Soil Heavy Metal Absorption Potential of Azolla pinnata and Lemna gibba with Arbuscular Mycorrhizal Fungi in Rice (Oryza sativa L.) Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Preparation of the Native AMF Inoculum
2.3. Azolla pinnata and Lemna gibba Collection
2.4. Crop Establishment and Management
2.4.1. Establishment of Treatment
2.4.2. Seedling Transplanting
2.5. Soil and Plant Sampling
2.6. Analysis of Available Heavy Metal Concentrations in Soil and Plant Samples
2.7. ICP -MS (Inductively Coupled Plasma Mass Spectrophotometer) Analysis
2.8. Quality Control
2.9. Percentage Arbuscular Mycorrhizal Colonization
2.10. Soil-to-Plant Transfer Factors
2.10.1. Bioaccumulation Factor (BAF)
2.10.2. Translocation Factor (TF)
2.11. Statistical Data Analysis
3. Results and Discussion
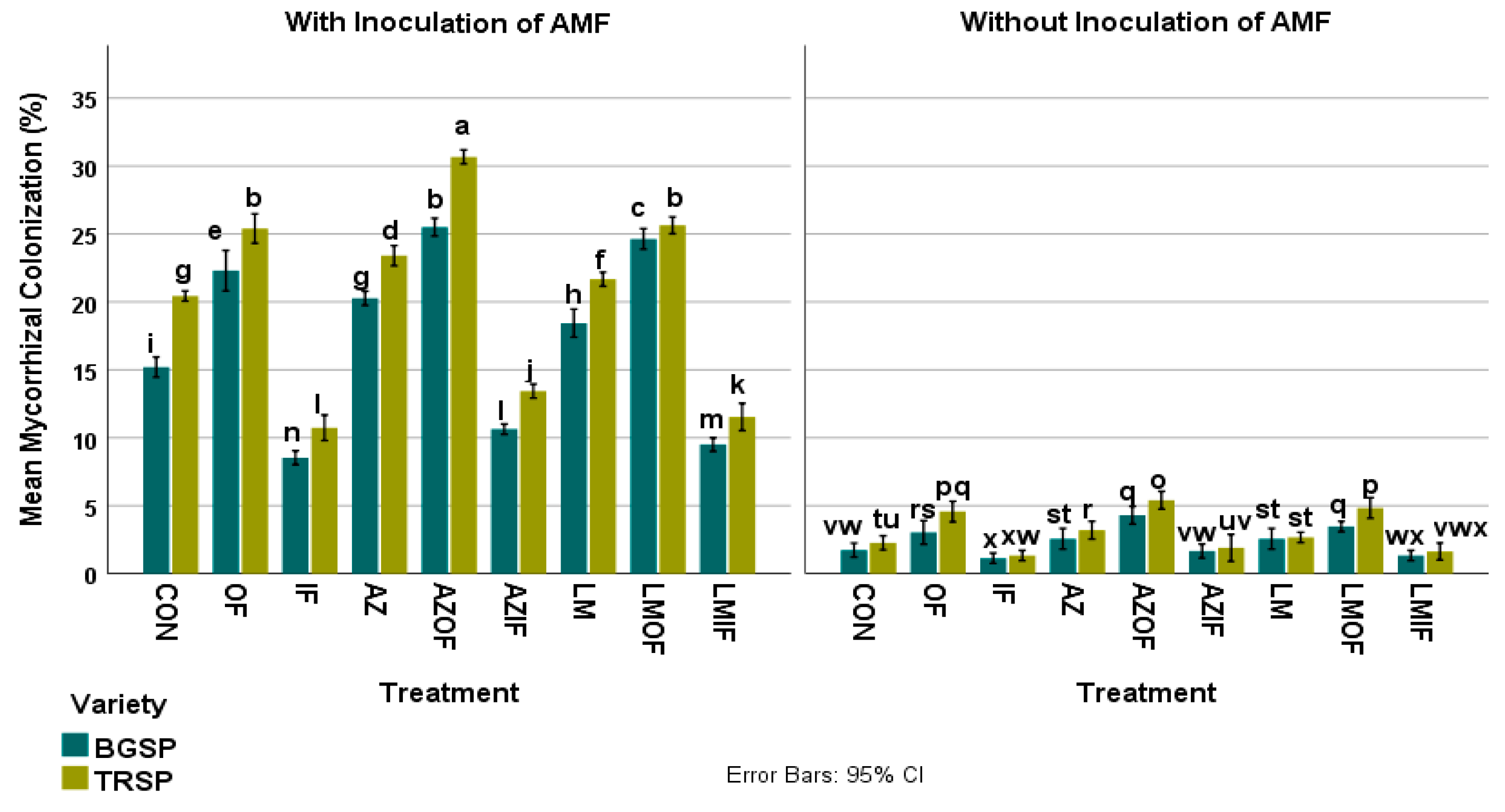
3.1. Percentage AMF Colonization of Rice Roots
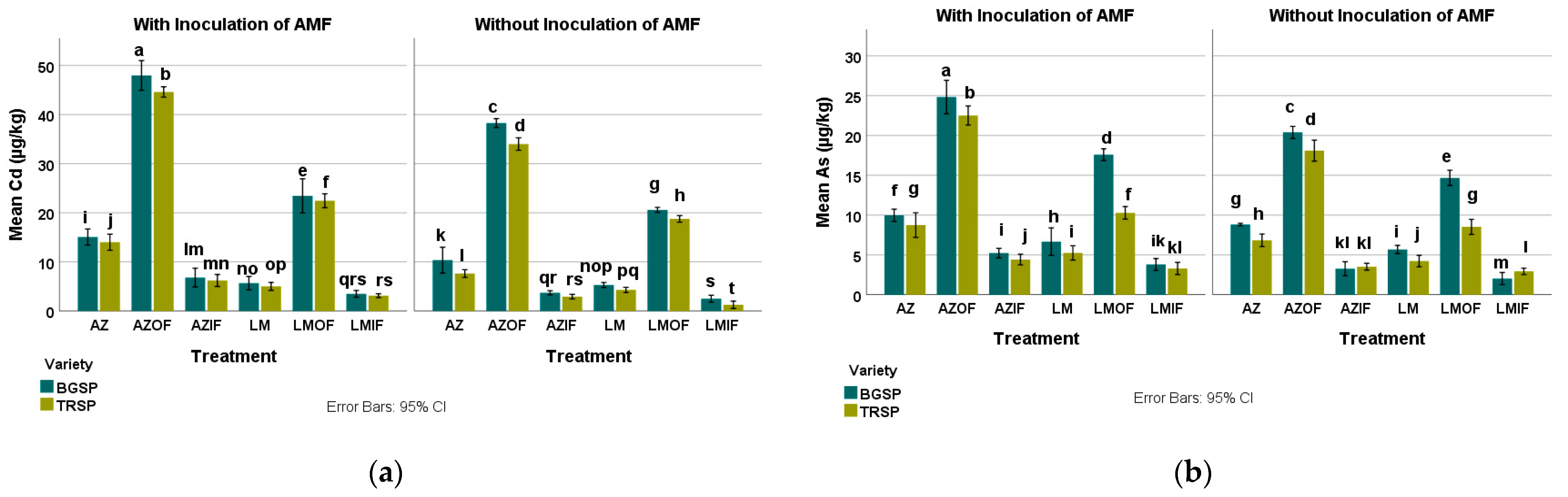
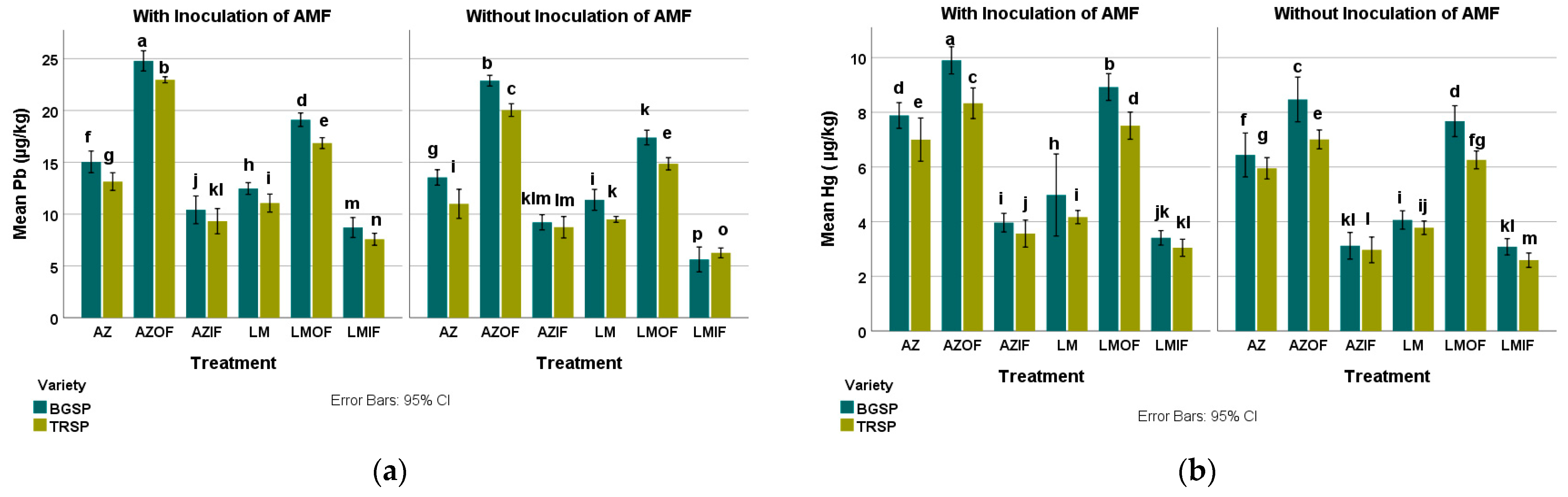
3.2. Heavy Metals in Rice Field Soil
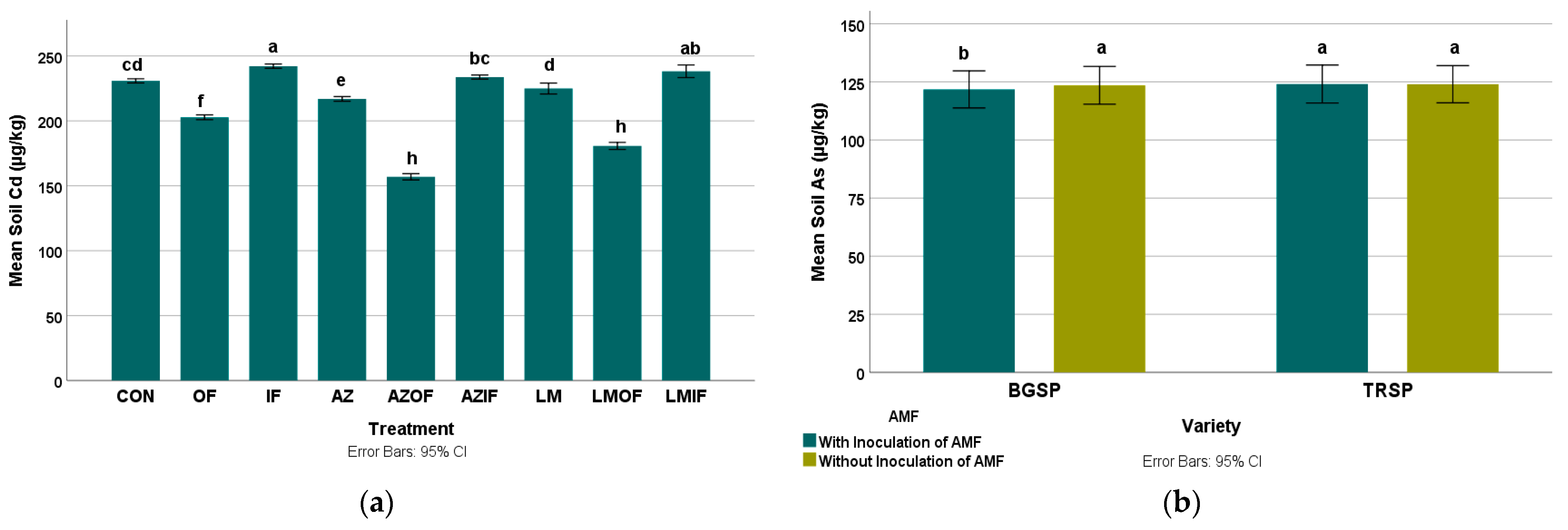
3.2.1. Cadmium in Rice Field Soil
3.2.2. Arsenic in Rice Field Soil
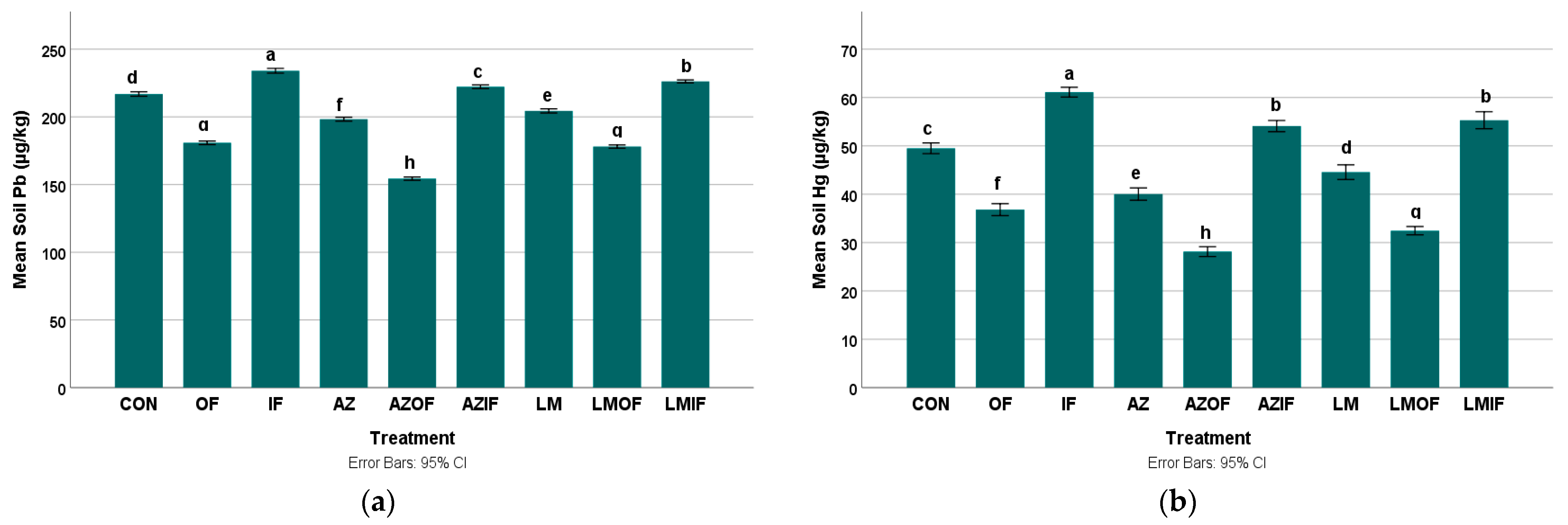
3.2.3. Lead in Rice Field Soil
3.2.4. Mercury in Rice Field Soil
3.3. Heavy Metals in Rice Roots
3.3.1. Cadmium in Rice Roots
3.3.2. Arsenic in Rice Roots
3.3.3. Lead in Rice Roots
3.3.4. Mercury in Rice Roots
3.4. Heavy Metals in Rice Shoots
3.4.1. Cadmium in Rice Shoots
3.4.2. Arsenic in Rice Shoots
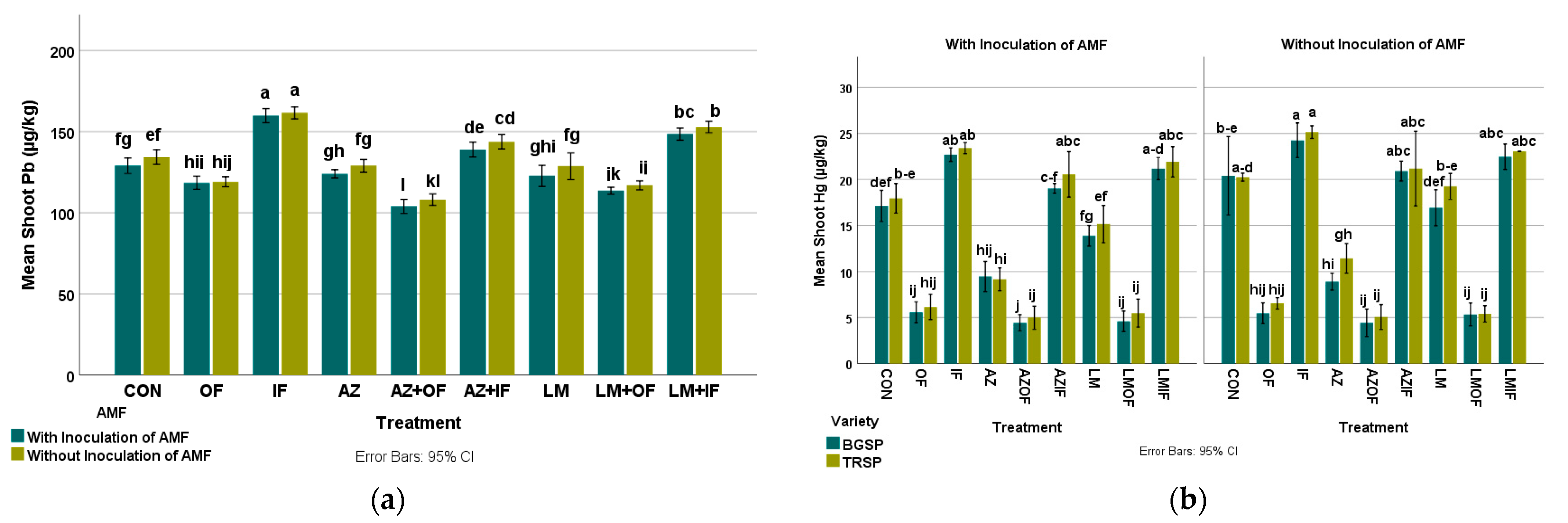
3.4.3. Lead in Rice Shoots
3.4.4. Mercury in Rice Shoots
3.5. Heavy Metals in Rice Grain
3.5.1. Cadmium in Rice Grain
3.5.2. Arsenic in Rice Grain
3.5.3. Lead in Rice Grain
3.5.4. Mercury in Rice Grain
3.6. Heavy Metals in Azolla pinnata and Lemna gibba
3.6.1. Cadmium in Azolla pinnata and Lemna gibba
3.6.2. Arsenic in Azolla pinnata and Lemna gibba
3.6.3. Lead in Azolla pinnata and Lemna gibba
3.6.4. Mercury in Azolla pinnata and Lemna gibba
3.7. Bioaccumulation Factor
3.8. Translocation Factor (TF)
3.8.1. Cadmium Translocation Factor
3.8.2. Arsenic Translocation Factor
3.8.3. Lead Translocation Factor
3.8.4. Mercury Translocation Factor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DOASL. Crop Recommendations. 2015. Available online: http://www.agridept.gov.lk/index.php/en/crop-recommendations/808. (accessed on 25 November 2022).
- Lombi, E.; Scheckel, K.G.; Pallon, J.; Carey, A.M.; Zhu, Y.G.; Meharg, A.A. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytol. 2009, 184, 193–201. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S. Heavy metals causing toxicity in animals and fishes. R. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Satpathy, D.; Reddy, M.V.; Dhal, S.P. Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. Biomed Res. Int. 2014, 2014, 545473. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Clark, R.Á.; Zeto, S.K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Jais, H.M. Interaction between arbuscular mycorrhiza and heavy metals in the rhizosphere and roots of Juniperus procera. Int. J. Agric. Biol. 2012, 14, 69–74. [Google Scholar]
