Abstract
Until the change in drug legislation in 2010, the Russian Federation (RF) tried to follow the world-recognised guidelines of the US and European food supplement and drug registration legislation. After introducing the new pharmaceutical law (NPL), a unique legal system was created that did not correspond to international practice. At the same time, legislation on food supplements (FSs) remained the same corresponding to worldwide accepted practice. Consequently, restructuring of regulatory authorities was implemented. There was no pathway or authority restructuring for FSs in the same period. The present study aimed to analyse the legislative change burden on the availability of food supplements and medicines in the RF during the most turbulent period from 2010 to 2012. Before the NPL, 20,836 drugs and 5000 food supplements (FSs) were registered. After the NPL, the number of registered drugs significantly fell, while the number of registered FSs grew, showing a 90% increase. During the observational period, the number of registered FSs grew, and the registration process was stable. Meanwhile, a reduced quantity of registered medicines was observed, and the decline was −21.25%. Moreover, decreased productivity of regulatory authorities was noted, and the drug MAs issued per year fell by −64.11%.
1. Introduction
Despite the complexity of this issue, the concept of strengthening national and regional regulatory systems as the foundation to ensure timely access to quality medicines is often not given due attention [1].
At the beginning of the study, we noted that there was a keen interest in the pharmaceutical industry in processes around the introduction of the new pharmaceutical law (NPL) in 2010 and food supplement (FS) registration. However, only a limited number of articles covered the topic in detail, particularly in English, at the time [2]. The FS segment was little covered by state regulation. Furthermore, it is only since the early 2000’s that the state paid serious attention to this segment of the pharmaceutical industry. Thus, in 2003, manufacturers were required to indicate on the packaging that FSs are not medicines; in 2006, advertising FSs as drugs was banned; finally, in 2010, mandatory declaration of conformity was introduced. Until 2010, a feature of the FS market in Russia was that the vast majority of supplements were produced in the Russian Federation. Imports were only 13% of the total number of packs and 29% of the total value. Simultaneously, the medicines market accounted for 75.8% imported and 24.2% domestic drugs. Table 1 shows the top five FSs and medicines manufacturers and the most sold products.

Table 1.
Leading FS, drug manufactures and products.
The Federal Law, introduced in 1998 [3], regulated the procedure of marketing authorisation on medicines in the RF. Other acts describe the standards in the technical processes of obtaining marketing authorisation (MA). However, it was its own unique regulatory system, which did not completely comply with the internationally recognised practices, but used its own general principles such as a unique dossier format, evaluation process, and pharmacovigilance requirements. No national clinical trials were mandatory for generics, and overall, the existing MA scheme was mostly in accordance with international practices.
With the declared intention to increase the availability of high-quality, safe, and clinically effective drugs for citizens, state health care stakeholders announced strategies for developing the pharmaceutical industry of the RF until 2020 [4]. One strategy was to improve the system for assessing the quality of medicines and eliminating excessive administrative barriers to the MAs of domestic drugs, as well as bringing Russian pharmaceutical standards in line with international ones to increase the competitiveness of the national pharmaceutical industry. As a result of legislation, the state registration of drugs was based on expert results and the ethical review of the possibility of clinical studies for the drug. The State registration consisted of two stages. The first stage entailed the examination of documents to obtain permission to conduct a clinical study of the drug. The second stage of registration assumed examination and employment of the proposed methods of quality control of the drug, as well as examination of the risk/benefit ratio of the drug’s usage based on clinical trial outcomes. Interestingly, clinical trials were initiated before the quality and safety testing of the drugs [4].
After the introduction of the NPL, the authorities issuing the MAs were reorganised and resubordinated. A new specialist staff member was employed, and the MA issue highly exceeded the time planned. Before the NPL, the executive functions of pharmaceutical registration were provided by Roszdravnadzor. With the entry into the enforcement of the NPL and other legal acts [5], the maintenance and control of drugs were assigned to the Department of State Regulation of Drug Circulation and the Department of Informatisation of the Ministry of Health and Social Development of Russia. The abovementioned and other normative acts [6] significantly changed both the order and responsible executors of the state register of medicines (SMR), its information structure, and its functional role. Thus, at the end of 2010, two crucial institutions taking care of provisions of medicine access were reorganised or excluded from the process.
