Guinea Grass (Megathyrsus maximus) Fibres as Sorbent in Diesel Bioremediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
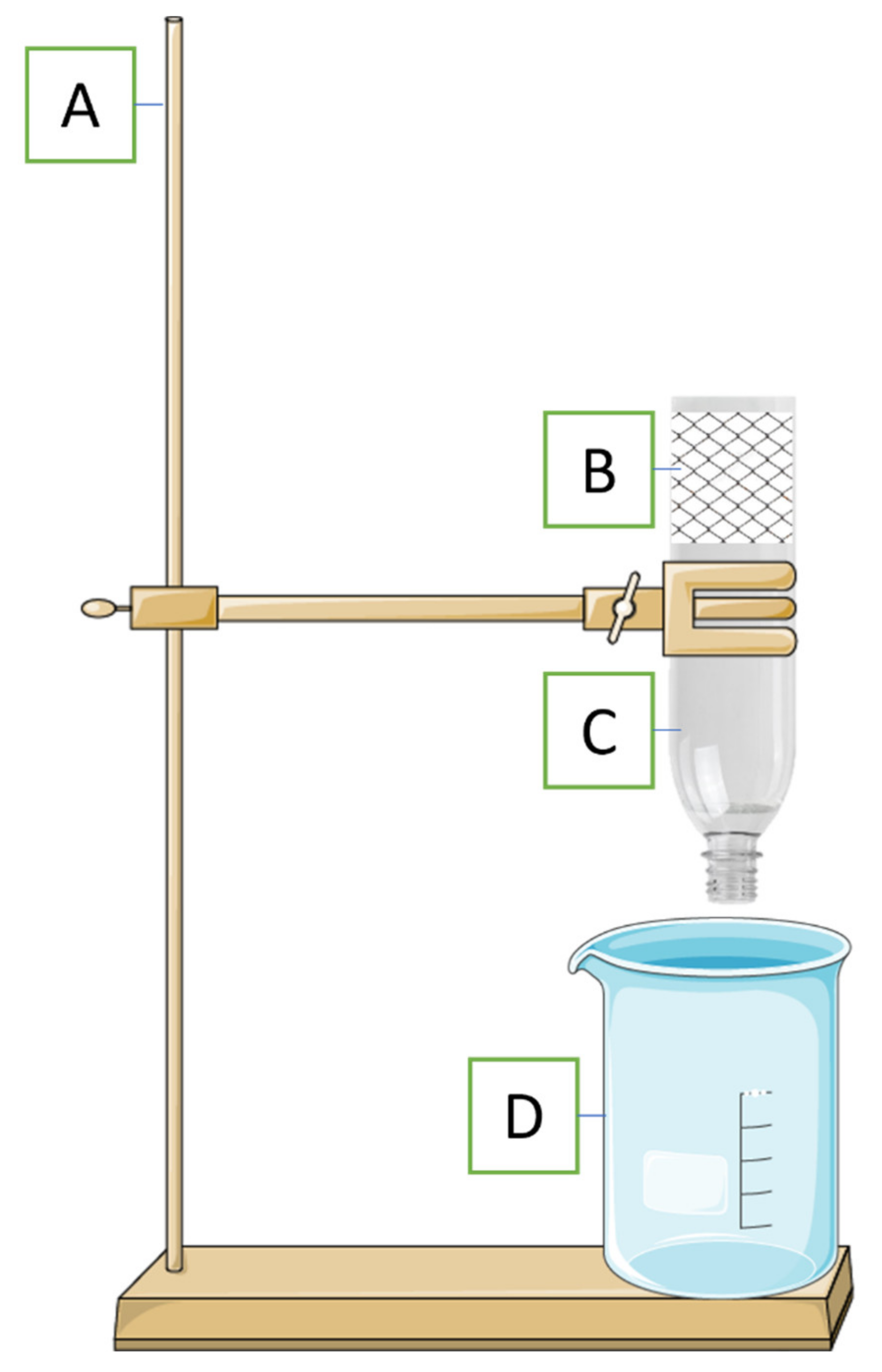
2.2. Experimental Setup and Sorbent Screening
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4. Conventional One Factor at a Time (OFAT) Optimisation
2.5. Statistical Respond Surface Methodology (RSM) Optimisation
2.5.1. Plackett Burman Design (PBD)
2.5.2. Central Composite Design (CCD)
3. Results and Discussion
3.1. Screening of Guinea Grass
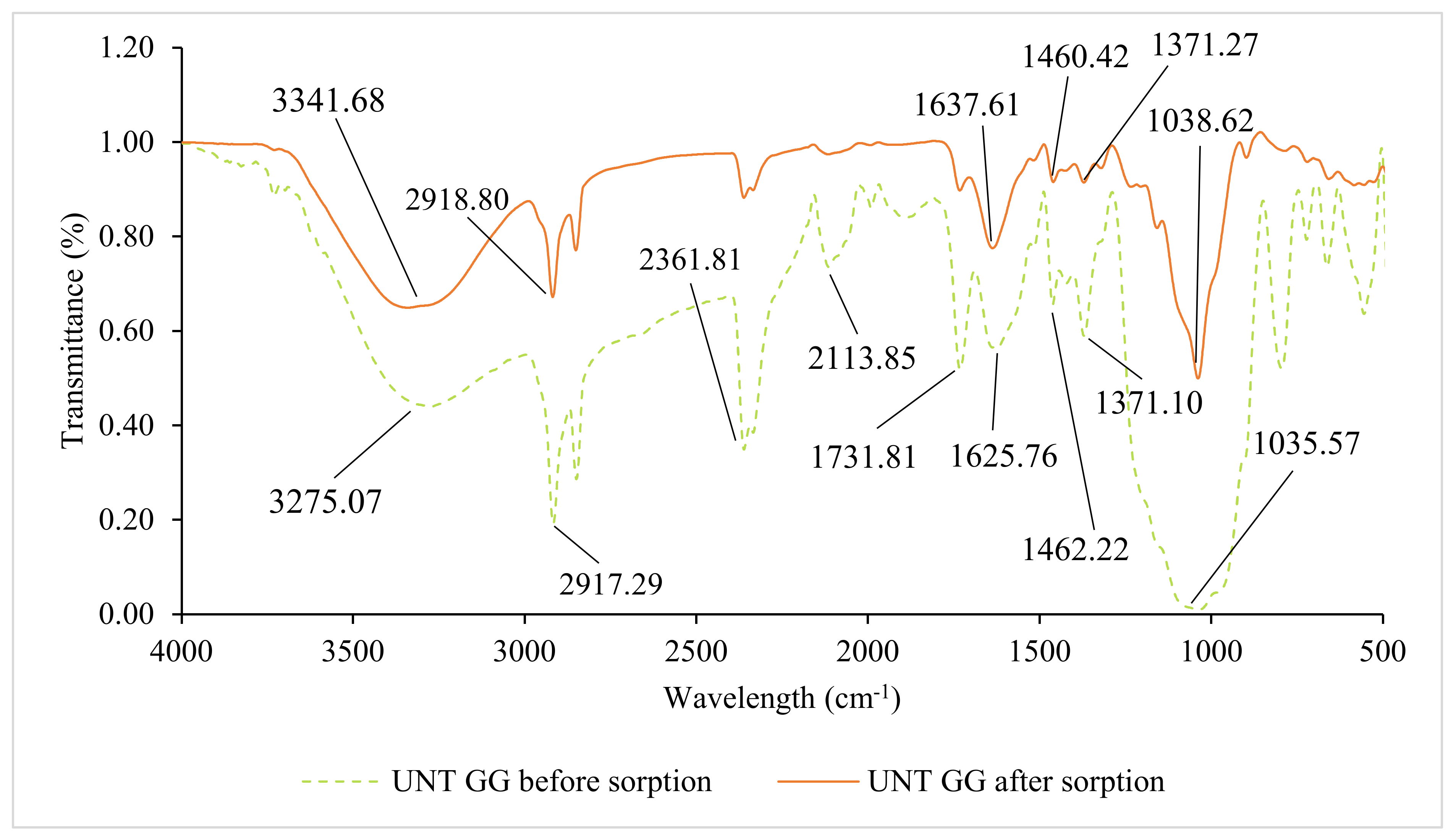
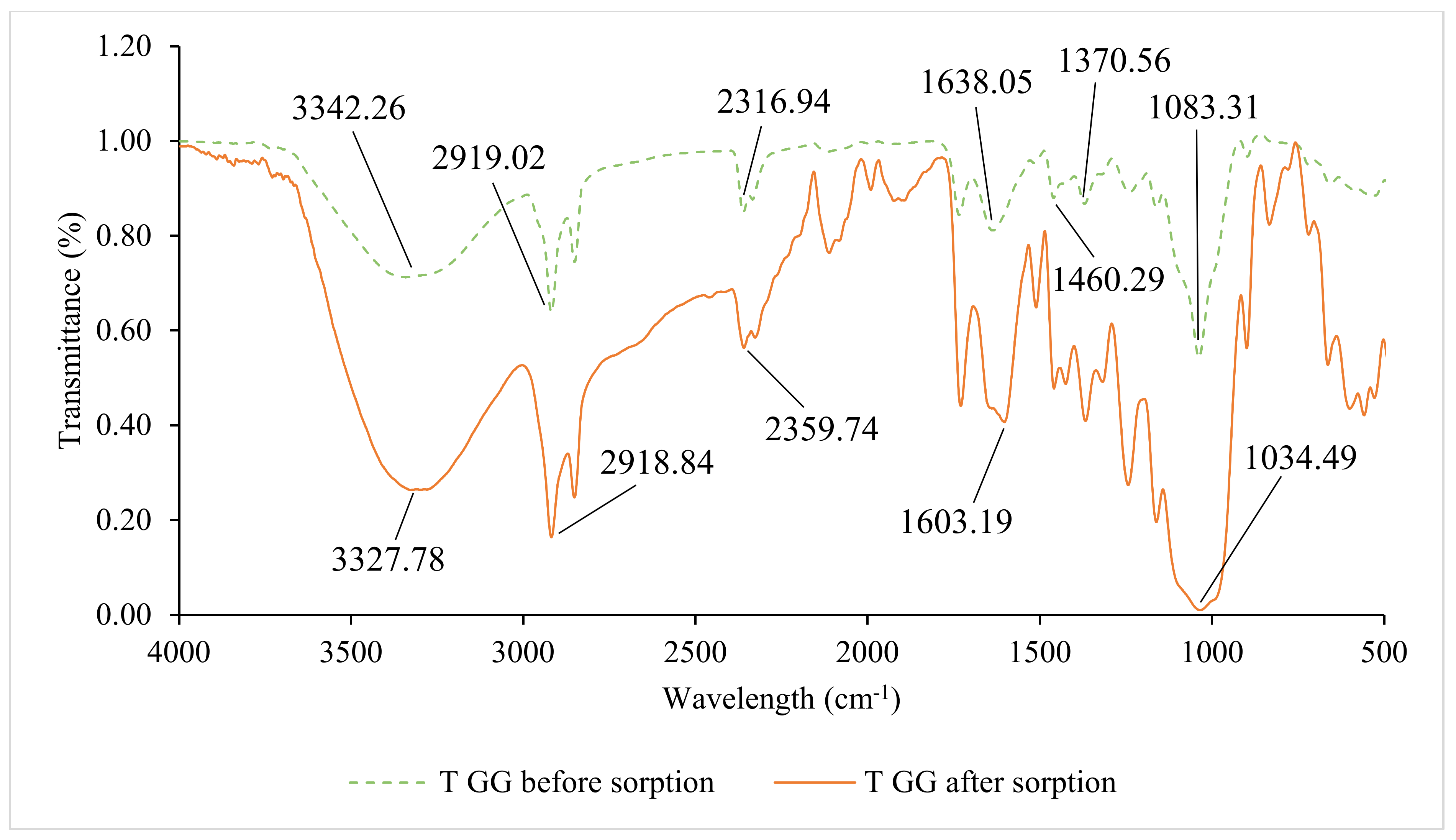
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Conventional OFAT Optimisation
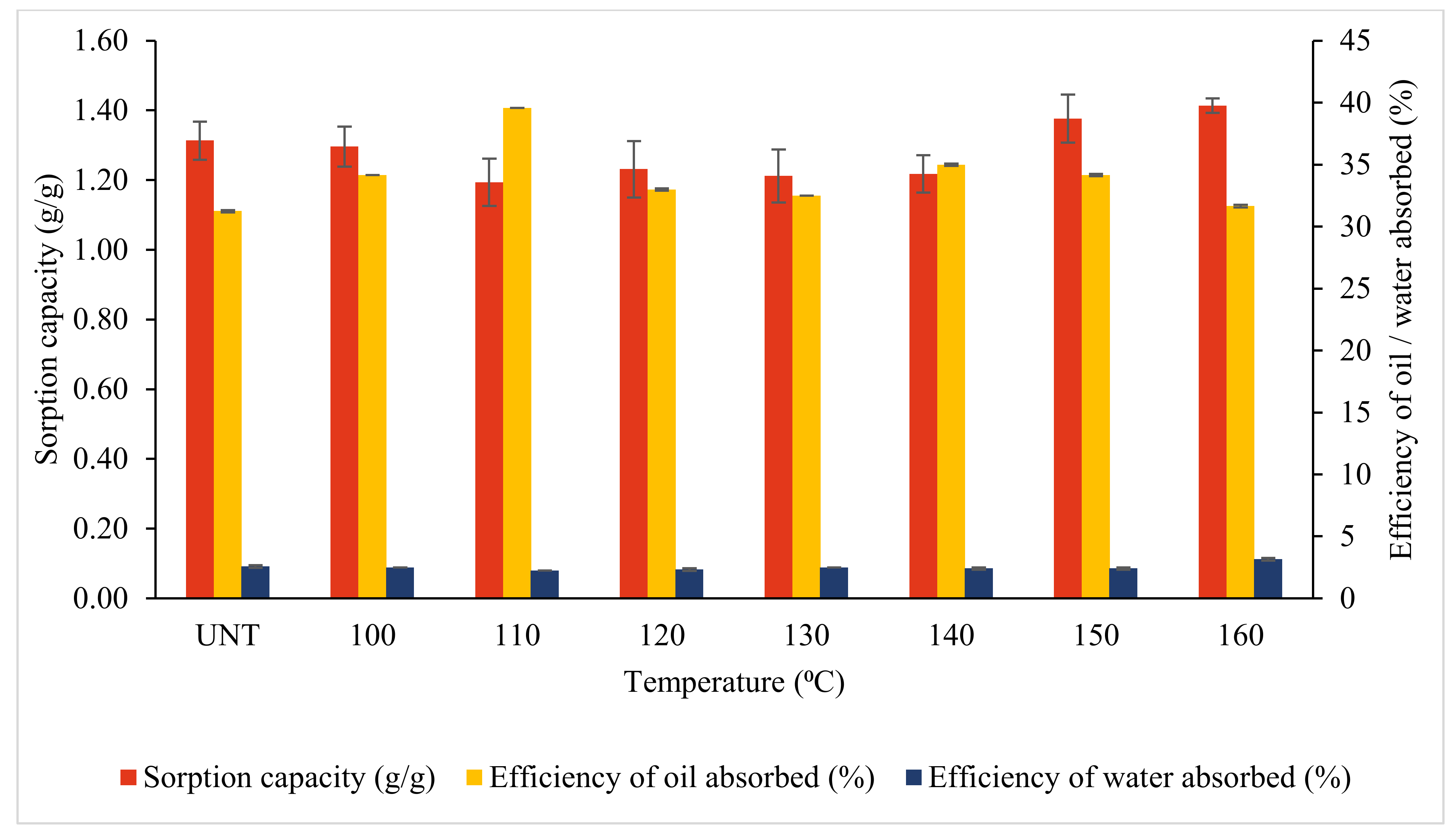
3.3.1. Effects of Temperature
3.3.2. Effects of Time
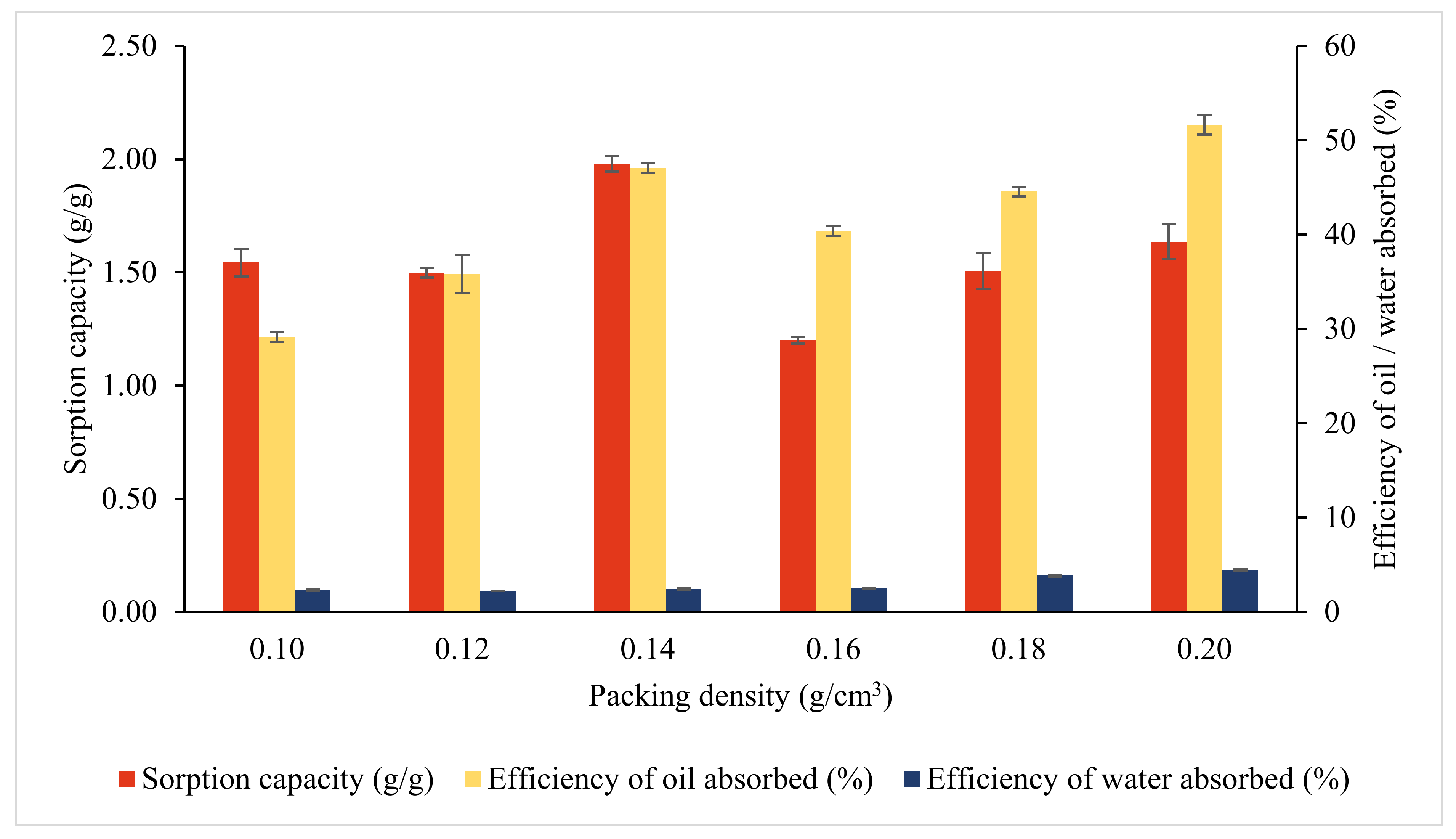
3.3.3. Effects of Packing Density
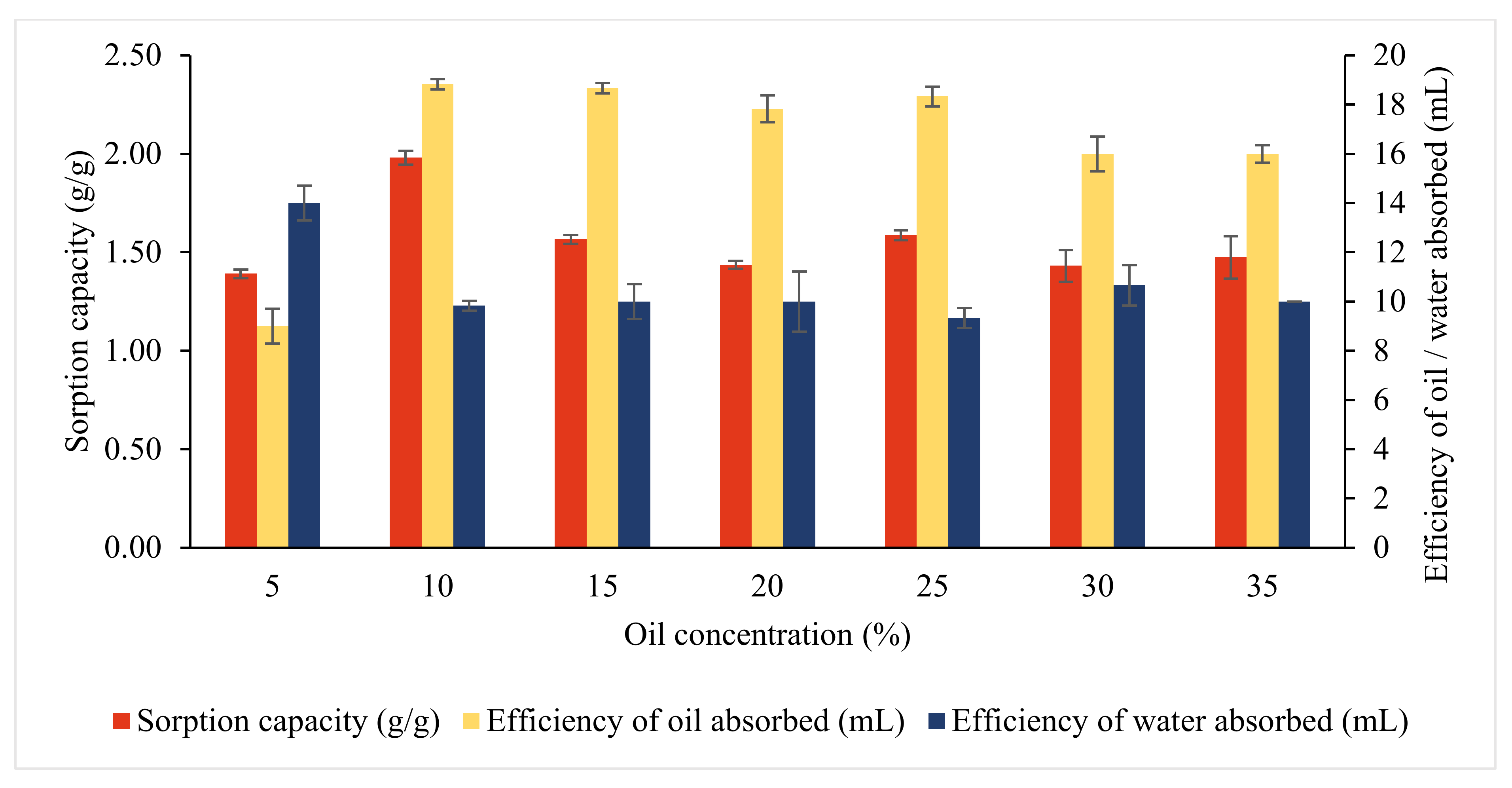
3.3.4. Effects of Oil Concentration
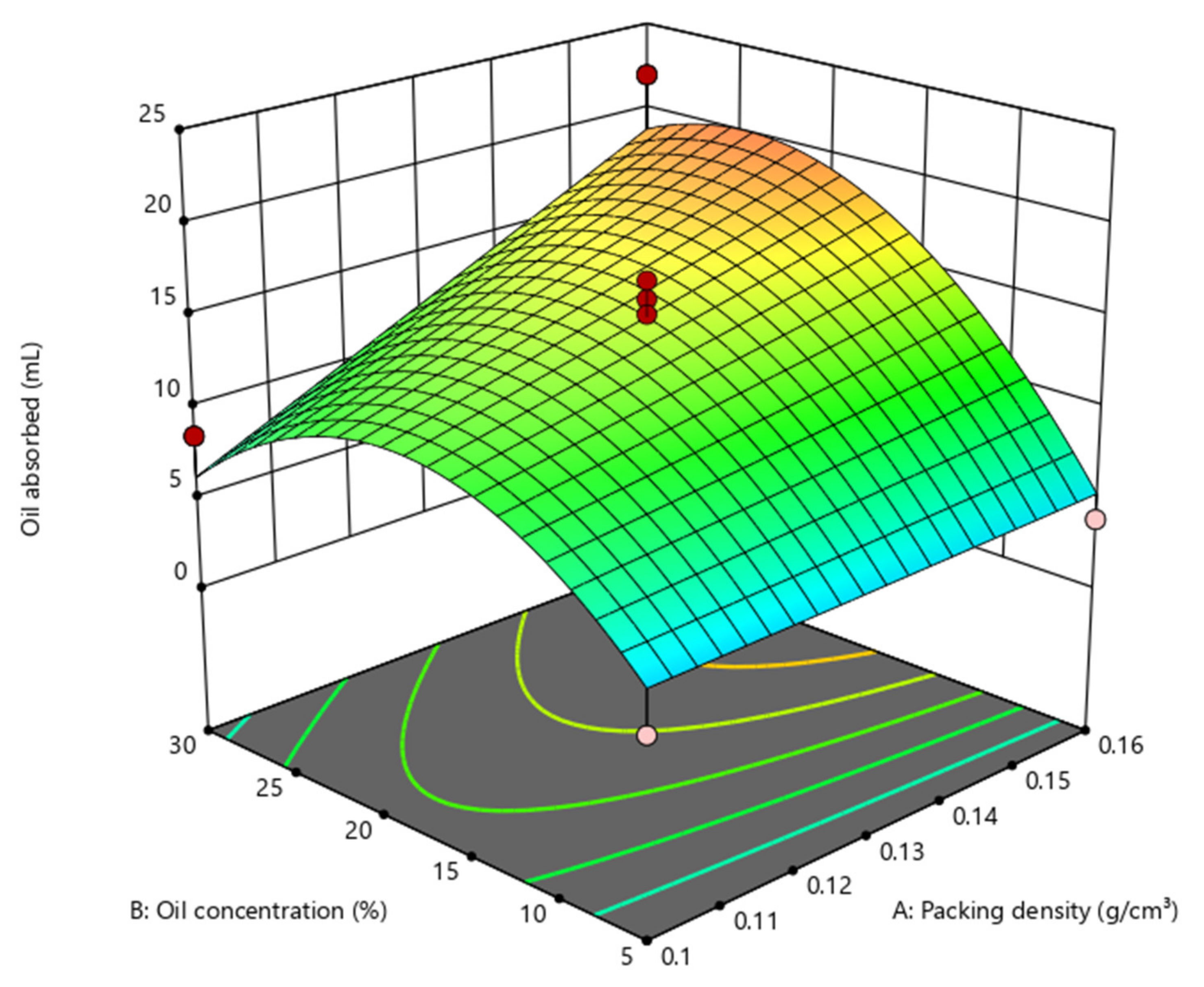
3.4. Response Surface Methodology (RSM) Optimisation
3.4.1. Plackett Burman Design
3.4.2. Central Composite Design
3.4.3. Model Validation Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Han, W.; Dong, S.; Fang, C.; Cheng, Y.; Liu, D.; Zhang, X. Constructing hydrothermal carbonization coatings on carbon fibers with controllable thickness for achieving tunable sorption of dyes and oils via a simple heat-treated route. J. Colloid Interface Sci. 2020, 559, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Anuzyte, E.; Vaisis, V. Natural oil sorbents modification methods for hydrophobicity improvement. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 147, pp. 295–300. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhaskarwar, A.N. A review on sorbent devices for oil-spill control. Environ. Pollut. 2018, 243, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi, D.A.; McPhedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- Yap, K.L.; Lee, C.M.; Gan, Y.L.; Tang, T.K.; Lee, Y.Y.; Tee, T.P.; Lai, O.M. High intrinsic biosorption efficiency of cattle manure on Cr (VI): A potential low-cost fibre-rich biosorbent. Pertanika J. Sci. Technol. 2018, 26, 193–214. [Google Scholar]