- Dodd, J.C.; Boddington, C.L.; Rodriguez, A.; Gonzalez-Chavez, C.; Mansur, I. Mycelium of arbuscular mycorrhizal fungi (AMF) from different genera: Form, function and detection. Plant Soil 2012, 226, 131–151. [Google Scholar] [CrossRef]
- Mathur, N.; Singh, J.; Bohra, S.; Vyas, A. Arbuscular mycorrhizal status of medicinal halophytes. Int. J. Soil Sci. 2007, 2, 119–127. [Google Scholar] [CrossRef]
- Xavier Martins, W.F.; Rodrigues, B.F. Identification of dominant arbuscular mycorrhizal fungi in different rice ecosystems. Agric. Res. 2020, 9, 46–55. [Google Scholar] [CrossRef]
- Vallino, M.; Greppi, D.; Novero, M.; Bonfante, P.; Lupotto, E. Rice root colonisation by mycorrhizal and endophytic fungi in aerobic soil. Ann. Appl. Biol. 2009, 154, 195–204. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Helmiyati, S.; Hapsari, M.; Setyaningrum, D.L. Rice in health and nutrition. Int. Food Res. J. 2014, 21, 13–24. [Google Scholar]
- Abeysekera, W.K.S.M.; Arachchige, S.P.G.; Ratnasooriya, W.D.; Chandrasekharan, N.V.; Bentota, A.P. Physicochemical and nutritional properties of twenty-three traditional rice (Oryza sativa L.) varieties of Sri Lanka. J. Coastal Life Med. 2017, 5, 343–349. [Google Scholar] [CrossRef]
- Abeysekera, W.K.S.M.; Gunasekara, U.K.D.S.S.; Arachchige, S.P.G.; Abeysekera, W.P.K.M. Antioxidant potential of brans of twenty-nine red and white rice (Oryza sativa L.) varieties of Sri Lanka. J. Coastal Life Med. 2017, 5, 480–485. [Google Scholar] [CrossRef]
- Prasantha, B.D.R. Glycemic index of four traditional red pigmented rice. Integr. Food Nutri. Metabol. 2018, 5, 1–3. [Google Scholar] [CrossRef]
- Wickramasekara, P. Labour Absorption in Paddy Cultivation in Sri Lanka; International Labour Organization: Bangkok, Thailand, 1980; pp. 179–251. [Google Scholar]
- Ekanayake, H.K.J. The impact of fertilizer subsidy on paddy cultivation in Sri Lanka. Staff Stud. 2009, 36, 73–101. [Google Scholar] [CrossRef]
- Amarasingha, R.P.R.K.; Galagedara, L.W.; Marambe, B.; Silva, G.L.L.P.; Punyawardena, R.; Nidumolu, U.; Howden, M.; Suriyagoda, L.D.B. Aligning sowing dates with onset of rains improve rice yields and water productivity: Modelling Oryza sativa L. in Maha season in the dry zone of Sri Lanka. Trop. Agric. Res. 2014, 25, 277–286. [Google Scholar] [CrossRef]
- Amarasingha, R.K.; Suriyagoda, L.D.B.; Marambe, B.; Galagedara, L.W.; Punyawardena, R. Impact of climate change on rice yield in Sri Lanka: A crop modelling approach using Agriculture Production System Simulator (APSIM). Sri Lanka J. Food Agric. 2018, 4, 21. [Google Scholar] [CrossRef]
