through Conventional and Emerging Extraction Methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct Biorefinery from Vine Shoots: Bio-Ethanol and Lignin Production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with Vine-Shoots. Modulating the Composition of Wines by Using Their Own Resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Wong, M.C.; Hendrikse, S.I.S.; Sherrell, P.C.; Ellis, A.V. Grapevine Waste in Sustainable Hybrid Particleboard Production. Waste Manag. 2020, 118, 501–509. [Google Scholar] [CrossRef]
- Jiménez, L.; Angulo, V.; Ramos, E.; De La Torre, M.J.; Ferrer, J.L. Comparison of Various Pulping Processes for Producing Pulp from Vine Shoots. Ind. Crops Prod. 2006, 23, 122–130. [Google Scholar] [CrossRef]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef]
- Benyei, P.; Cohen, M.; Gresillon, E.; Angles, S.; Araque-Jiménez, E.; Alonso-Roldán, M.; Espadas-Tormo, I. Pruning Waste Management and Climate Change in Sierra Mágina’s Olive Groves (Andalusia, Spain). Reg. Environ. Chang. 2018, 18, 595–605. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Cayuela, M.L.; Rasse, D.P.; Sánchez-Monedero, M.A. Biochars from Mediterranean Agroindustry Residues: Physicochemical Properties Relevant for C Sequestration and Soil Water Retention. ACS Sustain. Chem. Eng. 2019, 7, 4724–4733. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.-N.; Fu, S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea Mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Karer, J.; Wimmer, B.; Zehetner, F.; Kloss, S.; Soja, G. Biochar Application to Temperate Soils: Effects on Nutrient Uptake and Crop Yield under Field Conditions. Agric. Food Sci. 2013, 22, 390–403. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of Biochar and Its Application in Remediation of Contaminated Soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis Temperature Affects Pore Characteristics of Rice Straw and Canola Stalk Biochars and Biochar-Amended Soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482242300. [Google Scholar]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Pyrolysis Temperature Affects Phosphorus Availability of Rice Straw and Canola Stalk Biochars and Biochar-Amended Soils. J. Soils Sediments 2021, 21, 2817–2830. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Dalias, P.; Polycarpou, P.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Physicochemical and Structural Characterization of Biochar Derived from the Pyrolysis of Biosolids, Cattle Manure and Spent Coffee Grounds. J. Energy Inst. 2020, 93, 2063–2073. [Google Scholar] [CrossRef]