- Jmaa, S.B.; Kallel, A. Assessment of performance of Posidona oceanica (L.) as biosorbent for crude oil-spill cleanup in seawater. Biomed Res. Int. 2019, 2019, 6029654. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Surkova, T.Y.; Berkinbayeva, A.N.; Dosymbayeva, Z.D.; Mukhanova, A.A.; Abdikerim, B.E. Development of a method of modifying a natural sorbent for uranium extraction. J. Chem. Technol. Metall. 2020, 55, 1041–1046. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Singh, A.; Saini, P. Biosorption: A novel biotechnological application for removal of hazardous pollutants. In Innovations in Food Technology; Springer: Singapore, 2020; pp. 341–360. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Setiadi, T. Adsorption and desorption of copper (ii) ions onto garden grass. Bioresour. Technol. 2012, 121, 386–395. [Google Scholar] [CrossRef]
- Sindhu, N.; Shehrawat, P.S.; Singh, B. Agricultural waste utilization for healthy environment and sustainable lifestyle. Ann. Agric. Biol. Res. 2015, 20, 110–114. [Google Scholar]
- Adejumo, I.O.; Adebiyi, O.A. Agricultural solid wastes: Causes, effects, and effective management. In Strategies of Sustainable Solid Waste Management; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Gao, Y.; Yue, Q.; Gao, B.; Kong, W.; Zang, Y.; Jiang, W. Grass-modified graphene aerogel for effective oil-water separation. Process Saf. Environ. Prot. 2019, 129, 119–129. [Google Scholar] [CrossRef]
- Habermann, E.; de Oliveira, E.A.D.; Contin, D.R.; Delvecchio, G.; Viciedo, D.O.; de Moraes, M.A.; de Mello Prado, R.; de Pinho Costa, K.A.; Braga, M.R.; Martinez, C.A. Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiol. Plant. 2019, 165, 383–402. [Google Scholar] [CrossRef]