- Rambukwella, R.; Priyankara, E.A.C. Production and Marketing of Traditional Rice Varieties in Selected Districts in Sri Lanka: Present Status and Future Prospects; Hector Kobbekaduwa Agrarian Research and Training Institute: Colombo, Sri Lanka, 2016. [Google Scholar]
- Mason, H. Lemnaceae. Duckweed family in A Flora of the Marshes of California; University of California Press: Berkeley, CA, USA, 1957; pp. 327–343. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweed biomarkers for identifying toxic water contaminants? Environ. Sci. Pollut. Res. 2019, 26, 14797–14822. [Google Scholar] [CrossRef]
- Roy, D.C.; Pakhira, M.C.; Bera, S. A review on biology, cultivation and utilization of Azolla. ALS 2016, 5, 11–15. [Google Scholar]
- Vafaei, F.; Khataee, A.R.; Movafeghi, A.; Lisar, S.S.; Zarei, M. Bioremoval of an azo dye by Azolla filiculoides: Study of growth, photosynthetic pigments and antioxidant enzymes status. Int. Biodeterior. Biodegrad. 2012, 75, 194–200. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Q.; Liu, J.; Liu, W.; Wang, T.; Zhang, Q.; Jiang, G. High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 2008, 71, 1269–1275. [Google Scholar] [CrossRef]
- Wijesundara, W.M.G.D.; Nandasena, K.A.; Jayakody, A.N. Seasonal and spatial variations of N, P, K and Cd concentrations in water of the Mahakanumulla cascade in the dry zone of Sri Lanka. Trop. Agric. Res. 2015, 24, 279–288. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Grove, T.; Malajczuk, N. Examining mycorrhizal Association. In Working with Mycorrhizas in Forestry and Agriculture; Brundrett, M., Bougher, N., Dell, B., Grove, T., Malajczuk, N., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1982; pp. 141–171. ISBN 1-86320-181-5. [Google Scholar]
- Chaurasia, B.; Pandey, A.; Lok, M.; Palni, S. Distribution, colonization and diversity of arbuscular mycorrhizal fungi associated with central Himalayan rhododendrons. For. Ecol. Manag. 2005, 207, 315–324. [Google Scholar] [CrossRef]
- Okorie, A.; Entwistle, J.; Dean, J.R. The optimization of microwave digestion procedures and application to an evaluation of potentially toxic element contamination on a former industrial site. Talanta 2010, 82, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkani, M.F. Guideline of inductively coupled plasma mass spectrometry “ICP–MS”: Fundamentals, practices, determination of the limits, quality control, and method validation parameters. SN Appl. Sci. 2019, 1, 791. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. Comparative uptake and phytoextraction study of soil induced chromium by accumulator and high biomass weed species. Appl. Ecol. Environ. Res. 2005, 3, 67–79. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Decontamination and/or revegetation of fly ash dykes through naturally growing plants. J. Hazard. Mater. 2008, 153, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- FAO. Rice Market Monitor. 2018. Available online: https://www.fao.org/3/I9243EN/i9243en.pdf (accessed on 5 February 2023).
- Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.; Huang, Q. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy 2022, 12, 1277. [Google Scholar] [CrossRef]
- Ilag, L.L.; Rosales, A.M.; Elazegui, F.A.; Mew, T.W. Changes in the population of infective endomycorrhizal fungi in a rice-based cropping system. Plant Soil 1987, 103, 67–73. [Google Scholar] [CrossRef]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. J. Appl. Ecol. 2011, 21, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Z.; Wang, Y.T.; Olsson, P.A. Arbuscular mycorrhiza under water-carbon-phosphorus exchange between rice and arbuscular mycorrhizal fungi under different flooding regimes. Soil Boil. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- Yapa, N.; Dub, W.; Madhushan, A.; Yan, K.; Asad, S.; Karunarathna, S.C.; Bamunuarachchige, C. Potential of biofertilizers and natural soil amendments to mitigate heavy metal contents of soil in lowland rice (Oryza sativa L.) farming. Science 2022, 48, 326–334. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- Secilia, J.; Bagyaraj, D.J. Evaluation and first-year field testing of efficient vesicular arbuscular mycorrhizal fungi for inoculation of wetland rice seedlings. World J. Microbiol. Biotechnol. 1994, 10, 381–384. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Chhonkar, P.K. Influence of vesicular-arbuscular mycorrhizal fungi (Glomus etunicatum L.) on mobilization of zinc in wetland rice (Oryza sativa L.). Biol. Fert. Soils 2001, 33, 323–327. [Google Scholar] [CrossRef]
- Wangiyana, W.; Cornish, P.S.; Morris, E.C. Arbuscular mycorrhizal fungi dynamics in contrasting cropping systems on vertisol and regosol soils of Lombok, Indonesia. Exp. Agric. 2006, 42, 427–439. [Google Scholar] [CrossRef]
- Barber, N.A.; Kiers, E.T.; Theis, N.; Hazzard, R.V.; Adler, L.S. Linking agricultural practices, mycorrhizal fungi, and traits mediating plant–insect interactions. Ecol. Appl. 2013, 23, 1519–1530. [Google Scholar] [CrossRef]
- Doni, F.; Mispan, M.S.; Suhaimi, N.S.M.; Ishak, N.; Uphoff, N. Roles of microbes in supporting sustainable rice production using the system of rice intensification. Appl. Microbiol. Biot. 2019, 103, 5131–5142. [Google Scholar] [CrossRef]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biot. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Sawers, R.J.; Gutjahr, C.; Paszkowski, U. Cereal mycorrhiza: An ancient symbiosis in modern agriculture. Trends Plant Sci. 2008, 13, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K.; Allen, M.F. Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: A model and field test. New Phytol. 2002, 155, 507–515. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A.S.; Lemboye, K.; CS Chittoori, B.; Almajed, A. Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 2020, 12, 7019. [Google Scholar] [CrossRef]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Ouziad, F.; Hildebrandt, U.; Schmelzer, E.; Bothe, H. Differential gene expressions in arbuscular mycorrhizal-colonized tomato grown under heavy metal stress. J. Plant Physiol. 2005, 162, 634–649. [Google Scholar] [CrossRef]
- Punamiya, P.; Datta, R.; Sarkar, D.; Barber, S.; Patel, M.; Das, P. Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass Chrysopogon zizanioides L. J. Hazard. Mater. 2010, 177, 465–474. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. The symbionts forming arbuscular mycorrhizas. In Mycorrhizal Symbiosis; Smith, E.S., Read, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 13–41. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X.Q.; Luo, L.E.I.; Pei, Z.; Zhu, Y.G.; Liu, T.; Xie, Y.N.; Gault, A. Characterization of Pb, Cu, and Cd adsorption on particulate organic matter in soil. Environ. Toxicol. Chem. 2006, 25, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Seven, T.; Büşra, C.A.N.; Darende, B.N.; Sevda, O.C.A.K. Heavy metal pollution in air and soil. Natl. Environ. Sci. Res. J. 2018, 1, 91–103. [Google Scholar]
- Bandara, J.M.R.S.; Senevirathna, D.M.A.; Dasanayake, D.M.R.S.V.; Herath, V.; Bandara, J.M.R.P. Chronic renal failure in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and fresh water fish (Tilapiya). Environ. Geochem. Health 2008, 30, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, H.M.P.L.; Hettiarachchi, G.M.; Indraratne, S.P. Trace Metal concentration in crops and soils collected from intensively cultivated areas of Sri Lanka. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 230–240. [Google Scholar]
- Nuray, D.A.Ğ.; ARICI, Ö.K. Heavy metals in soils Pb (Lead), Hg (Mercury), Cd (Cadmium), As (Arsenic) effects on human health. Int. J. Environ. Res. 2021, 5, 48–59. [Google Scholar]
- Ullah, S.; Dahlawi, S.; Naeem, A.; Iqbal, M.; Farooq, M.A.; Bibi, S.; Rengel, Z. Opportunities and challenges in the use of mineral nutrition for minimizing arsenic toxicity and accumulation in rice: A critical review. Chemosphere 2018, 194, 171–188. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, S.K.; Pathak, R.K.; Gupta, V. Accumulation of heavy metals in soil and paddy crop (Oryza sativa), irrigated with water of Ramgarh Lake, Gorakhpur, UP, India. Toxicol. Environ. Chem. 2011, 93, 462–473. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.S.; Lee, J.Y.; Jeon, J.S.; Kim, J.W.; Russo, R.E.; Gonzalez, J.; Yoo, J.H.; Kim, K.S.; Yang, J.S.; et al. Analysis of arsenic in rice grains using ICP-MS and fs LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1233–1237. [Google Scholar] [CrossRef]
- Song, D.; Zhuang, D.; Jiang, D.; Fu, J.; Wang, Q. Integrated health risk assessment of heavy metals in Suxian county, South China. Int. J. Environ. Res. Public Health 2015, 12, 7100–7117. [Google Scholar] [CrossRef]
- Payus, C.; Talip, A.F.A.; Hsiang, T.W. Heavy metals accumulation in paddy cultivation area of Kompipinan, Papar District, Sabah. J. Sustain. Sci. Manag. 2015, 10, 76–86. [Google Scholar]
- Ahmad, R.; Hadi, F.; Jan, A.U.; Ditta, A. Straw incorporation in contaminated soil enhances drought tolerance but simultaneously increases the accumulation of heavy metals in rice. Sustainability 2022, 14, 10578. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Juang, K.W.; Ho, P.C.; Yu, C.H. Short-term effects of compost amendment on the fractionation of cadmium in soil and cadmium accumulation in rice plants. Environ. Sci. Pollut. Res. 2012, 19, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.A.J.; Chandrasekara, G.A.P.; Pulenthiraj, U.D.G.N.G.; Chandrasekara, C.M.N.R.; Wijesinghe, D.G.N.G. Mineral contents of Sri Lankan rice varieties as affected by inorganic fertilization. Trop. Agric. Res. 2019, 30, 89–96. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, H.; Zhang, Y.; Dong, Q.; Meng, S.; Cao, L.; Shao, G.; Shen, X. Changes in cadmium concentration in rice plants under different cadmium levels and expression analysis of genes related to cadmium regulation. Chin. J. Rice Sci. 2016, 30, 380–388. [Google Scholar]
- Chai, M.W.; Shi, F.C.; Li, R.L.; Liu, F.C.; Qiu, G.Y.; Liu, L.M. Effect of NaCl on growth and Cd accumulation of halophyte Spartina alterniflora under CdCl2 stress. S. Afr. J. Bot. 2013, 85, 63–69. [Google Scholar] [CrossRef]