- Dias, F.A.N.; Mota, R.V.D.; Souza, C.R.D.; Pimentel, R.M.D.A.; Souza, L.C.D.; Souza, A.L.D.; Regina, M.D.A. Rootstock on Vine Performance and Wine Quality of ‘Syrah’ under Double Pruning Management. Sci. Agric. 2017, 74, 134–141. [Google Scholar] [CrossRef]
- Manyà, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimental Study on the Effect of Pyrolysis Pressure, Peak Temperature, and Particle Size on the Potential Stability of Vine Shoots-Derived Biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Sadaka, S.; Sharara, M.A.; Ashworth, A.; Keyser, P.; Allen, F.; Wright, A. Characterization of Biochar from Switchgrass Carbonization. Energies 2014, 7, 548–567. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management : An Introduction. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan Publications Ltd.: Oxford, UK, 2009; ISBN 9781844076581. [Google Scholar]
- Wang, X.; Ma, D.; Jin, Q.; Deng, S.; Stančin, H.; Tan, H.; Mikulčić, H. Synergistic Effects of Biomass and Polyurethane Co-Pyrolysis on the Yield, Reactivity, and Heating Value of Biochar at High Temperatures. Fuel Process. Technol. 2019, 194, 106127. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.A.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar Characterization of Invasive Pennisetum Purpureum Grass: Effect of Pyrolysis Temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- Şensöz, S.; Can, M. Pyrolysis of Pine (Pinus Brutia Ten.) Chips: 1. Effect of Pyrolysis Temperature and Heating Rate on the Product Yields. Energy Sources 2002, 24, 347–355. [Google Scholar] [CrossRef]
- Amonette, J.; Stephen, J. Characteristics of Biochar: Microchemical Properties (Book)|OSTI.GOV. In Biochar for Environmental Management; Routledge: London, UK, 2009. [Google Scholar]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of Biochar from Fast Pyrolysis and Gasification Systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality Variations of Poultry Litter Biochar Generated at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Krack, K.; Clay, S.A.; Clay, D.E.; Schumacher, T. Impact of Biochar Application on Soil Properties and Herbicide Sorption. Proc. S. Dak. Acad. Sci. 2015, 93, 208. [Google Scholar]
