Abstract
Several kinds of food can be analyzed by the human sensory organs. In this review, we demonstrate the relation and importance of the color and bioactive molecules of food and their health effects. This work focuses on black foods, which can be found in both natural and processed forms, present in our daily life for several years without being noticed. Besides, the chemistry underlying the black color of black foods has not yet been fully understood. More than 130 black foods are reported in the current review, which belong to 3 main groups and 12 sub-groups. In studied black foods, melanins and anthocyanins are the primary pigments, along with other pigments such as chlorophylls, carotenoids, and tannins. The health potential of black foods is also discussed. Due to their high concentration of phytochemical and phenolic compounds, black-colored foods are beneficial in preventing diseases and boosting the immune system. As a promising natural pigment and antioxidant compound source, black foods could be used as functional foods. Several questions on black foods are still open and need more investigation, especially the mechanisms by which the black color is formed in fruits and vegetables.
1. Introduction
Agri-foods are the foods in different colors that can produce from the animal and plant farming. The color of foods has a massive effect on the food choice of customers by affecting taste thresholds, sweetness perception, food preference, pleasantness, and acceptability [1]. Foods with a natural black color are one of the foods that should be included in the list, with high pigment content and a unique, attractive color, given the fact that natural black color is present in numerous types of foods from fruits, vegetables, and sea-foods to spices and even processed foods. For most of these pigments, structural formulations have been studied; melanins are major phenolic compounds that give black food its color. Melanin is a common biological pigment that can be found in skin, hair, and eyes. It is present in many different biological systems including plants, insects, seafood, and even bird feathers [2]. In plants, melanin is a high molecular weight pigment producing black color through the processes of the oxidation and polymerization of phenols [3]. Several black-colored foods are found with melanin content, such as black garlic and sepia ink [4]; black chicken [5]; black sesame seeds [6]; black oat [7]; or black tea [8].
The black color does not only come from melanins but also anthocyanins. As water-soluble pigments, anthocyanins can form colors ranging from red to purple to black [9]. A high number of anthocyanins can form black color in black fruits and flowers [10]. The concentration of anthocyanins in blackcurrants is up to four-fold greater than other common fruits [11]. Up to now, there have been almost 600 anthocyanins reported in fruits, vegetables, and plants. Anthocyanins in foods are formed from six main anthocyanidins including cyanidin, delphinidin, peonidin, pelargonidin, petunidin, and malvidin [10,12,13]. Cyanidin-based anthocyanins are reported to cause black color in plants by high accumulation [14]. Furthermore, cyanidin-3-glucoside is the most abundant pigment in black foods such as black rice [15], black bean [16], blackberry [17], and black carrot [18,19]. Besides the cultivars, the presence of black anthocyanins is described as a natural defense of the plants in harsh environments by increasing internal temperature and attracting more pollinators [10]. Furthermore, the Maillard reaction and caramelization are involved in the black formation of processed foods and restaurant foods such as black garlic [20], black coffee [21], black sugar [22], or the famous soft drink Coca Cola [23].
Besides color, black-colored foods bring actual benefits to human health [24]. Black foods are the potential sources of phytochemicals that can have a remarkable impact in various major metabolic syndromes including cardiovascular disease, diabetes, obesity, cancer, osteoporosis, and menopausal conditions [25]. Moreover, black foods contain a high number of anthocyanins with high antioxidant properties, which bring anti-inflammatory, vasoprotective, antineoplastic, radiation-protective, chemo- and hepatoprotective benefits [26,27,28]. In some cases, black foods frequently have higher levels of antioxidant activity than those of other colors in the same species, which can apply in food, pharmaceuticals, and the cosmetic industries. In terms of eggplants, black and purple cultivars are reported with greater antioxidant properties and total phenolic contents compared with white cultivars [29]. In Europe and North America, black eggplants are the most important cultivar in the eggplant group, and due to the good effects of black eggplant in terms of hypolipidemic and anti-angiogenic activities, the interest in developing black-colored eggplants is rising day by day [30]. With the abundant sources of phenolic compounds, black-pigmented and black foods hold potential as functional foods and food colorants. Black-colored foods are used as functional foods in several countries. In China, black sesame seeds and mulberries were approved as functional foods due to their health potential [31]. The color extracted from black-colored foods has been used in several dishes over the world. In Asian countries, black rice has been used as a food colorant in bread, ice cream, yogurts, and several other food products. For example, Thai pork sausage is made by using black rice bran as a food colorant [32].
