Eco-Friendly Valorization and Utilization of Plant Waste as a Source of Tannin for Leather Tanning
Abstract
1. Introduction
- Optimization of extraction process from barks of selected plants for variables such as solvent, temperature, time and extraction technique to obtain maximum tannin in extract;
- Application of extract on a pickle and wet blue leather to evaluate its suitability as a tanning/re-tanning agent against mimosa: a most widely used vegetable tanning agent in leather industry;
- The suitability and efficacy of tannin extract against mimosa, by performing different physical tests on tanned/re-tanned pickle and wet blue leather.
2. Material and Methods
2.1. Materials
2.2. Instruments and Apparatus
2.3. Treatment of Bark Samples
2.4. Premiliinnaory Extraction of Bark in Aqueous Medium
2.5. Identification Test for Total Phenolic Contents (TPC)
2.5.1. Ferric Chloride Test
2.5.2. Lead Acetate Test
2.5.3. Gelatin Test
2.6. Optimization of Extraction Process
2.6.1. Extraction Solvent
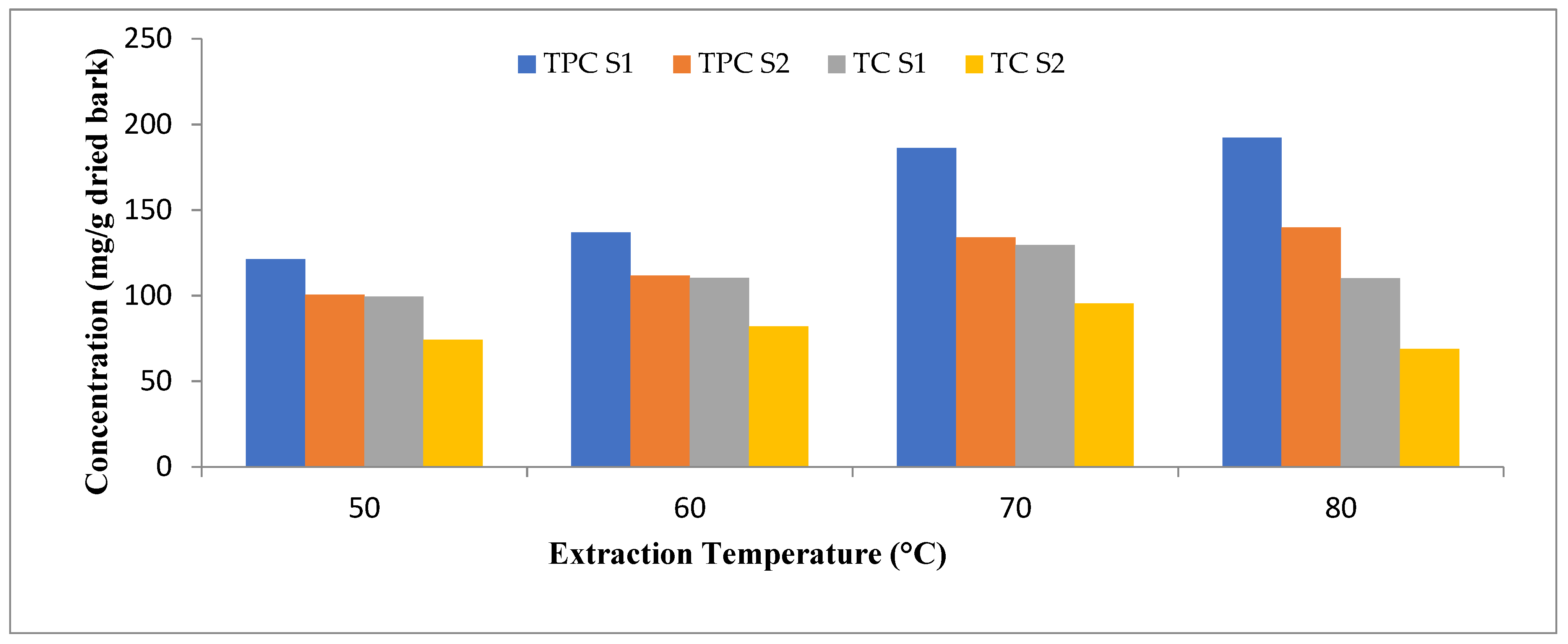
2.6.2. Extraction Temperature
2.6.3. Extraction Time
2.6.4. Extraction Technique
2.6.5. Ultrasonic Extraction at Variable Watts
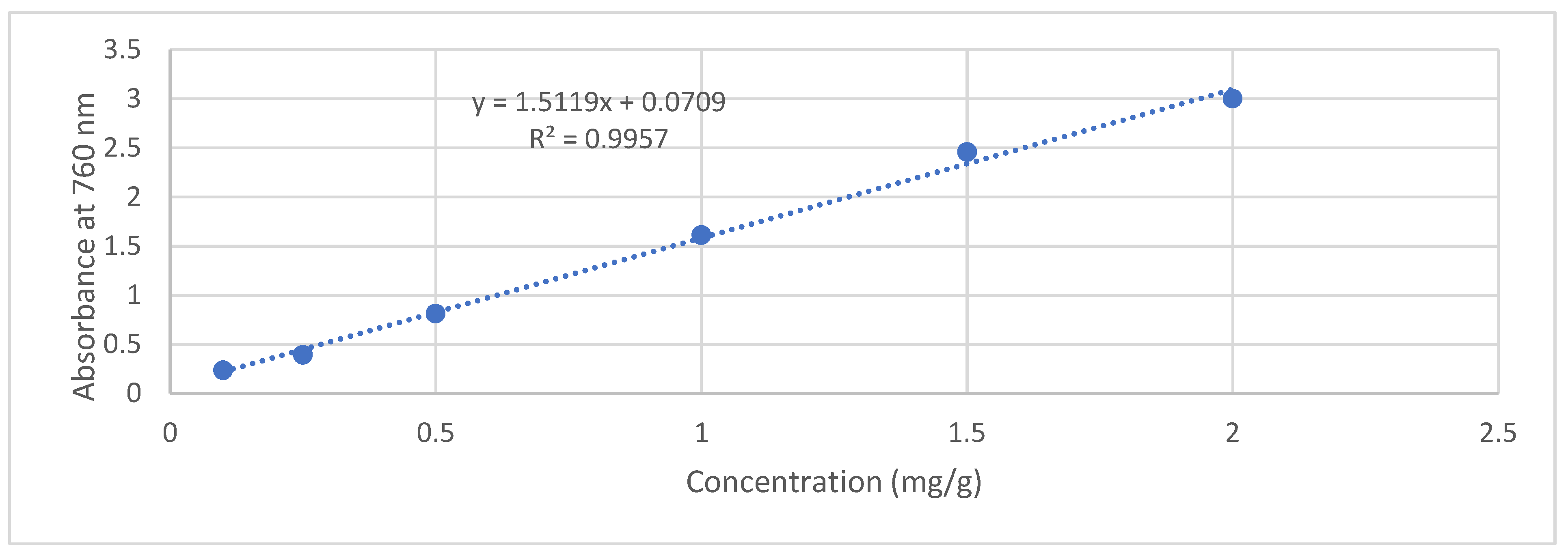
2.7. Total Phenolic Content (TPC) in Extracts
2.8. Tannin Contents (Hide Powder Method)
2.9. FTIR of Dried Extracts
2.10. Application of Extract on Wet Blue and Pickle
2.10.1. Re-Tanning of Wet Blue Leather
2.10.2. Tanning of Pickle Hide
2.10.3. Re-Tanning of Tanned Pickle
2.11. Physical Properties of Re-Tanned Wet Blue and Tanned Pickle Samples
3. Results and Discussions
3.1. Optimization of Extraction Parameters
3.2. Study of FT-IR Spectra and Comparison with Mimosa
3.3. Application of Tannin Extract
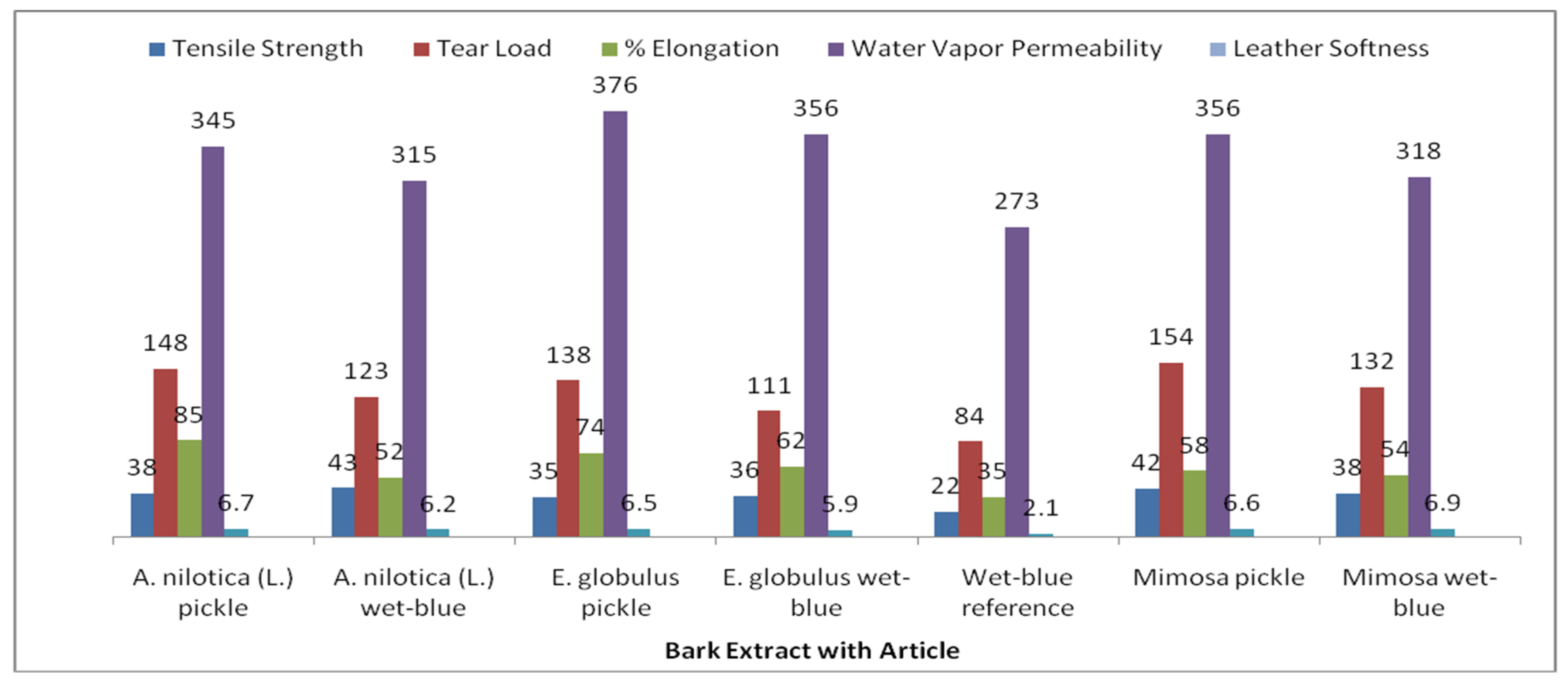
3.4. Evaluation of Properties of Tanned Leather
3.5. Environmental Advantages of Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kautish, P.; Paul, J.; Sharma, R. The moderating influence of environmental consciousness and recycling intentions on green purchase behavior. J. Clean. Prod. 2019, 228, 1425–1436. [Google Scholar] [CrossRef]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable phenolic fractions as basis for furfuryl alcohol-based co-polymers and their use as wood adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Adeel, S.; Anjum, F.; Zuber, M.; Hussaan, M.; Amin, N.; Ozomay, M. Sustainable extraction of colourant from harmal seeds (Peganum harmala) for dyeing of bio-mordanted wool fabric. Sustainability 2022, 14, 12226. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Jackson, J.B.; Bowen, J.; Walker, G.; Labaune, J.; Mourou, G.; Menu, M.; Fukunaga, K. A survey of terahertz applications in cultural heritage conservation science. IEEE Trans. Terahertz Sci. Technol. 2011, 1, 220–231. [Google Scholar] [CrossRef]
- Adeel, S.; Habiba, M.; Kiran, S.; Iqbal, S.; Abrar, S.; Hassan, C.M. Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability 2022, 14, 11758. [Google Scholar] [CrossRef]
- Kohan, L.; Martins, C.R.; Santos, H.N.D.; Fernandes, P.R.B.; Brandao, F.; Baruque-Ramos, J. Brazilian Sustainability Outlook in Footwear Sector. In Leather and Footwear Sustainability: Manufacturing, Supply Chain, and Product Level Issues; Springer: Singapore, 2020; pp. 199–260. [Google Scholar]
- Ocak, B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci. Pollut. Res. 2018, 25, 4643–4655. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Sun, Y.; Xu, J.; Kim, E.J. Zirconium dioxide loaded montmorillonite composites as high-efficient adsorbents for the removal of Cr3+ ions from tanning wastewater. J. Solid State Chem. 2019, 277, 502–509. [Google Scholar]
- Pimentel, P.R.S.; Pellegrini, C.B.; Lanna, D.P.D.; Brant, L.M.S.; Ribeiro, C.V.D.M.; Silva, T.M.; Oliveira, R.L. Effects of Acacia mearnsii extract as a condensed-tannin source on animal performance, carcass yield and meat quality in goats. Anim. Feed Sci. Technol. 2021, 271, 114733. [Google Scholar] [CrossRef]
- Rolence, C. An Eco-Friendly Tanning Method using Plant Barks and Their Combination with Aluminium Sulphate from Kaolin for Leather Industry. Ph.D Thesis, NM-AIST, Arusha, Tanzania, 2021. [Google Scholar]
- Sundar, V.J.; Muralidharan, C.; Mandal, A.B. Eco-benign stabilization of skin protein role of Jatropha curcas oil as a co-tanning agent. Ind. Crops Prod. 2013, 47, 227–231. [Google Scholar] [CrossRef]
- Ramya, K.R.; Sathish, M.; Madhan, B.; Jaisankar, S.N.; Saravanan, P. Effective utilization of tannery hair waste to develop a high-performing re-tanning agent for cleaner leather manufacturing. J. Environ. Manag. 2022, 302, 114029. [Google Scholar] [CrossRef]