- Li, C.; Xiong, Y.; Qu, Z.; Xu, X.; Huang, Q.; Huang, G. Impact of Biochar Addition on Soil Properties and Water-Fertilizer Productivity of Tomato in Semi-Arid Region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Ion, V.A.; Bucharest, V.M.; Mot, A.; Popa, V.I.; Badulescu, L. Physicochemical Characterisation of Vine Waste Used for Producing Biochar. Sci. Papers Ser. B Hortic. 2021, 65, 268–273. [Google Scholar]
- Nunes, L.J.R.; Rodrigues, A.M.; Matias, J.C.O.; Ferraz, A.I.; Rodrigues, A.C. Production of Biochar from Vine Pruning: Waste Recovery in the Wine Industry. Agriculture 2021, 11, 489. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, T.; Gao, F.; Wei, R.; Wang, Z.; Li, H.; Wang, H. Carbon Storage Distribution Characteristics of Vineyard Ecosystems in Hongsibu, Ningxia. Plants 2021, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes 2022, 10, 37. [Google Scholar] [CrossRef]
- Yang, F.; Lee, X.q.; Wang, B. Characterization of Biochars Produced from Seven Biomasses Grown in Three Different Climate Zones. Chin. J. Geochem. 2015, 34, 592–600. [Google Scholar] [CrossRef]
- EBC. Guidelines for a Sustainable Production of Biochar; EBC: Arbaz, Switzerland, 2012. [Google Scholar]
- Werkelin, J.; Skrifvars, B.J.; Hupa, M. Ash-Forming Elements in Four Scandinavian Wood Species. Part 1: Summer Harvest. Biomass Bioenergy 2005, 29, 451–466. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.; Sinha, K. Morpho-Mineralogical Exploration of Crop, Weed and Tree Derived Biochar. J. Hazard. Mater. 2021, 407, 124370. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tsai, W.T.; Chen, J.W.; Lin, Y.Q.; Chang, Y.M. Characterization of Biochar Prepared from Biogas Digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- European Commission. ODLUKA KOMISIJE (EU) 2015/2099 Od 18. Studenoga 2015. o Utvrđivanju Ekoloških Mjerila Za Dodjelu Znaka Za Okoliš EU-a Za Uzgojne Supstrate, Poboljšivače Tla i Malč; European Commission: Zagreb, Croatia, 2015. [Google Scholar]