FS registration procedures and requirements during the observational period did not face any significant legislative changes until late 2012. Overall, FSs must undergo mandatory state registration and have to obtain an appropriate certificate prior to sale in Russia in order to initiate the free trade. However, conclusions about the efficacy of FSs and the claims of positive effects on the human body remained outside of the state registration process. To prevent consumers from engaging with misleading advertising and subsequent legal consequences for the manufacturers, the voluntary certification system (VCS) had been established. VCS was an official system that applied to goods, services, or equipment if their quality assurance was not a mandatory requirement of the law [7]. That somehow mimicked indications for medicines. Therefore, the certificate of state registration of food supplements had no information about the efficacy of FSs. For the purpose of advertising in the media, a manufacturer might submit information about features of a product, including composition, properties, effects on human health, and conditions of use according to the instruction of use as approved during the state registration. Thus, the legal pathways of FS marketing authorisation followed key general steps of pharmaceuticals such as safety and quality examinations. Hence, FS authorisations issuing authorities were chosen as a suitable control unit [7]. Generally, in the same jurisdiction, there were two institutions performing licensing with and without undergoing administrative changes. Overall, the comparison of two MA pathways before and after 2010 is described in the Figure 1, Figure 2 and Figure 3 below.
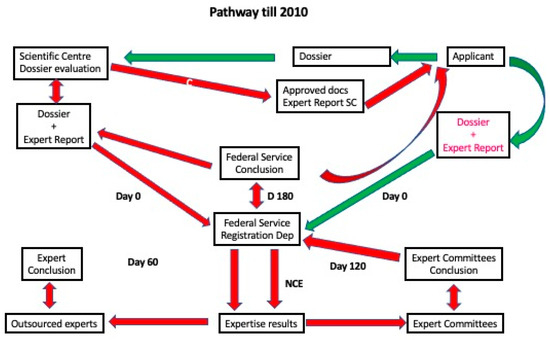
Figure 1.
MA Pathway until 2010. Red arrows indicate marketing authorisation holder (MAH) action, black arrows denote institutional actions, and blue marks refer to decisions made by the State Authority.
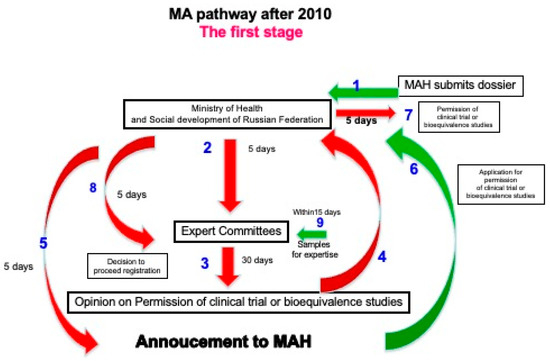
Figure 2.
MA Pathway after 2010. Stage 1. Green arrows indicate marketing MAH action, red arrows refer to institutional activities, and blue numbers represent each step in the pathway.
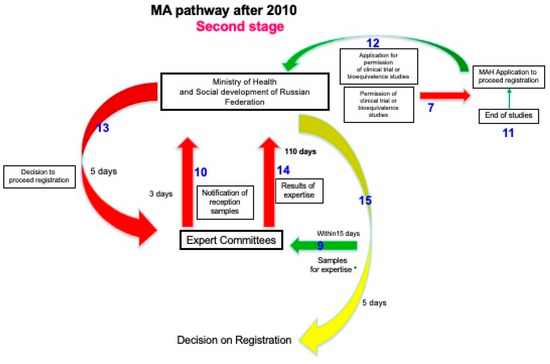
Figure 3.
MA Pathway after 2010. Stage 2. Green arrows denote marketing MAH action, red arrows represent institutional activities, and blue numbers embody the order of each step in the pathway (if the action concerns both stages of the pathway, they are presented in Figure 2 and Figure 3 simultaneously). The yellow arrow refers to the decision of the State Authority.
Despite initially declared improvements, the MA system experienced a lack of transparency concerning the procedures, leading to frequent amendments. At the same time, the legislation of the Customs Union and the Eurasian Economic Union (EAEU) regarding requirements and transparency in the regulation of nutritional supplements came even closer to world and EU practices.