- Aswanimiyuni, A.; Mohamad, N.I.; Haryani, H.; Norfadzrin, F. A comparison of feed intake and growth performance of goats fed guinea grass and napier grass. Malays. J. Vet. Res. 2018, 9, 13–18. [Google Scholar]
- Soti, P.; Thomas, V. Review of the invasive forage grass, guinea grass (Megathyrsus maximus): Ecology and potential impacts in arid and semi-arid regions. Weed Res. 2022, 62, 68–74. [Google Scholar] [CrossRef]
- Rhodes, A.C.; Plowes, R.M.; Goolsby, J.A.; Gaskin, J.F.; Musyoka, B.; Calatayud, P.A.; Cristofaro, M.; Grahmann, E.D.; Martins, D.J.; Gilbert, L.E. The dilemma of guinea grass (Megathyrsus maximus): A valued pasture grass and a highly invasive species. Biol. Invasions 2021, 23, 3653–3669. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Elfalleh, W. Forage potential of non-native guinea grass in north african agroecosystems: Genetic, agronomic, and adaptive traits. Agronomy 2021, 11, 1071. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Ahmad, R.; Jonathan, S.G. Synergistic action of rhizospheric fungi with Megathyrsus maximus root speeds up hydrocarbon degradation kinetics in oil polluted soil. Chemosphere 2017, 187, 1–10. [Google Scholar] [CrossRef]
- Olatunji, S.O.; Ximba, J.B.; Fatoki, S.O.; Opeolu, O.B. Assessment of the phytoremediation potential of Panicum maximum (guinea grass) for selected heavy metal removal from contaminated soils. Afr. J. Biotechnol. 2014, 13, 1979–1984. [Google Scholar] [CrossRef]
- Abad, I.M.G.; Capundag, R.O., Jr.; Gamalo, J.C.; Cordulo, P.A.I.; Lacang, G.C. Phytoremediation potential of selected grass species in the mine tailing pond of Sitio Manlauyan, Gango, Libona. J. Biodivers. Environ. Sci. 2017, 11, 321–328. [Google Scholar]