- de la Rosa, J.M.; Rosado, M.; Paneque, M.; Miller, A.Z.; Knicker, H. Effects of Aging under Field Conditions on Biochar Structure and Composition: Implications for Biochar Stability in Soils. Sci. Total Environ. 2018, 613–614, 969–976. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Horn, R. Comparing the Potentials of Clay and Biochar in Improving Water Retention and Mechanical Resilience of Sandy Soil. Int. Agrophysics 2016, 30, 391–399. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase-Mineral and Chemical Composition and Classification. Fuel 2013, 89, 913–933. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y.; Cho, T.S.; Choi, J.W. Influence of Pyrolysis Temperature on Physicochemical Properties of Biochar Obtained from the Fast Pyrolysis of Pitch Pine (Pinus Rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zong, Y. Pore Structure and Environmental Serves of Biochars Derived from Different Feedstocks and Pyrolysis Conditions. Environ. Sci. Pollut. Res. 2018, 25, 30401–30409. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of Biochar as Fuel and Catalyst in Energy Recovery Technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Marshall, J.; Muhlack, R.; Morton, B.J.; Dunnigan, L.; Chittleborough, D.; Kwong, C.W. Pyrolysis Temperature Effects on Biochar–water Interactions and Application for Improved Water Holding Capacity in Vineyard Soils. Soil Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov Vladimir, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of Pyrolysis Temperature on Production and Nutrient Properties of Wastewater Sludge Biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting Biochar Properties and Functions Based on Feedstock and Pyrolysis Temperature: A Review and Data Syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
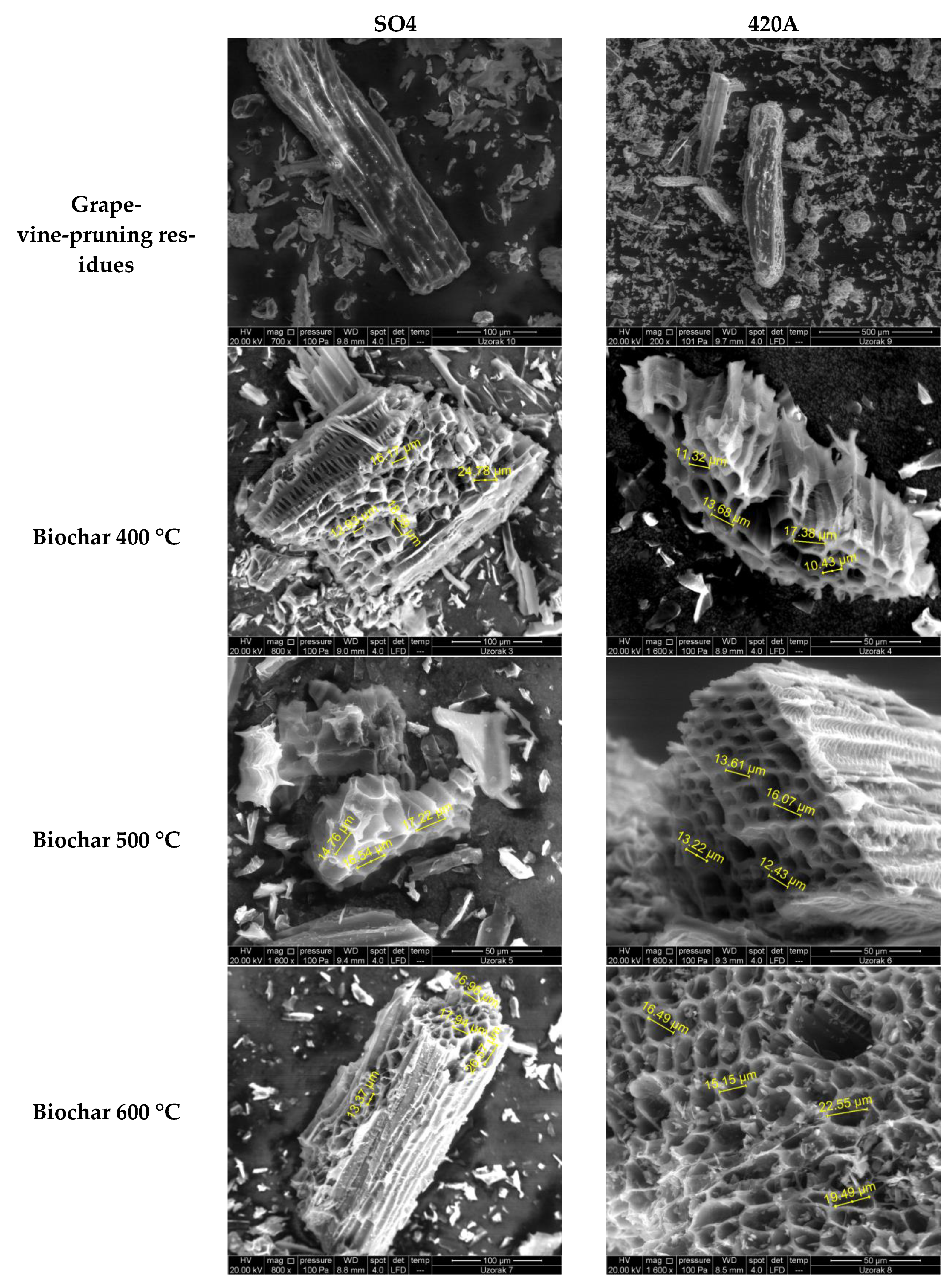
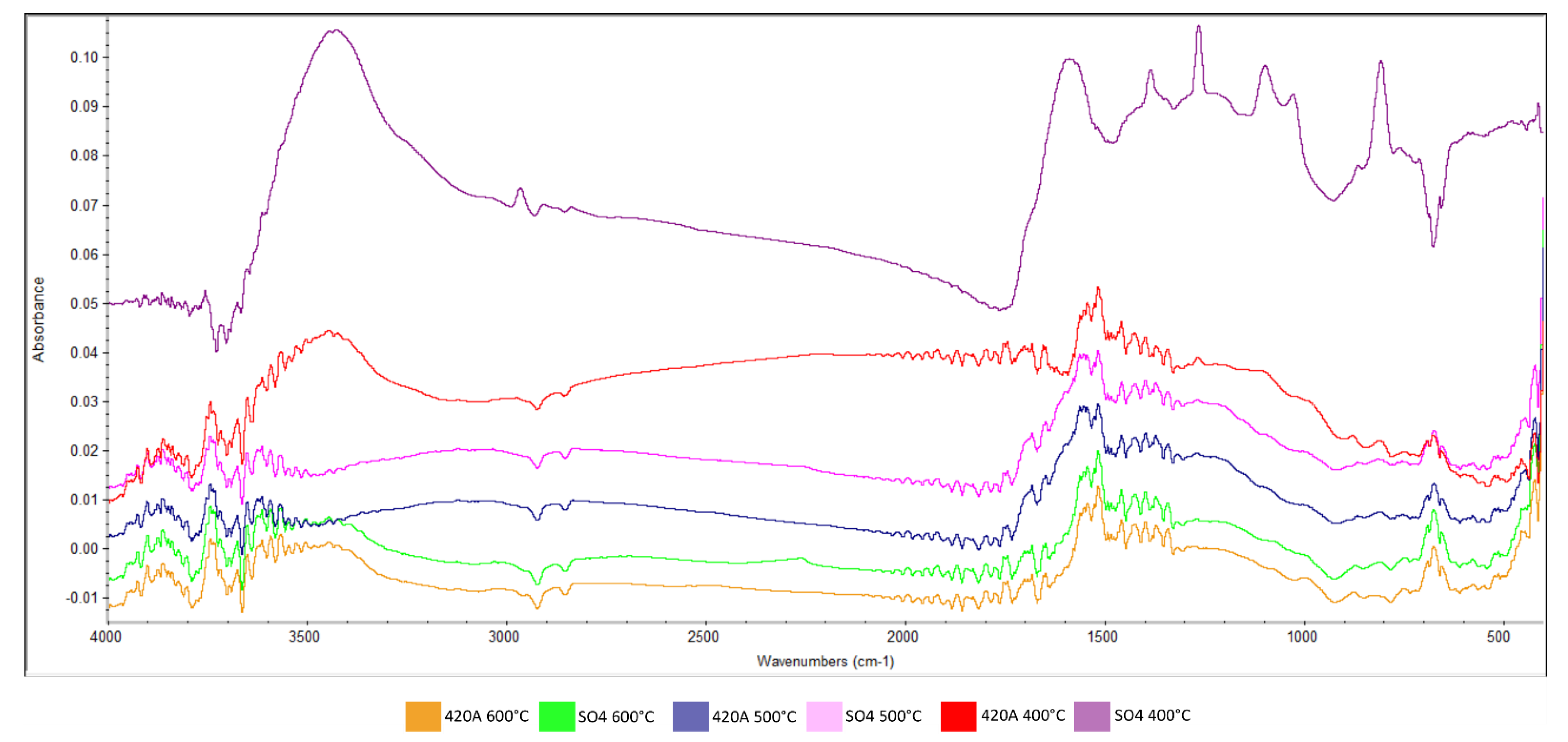
| Parameter | Unit | Rootstock | Significance | |
|---|---|---|---|---|
| SO4 | 420A | |||
| ash | % | 3.71 ± 0.01 | 3.27 ± 0.01 | *** |
| pH | / | 5.21 ± 0.02 | 5.34 ± 0.02 | ** |
| EC | µS/cm | 2240 ± 70.0 | 2456 ± 8.82 | * |
| TC | % | 44.4 ± 0.32 | 44.5 ± 0.42 | n.s. |
| N | % | 0.61 ± 0.05 | 0.61 ± 0.06 | n.s. |
| P | g/kg | 0.68 ± 0.04 | 0.63 ± 0.02 | n.s. |
| K | g/kg | 9.14 ± 0.63 | 10.9 ± 1.12 | n.s. |
| S | g/kg | 0.35 ± 0.02 | 0.29 ± 0.02 | n.s. |
| Ca | g/kg | 5.91 ± 0.95 | 5.19 ± 0.57 | n.s. |
| Mg | g/kg | 0.95 ± 0.10 | 0.85 ± 0.05 | n.s. |
| Na | g/kg | 34.2 ± 2.48 | 41.5 ± 6.04 | n.s. |
| As | mg/kg | n.d. | n.d. | / |