- Manousaki, E.; Kadukova, J.; Papadantonakis, N.; Kalogerakis, N. Phytoextraction and phytoexcretion of Cd by the leaves of Tamarix smyrnensis growing on contaminated non-saline and saline soils. Environ. Res. 2008, 106, 326–332. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Zhao, S.; Li, S.; Lei, X.; Qin, L.; Sun, X.; Chen, S. Manganese facilitates cadmium stabilization through physicochemical dynamics and amino acid accumulation in rice rhizosphere under flood-associated low pe+pH. J. Hazard. Mater. 2021, 416, 126079. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, W.; Zou, J.; Zhou, H.; Zeng, Q.; Liao, B. Effects of calcium magnesium phosphate fertilizer on Cd bioavailability in soil and Cd contents in rice. Acta Sci. Circumst. 2017, 37, 2322–2330. [Google Scholar] [CrossRef]
- Shao, J.F.; Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Silicon reduces cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in rice. J. Exp. Bot. 2017, 68, 5641–5651. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Looi, L.Y.; Aris, A.Z.; Lim, W.Y.; Haris, H. Bioconcentration and translocation efficiency of metals in paddy (Oryza sativa): A case study from Alor Setar, Kedah, Malaysia. Sains Malays. 2014, 43, 521–528. [Google Scholar]
- Muehe, E.M.; Wang, T.; Kerl, C.F.; Planer-Friedrich, B.; Fendorf, S. Rice production threatened by coupled stresses of climate and soil arsenic. Nat. Commun. 2019, 10, 4985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.D.; Zhang, G.P.; Yao, H.G.; Wu, W.; Xu, M. Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. J. Zhejiang Univ. Sci. B 2006, 7, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Noman, M.; Jiang, H.; Shahid, M.; Ma, C.; Wu, Z.; Nazir, M.M.; Ali, M.A.; White, J.C.; Chen, J.; et al. Bioengineered chitosan-iron nanocomposite controls bacterial leaf blight disease by modulating plant defense response and nutritional status of rice (Oryza sativa L.). Nano Today 2022, 45, 101547. [Google Scholar] [CrossRef]
- Jarvis, S.C.; Jones, L.H.P.; Hopper, M.J. Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil 1976, 44, 179–191. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Tukendorf, A. The effect of EDTA on maize seedlings response to Cd-induced stress. Z. Naturforsch. 1999, 54, 754–758. [Google Scholar] [CrossRef]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, USA. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Alimentarius Commission, Food Additives and Contaminants. 2001. Joint FAO/WHO Food Standards Programme, ALINORM 01/12A: 1-289, Food and Agriculture. Available online: http://www.fao.org (accessed on 20 November 2022).
- Banuelos, G.S.; Ajwa, H.A. Trace elements in soils and plants: An overview. J. Environ. Sci. Health A 1999, 34, 951–974. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Q.; Zhang, Z.; Xu, J.; Yang, J.; Wong, M.H. Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J. Sci. Food Agric. 2005, 85, 147–153. [Google Scholar] [CrossRef]
- Murakami, M.; Nakagawa, F.; Ae, N.; Ito, M.; Arao, T. Phytoextraction by rice capable of accumulating Cd at high levels: Reduction of Cd content of rice grain. Environ. Sci. Technol. 2009, 43, 5878–5883. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Li, X.; Chen, A.Y.; Zhang, L.J.; Zhao, H.M.; Xiang, L.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; Li, H. Does arbuscular mycorrhizal fungus affect cadmium uptake and chemical forms in rice at different growth stages? Sci. Total Environ. 2017, 599, 1564–1572. [Google Scholar] [CrossRef]
- Dai, J.C.; Wu, X.T.; Fu, Z.Y.; Cui, C.P.; Hu, S.M.; Du, W.X.; Wu, L.M.; Zhang, H.H.; Sun, R.Q. Synthesis, structure, and fluorescence of the novel cadmium (II)− trimesate coordination polymers with different coordination architectures. Inorg. Chem. 2002, 41, 1391–1396. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.; Li, G.; Zhao, F.J.; McGrath, S. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef]
- Shahriar, S.; Rahman, M.M.; Naidu, R. Geographical variation of cadmium in commercial rice brands in Bangladesh: Human health risk assessment. Sci. Total Environ. 2020, 716, 137049. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.T.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from Smelting in China (China Cad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef]
- Areo, T.; Ae, N. Genotypic variations in cadmium levels of rice grain. Soil Sci. Plant Nutr. 2003, 49, 473–479. [Google Scholar] [CrossRef]
- Shi, Z.; Carey, M.; Meharg, C.; Williams, P.N.; Signes-Pastor, A.J.; Triwardhani, E.A.; Pandiangan, F.I.; Campbell, K.; Elliott, C.; Marwa, E.M. Rice grain cadmium concentrations in the global supply-chain. Expo. Health 2020, 12, 869–876. [Google Scholar] [CrossRef]
- Navarathna, C.; Pathiratne, S.; De Silva, D.S.M.; Rinklebe, J.; Mohan, D.; Mlsna, T. Intrusion of heavy metals/metalloids into rice (Oryza sativa L.) in relation to their status in two different agricultural management systems in Sri Lanka. Groundw. Sustain. Dev. 2021, 14, 100619. [Google Scholar] [CrossRef]
- Herath, H.M.D.A.K.; Bandara, D.C.; Weerasinghe, P.A.; Iqbal, M.C.M.; Wijayawardhana, H.C.D. Effect of cadmium on growth parameters and plant accumulation in different rice (Oryza sativa L.) varieties in Sri Lanka. Trop. Agric. Res. 2015, 25, 532–542. [Google Scholar] [CrossRef]
- Arao, T.; Makino, T.; Kawasaki, A.; Akahane, I.; Kiho, N. Effect of air temperature after heading of rice on the arsenic concentration of grain. Soil Sci. Plant Nutr. 2018, 64, 433–437. [Google Scholar] [CrossRef]
- Cattani, I.; Romani, M.; Boccelli, R. Effect of cultivation practices on cadmium concentration in rice grain. Agron. Sustain. Dev. 2008, 28, 265–271. [Google Scholar] [CrossRef]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to Wheat and Barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in rice-producing soils of Hunan Province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Yang, J.Y.; Yoon, H.J.; Park, K.S. Safety assessment of heavy metals in rice, cultivated habitats (soil and water, etc.) and cooked rice that may arise from environment. Int. J. Res. Chem. Metall. Civil Eng. 2015, 2, 105–110. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.; Wu, P.; Han, J.; Chen, Q. Health Risk assessment of heavy metals in rice to the population in Zhejiang, China. PLoS ONE 2013, 8, e75007. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zhang, C.; Li, Y.; Zou, B.; Mo, H.; Wu, K.; Wu, J.; Li, Z. Assessment of influences of cooking on cadmium and arsenic bio-accessibility in rice, using an in vitro physiologically-based extraction test. Food Chem. 2016, 213, 206–214. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, M.; Zhou, X.; Liao, B.H.; Peng, P.Q.; Hu, M.; Zhu, W.; Wu, Y.J.; Zou, Z.J. Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil 2015, 386, 317–329. [Google Scholar] [CrossRef]
- GB2762National Committee of Standardization. Maximum Levels of Contaminants in Foods.; Ministry of Health, PRC, Standards Press of China: Beijing, China, 2005. Available online: https://apps.fas.usda.gov (accessed on 10 November 2022).