In a recent study related to the MA of original drugs in Russia after the NPL, it was found that due to impacts of market factors and the imperfections of state regulation, foreign drugs enter the Russian market a few years after their registration in the US, the European Union (EU), and Japan. The authors concluded that to attain the goal of adequate supplies for the population of the most up-to-date drugs, Russia must upgrade the existing system of original drug registration [8].
Although the above legislative burden seems to have been overcome, there is no analysis of whether there has been an impact on the availability of medicines. Therefore, the present study aimed to analyse the legislative change burden on the availability of medicines versus FSs in the RF during the most turbulent period, from 2010–2012. In this study, for the first time, legislative performance was statistically evaluated, and study confounders were controlled based on the number of authorised medicines.
The analysis of this unique situation and consequences will be useful for process modelling and anticipation of the development options of planned legislative changes, their possible influence on the efficiency of the responsible institutions, and their impact on the availability of medicines or FSs.
2. Materials and Methods
The present research is based on a case study approach, which is particularly useful when there is a need to obtain an in-depth appreciation of an issue, event, or phenomenon of interest in its natural, real-life context [9]. We performed analysis of the regulatory framework governing the scope of dossier examination of medicines during their state registration in the RF from 2008–2017 based on a systematic literature review. The outcomes were regularly published. We investigated all the norms of the legislation of the RF and Eurasian EAEU in terms of regulating the medicines or FSs in the consumer market of the RF, the practice of applying such norms, and scientific and theoretical work on the topic of the present research. We extracted data from the papers identified using a structured data extraction form.
A functional efficacy assessment of institutions involved in the MA by statistically comparing them with a control unit was carried out to assess the impact of structural reforms on NPL performance. The approach of legislative performance evaluation, mentioned in the literature, is to compare items of interest with control units from different companion jurisdictions (in a territorial sense). Unfortunately, comparing the Russian drug MA authorities with companions in other countries would not deal with national, specific issues.
2.1. Indicators of Regulatory Performance
By evaluating pharmaceutical legislation, the research presented must determine whether a legislative act can ensure the reduction of a stated problem [10]. Thus, the performance indicator concerned was the impact of regulation on the availability of medicines in terms of the total and per year quantity of registered drugs during implementation of the NPL.
2.2. Data Availability
We had available and compiled data in our possession. The data already existed in an available dataset and were considered the easiest to use [11]. A longitudinal collection of Excel sheets issued by the State Medicines register of the RF, counting all authorised medicines, was available for a defined number of years before and after the introduction of the NPL.
We took data on the parallel trend assumption from available data in the literature.
2.3. Causal Attribution to MA Regulation
To establish whether the NPL led to positive improvements concerning access to medicine, we used datasets before and after the introduction of the NPL. That is, before and after the adoption of regulations. Neither data group was randomly assigned and could not be viewed as equivalent. Therefore, the confounders were controlled.
2.4. Control of Confounders
To account for confounders in the regression analysis, we used DiD estimation. We used a quasi-experimental design with longitudinal data from treatment (NPL impact) and control groups to obtain an appropriate counterfactual to estimate a causal effect for the MA numbers. Therefore, the following control group was chosen: for FSs registered as medicinal products, FSs registered in the RF were chosen. This control group was found to be appropriate because the MA schemes for both product groups were similar until the adoption of the NPL.
For DiD to be a valid statistical method, several assumptions were considered. To estimate any causal effect, the attention was drawn to three concerns: exchangeability, positivity, and stable unit treatment value assumption, or the potential outcome observation [12] on one FS unit, which should be unaffected by the particular assignment of treatment (NPL) to the pharmaceutical legislation. Thus, the treatment (impacted by legislation—NPL) was unrelated to an outcome at baseline (allocation of the intervention was not determined by outcome), as FS and pharmaceutical MA systems do not interfere with one another. The assumption of parallel trends implies that if untreated, the results for the NPL treatment and control groups are expected to change at the same rate. Hence, any difference in the discrepancies in results between groups can be explained by policy, rather than by differentiated pre-existing trends in results [13].