- Hansen, É.; Cardoso, J.K.; Gutterres, M.; de Aquim, P.M. Scale-up testing for reducing pollution load of chemicals in wastewater of leather post-tanning. Process Safe. Environ. Prot. 2021, 155, 466–472. [Google Scholar]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant. Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Missio, A.L.; Gatto, D.A.; Tondi, G. Exploring tannin extracts: Introduction to new bio-based materials. Rev. Ciência Madeira 2019, 10, 88–102. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.H.; Sun, K.F.; Chang, C.; Liu, W.F. Effects of covalent interactions and gel characteristics on soy protein-tannic acid conjugates prepared under alkaline conditions. Food Hydrocoll. 2021, 112, 106293. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Liao, J.; Amirou, S.; Abdalla, S. Glutaraldehyde-wheat gluten protein adhesives for wood bonding. J. Adhes. 2021, 97, 88–100. [Google Scholar] [CrossRef]
- Oktay, S.; Kızılcan, N.; Bengü, B. Development of bio-based cornstarch-Mimosa tannin-sugar adhesive for interior particleboard production. Ind. Crops Prod. 2021, 170, 113689. [Google Scholar] [CrossRef]
- Sahakyan, N.; Bartoszek, A.; Jacob, C.; Petrosyan, M.; Trchounian, A. Bioavailability of tannins and other oligomeric polyphenols: A still to be studied phenomenon. Curr. Pharmacol. Rep. 2020, 6, 131–136. [Google Scholar] [CrossRef]
- Zarin, M.A.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef]
- Kilicarislan, C.; Ozgunay, H. Ultrasound extraction of valonea tannin. J. Am. Leather Chem. Assoc. 2013, 108, 63–71. [Google Scholar]
- Grigsby, W.J. Photooxidative stability provided by condensed tannin additives in acrylic-based surface coatings on exterior exposure. J. Coat. Technol. Res. 2018, 15, 1273–1282. [Google Scholar] [CrossRef]
- Onem, E.; Gulumser, G.; Akay, S.; Yesil-Celiktas, O. Optimization of tannin isolation from acorn and application in leather processing. Ind. Crops Prod. 2014, 53, 16–22. [Google Scholar] [CrossRef]
- Win, K.H.; Khaing, T.; Naing, H.H.; Khaing, Y.K. Extraction of Tannin as a Natural Mordant from Tea Leaf for Dyeing of Cotton cloth. Ph.D Thesis, MERAL Portal, Jharkhand, India, 2020. [Google Scholar]
- Abbasian, K.; Asgarpanah, J.; Ziarati, P. Chemical composition profile of A. nilotica seed growing wild in South of Iran. Orient. J. Chem. 2015, 31, 1027–1033. [Google Scholar] [CrossRef]
