Drought Stress Restoration Frequencies of Phenotypic Indicators in Early Vegetative Stages of Soybean (Glycine max L.)
Abstract
1. Introduction
2. Results
2.1. Morphological Traits
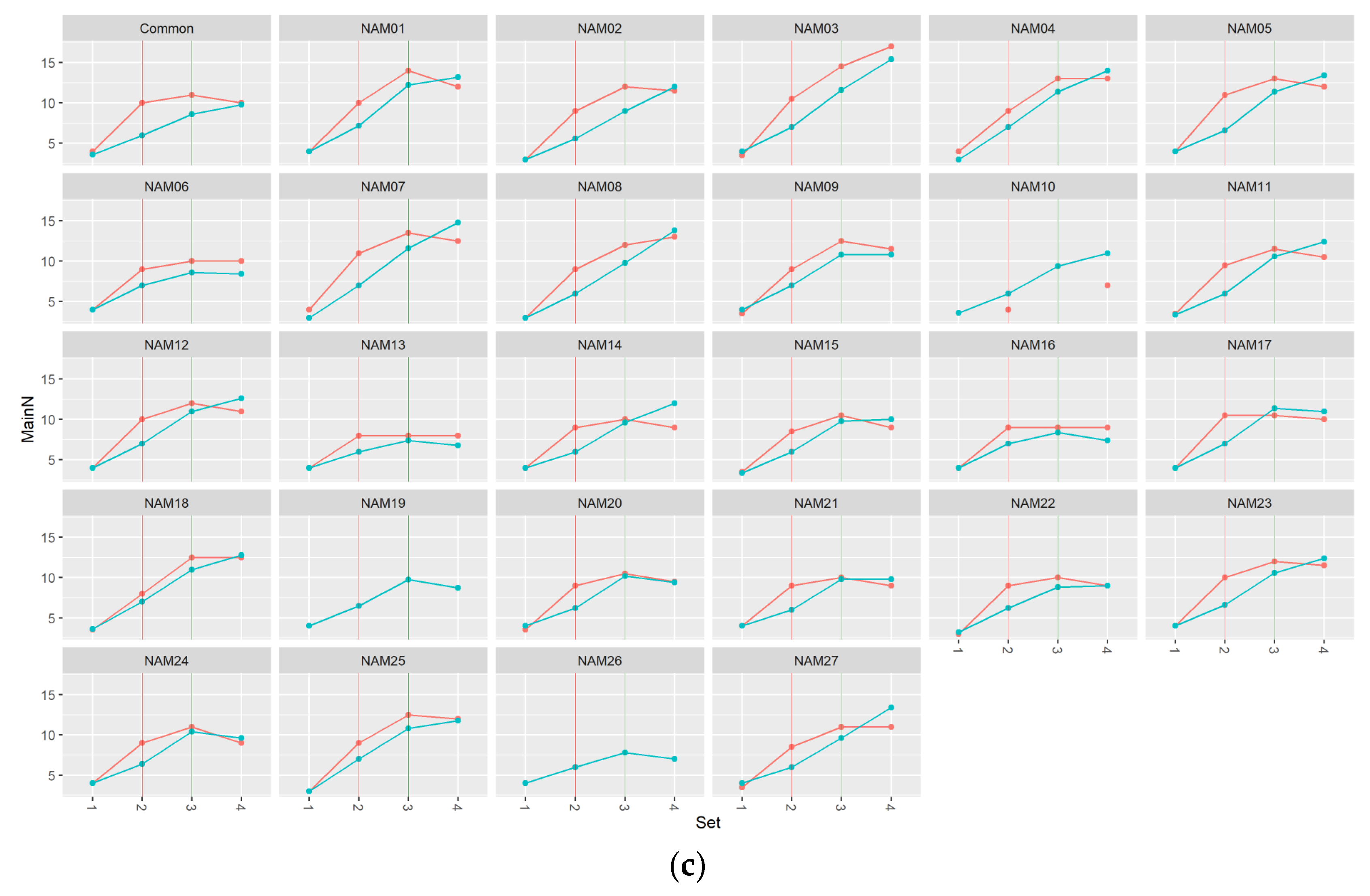
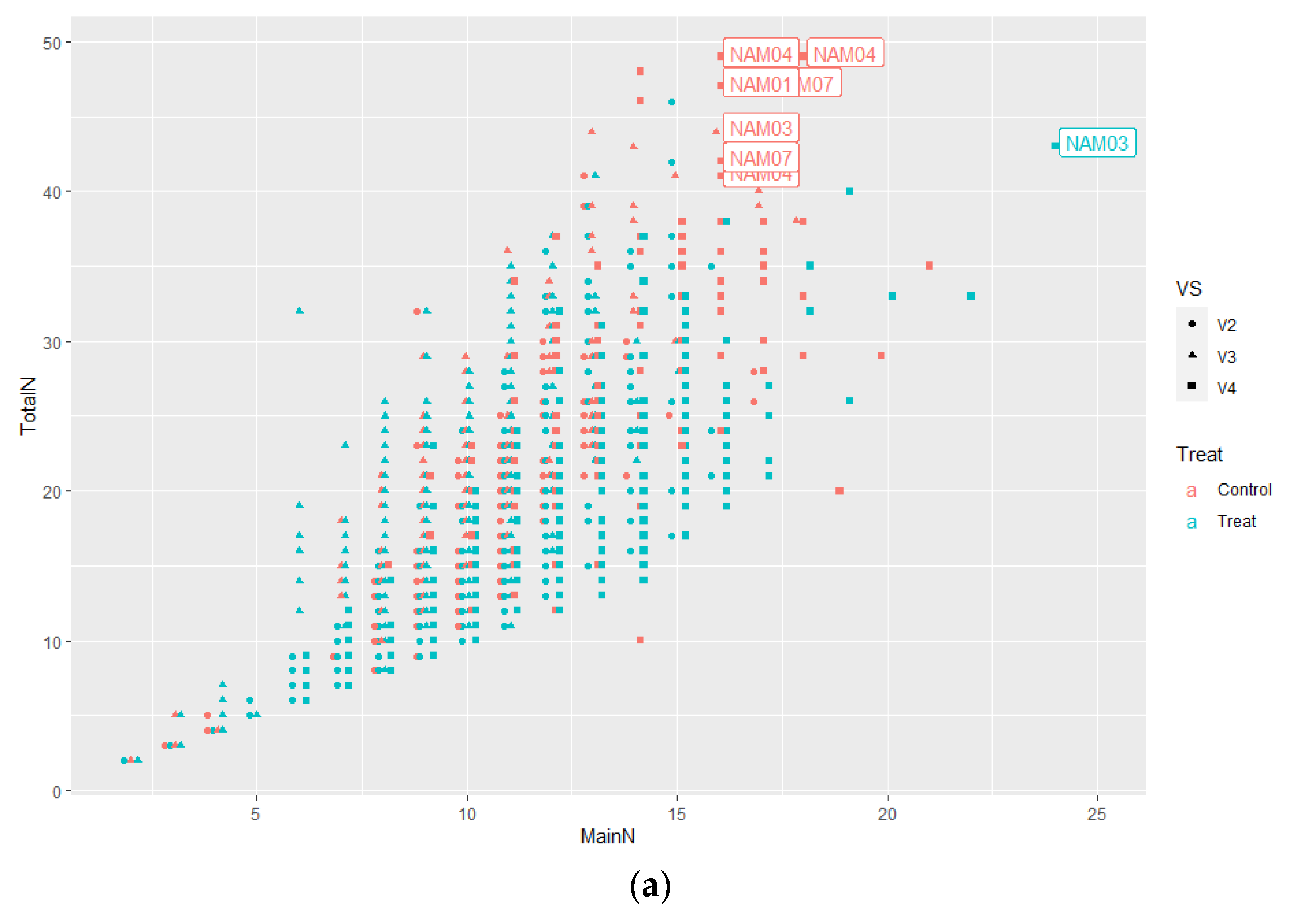
2.1.1. Number of Main Stem Nodes
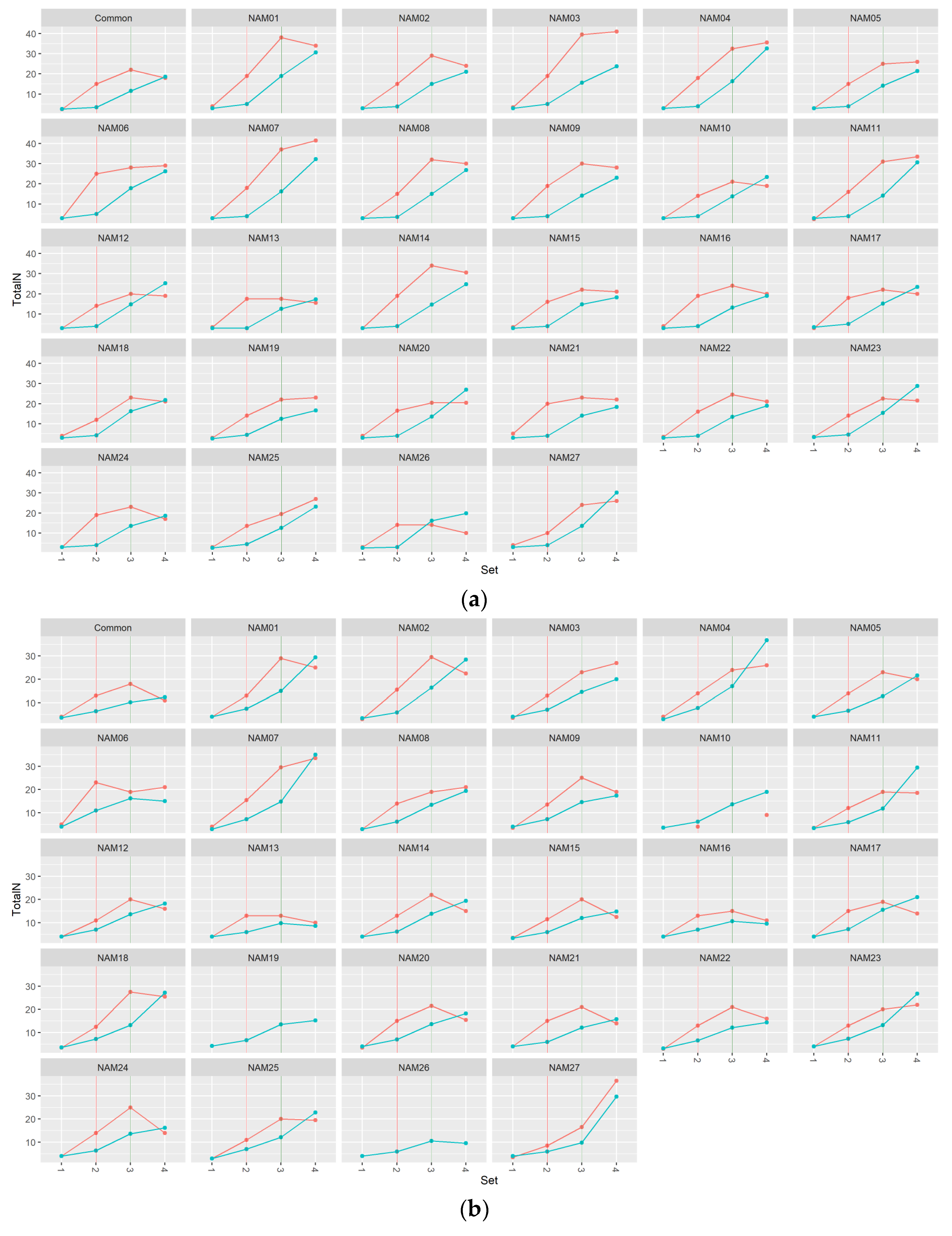
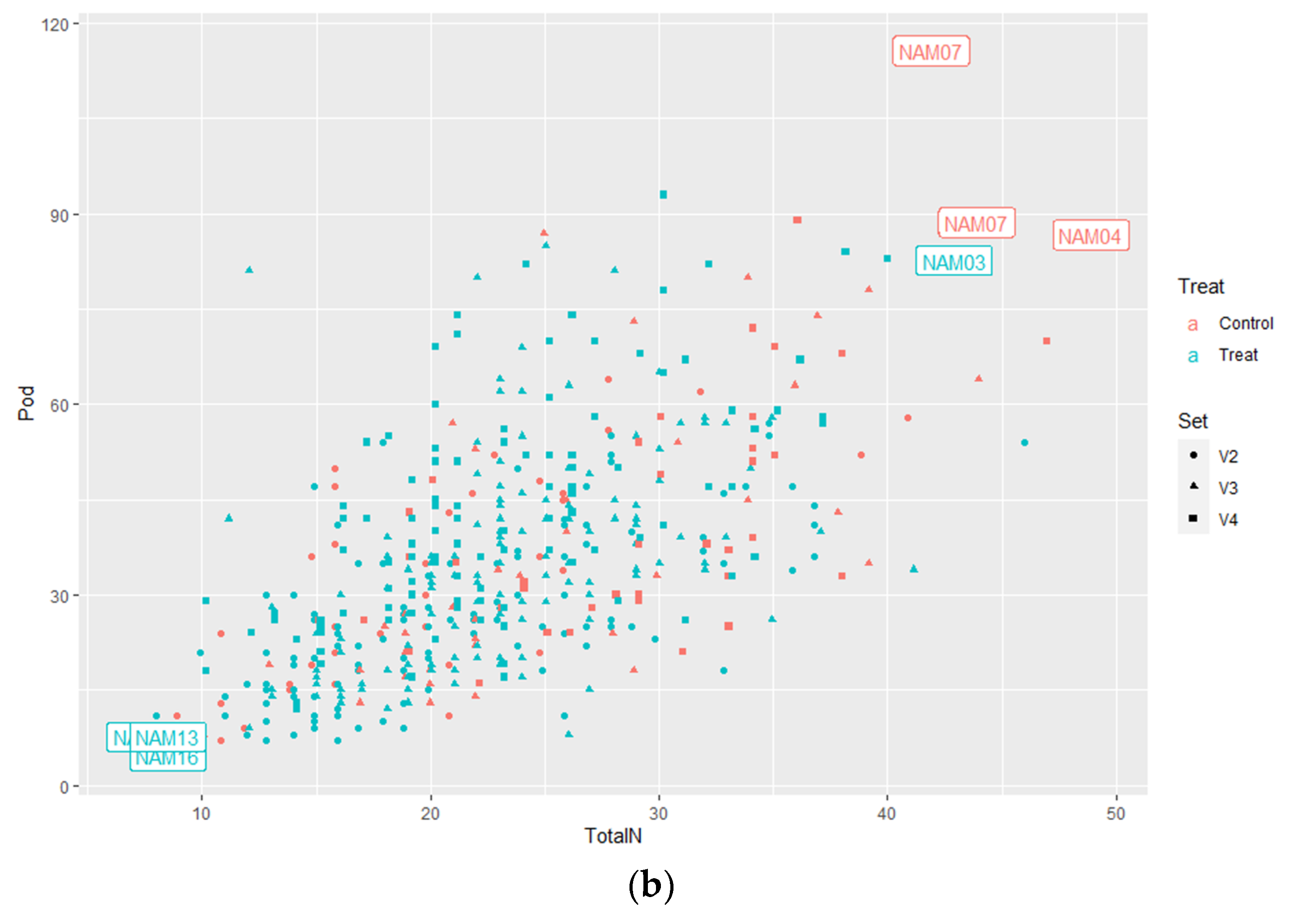
2.1.2. Number of Total Nodes
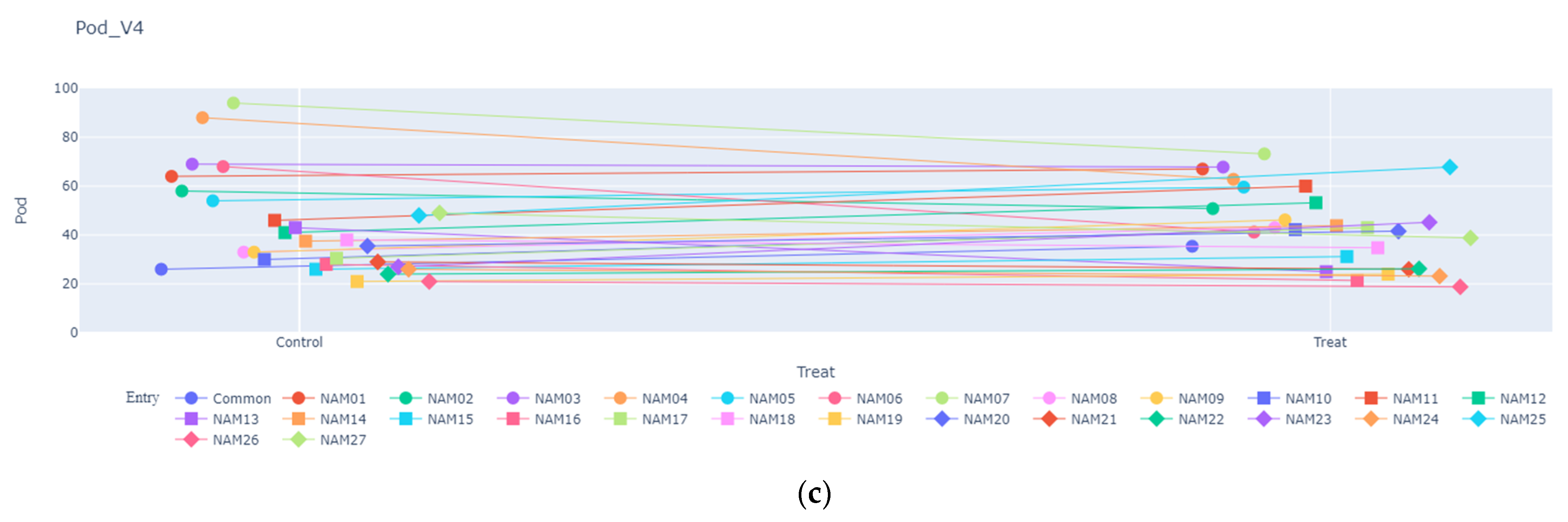
2.1.3. Number of Pods
2.1.4. Area
2.2. Statistical Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Drought Treatments
5.3. Measurements
5.4. Image Data
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Mokhtar, M.A.; Anli, M.; Laouane, R.B.; Boutasknit, A.; Boutaj, H.; Draoui, A.; Zarik, L.; Fakhech, A. Food security and climate change. In Research Anthology on Environmental and Societal Impacts of Climate Change; Information Resources Management Association, Ed.; lgi Global: Hershey, PA, USA, 2022; pp. 44–63. [Google Scholar] [CrossRef]