3. Results
3.1. Marketing Authorisation Pathways Prior to and Post NPL
The quality and safety of both medicines and FSs were tested during registration. All administrative activities were performed within one ministry and a single institution subordinate to it. Many national market research publications also mention the FS market as a segment of the overall pharmaceutical market [14]. In contrast, after the NPL, the authorisation process of FSs did not change, but the confounders were deemed important for DiD analysis—they influenced the dependent variable (the number of registered medicines) and the independent variable (the number of FSs)—as they were similar and nationally specific. Most importantly, during the observational period of the present study, FS registration and the MA of medicines were carried out under supervision of the same ministry. The registration pathway comparison between FSs and drugs is shown in the Figure 4 and Figure 5 below.
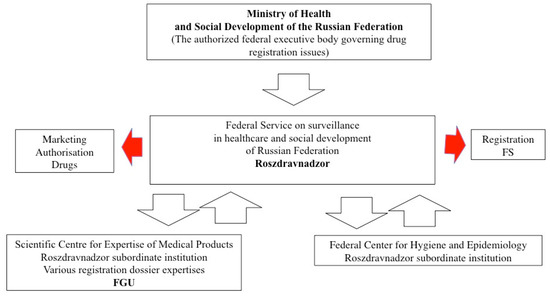
Figure 4.
MA and FS pathways before the NPL.
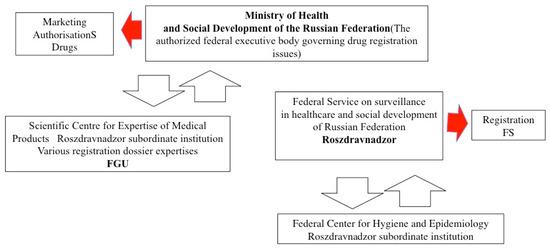
Figure 5.
MA and FS pathways after the NPL.
As seen from the schemes above, the administration of the MA changed, whereas for FSs it remained the same, as presented in the schemes above. Regarding registration procedures and requirements during the observational period, FSs did not face any legislative changes until late 2012, when EAEU norms were introduced, but that did not influence registration pathways in Russia. Generally, in the same jurisdiction, two institutions performed licensing procedures with and without administrative changes.
Therefore, it was assumed that both would be comparable to assess their performance due to organisational changes.
3.2. Parallel Trend Assumption for Medicines and FSs Prior to the NPL
For the state institution performance assessment under the NPL, two efficacy indicators were evaluated: the number of registered medicines and issued medicine MAs per year versus the same for FSs. Therefore, the treatment (impacted by legislation—NPL) was unrelated to an outcome at baseline (allocation of the intervention was not determined by an outcome), as FS and pharmaceutical MA systems do not interfere with one another. Treatment and control groups comparing the quantity of registered medicines, as seen from the trend lines in Figure 6, could be considered parallel in the outcomes prior to the intervention.
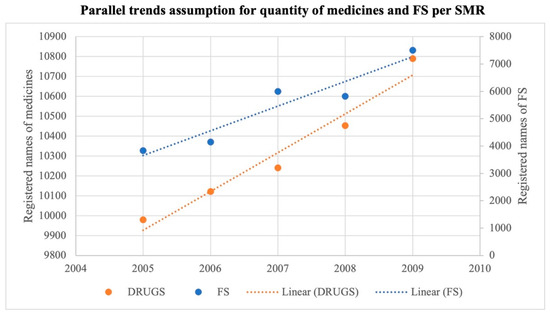
Figure 6.
Parallel trends assumption quantity.
The literature data show a steady rise in the number of medicine names due to active MA efforts in the Russian pharmaceutical market; in 2009, it reached 10,790 units [15].
We derived the numbers of registered FSs from other literature data [16]. According to Figure 6, the composition of the treatment and control groups was stable for repeated cross-sectional design (according to SUTVA). The quantity of issued MAs per year for drugs and FSs prior to the NPL shows a parallel trend. The graphic presentation of parallel trends is depicted in Figure 7 below.
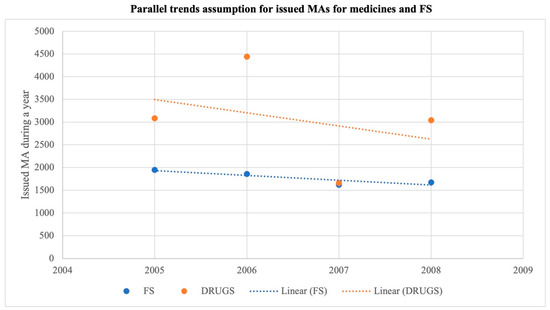
Figure 7.