- Karim, A.A.; Azlan, A. Fruit pod extracts as a source of nutraceuticals and pharmaceuticals. Molecules 2012, 17, 11931–11946. [Google Scholar] [CrossRef]
- Ojha, S.; Raj, A.; Roy, A.; Roy, S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. Leaves and their relation with antioxidant activity. Pharmacogn. J. 2018, 10, 541–547. [Google Scholar] [CrossRef]
- Hossain, M.A.; Disha, N.K.; Shourove, J.H.; Dey, P. Determination of antioxidant activity and total tannin from drumstick (Moringa oleifera lam.) leaves using different solvent extraction methods. Turk. J. Agri. Food Sci. Technol. 2020, 8, 2749–2755. [Google Scholar] [CrossRef]
- Mughal, T.A.; Abid, U.; Saddique, Z.; Nosheen, S.; Pervaiz, S. Qualitative and quantitative evaluation of tannins in bark extracts of some indigenous plants of Pakistan. Int. J. Biosci. 2018, 12, 78–84. [Google Scholar]
- Society of Leather Technologists and Chemists. SLC 117: Determination of Tannin Matter Absorbable by Hide Powder; Society of Leather Technologists and Chemists: London, UK, 2001. [Google Scholar]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef] [PubMed]
- Ibrahi, S.L.; Hassen, A. Effects of graded levels of mimosa (Acacia mearnsii) tannin purified with organic solvents on gas, methane, and in vitro organic matter digestibility of eragrostis curvula Hay. Animals 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- ISO 3380 IULTCS/IUP 16; Leather-Physical and Mechanical Tests-Determination of Shrinkage Temperature Up to 100 °C. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 3376 IULTCS/IUP 6; Leather-Physical and Mechanical Tests-Determination of Tensile Strength and Percentage Elongation. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 21420; Leather-Protective Gloves-General Requirements and Tests. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 17235; Leather-Physical and Mechanical Tests-Determination of Softness. International Organization for Standardization: Geneva, Switzerland, 2015.
- Hazi Naima, M.; Oumama, H.; Hannachea, A.; Sesboue, B.; Charrie, A.; Pizzi, F. Charrier—El Bouhtoury. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Missio, A.L.; Tischer, B.; dos Santos, P.S.; Codevilla, C.; de Menezes, C.R.; Barin, J.S.; Tondi, G. Analytical characterization of purified mimosa (Acacia mearnsii) industrial tannin extract: Single and sequential fractionation. Sep. Purif. Technol. 2017, 186, 218–225. [Google Scholar] [CrossRef]