- da Silva, E.C.; Nogueira, R.J.M.C.; da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin, Germany, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Y.; Zhang, X.; Wu, X.; Zhou, X.; Ren, B.; Zeng, J.; Wang, Q. Spatiotemporal variability in annual drought severity, duration, and frequency from 1901 to 2020. Clim. Res. 2022, 87, 81–97. [Google Scholar] [CrossRef]
- Tuberosa, R.; Salvi, S. Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci. 2006, 11, 405–412. [Google Scholar] [CrossRef]
- Bernier, J.; Atlin, G.N.; Serraj, R.; Kumar, A.; Spaner, D. Breeding upland rice for drought resistance. J. Sci. Food Agric. 2008, 88, 927–939. [Google Scholar] [CrossRef]
- Ku, Y.S.; Au-Yeung, W.K.; Yung, Y.L.; Li, M.W.; Wen, C.Q.; Liu, X.; Lam, H.M. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J., Ed.; IntechOpen: London, UK, 2013; pp. 209–237. [Google Scholar] [CrossRef]
- Vogel, J.T.; Liu, W.; Olhoft, P.; Crafts-Brandner, S.J.; Pennycooke, J.C.; Christiansen, N. Soybean yield formation physiology–a foundation for precision breeding based improvement. Front. Plant Sci. 2021, 12, 719706. [Google Scholar] [CrossRef]
- Kantolic, A.G.; Slafer, G.A. Reproductive development and yield components in indeterminate soybean as affected by post-flowering photoperiod. Field Crops Res. 2005, 93, 212–222. [Google Scholar] [CrossRef]