| Cu | mg/kg | 3.71 ± 0.95 | 3.37 ± 0.57 | n.s. |
| Fe | mg/kg | 92.8 ± 41.5 | 27.7 ± 3.76 | n.s. |
| Mn | mg/kg | 25.1 ± 3.83 | 12.1 ± 1.84 | * |
| Mo | mg/kg | 0.19 ± 0.01 | 0.17 ± 0.02 | n.s. |
| Ni | mg/kg | 0.24 ± 0.10 | 0.04 ± 0.02 | n.s. |
| Pb | mg/kg | n.d. | n.d. | / |
| Se | mg/kg | 0.02 ± 0.24 | 0.06 ± 0.23 | n.s. |
| Zn | mg/kg | 8.64 ± 3.13 | 4.66 ± 1.44 | n.s. |
| SSA | m2g−1 | 1.02 ± 0.01 | 1.05 ± 0.01 | n.s. |
| Yield | pH | EC | Ash | TC | |
|---|---|---|---|---|---|
| % | µS/cm | % | % | ||
| Rootstock | |||||
| SO4 | 30.6 ± 0.01 | 9.53 ± 0.13 a | 1727 ± 257 a | 9.51 ± 0.01 a | 75.60 ± 0.64 |
| 420A | 31.1 ± 0.01 | 9.38 ± 0.12 b | 1094 ± 233 b | 8.29 ± 0.01 b | 75.29 ± 0.73 |
| p value | n.s. | ** | *** | *** | n.s. |
| Temperature | |||||
| 400 °C | 34.0 ± 0.01 a | 9.79 ± 0.05 a | 792 ± 65.9 c | 8.36 ± 0.01 c | 73.1 ± 0.43 c |
| 500 °C | 29.9 ± 0.01 b | 9.00 ± 0.03 c | 1123 ± 249 b | 8.81 ± 0.01 b | 75.8 ± 0.26 b |
| 600 °C | 28.7 ± 0.01 c | 9.58 ± 0.10 b | 2318 ± 158 a | 9.52 ± 0.01 a | 77.4 ± 0.23 a |
| p value | *** | *** | *** | *** | *** |
| Rootstock × Temperature | 400 °C | ||||
| SO4 | 33.9 ± 0.01 | 9.78 ± 0.46 a | 921 ± 80.9 | 9.03 ± 0.01 | 73.5 ± 0.65 |
| 420A | 33.9 ± 0.1 | 9.79 ± 0.19 a | 663 ± 93.5 | 7.69 ± 0.01 | 72.7 ± 1.39 |
| 500 °C | |||||
| SO4 | 29.6 ± 0.01 | 9.02 ± 0.95 c | 1595 ± 196 | 9.49 ± 0.01 | 75.6 ± 0.68 |
| 420A | 30.1 ± 0.01 | 8.97 ± 0.05 c | 651 ± 470 | 8.13 ± 0.01 | 76.0 ± 0.65 |
| 600 °C | |||||
| SO4 | 28.3 ± 0.01 | 9.79 ± 0.92 a | 2666 ± 55.1 | 10.0 ± 0.01 | 77.7 ± 0.71 |
| 420A | 29.1 ± 0.01 | 9.37 ± 0.70 b | 1969 ± 95.9 | 9.04 ± 0.01 | 77.2 ± 0.37 |
| p value | n.s. | ** | n.s. | n.s. | n.s. |
| N | P | K | S | Ca | Mg | Na | |
|---|---|---|---|---|---|---|---|
| % | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | |
| Rootstock | |||||||
| SO4 | 1.07 ± 0.01 | 24.3 ± 1.02 | 43.8 ± 4.81 | 14.8 ± 0.53 a | 224 ± 12.4 | 31.0 ± 1.46 | 15.0 ± 1.80 |
| 420A | 1.04 ± 0.05 | 25.1 ± 0.69 | 42.1 ± 4.45 | 13.1 ± 0.37 b | 212 ± 9.36 | 33.1 ± 1.23 | 15.3 ± 1.58 |
| p-value | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. |
| Temperature | |||||||
| 400 °C | 1.06 ± 0.01 | 22.0 ± 0.82 b | 28.6 ± 0.43 b | 12.6 ± 0.32 b | 182 ± 3.79 b | 27.7± 0.96 b | 9.65 ± 0.47 b |
| 500 °C | 1.03 ± 0.03 | 25.3 ± 0.72 a | 45.9 ± 5.76 a | 14.2 ± 0.68 a | 227 ± 9.26 a | 33.4 ± 0.82 a | 16.4 ± 1.45 a |