Parallel trends assumption MAs per year.
3.3. Regulatory Performance Evaluation for the Number of Medicines Versus FSs in 2008 and 2012
Before the NPL, the number of drugs per SMR was 20,836, and the number of FSs was 5000 [14]. Further, in 2012, there were 16,409 drugs and 9500 FSs. The number of registered FSs significantly grew from 5000 in 2008 to 9500 in 2012, showing a massive 90% increase. The Figure 8 below illustrates slopes of numbers of drugs versus FSs during two observational periods.
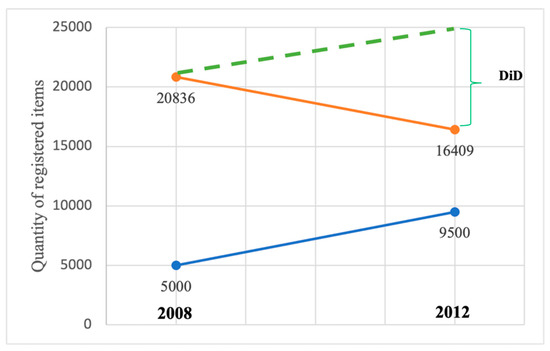
Figure 8.
Graphic view of registered medicines per SMR and FSs during two observational periods and DiD. Red line denotes drugs, blue FSs. Dashed green line shows DiD slope.
After the NPL, there were 16,409 drugs in the SMR versus 9500 FSs. The difference was −4427 drugs according to DiD, considering the FSs trend of −8927. The reported decline was −21.25%. Thus, simply due to weak regulatory performance, there was a significant decrease in availability in terms of their total quantity per SMR is noted.
3.4. Regulatory Performance Evaluation for Medicine Mas vs. FSs in 2008 and 2012
The difference in average outcome in the impacted group before and after the NPL, minus the difference in average outcome in the control group before and after the legislative impact, shows a significant decline in issued Mas per year for medicines, whereas the number of Mas for FSs slightly increased. This came as a justification for the authority’s decreased productivity after the implementation of the NPL. Before the NPL, the number of Mas issued for drugs was twice as high as for FSs, which, after NPL’s introduction, was bound to fall to a much lower level. Before the NPL in 2008, 3043 drug Mas were issued per year, whereas two years post-intervention in 2012, that figure was 1092. The difference was −1975 Mas/year, where DiD assumed the FSs trend to be 1978. The fall was obvious at −64.11%. The yearly activities of FS registration remained stable at 1675 in 2008 and 1702 in 2012, accounting for a 1.61% increase. The Figure 9 below shows the slopes of issued MAs during two observational periods.
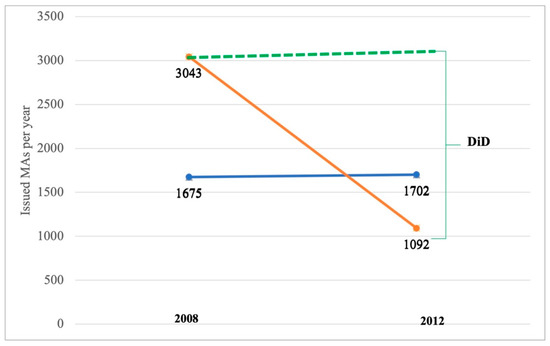
Figure 9.
Issued MAs/year during two observational periods and DiD estimator. Red line denotes drugs, blue FS. Dashed green line shows DiD slope.
According to the methods of the present research, a two-group, two-period DiD design [17] was used. Thus, the only evaluated numbers were from pre-NPL year 2008 and post-NPL year 2012. However, for example, the number of MAs issued per year was 1282 in 2011 [18], showing a negative trend after the NPL. The abovementioned regression calculations again stress the low productivity of the state authorities involved in the MA of medicines after the introduction of the NPL, affecting access to medicines. The NPL performance, in terms of the authority’s productivity concerning issued MAs per year, is insufficient.