| Extraction Solvent | Extraction Conditions | Total Phenolic Contents (mg GAE/g Bark) S1 (A. nilotica) | Total Phenolic Contents (mg GAE/g Bark) S2 (E. globulus) | Tannin Contents (Hide Powder Method mg/g) S1 (A. nilotica) | Tannin Contents (Hide Powder Method mg/g) S2 (E. globulus) |
|---|---|---|---|---|---|
| Water (150 mL) | Maceration (150 rpm)-5 g 150 mL solvent-2 h-RT | 91.7 | 58.9 | 66.4 | 40.6 |
| MoH:Water (50:50) | 100.2 | 62.3 | 71.7 | 46.2 | |
| Ac:Water (50:50) | 107.5 | 68.4 | 85.7 | 51.6 | |
| Ac:Water (70:30) | 116.6 | 71.6 | 96.3 | 54.2 |
| Extraction Time | Extraction Conditions | Total Phenolic Contents (mg GAE/g Bark) S1 (A. nilotica) | Total Phenolic Contents (mg GAE/g Bark) S2 (E. globulus) | Tannin Contents (Hide Powder Method mg/g) S1 (A. nilotica) | Tannin Contents (Hide Powder Method mg/g) S2 (E. globulus) |
|---|---|---|---|---|---|
| 2 h | Maceration (shaking water bath Ac:Water(70:30)-5 g bark-150 mL solvent-70 °C | 106 | 53.9 | 69.6 | 52.4 |
| 3 h | 129 | 64.7 | 84.3 | 60.9 | |
| 5 h | 195 | 88.9 | 107.6 | 81.4 | |
| 6 h | 199 | 89.4 | 110.4 | 80.3 |
| Extraction Technique | Extraction Conditions | Total Phenolic Contents (mg GAE/g Bark) S1 (A. nilotica) | Total Phenolic Contents (mg GAE/g Bark) S2 (E. globulus) | Tannin Contents (Hide Powder Method mg/g) S1 (A. nilotica) | Tannin Contents (Hide Powder Method mg/g) S2 (E. globulus) |
|---|---|---|---|---|---|
| Ultrasonic Extraction-100 W | Ac: Water (70:30)-70 °C 5 g bark-150 mL solvent-5 h | 228.6 | 106.4 | 131.4 | 102.3 |
| Ultrasonic Extraction-300 W | 276.7 | 124.9 | 169.4 | 111.9 | |
| Ultrasonic Extraction-400 W | 318.7 | 199.1 | 196.1 | 125.2 | |
| Ultrasonic Extraction-500 W | 298.6 | 170.5 | 181.4 | 102.3 |
| Process | Amount (o.w.m) Leather | Chemicals | Temperature (°C) | Time (min) | Remarks |
|---|---|---|---|---|---|
| Re-tanning of Wet Blue (50 g) | |||||
| Washing | Plenty of water | Water | Room Temperature | 20 min | - |
| Neutralization | 2 50 | Neutralizer N Water | Room Temperature | 2.45 h Rotation | pH 5.5–6.0 drain |
| Re-Tanning | 350 mL | Bark Extract | RT | 4–5 h Rotation | - |
| Soaking | - | Bark Extract | RT | Overnight (Without Rotation) | - |
| Re-Tanning | 30 | Fatliquor, Formic Acid to adjust pH at 3.5 | RT | 45 min, Rotation | Drain |