- Wang, S.X.; Zhang, L.; Li, G.H.; Wu, Y.; Hao, J.M.; Pirrone, N.; Sprovieri, F.; Ancora, M.P. Mercury emission and speciation of coal-fired power plants in China. Atmospheric Chem. Phys. 2010, 10, 1183–1192. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Feng, X.; Dong, B.; Shang, L.; Yin, R.; Yuan, X. Characterization of mercury species in brown and white rice (Oryza sativa L.) grown in water-saving paddies. Environ. Pollut. 2011, 159, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Amare, E.; Kebede, F.; Mulat, W. Wastewater treatment by Lemna minor and Azolla filiculoides in tropical semi-arid regions of Ethiopia. Ecol. Eng. 2018, 120, 464–473. [Google Scholar] [CrossRef]
- Bennicelli, R.; Stępniewska, Z.; Banach, A.; Szajnocha, K.; Ostrowski, J. The ability of Azolla caroliniana to remove heavy metals (Hg (II), Cr (III), Cr (VI)) from municipal waste water. Chemosphere 2004, 55, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Phytoremediation of Hg and Cd from industrial effluents using an aquatic free floating macrophyte Azolla pinnata. Int. J. Phytoremediation 2008, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int. J. Phytoremediation 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, A.J.; Zhao, F.J.; Xu, G.Z.; Duan, G.L.; Zhu, Y.G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155. [Google Scholar] [CrossRef]
- Axtell, N.R.; Sternberg, S.P.; Claussen, K. Lead and nickel removal using Microspora and Lemna minor. Bioresour. Technol. 2003, 89, 41–48. [Google Scholar] [CrossRef]
- Elmacı, A.; Özengin, N.; Yonar, T. Removal of chromium (III), copper (II), lead (II) and zinc (II) using Lemna minor L. Fresenius Environ. Bull. 2009, 18, 538–542. [Google Scholar]
- Miretzky, P.; Saralegui, A.; Cirelli, A.F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 2004, 57, 997–1005. [Google Scholar] [CrossRef]
- Cvjetko, P.; Tolić, S.; Šikić, S.; Balen, B.; Tkalec, M.; Vidaković-Cifrek, Ž. Effect of copper on the toxicity and genotoxicity of cadmium in duckweed (Lemna minor L.). Arh. Hig. Rada. Toksikol. 2010, 61, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Tripathi, B.D. Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from GB Pant Sagar of Singrauli Industrial region, India. Environ. Monit. Assess. 2009, 148, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Khan, M.Q.; Zia, M.S. Sugar industry press mud as alternate organic fertilizer source. Intern. J. Environ. Waste Manag. 2012, 9, 41–55. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Chowdhury, M.A.Z.; Fardous, Z.; Alam, M.K.; Hossain, M.S.; Fakhruddin, A.N.M. Ecological risk posed by heavy metals contamination of ship breaking yards in Bangladesh. Int. J. Environ. Res. 2014, 8, 469–478. [Google Scholar]
- Ahmad, J.U.; Goni, M.A. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ. Monit. Assess. 2010, 166, 347–357. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zheng, Y.; Tang, L.; Lai, Z.; Liu, N.; Li, H. The accumulation characteristics of different heavy metals in sea rice. Appl. Sci. 2022, 12, 9718. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef]
- Liu, Z.G. Study on geotechnical investigation and foundation treatment of karst foundation. Resour. Inf. Eng. 2016, 31, 103–104. [Google Scholar]
- Hang, X.; Wang, H.; Zhou, J.; Ma, C.; Du, C.; Chen, X. Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ. Pollut. 2009, 157, 2542–2549. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Yu, L.; Yang, M.; Zou, X.; Yin, C.; Lin, Y. Research advances in cadmium uptake, transport and resistance in rice (Oryza sativa L.). Cells 2022, 11, 569. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.X.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and intervascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Kolahchi, Z.; Charkhabi, A. Uptake and translocation of some heavy metals by rice crop (Oryza sativa) in paddy soils. Agriculture (Pol’nohospodárstvo) 2017, 63, 163–175. [Google Scholar] [CrossRef]
- Rubio, M.I.; Escrig, I.; Martínez-Cortina, C.; Lo´pez-Benet, F.J.; Sanz, A. Cadmium and nickel accumulation in rice plant. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul. 1994, 14, 151–157. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.; Liu, X.; Sun, S.B.; Xu, G.H.; Liu, Y.; Chen, Y.; Ma, L.Q. Knocking out OsPT4 gene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [CrossRef]
- Kamiya, T.; Islam, M.R.; Duan, G.; Uraguchi, S.; Fujiwara, T. Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in as accumulation in shoots of rice. Soil Sci. Plant Nutr. 2013, 59, 580–590. [Google Scholar] [CrossRef]
- Wang, P.T.; Zhang, W.W.; Mao, C.Z.; Xu, G.H.; Zhao, F.J. The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J. Exp. Bot. 2016, 67, 6051–6059. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, P.; Xu, T.; Zeng, L.; Cheng, D.; Yang, M.; Luo, J.; Lian, X. OsPT4 contributes to arsenate uptake and transport in rice. Front Plant Sci. 2017, 8, 2197. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.F.; Zhou, H.; Tang, H.L.; Yang, W.T.; Zeng, M.; Liu, Z.M.; Peng, P.Q.; Liao, B.H. Cadmium and arsenic accumulation during the rice growth period under in-situ remediation. Ecotoxicol. Environ. Saf. 2019, 171, 451–459. [Google Scholar] [CrossRef] [PubMed]