The EU member state Ireland can be mentioned as an external comparison of the stable legislative and structural situation [19,20]. Figure 10 shows the regulatory performance of the Irish Medicines Board (IMB) during the observational period. At that time, the IBM was not exposed to any structural or legislative reform.
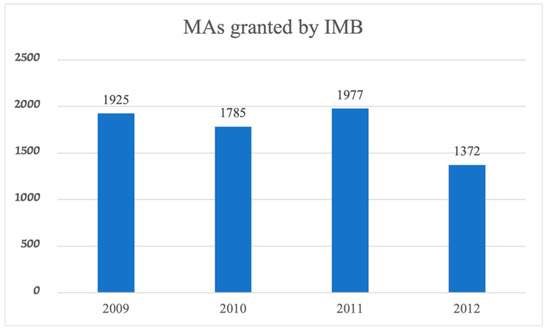
Figure 10.
Issued MAs/year during the observational period by the IMB.
Although the number of MAs issued by the IMB in 2009 and 2012 decreased slightly, it was certainly not by 64%.
4. Discussion
This paper, as seen in Figure 6, Figure 7, Figure 8 and Figure 9, describes the impact of a unique authorisation system created in the RF in 2010 on the availability of medicines in comparison to FSs. The market situation before the legislative burden is seen in Table 1. After the introduction of the NPL, the dossier evaluation process was completely renewed and administered by the new ministry and subordinating institutions (please refer to Figure 4 and Figure 5). New and inexperienced authority employees were involved in the MA process, which created a bottleneck in the dossier evaluation flow. Conversely, no legislation or structural changes in the institutions handling FS registration was observed (Figure 4 and Figure 5). The drug registration procedure had two stages: the first dossier evaluation for obtaining clinical trial (CT) permission, and the second quality control of medicines (Figure 1 and Figure 2). Thus, irrespective of whether a medicine is a novel chemical entity or generic, a CT is mandatory. Thus, the CT requirement can be defined as another obstacle that decreases MA process productivity.
In recent research, some authors have recommended expanding the list of drugs required for bioequivalence studies and using Biopharmaceutics Classification System-based biowaivers for some drugs at the time of generic registration, if scientifically justified. Moreover, some authorities in low-income countries have regulatory systems that rely on the review process already done by the International Council on Harmonisation (ICH) member countries or ICH observer countries. This process would be easier if a common dossier format were used during regulatory submission [21].
The duration of registration in RF after 2010 highly exceeded defined timeframes. The key reasons for the delay mentioned by local regulatory staff were mandatory local clinical trials. At the same time, there was no unexpected prolongation of FS registration duration.
Other authors have referred to the same historical time, stating that due to impacts of market factors and imperfections in the state regulation, original drugs developed abroad enter the Russian market a few years after their registration in the US, the EU, and Japan. The average time from the moment a drug is initially approved in the aforementioned countries and jurisdictions to the moment of registration in Russia, is 4 years and 8 months, with a median value of 2.5 years [8].
During the period of turmoil caused by the NPL, the local pharmaceutical industry endured serious challenges. Given the high level of self-organisation, excellent problem-solving skills, and people’s perseverance, a significant negative impact of these regulatory affairs system changes was prevented. However, before the present study, the impact thought to have been overcome by the aforementioned efforts was not justified by controlled statistical calculations. Our current research shows the weak performance of the NPL, and a decrease in the number of medicines registered while maintaining stable growth of FSs. The impact of the activity on the general public and pharmaceutical industry representatives led to a process in which the latest legislation changes were implemented at several sites. This process included the Analytical Centre of the Russian Government, representatives of patient and medical communities, businesses, and the 20 largest professional associations [4].
In health systems, access to medicines depends on five main factors: availability, affordability, accessibility, acceptability, and quality [22]. In our study, it was found that due to the regulatory burden after the introduction of the NPL, the availability and accessibility of medicines in the RF were impacted. Our findings correspond to the statement that national regulatory authorities are the key government institutions that promote access to quality-assured medicines and to combat falsified medical products; however, despite progress, regulatory capacity in low-income countries is still insufficient [23].