- Khalid, F.E.; Ahmad, S.A.; Zakaria, N.N.; Shaharuddin, N.A.; Sabri, S.; Azmi, A.A.; Khalil, K.A.; Verasoundarapandian, G.; Gomez-Fuentes, C.; Zulkharnain, A. Application of cogon grass (Imperata cylindrica) as biosorbent in diesel-filter system for oil spill removal. Agronomy 2021, 11, 2273. [Google Scholar] [CrossRef]
- ASTM. Sorbent Test Program 1999–2000; SAIC: Ottawa, ON, Canada, 2000; pp. 1–24. [Google Scholar]
- Venkataraghavan, R.; Thiruchelvi, R.; Sharmila, D. Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using plackett-burman design and response surface methodology. Heliyon 2020, 6, e05219. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; Taitai, A. Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef]
- Onwuka, J.C.; Agbaji, E.B.; Ajibola, V.O.; Okibe, F.G. Treatment of crude oil-contaminated water with chemically modified natural fiber. Appl. Water Sci. 2018, 8, 86. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Morphology, structure, and mechanical properties of natural cellulose fiber from mendong grass (Fimbristylis globulosa). J. Nat. Fibers 2014, 11, 333–351. [Google Scholar] [CrossRef]
- Klavins, M.; Porshnov, D. Development of a new peat-based oil sorbent using peat pyrolysis. Environ. Technol. 2013, 34, 1577–1582. [Google Scholar] [CrossRef]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non. Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Cao, S.; Dong, T.; Xu, G.; Wang, F. Study on structure and wetting characteristic of cattail fibers as natural materials for oil sorption. Environ. Technol. 2016, 37, 3193–3199. [Google Scholar] [CrossRef]
- Rengasamy, R.S.; Das, D.; Karan, C.P. Study of oil sorption behavior of filled and structured fiber assemblies made from polypropylene, kapok and milkweed fibers. J. Hazard. Mater. 2011, 186, 526–532. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Wang, F. Oil spill cleanup by structured natural sorbents made from cattail fibers. Ind. Crop. Prod. 2015, 76, 25–33. [Google Scholar] [CrossRef]