- Xiong, R.; Liu, S.; Considine, M.J.; Siddique, K.H.; Lam, H.M.; Chen, Y. Root system architecture, physiological and transcriptional traits of soybean (Glycine max L.) in response to water deficit: A review. Physiol. Plant. 2021, 172, 405–418. [Google Scholar] [CrossRef]
- Desclaux, D.; Roumet, P. Impact of drought stress on the phenology of two soybean (Glycine max L. Merr) cultivars. Field Crops Res. 1996, 46, 61–70. [Google Scholar] [CrossRef]
- Kron, A.P.; Souza, G.M.; Ribeiro, R.V. Water deficiency at different developmental stages of Glycine max can improve drought tolerance. Bragantia 2008, 67, 43–49. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Funct. Plant Biol. 2003, 30, 65–73. [Google Scholar] [CrossRef]
- Zhang, J.; Smith, D.L.; Liu, W.; Chen, X.; Yang, W. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr. J. Biotechnol. 2011, 10, 14392–14398. [Google Scholar] [CrossRef]
- Liu, F.; Andersen, M.N.; Jensen, C.R. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Funct. Plant Biol. 2003, 30, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef]
- Sarwat, M.; Tuteja, N. Hormonal signaling to control stomatal movement during drought stress. Plant Gene 2017, 11, 143–153. [Google Scholar] [CrossRef]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Synková, H.; Van Loven, K.; Pospíšilová, J.; Valcke, R. Photosynthesis of transgenic Pssu-ipt tobacco. J. Plant Physiol. 1999, 155, 173–182. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Vankova, R.; Tanaka, M.; Seki, M.; Ham, L.H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Identification and expression analysis of cytokinin metabolic genes in soybean under normal and drought conditions in relation to cytokinin levels. PLoS ONE 2012, 7, e42411. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]







| NAM Number | Variety |
|---|---|
| Common | Daepung |
| NAM 01 | Bangsa |
| NAM 02 | Pungwon |
| NAM 03 | Hannam |
| NAM 04 | Sowon |
| NAM 05 | Galche |
| NAM 06 | Somyeong |
| NAM 07 | Sinhwa |
| NAM 08 | Pureun |
| NAM 09 | Taegwang |
| NAM 10 | Wuram |
| NAM 11 | Danbek |
| NAM 12 | PI96983 |
| NAM 13 | Haman |
| NAM 14 | Willians82 |
| NAM 15 | Saedanbek |
| NAM 16 | Daewon |
| NAM 17 | Hwanggeum |
| NAM 18 | Chungja |