| Instrument Parameter | Standard | Helium KED | Oxygen DRC |
|---|---|---|---|
| Torch | Quartz single pieces touch | ||
| Nebulizer gas flow (L min−1) | 0.98 | 0.98 | 0.98 |
| Nebulizer | Meinhard Concentric | ||
| ICP RF power (W) | 1600 | 1600 | 1600 |
| Gas flow (L min−1) | 0 | 3.8 | 0.6 |
| Plasma gas flow (L min−1) | 15 | 15 | 15 |
| Auxiliary gas flow (L min−1) | 1.2 | 1.2 | 1.2 |
| Outer gas flow | 0.2 | 0.2 | 0.2 |
| Sample uptake rate (rpm) | 35 | 35 | 35 |
| Number of replicates | 3 | 3 | 3 |
| Element | Normal Sample | Spike Sample | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Plant (mg kg−1) | Soil (mg kg−1) | Plant (mg kg−1) | Soil (mg kg−1) | Plant | Soil | |
| Cd | 0.21 ± 0.02 | 0.23 ± 0.04 | 5.07 ± 0.51 | 5.12 ± 0.45 | 95.86 | 95.51 |
| As | 0.13 ± 0.04 | 0.15 ± 0.02 | 2.59 ± 0.39 | 2.72 ± 0.64 | 94.98 | 94.49 |
| Pb | 0.15 ± 0.01 | 0.18 ± 0.03 | 2.68 ± 0.29 | 2.75 ± 0.18 | 94.40 | 93.45 |
| Hg | 0.04 ± 0.01 | 0.05 ± 0.02 | 1.35 ± 0.15 | 1.39 ± 0.1 | 97.01 | 96.40 |
| AMF | Rice Variety | Treatment | Cd | As | Pb | Hg |
|---|---|---|---|---|---|---|
| With inoculation of AMF | BGSP | CON | 0.392 ± 0.014k | 0.782 ± 0.045k | 0.519 ± 0.006h–k | 0.210 ± 0.007g–j |
| OF | 0.218 ± 0.012s–u | 0.226 ± 0.022st | 0.538 ± 0.015g–i | 0.116 ± 0.006no | ||
| IF | 0.506 ± 0.007h | 1.024 ± 0.017fg | 0.542 ± 0.011f–j | 0.273 ± 0.009de | ||
| AZ | 0.265 ± 0.012o–s | 0.431 ± 0.018h | 0.502 ± 0.017i–m | 0.189 ± 0.01i–k | ||
| AZOF | 0.260 ± 0.009o–s | 0.268 ± 0.016rs | 0.593 ± 0.014b–d | 0.123 ± 0.011mn | ||
| AZIF | 0.439 ± 0.008j | 0.885 ± 0.014ij | 0.523 ± 0.01h–k | 0.216 ± 0.009g–i | ||
| LM | 0.306 ± 0.012m–o | 0.625 ± 0.015l | 0.518 ± 0.012h–k | 0.208 ± 0.01g–j | ||
| LMOF | 0.235 ± 0.006q–u | 0.244 ± 0.02st | 0.523 ± 0.013h–k | 0.110 ± 0.009no | ||
| LMIF | 0.495 ± 0.010hi | 0.962 ± 0.015g | 0.557 ± 0.011d–h | 0.225 ± 0.015gh | ||
| TRSP | CON | 0.248 ± 0.020p–t | 0.603 ± 0.027l | 0.450 ± 0.006o | 0.087 ± 0.002o | |
| OF | 0.199 ± 0.005u | 0.191 ± 0.024t | 0.491 ± 0.01k–h | 0.086 ± 0.01o | ||
| IF | 0.357 ± 0.01kl | 0.824 ± 0.021jk | 0.475 ± 0.007m–o | 0.082 ± 0.002o | ||
| AZ | 0.226 ± 0.011r–u | 0.435 ± 0.027n | 0.469 ± 0.007m–o | 0.095 ± 0.011no | ||
| AZOF | 0.249 ± 0.012p–t | 0.235 ± 0.027st | 0.580 ± 0.018b–f | 0.105 ± 0.006no | ||
| AZIF | 0.305 ± 0.008m–o | 0.667 ± 0.003l | 0.454 ± 0.014no | 0.082 ± 0.006o | ||
| LM | 0.250 ± 0.01p–t | 0.534 ± 0.019m | 0.464 ± 0.009m–o | 0.090 ± 0.003no | ||
| LMOF | 0.212 ± 0.017t–u | 0.196 ± 0.018t | 0.500 ± 0.016j–m | 0.115 ± 0.005no | ||
| LMIF | 0.332 ± 0.01l–n | 0.784 ± 0.022k | 0.477 ± 0.01l–o | 0.089 ± 0.007no | ||
| Without inoculation of AMF | BGSP | CON | 0.645 ± 0.011f | 1.052 ± 0.018f | 0.599 ± 0.014bc | 0.301 ± 0.008cd |
| OF | 0.268 ± 0.011o–r | 0.350 ± 0.013o–q | 0.556 ± 0.007d–h | 0.190 ± 0.016i–k | ||
| IF | 1.013 ± 0.01a | 1.600 ± 0.013a | 0.669 ± 0.007a | 0.417 ± 0.011a | ||
| AZ | 0.359 ± 0.01kl | 0.502 ± 0.018m | 0.546 ± 0.014e–h | 0.240 ± 0.009fg | ||
| AZOF | 0.341 ± 0.017lm | 0.400 ± 0.035no | 0.612 ± 0.013b | 0.200 ± 0.018h–j | ||
| AZIF | 0.762 ± 0.017d | 1.119 ± 0.010e | 0.612 ± 0.014b | 0.374 ± 0.012b | ||
| LM | 0.458 ± 0.014ij | 0.832 ± 0.023jk | 0.571 ± 0.014c–g | 0.276 ± 0.016de | ||
| LMOF | 0.278 ± 0.007o–q | 0.353 ± 0.030o–q | 0.540 ± 0.015f–j | 0.183 ± 0.018i–k | ||
| LMIF | 0.913 ± 0.042b | 1.506 ± 0.014b | 0.675 ± 0.008a | 0.421 ± 0.013a | ||
| TRSP | CON | 0.645 ± 0.014f | 0.912 ± 0.011hi | 0.586 ± 0.009b–e | 0.261 ± 0.004ef | |
| OF | 0.229 ± 0.009r–u | 0.310 ± 0.025qr | 0.532 ± 0.018g–k | 0.150 ± 0.012lm | ||
| IF | 0.849 ± 0.014c | 1.316 ± 0.037c | 0.616 ± 0.023b | 0.369 ± 0.01b | ||
| AZ | 0.450 ± 0.008j | 0.638 ± 0.017l | 0.547 ± 0.01e–h | 0.186 ± 0.006i–k | ||
| AZOF | 0.294 ± 0.013n–p | 0.386 ± 0.028n–p | 0.603 ± 0.009bc | 0.177 ± 0.017j–l | ||
| AZIF | 0.709 ± 0.011e | 1.026 ± 0.011f–g | 0.598 ± 0.01bc | 0.283 ± 0.015de | ||
| LM | 0.553 ± 0.034g | 0.763 ± 0.031k | 0.596 ± 0.006b–d | 0.226 ± 0.003gh | ||
| LMOF | 0.254 ± 0.012p–t | 0.324 ± 0.022p–r | 0.516 ± 0.021h–l | 0.157 ± 0.003kl | ||
| LMIF | 0.777 ± 0.004d | 1.239 ± 0.015d | 0.615 ± 0.007b | 0.328 ± 0.005c |
| AMF | Cd | As | Pb | Hg |
|---|---|---|---|---|
| With inoculation of AMF | 0.79 ± 0.02b | 0.82 ± 0.02b | 0.78 ± 0.01b | 0.63 ± 0.01b |
| Without inoculation of AMF | 0.89 ± 0.01a | 0.91 ± 0.01a | 0.90 ± 0.01a | 0.79 ± 0.01a |
| AMF | Treatment | Cd | As | Pb | Hg |
|---|---|---|---|---|---|
| With inoculation of AMF | CON | 0.50 ± 0.02g | 0.74 ± 0.02f | 0.67 ± 0.01i | 0.33 ± 0.01j |
| OF | 0.38 ± 0.03hi | 0.51 ± 0.01k | 0.51 ± 0.04k | 0.31 ± 0.06l | |
| IF | 0.71 ± 0.04b | 0.83 ± 0.05d | 0.71 ± 0.02d | 0.49 ± 0.09d | |
| AZ | 0.35 ± 0.05j | 0.63 ± 0.06i | 0.50 ± 0.06l | 0.30 ± 0.04m | |
| AZOF | 0.30 ± 0.01k | 0.45 ± 0.01m | 0.43 ± 0.02m | 0.24 ± 0.01n | |
| AZIF | 0.62 ± 0.02de | 0.82 ± 0.04e | 0.62 ± 0.07g | 0.47 ± 0.02e | |
| LM | 0.39 ± 0.03i | 0.69 ± 0.06g | 0.52 ± 0.02j | 0.39 ± 0.06i | |