- Balogun, A.O.; Adeleke, A.A.; Ikubanni, P.P.; Adegoke, S.O.; Alayat, A.M.; McDonald, A.G. Study on combustion characteristics and thermodynamic parameters of thermal degradation of guinea grass (Megathyrsus maximus) in N2-Pyrolytic and Oxidative Atmospheres. Sustainability 2022, 14, 112. [Google Scholar] [CrossRef]
- Alassod, A.; Gibril, M.; Islam, S.R.; Huang, W.; Xu, G. Polypropylene/lignin blend monoliths used as sorbent in oil spill cleanup. Heliyon 2020, 6, e04591. [Google Scholar] [CrossRef] [PubMed]
- Onwuka, J.C.; Agbaji, E.B.; Ajibola, V.O.; Okibe, F.G. Kinetic studies of surface modification of lignocellulosic Delonix regia pods as sorbent for crude oil spill in water. J. Appl. Res. Technol. 2016, 14, 415–424. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ang, H.M.; Wang, S. Removal of emulsified food and mineral oils from wastewater using surfactant modified barley straw. Bioresour. Technol. 2009, 100, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, L.; Li, X.; Wu, Q.; Liu, J. Effect of temperature on color changes and mechanical properties of poplar/bismuth oxide wood alloy during warm-press forming. J. Wood Sci. 2022, 68, 25. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Van Acker, J.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Rocha, C.G.; Zaia, D.A.M.; da Silva Alfaya, R.V.; da Silva Alfaya, A.A. Use of rice straw as biosorbent for removal of Cu(ii), Zn(ii), Cd(ii) and Hg(ii) ions in industrial effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Zakaria, N.N.; Shaharuddin, N.A.; Khalil, K.A.; Puasa, N.A.; Azmi, A.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Wong, C.Y.; Rahman, M.F.; et al. Coco peat as agriculturalwaste sorbent for sustainable diesel-filter system. Plants 2021, 10, 2468. [Google Scholar] [CrossRef]
- Bazargan, A.; Tan, J.; Hui, C.W.; McKay, G. Utilization of rice husks for the production of oil sorbent materials. Cellulose 2014, 21, 1679–1688. [Google Scholar] [CrossRef]