| Washing | Plenty of water | Water | RT | 20 min, Rotation | Re-tanned Wet Blue |
| Tanning of Pickle (50 g) | |||||
| De-pickle | 250 | NaCl Solution Baume 6–7 | RT | 4 h Rotation | Adjust pH 2.5–3 using Formic Acid |
| 1.5 | Sodium Formate to adjust pH at 22.5–3.0 | ||||
| 4 | Degreasing Agent | ||||
| 0.5 | Sodium Carbonate | ||||
| Washing | Plenty of water | Water | RT | 30 min, Rotation | Drain |
| Tanning | 350 mL | Bark Extract | RT | 4–5 h, Rotation | - |
| Soaking | - | Bark Extract | RT | Overnight (Without Rotation) | - |
| Tanning | - | Adjust pH 4 with Formic Acid | RT | 45 min, Rotation | Drain |
| Re-Tanning | Using Same Steps as used for Re-Tanning of Wet Blue | ||||
| Bark with Article Description | Tensile Strength IUP 6 (N/mm2) | Breaking Force IUP 6 (N) | % Elongation IUP 6 (%Age) | Shrinkage Temperature (°C) |
|---|---|---|---|---|
| A. nilotica pickle | 38 | 148 | 85 | 80 °C |
| A. nilotica wet blue | 43 | 123 | 52 | >100 °C |
| E. globulus pickle | 35 | 138 | 74 | 75 °C |
| E. globulus wet blue | 36 | 111 | 62 | >100 °C |
| Wet blue reference | 22 | 84 | 35 | >100 °C |
| Mimosa pickle | 42 | 154 | 58 | 76 °C |
| Mimosa wet blue | 38 | 132 | 54 | >100 °C |
| No. | Test | A. nilotica (S1) | E. globulus (S2) | Total Phenolic Content (TPC) Presence |
|---|---|---|---|---|
| 1 | Ferric Chloride test | +++ | +++ | Positive |
| 2 | Lead Acetate test | +++ | +++ | Positive |
| 3 | Gelatin test | +++ | +++ | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.R.; Khan, S.M.; Khan, R.U. Eco-Friendly Valorization and Utilization of Plant Waste as a Source of Tannin for Leather Tanning. Sustainability 2023, 15, 3884. https://doi.org/10.3390/su15053884
Khan SR, Khan SM, Khan RU. Eco-Friendly Valorization and Utilization of Plant Waste as a Source of Tannin for Leather Tanning. Sustainability. 2023; 15(5):3884. https://doi.org/10.3390/su15053884
Chicago/Turabian StyleKhan, Shahid Rehman, Shahzad Maqsood Khan, and Rafi Ullah Khan. 2023. "Eco-Friendly Valorization and Utilization of Plant Waste as a Source of Tannin for Leather Tanning" Sustainability 15, no. 5: 3884. https://doi.org/10.3390/su15053884
APA StyleKhan, S. R., Khan, S. M., & Khan, R. U. (2023). Eco-Friendly Valorization and Utilization of Plant Waste as a Source of Tannin for Leather Tanning. Sustainability, 15(5), 3884. https://doi.org/10.3390/su15053884