In overcoming the burden caused by the NPL, the MA system slightly recovered, and in March 2019, there were 20,192 entries on the authorised medicines list versus 20,836 entries in 2008. As mentioned earlier, the NPL was amended many times before 2020, and neither the CTD format nor national CT issues were solved. To be in accordance with international regulatory approaches—which are definitely beneficial for companies in the domestic pharmaceutical industry that want to be successful exporters—a harmonised position of the legislation was considered. To address such issues in the future, common EAEU legislation was introduced. However, there were almost no legal obstacles to implementing common EAEU pharmaceutical legislation to start a common market [24]. However, at the end of 2017, despite an enormous workload with the intention of an easy market entrance and overall unification, all the efforts led nowhere, and the Russian MA system proceeded to function autonomously [24].
In 2011, the additional FS legislation of EAEU came into force, namely “Unified sanitary and epidemiological and hygienic requirements for goods subject to sanitary and epidemiological surveillance (control)” [25]; however, practical implications of the Customs Union (CU) legislation became significant after 2012 [25]. Overall, the scheme of FS marketing authorisation and Voluntary certification scheme acknowledges its suitability to be used as a control model to evaluate the performance of pharmaceutical legislation changes which took place in 2010. Further, in statistical analysis of the present study related to legislation performance, particularly parallel trends assumption, it can be seen that the number of registered products follow the same trends before the adoption. The introduction of the CU legislation followed by implementation in late 2011 maintained the tendency of growth in terms of registered FSs amount.
5. Conclusions
This is the first study to statistically assess the impact of drug regulatory changes due to the introduction of the NPL in the RF in 2010. Quantitative research was performed via statistical evaluation of the controlling confounders. During the observational period, a reduced quantity of registered medicines was observed, and the decline was −21.25% while FSs grew by 90%. Additionally, decreased productivity of drug regulatory authorities was noted, and the drug MAs issued per year fell by −64.11% vs. 1.61% of FSs. Thus, the aims of the drug legislation to increase medicine availability and admission to the market were not achieved. The analysis of this unique situation and its consequences will be useful for process modelling and anticipation of the development options of planned legislative changes, their possible influence on the efficiency of the responsible institutions, and their impact on the availability and safety of medicines. A similar design of future studies is necessary to justify the value of the research methods and their applicability in the appropriate scientific evaluation.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karrarm, K. Functioning Regulatory Systems: The Cure for Lack of Access to Medicines in low- and Middle-Income Countries. ReliefWeb. 2019. Available online: https://reliefweb.int/terms-conditions (accessed on 23 October 2020).
- Tyupa, V. Russian Law on Circulation of Medicines Significant Amendments to Come into Force on 1 July 2015. Available online: https://seamless.legal/en/rus/publication/russian- (accessed on 20 May 2022).
- The Federal Law. About Medicines. Pravo.gov.ru. 1998; pp. 1–21. Available online: http://pravo.gov.ru/proxy/ips/?docview&page=1&print=1&nd=102053738&rdk=9&&empire= (accessed on 27 October 2020).
- Lozda, R. Regulatory system changes in russia: A historical review and future perspectives. Ther. Innov. Regul. Sci. 2016, 50, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Social Development. Order of the Ministry of Health and Social Development of the Russian Federation of August 26, 2010 N 746n On approval of the procedure for maintaining the state register of medicines for medical use. 2010. Available online: https://legalacts.ru/doc/prikaz-minzdravsotsrazvitija-rf-ot-26082010-n-746n/ (accessed on 27 October 2020).
- Government of Russian Federation. Decree of the Government of the Russian Federation of 04.08.2010 N 1316-r. On the Assignment to the Jurisdiction of the Ministry of Health and Social Development of Russia of the Federal State Institution “Scientific Center for. 2010. Available online: https://rulaws.ru/goverment/Rasporyazhenie-Pravitelstva-RF-ot-04.08.2010-N-1316-r/ (accessed on 27 October 2020).
- Lozda, R. Regulatory Issues of Voluntary Certification of Food Supplements in Russia. Ther. Innov. Regul. Sci. 2020, 54, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chaplenko, A.; Gildeeva, G.; Vlassov, V. The entry lag of innovative drugs in russia, 2010–2019. Int. J. Environ. Res. Public Health 2021, 18, 5052. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.; Cresswell, K.; Robertson, A.; Huby, G.; Avery, A.; Sheikh, A. The case study approach. BMC Med. Res. Methodol. 2011, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Coglianese, C. Measuring Regulatory Performance—Evaluating the Impact of Regulation and Regulatory Policy. Organ. Econ. Co-Oper. Dev. 2012. Available online: http://www.oecd.org/gov/regulatory-policy/1_coglianese%web.pdf (accessed on 20 February 2023).