| 600 °C | 1.07 ± 0.07 | 26.8 ± 0.36 a | 54.3 ± 1.33 a | 15.1 ± 0.49 a | 246 ± 8.13 a | 35.1 ± 1.34 a | 19.4 ± 1.24 a |
| p-value | n.s. | *** | *** | *** | *** | *** | *** |
| Rootstock × Temperature | |||||||
| 400 °C | |||||||
| SO4 | 1.07 ± 0.01 | 20.5 ± 0.87 d | 28.7 ± 1.17 | 12.9 ± 0.86 | 182 ± 12.5 | 26.4 ± 1.82 | 9.43 ± 0.92 |
| 420A | 1.04 ± 0.01 | 23.5 ± 1.51 c | 28.6 ± 1.16 | 12.24 ± 0.69 | 181 ± 7.57 | 29.1 ± 2.29 | 9.88 ± 1.51 |
| 500 °C | |||||||
| SO4 | 1.08 ± 0.01 | 26.1 ± 1.74 ab | 46.2 ± 15.3 | 15.7 ± 0.39 | 242 ± 23.7 | 33.2 ± 2.95 | 16.4 ± 4.36 |
| 420A | 0.98 ± 0.08 | 24.4 ± 1.56 bc | 45.7 ± 16.2 | 12.7 ± 0.53 | 211 ± 1.94 | 33.5 ± 1.10 | 16.4 ± 3.54 |
| 600 °C | |||||||
| SO4 | 1.05 ± 0.05 | 26.4 ± 0.46 ab | 56.4 ± 2.82 | 15.9 ± 1.06 | 248 ± 23.7 | 33.5 ± 4.03 | 19.3 ± 4.41 |
| 420A | 1.09 ± 0.27 | 27.3 ± 1.06 a | 52.1 ± 2.08 | 14.3 ± 0.79 | 244 ± 12.0 | 36.8 ± 1.57 | 19.4 ± 1.86 |
| p-value | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. |
| As | Cu | Fe | Mn | Mo | Ni | Pb | Se | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| Rootstock | |||||||||
| SO4 | 0.10 ± 0.03 | 5.05 ± 0.15 | 6.38 ± 0.37 | 6.99 ± 0.26 a | 0.10 ± 0.01 | 0.14 ± 0.03 | 0.65 ± 0.08 | 0.07 ± 0.02 | 3.82 ± 0.42 |
| 420A | 0.33 ± 0.14 | 5.15 ± 0.44 | 6.74 ± 0.47 | 3.01 ± 0.08 b | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.65 ± 0.08 | 0.12 ± 0.07 | 3.99 ± 0.16 |
| p-value | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. | n.s. |
| Temperature | |||||||||
| 400 °C | 0.08 ± 0.04 | 4.36 ± 0.34 b | 6.60 ± 0.84 | 4.89 ± 0.88 | 0.09 ± 0.01 | 0.05 ± 0.01 c | 0.49 ± 0.04 | 0.05 ± 0.01 | 3.59 ± 0.31 |
| 500 °C | 0.19 ± 0.14 | 5.16 ± 0.27 ab | 6.34 ± 0.40 | 4.94 ± 0.97 | 0.10 ± 0.02 | 0.12 ± 0.02 b | 0.65 ± 0.11 | 0.05 ± 0.01 | 4.48 ± 0.38 |
| 600 °C | 0.36 ± 0.17 | 5.77 ± 0.37 a | 6.74 ± 0.09 | 5.16 ± 0.91 | 0.08 ± 0.01 | 0.23 ± 0.03 a | 0.80 ± 0.08 | 0.19 ± 0.10 | 3.63 ± 0.38 |
| p-value | n.s. | ** | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. |
| Rootstock × temperature | 400 °C | ||||||||
| SO4 | 0.12 ± 0.12 | 4.73 ± 0.29 | 5.61 ± 1.37 | 6.76 ± 1.02 | 0.08 ± 0.02 b | 0.05 ± 0.00 | 0.49 ± 0.09 | 0.05 ± 0.00 | 3.10 ± 0.28 b |
| 420A | 0.05 ± 0.04 | 3.98 ± 1.11 | 7.58 ± 2.39 | 3.03 ± 0.29 | 0.09 ± 0.01 ab | 0.05 ± 0.00 | 0.48 ± 0.12 | 0.05 ± 0.00 | 4.08 ± 0.77 ab |