- Wong, C.; McGowan, T.; Bajwa, S.G.; Bajwa, D.S. Impact of fiber treatment on the oil absorption characteristics of plant fibers. Bioresources 2016, 11, 6452–6463. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. Continuous fixed-bed biosorption process: A review. Chem. Eng. J. Adv. 2021, 8, 100188. [Google Scholar] [CrossRef]
- Xu, Y.; Su, Q.; Shen, H.; Xu, G. Physicochemical and sorption characteristics of poplar seed fiber as a natural oil sorbent. Text. Res. J. 2019, 89, 4186–4194. [Google Scholar] [CrossRef]
- Lim, T.T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic-oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Ngaini, Z.; Nourouzi, M.M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Huang, X.; Lim, T.T. Performance and mechanism of a hydrophobic-oleophilic kapok filter for oil/water separation. Desalination 2006, 190, 295–307. [Google Scholar] [CrossRef]


| Code | Name | Units | Experimental Value | |
|---|---|---|---|---|
| Minimum (−1) | Maximum (+1) | |||
| A | Temperature | (°C) | 100.00 | 120.00 |
| B | Time | min | 5.00 | 30.00 |
| C | Packing density | g/cm3 | 0.10 | 0.26 |
| D | Oil concentration | % (v/v) | 5.00 | 30.00 |
| Code | Variables | Units | Experimental Value | ||||
|---|---|---|---|---|---|---|---|
| (−2) | (−1) | (0) | (+1) | (+2) | |||
| A | Packing density | g/cm3 | 0.088 | 0.10 | 0.13 | 0.16 | 0.172 |
| B | Oil concentration | % (v/v) | −0.178 | 5.00 | 17.50 | 30.00 | 35.178 |
| Run | A | B | C | D | Oil Absorption (mL) |
|---|---|---|---|---|---|
| 1 | 110 | 17.5 | 0.13 | 17.5 | 15.83 |
| 2 | 110 | 17.5 | 0.13 | 17.5 | 19.00 |
| 3 | 120 | 30.0 | 0.16 | 5.0 | 11.67 |
| 4 | 100 | 5.0 | 0.10 | 5.0 | 6.33 |
| 5 | 120 | 5.0 | 0.16 | 30.0 | 22.33 |
| 6 | 120 | 30.0 | 0.10 | 5.0 | 6.50 |
| 7 | 110 | 17.5 | 0.13 | 17.5 | 18.83 |
| 8 | 100 | 30.0 | 0.10 | 30.0 | 14.33 |