- Inforeg. Index. 2020. Available online: https://inforeg.eu/ (accessed on 5 November 2020).
- Schwartz, S.; Gatto, N.M.; Campbell, U.B. Extending the sufficient component cause model to describe the Stable Unit Treatment Value Assumption (SUTVA). Epidemiol. Perspect. Innov. 2012, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M. Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv. Res. 2009, 44, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Turovskaya, K. Improper Sale of Dietary Supplements and Administrative Responsibility for it. 2010. Available online: https://center-bereg.ru/f880.html (accessed on 27 October 2020).
- Yavorsky, D. Dissertation on the Topic. Development of a Strategy for the Formation of Rational Drug Names Based on the Concept of a Multidimensional Decision Space. 2010. Available online: https://www.dissercat.com/content/razrabotka-strategii-formirovaniya-ratsionalnykh-naimenovanii-lekarstvennykh-preparatov-na-o (accessed on 27 October 2020).
- Pronchenko, G. Journey into the world of pharmacognosy. ГЭОТАР-Медиа. 2010. Available online: https://library.geotar.ru/book/ISBN9785970417249.html (accessed on 27 October 2020).
- Wing, C.; Simon, K.; Bello-Gomez, R.A. Designing Difference in Difference Studies: Best Practices for Public Health Policy Research. Annu. Rev. Public Health 2018, 39, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Social Development. Provision of High-Quality and Safe Medicines and Medical Products. Fragment of the Annual Report of the Ministry of Health and Social Development of Russia for 2011. 2012. Available online: https://cyberleninka.ru/article/n/obespechenie-kachestvennymi-i-bezopasnymi-lekarstvennymi-preparatami-i-izdeliyami-meditsinskogo-naznacheniya/viewer (accessed on 20 February 2023).
- HPRA. Irish Medicines Board Annual Report 2011. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwibk4G11YX9AhXJmYsKHUoqA3gQFnoECBIQAQ&url=https%3A%2F%2Fwww.hpra.ie%2Fdocs%2Fdefault-source%2Fpublications-forms%2Fcorporate-policy-documents%2Fimb-annual-report-2011 (accessed on 8 February 2023).
- HPRA. Irish Medicines Board Annual Report. 2013. Available online: https://www.hpra.ie/homepage/about-us/publications-forms/corporate-and-policy-documents/item?id=c0aa0226-9782-6eee-9b55-ff00008c97d0&t=/docs/default-source/publications-forms/corporate-policy-documents/annual-report-2013 (accessed on 8 February 2023).
- Thambavita, D.; Galappatthy, P.; Jayakody, R.L. Regulatory requirements for the registration of generic medicines and format of drug dossiers: Procedures in Sri Lanka in comparison with selected regulatory authorities. J. Pharm. Policy Pract. 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Tiguman, G.M.B.; Silva, M.T.; Galvão, T.F. Consumption and Lack of Access to Medicines and Associated Factors in the Brazilian Amazon: A Cross-Sectional Study, 2019. Front. Pharm. 2020, 11, 586559. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Bempong, D.; Babigumira, J.B.; Banoo, S.; Cooke, E.; Jeffreys, D.; Kasonde, L.; Leufkens, H.G.; Lim, J.C.; Lumpkin, M.; et al. Expanding global access to essential medicines: Investment priorities for sustainably strengthening medical product regulatory systems. Global Health 2018, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Lozda, R. The Common Pharmaceutical Market of the Eurasian Economic Union: A Regulatory Review. Ther. Innov. Regul. Sci. 2017, 51, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Customs Union Commission. Uniform Sanitary-Epidemiological and Hygienic Requirements for Goods Subject to Sanitary-epidemiological Supervision (Control). 2010. Available online: http://www.eurasiancommission.org/en/act/texnreg/depsanmer/sanmeri/Pages/P2_299.aspx (accessed on 27 October 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).