| NAM 19 | Chungja 3ho |
| NAM 20 | Sochung 2ho |
| NAM 21 | Ilpumgeomjung |
| NAM 22 | Daeheuk |
| NAM 23 | Josangseori |
| NAM 24 | Yeunpung |
| NAM 25 | Chunal |
| NAM 26 | Heukchung |
| NAM 27 | Seoritae |
| Vegetative Stage | Variables (1) | df | p (<0.05) | |
|---|---|---|---|---|
| V2 | Main nodes | Treatment | 1 | <0.005 * (2) |
| Varieties | 27 | <0.005 * | ||
| Total nodes | Treatment | 1 | <0.005 * | |
| Varieties | 27 | 0.2503 | ||
| Pods | Treatment | 1 | 0.01479 * | |
| Varieties | 27 | <0.005 * | ||
| Area | Treatment | 1 | <0.005 * | |
| Varieties | 27 | 0.9673 | ||
| V3 | Main nodes | Treatment | 1 | <0.005 * |
| Varieties | 27 | <0.005 * | ||
| Total nodes | Treatment | 1 | <0.005 * | |
| Varieties | 27 | 0.7901 | ||
| Pods | Treatment | 1 | 0.6713 | |
| Varieties | 27 | <0.005 * | ||
| Area | Treatment | 1 | <0.005 * | |
| Varieties | 27 | 0.3360 | ||
| V4 | Main nodes | Treatment | 1 | <0.005 * |
| Varieties | 27 | <0.005 * | ||
| Total nodes | Treatment | 1 | <0.005 * | |
| Varieties | 27 | <0.005 * | ||
| Pods | Treatment | 1 | 0.6582 | |
| Varieties | 27 | <0.005 * | ||
| Area | Treatment | 1 | <0.005 * | |