| 9 | 100 | 5.0 | 0.16 | 5.0 | 11.00 |
| 10 | 120 | 5.0 | 0.16 | 30.0 | 22.33 |
| 11 | 120 | 30.0 | 0.10 | 30.0 | 14.00 |
| 12 | 100 | 5.0 | 0.10 | 30.0 | 13.33 |
| 13 | 100 | 30.0 | 0.16 | 30.0 | 23.67 |
| 14 | 110 | 17.5 | 0.13 | 17.5 | 15.33 |
| 15 | 100 | 30.0 | 0.16 | 5.0 | 10.83 |
| 16 | 120 | 5.0 | 0.10 | 5.0 | 8.00 |
| 17 | 110 | 17.5 | 0.13 | 17.5 | 19.83 |
| 18 | 110 | 17.5 | 0.13 | 17.5 | 15.83 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 389.98 | 4 | 97.50 | 31.63 | <0.0001 *** |
| A-Temperature | 2.37 | 1 | 2.37 | 0.7690 | 0.3978 |
| B-Time | 0.4537 | 1 | 0.4537 | 0.1472 | 0.7080 |
| C-Packing density | 128.93 | 1 | 128.93 | 41.82 | <0.0001 *** |
| D-Oil concentration | 258.23 | 1 | 258.23 | 83.77 | <0.0001 *** |
| Residual | 36.99 | 12 | 3.08 | ||
| Lack of Fit | 17.29 | 6 | 2.88 | ||
| Pure Error | 19.70 | 6 | 3.28 | ||
| Cor Total | 483.22 | 17 | |||
| Std. Dev. | 1.76 | R2 | 0.9134 | ||
| Mean | 14.94 | Adjusted R2 | 0.8845 | ||
| C.V. | 11.75 | Predicted R2 | 0.8146 | ||
| Adequate Precision | 16.4967 |
| Run | A | D | Oil Absorption (mL) | |
|---|---|---|---|---|
| Experimental Value | Predicted Value | |||
| 1 | 0.088 | 17.50 | 10.67 | 10.43 |
| 2 | 0.10 | 5.00 | 2.33 | 4.71 |
| 3 | 0.10 | 30.00 | 8.50 | 6.21 |
| 4 | 0.13 | −0.18 | 0.00 | 0.00 |
| 5 | 0.13 | 17.50 | 15.17 | 15.03 |
| 6 | 0.16 | 5.00 | 3.83 | 5.29 |
| 7 | 0.13 | 17.50 | 14.00 | 15.03 |
| 8 | 0.13 | 17.50 | 16.00 | 15.03 |
| 9 | 0.17 | 17.50 | 18.67 | 19.74 |
| 10 | 0.13 | 17.50 | 17.00 | 15.03 |
| 11 | 0.16 | 30.00 | 22.00 | 18.79 |
| 12 | 0.13 | 35.18 | 4.00 | 7.72 |
| 13 | 0.13 | 17.50 | 13.00 | 15.03 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 516.90 | 5 | 103.38 | 12.76 | 0.0021 ** |
| A-Packing density | 86.55 | 1 | 86.55 | 10.68 | 0.0137 * |
| B-Oil concentration | 112.43 | 1 | 112.43 | 13.88 | 0.0074 ** |
| AB | 36.00 | 1 | 36.00 | 4.44 | 0.0730 |