| 500 °C | |||||||||
| SO4 | 0.05 ± 0.00 | 5.30 ± 0.98 | 6.69 ± 1.29 | 7.06 ± 0.83 | 0.13 ± 0.04 a | 0.11 ± 0.04 | 0.67 ± 0.27 | 0.05 ± 0.00 | 5.16 ± 0.89 a |
| 420A | 0.23 ± 0.48 | 5.02 ± 1.02 | 5.99 ± 0.64 | 2.82 ± 0.21 | 0.07 ± 0.01 b | 0.12 ± 0.05 | 0.64 ± 0.34 | 0.05 ± 0.00 | 3.80 ± 0.18 b |
| 600 °C | |||||||||
| SO4 | 0.11 ± 0.13 | 5.11 ± 0.69 | 6.84 ± 0.29 | 7.14 ± 0.80 | 0.08 ± 0.03 b | 0.26 ± 0.07 | 0.78 ± 0.26 | 0.12 ± 0.13 | 3.19 ± 1.14 b |
| 420A | 0.61 ± 0.45 | 6.43 ± 0.55 | 6.64 ± 0.12 | 3.19 ± 0.15 | 0.08 ± 0.02 b | 0.19 ± 0.02 | 0.82 ± 0.14 | 0.26 ± 0.36 | 4.07 ± 0.51 ab |
| p-value | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | * |
| SSA (m2g−1) | |
|---|---|
| Rootstock | |
| SO4 | 1.60 ± 0.20 a |
| 420A | 1.36 ± 0.10 b |
| p-value | *** |
| Temperature | |
| 400 °C | 2.07 ± 0.14 a |
| 500 °C | 1.14 ± 0.02 b |
| 600 °C | 1.25 ± 0.02 b |
| p-value | *** |
| Rootstock × Temperature | 400 °C |
| SO4 | 2.38 ± 0.02 a |
| 420A | 1.76 ± 0.02 b |
| 500 °C | |
| SO4 | 1.15 ± 0.05 e |
| 420A | 1.12 ± 0.03 e |
| 600 °C | |
| SO4 | 1.28 ± 0.01 c |
| 420A | 1.21 ± 0.01 d |
| p-value | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anđelini, D.; Cvitan, D.; Prelac, M.; Pasković, I.; Černe, M.; Nemet, I.; Major, N.; Goreta Ban, S.; Užila, Z.; Zubin Ferri, T.; et al. Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability 2023, 15, 4851. https://doi.org/10.3390/su15064851
Anđelini D, Cvitan D, Prelac M, Pasković I, Černe M, Nemet I, Major N, Goreta Ban S, Užila Z, Zubin Ferri T, et al. Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability. 2023; 15(6):4851. https://doi.org/10.3390/su15064851
Chicago/Turabian StyleAnđelini, Dominik, Danko Cvitan, Melissa Prelac, Igor Pasković, Marko Černe, Ivan Nemet, Nikola Major, Smiljana Goreta Ban, Zoran Užila, Tea Zubin Ferri, and et al. 2023. "Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature" Sustainability 15, no. 6: 4851. https://doi.org/10.3390/su15064851
APA StyleAnđelini, D., Cvitan, D., Prelac, M., Pasković, I., Černe, M., Nemet, I., Major, N., Goreta Ban, S., Užila, Z., Zubin Ferri, T., Njegić Džakula, B., Petek, M., Ban, D., & Palčić, I. (2023). Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability, 15(6), 4851. https://doi.org/10.3390/su15064851