| Varieties | 27 | 0.2394 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, C.; Park, J.-E.; Mansoor, S.; Chung, Y.S.; Kim, K. Drought Stress Restoration Frequencies of Phenotypic Indicators in Early Vegetative Stages of Soybean (Glycine max L.). Sustainability 2023, 15, 4852. https://doi.org/10.3390/su15064852
Kim J, Lee C, Park J-E, Mansoor S, Chung YS, Kim K. Drought Stress Restoration Frequencies of Phenotypic Indicators in Early Vegetative Stages of Soybean (Glycine max L.). Sustainability. 2023; 15(6):4852. https://doi.org/10.3390/su15064852
Chicago/Turabian StyleKim, JaeYoung, Chaewon Lee, Ji-Eun Park, Sheikh Mansoor, Yong Suk Chung, and Kyunghwan Kim. 2023. "Drought Stress Restoration Frequencies of Phenotypic Indicators in Early Vegetative Stages of Soybean (Glycine max L.)" Sustainability 15, no. 6: 4852. https://doi.org/10.3390/su15064852
APA StyleKim, J., Lee, C., Park, J.-E., Mansoor, S., Chung, Y. S., & Kim, K. (2023). Drought Stress Restoration Frequencies of Phenotypic Indicators in Early Vegetative Stages of Soybean (Glycine max L.). Sustainability, 15(6), 4852. https://doi.org/10.3390/su15064852