| A2 | 0.0043 | 1 | 0.0043 | 0.0005 | 0.9822 |
| B2 | 276.84 | 1 | 276.84 | 34.17 | 0.0006 *** |
| Residual | 56.71 | 7 | 8.10 | ||
| Lack of Fit | 46.69 | 3 | 15.56 | 6.21 | 0.0550 |
| Pure Error | 10.02 | 4 | 2.51 | ||
| Cor Total | 573.61 | 12 | |||
| Std. Dev. | 2.85 | R2 | 0.9011 | ||
| Mean | 11.17 | Adjusted R2 | 0.8305 | ||
| C.V. | 25.49 | Predicted R2 | 0.3939 | ||
| Adequate Precision | 11.6977 |
| Optimised Parameters | Value | Predicted Value | Experimental Value | Efficiency |
|---|---|---|---|---|
| Packing density | 0.14 g/cm3 | 16.64 mL | 22.33 mL | 74.52% |
| Oil concentration | 21.12% (v/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, F.E.; Zakaria, N.N.; Azmi, A.A.; Shaharuddin, N.A.; Sabri, S.; Khalil, K.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Lim, S.; Ahmad, S.A. Guinea Grass (Megathyrsus maximus) Fibres as Sorbent in Diesel Bioremediation. Sustainability 2023, 15, 3904. https://doi.org/10.3390/su15053904
Khalid FE, Zakaria NN, Azmi AA, Shaharuddin NA, Sabri S, Khalil KA, Gomez-Fuentes C, Zulkharnain A, Lim S, Ahmad SA. Guinea Grass (Megathyrsus maximus) Fibres as Sorbent in Diesel Bioremediation. Sustainability. 2023; 15(5):3904. https://doi.org/10.3390/su15053904
Chicago/Turabian StyleKhalid, Farah Eryssa, Nur Nadhirah Zakaria, Alyza Azzura Azmi, Noor Azmi Shaharuddin, Suriana Sabri, Khalilah Abdul Khalil, Claudio Gomez-Fuentes, Azham Zulkharnain, Sooa Lim, and Siti Aqlima Ahmad. 2023. "Guinea Grass (Megathyrsus maximus) Fibres as Sorbent in Diesel Bioremediation" Sustainability 15, no. 5: 3904. https://doi.org/10.3390/su15053904
APA StyleKhalid, F. E., Zakaria, N. N., Azmi, A. A., Shaharuddin, N. A., Sabri, S., Khalil, K. A., Gomez-Fuentes, C., Zulkharnain, A., Lim, S., & Ahmad, S. A. (2023). Guinea Grass (Megathyrsus maximus) Fibres as Sorbent in Diesel Bioremediation. Sustainability, 15(5), 3904. https://doi.org/10.3390/su15053904