| LMOF | 0.39 ± 0.07h | 0.50 ± 0.01i | 0.50 ± 0.06l | 0.30 ± 0.08kl | |
| LMIF | 0.59 ± 0.06e | 0.82 ± 0.05e | 0.68 ± 0.08f | 0.48 ± 0.04de | |
| Without inoculation of AMF | CON | 0.63 ± 0.05d | 0.88 ± 0.03c | 0.65 ± 0.09e | 0.43 ± 0.09f |
| OF | 0.51 ± 0.03fg | 0.63 ± 0.07g | 0.61 ± 0.02gh | 0.41 ± 0.04g | |
| IF | 0.82 ± 0.02a | 0.98 ± 0.02a | 0.81 ± 0.01a | 0.60 ± 0.01a | |
| AZ | 0.51 ± 0.05f | 0.82 ± 0.09d | 0.57 ± 0.06i | 0.42 ± 0.06h | |
| AZOF | 0.39 ± 0.06i | 0.82 ± 0.06d | 0.51 ± 0.08k | 0.31 ± 0.02jk | |
| AZIF | 0.73 ± 0.09bc | 0.62 ± 0.06j | 0.74 ± 0.06c | 0.55 ± 0.09b | |
| LM | 0.52 ± 0.04f | 0.85 ± 0.01cd | 0.61 ± 0.02gh | 0.52 ± 0.06c | |
| LMOF | 0.52 ± 0.02f | 0.68 ± 0.05gh | 0.51 ± 0.06k | 0.42 ± 0.03fg | |
| LMIF | 0.71 ± 0.04c | 0.95 ± 0.04b | 0.77 ± 0.03b | 0.54 ± 0.04b |
| AMF | Rice Variety | Treatment | Cd | As | Pb | Hg |
|---|---|---|---|---|---|---|
| With inoculation of AMF | BGSP | CON | 0.76 ± 0.02ef | 0.62 ± 0.02g | 0.76 ± 0.01f | 0.77 ± 0.01de |
| OF | 0.69 ± 0.03j | 0.59 ± 0.05h | 0.67 ± 0.02h | 0.74 ± 0.06fg | ||
| IF | 0.83 ± 0.02b | 0.80 ± 0.03b | 0.85 ± 0.06b | 0.82 ± 0.06b | ||
| AZ | 0.64 ± 0.03kl | 0.57 ± 0.04i | 0.64 ± 0.01ij | 0.68 ± 0.04h | ||
| AZOF | 0.61 ± 0.05m | 0.52 ± 0.02k | 0.62 ± 0.05l | 0.63 ± 0.01j | ||
| AZIF | 0.80 ± 0.03cd | 0.64 ± 0.06f | 0.80 ± 0.06de | 0.79 ± 0.02cd | ||
| LM | 0.72 ± 0.01g | 0.62 ± 0.05gh | 0.75 ± 0.04g | 0.75 ± 0.06f | ||
| LMOF | 0.62 ± 0.05l | 0.56 ± 0.06j | 0.62 ± 0.02k | 0.66 ± 0.09i | ||
| LMIF | 0.82 ± 0.06bc | 0.72 ± 0.07d | 0.83 ± 0.06c | 0.82 ± 0.07b | ||
| TRSP | CON | 0.74 ± 0.08fg | 0.62 ± 0.02gh | 0.75 ± 0.06g | 0.76 ± 0.04ef | |
| OF | 0.67 ± 0.04jk | 0.58 ± 0.06hi | 0.65 ± 0.04i | 0.72 ± 0.06gh | ||
| IF | 0.82 ± 0.02bc | 0.72 ± 0.04d | 0.80 ± 0.02de | 0.82 ± 0.02b | ||
| AZ | 0.62 ± 0.03lm | 0.56 ± 0.08ij | 0.62 ± 0.03k | 0.67 ± 0.04hi | ||
| AZOF | 0.60 ± 0.03n | 0.50 ± 0.02l | 0.60 ± 0.02n | 0.62 ± 0.01l | ||
| AZIF | 0.78 ± 0.08de | 0.62 ± 0.01g | 0.76 ± 0.08f | 0.77 ± 0.01de | ||
| LM | 0.70 ± 0.02hi | 0.59 ± 0.06h | 0.66 ± 0.01hi | 0.73 ± 0.02g | ||
| LMOF | 0.62 ± 0.06lm | 0.52 ± 0.09k | 0.62 ± 0.06m | 0.63 ± 0.03j | ||
| LMIF | 0.81 ± 0.02c | 0.68 ± 0.04de | 0.79 ± 0.01e | 0.79 ± 0.08cd | ||
| Without inoculation of AMF | BGSP | CON | 0.77 ± 0.05e | 0.64 ± 0.05f | 0.78 ± 0.05ef | 0.79 ± 0.06cd |
| OF | 0.71 ± 0.03h | 0.62 ± 0.02g | 0.75 ± 0.07g | 0.75 ± 0.04f | ||
| IF | 0.89 ± 0.05a | 0.85 ± 0.10a | 0.88 ± 0.03a | 0.85 ± 0.04a | ||
| AZ | 0.67 ± 0.01jk | 0.59 ± 0.04h | 0.67 ± 0.06h | 0.72 ± 0.06gh | ||
| AZOF | 0.63 ± 0.09l | 0.54 ± 0.03jk | 0.63 ± 0.09k | 0.66 ± 0.04i | ||
| AZIF | 0.82 ± 0.02bc | 0.67 ± 0.05e | 0.84 ± 0.04bc | 0.80 ± 0.06c | ||
| LM | 0.76 ± 0.02ef | 0.63 ± 0.03fg | 0.76 ± 0.01f | 0.76 ± 0.08e | ||
| LMOF | 0.64 ± 0.05kl | 0.57 ± 0.07i | 0.68 ± 0.03ij | 0.68 ± 0.06h | ||
| LMIF | 0.83 ± 0.06b | 0.75 ± 0.03c | 0.84 ± 0.06bc | 0.82 ± 0.09b | ||
| TRSP | CON | 0.75 ± 0.06f | 0.63 ± 0.06fg | 0.75 ± 0.06g | 0.76 ± 0.05e | |
| OF | 0.70 ± 0.04hi | 0.59 ± 0.04h | 0.67 ± 0.08h | 0.73 ± 0.01g | ||
| IF | 0.86 ± 0.06ab | 0.83 ± 0.06ab | 0.85 ± 0.04b | 0.82 ± 0.02b | ||
| AZ | 0.65 ± 0.06k | 0.58 ± 0.03hi | 0.65 ± 0.05i | 0.68 ± 0.06h | ||
| AZOF | 0.62 ± 0.05lm | 0.52 ± 0.09k | 0.63 ± 0.06j | 0.63 ± 0.03i | ||
| AZIF | 0.79 ± 0.02d | 0.64 ± 0.07f | 0.81 ± 0.07d | 0.78 ± 0.05d | ||
| LM | 0.75 ± 0.06f | 0.62 ± 0.06gh | 0.75 ± 0.02g | 0.75 ± 0.04f | ||
| LMOF | 0.62 ± 0.09lm | 0.56 ± 0.01j | 0.61 ± 0.01kl | 0.63 ± 0.07k | ||
| LMIF | 0.83 ± 0.02b | 0.75 ± 0.03c | 0.82 ± 0.06cd | 0.81 ± 0.05bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath, B.M.; Bamunuarachchige, C.; Stephenson, S.L.; Elgorban, A.M.; Asad, S.; Kumla, J.; Suwannarach, N.; Karunarathna, S.C.; Yapa, P.N. Soil Heavy Metal Absorption Potential of Azolla pinnata and Lemna gibba with Arbuscular Mycorrhizal Fungi in Rice (Oryza sativa L.) Farming. Sustainability 2023, 15, 4320. https://doi.org/10.3390/su15054320
Herath BM, Bamunuarachchige C, Stephenson SL, Elgorban AM, Asad S, Kumla J, Suwannarach N, Karunarathna SC, Yapa PN. Soil Heavy Metal Absorption Potential of Azolla pinnata and Lemna gibba with Arbuscular Mycorrhizal Fungi in Rice (Oryza sativa L.) Farming. Sustainability. 2023; 15(5):4320. https://doi.org/10.3390/su15054320
Chicago/Turabian StyleHerath, Bimal Manuranga, Chaturanga Bamunuarachchige, Steven L. Stephenson, Abdallah M. Elgorban, Suhail Asad, Jaturong Kumla, Nakarin Suwannarach, Samantha C. Karunarathna, and Pinnaduwage Neelamanie Yapa. 2023. "Soil Heavy Metal Absorption Potential of Azolla pinnata and Lemna gibba with Arbuscular Mycorrhizal Fungi in Rice (Oryza sativa L.) Farming" Sustainability 15, no. 5: 4320. https://doi.org/10.3390/su15054320
APA StyleHerath, B. M., Bamunuarachchige, C., Stephenson, S. L., Elgorban, A. M., Asad, S., Kumla, J., Suwannarach, N., Karunarathna, S. C., & Yapa, P. N. (2023). Soil Heavy Metal Absorption Potential of Azolla pinnata and Lemna gibba with Arbuscular Mycorrhizal Fungi in Rice (Oryza sativa L.) Farming. Sustainability, 15(5), 4320. https://doi.org/10.3390/su15054320