However, the chemistry underlying the black color of black foods has not yet been fully understood. This review paper presents comprehensive knowledge of black foods with real pictures as well as their benefits for human health. It also discusses the chemistry of the black hue in black foods and its potential in various aspects of life.
2. Methodology of This Review and Its Justification
Is the color of food an important indicator for evaluating any agricultural product? Can the science depend on the human senses in the recognition of positive and negative attributes in agro-products instead of laboratory devices? If yes, to what extent is this approach a crucial tool? At first glance, many human senses can be used successfully in the field of food processing, such as smell (olfaction), taste (gustation), and sight or the visual analysis of color. Therefore, research should depend on an integrated approach (human senses and laboratory testing) for determining the quality chain “from field to fork” of various agri-products. Regarding black color, it is a color if we define color not as a selective absorption, but the complete absorption of visible light. Something is black if it absorbs the visible light in the whole range of the wavelength (400–800 nm). Foods communicate with us with their color, smell, texture, and taste. Researchers understand that colors send information about the structure, function, and reactivity of molecules. Therefore, it is very important to understand the relationship between colors, molecular structure, and functions. Life and chemistry are written in the language of electrons, and molecules send messages to us through electromagnetic waves that we should understand. In this review, we explain the connection between the active ingredients of black foods and their health effects. Free radicals are molecules or atoms that violently and at all costs want to acquire electrons. These are taken away from any molecule, and if it is a biologically important molecule, its function is impaired. Antioxidants are compounds that easily and willingly transfer their electrons. They protect important molecules. Antioxidants donate their electrons easily, so their outer electrons can be easily excited. This means that they can absorb lower-energy electromagnetic radiation, such as visible light. Therefore, a significant part of an antioxidant has visible color. Colors indicate the presence of antioxidants in foods. Red fruits absorb green light; orange fruits absorb blue. Black foods absorb at all wavelengths, i.e., they contain many types of very effective antioxidants, and electron donors.
This review attempts to concentrate on listing black foods at present and pigments creating black colors. The database and materials were collected from the most widely used scientific websites including the databases of MDPI, PubMed, SpringerLink, and ScienceDirect, and the specific databases of the European Commission and United States Department of Agriculture (USDA). The information on black foods was collected, analyzed, and listed in tables. All possible black foods were collected and pictures were taken at high resolution, as shown in the figures. The overall knowledge on pigment formation and the health benefits of black foods was elaborated from previous studies with a focus on recent publications.
3. Definition of Black Foods
For a definition of black foods, black color should be talked about first. As the definition of color in physics, black is on the visible spectrum of color because it does not reflect light [33]. Black is formed from two seemingly incompatible but complementary states of the description of black: (1) black results from the absence of visible light; (2) black forms by the complete absorption of visible light. Furthermore, the mixture of three primary pigments at appropriate proportions can result in “black” due to the limited reflection of light. However, it is rare to have complete black in nature. In September 2019, MIT engineers made the darkest material from vertically aligned carbon nanotubes, which was reported to have a 99.995% absorption rate of any incoming light [34].
4. Classification of Black Foods
Black foods can be found anywhere, from berries to meat and even fungi so their presence takes a significant role in biological systems [2]. After screening several types of foods, around one-hundred and fifty foods are listed in Table 1 and Table 2. In terms of the origin of black color, black foods are classified into three main types: (1) natural black food with a black color from origin, (2) black processed foods that have a black color due to processing, and (3) black dishes (gastronomy) that obtain a black color from cooking. This classification is illustrated in Figure 1.
Information in this table was collected from varied sources including of European Commission [35], Food Data Central of United States Department of Agriculture (USDA) [36].

Table 1.
List of natural black foods.
Table 1.
List of natural black foods.
| Groups | Family | Scientific Name | Common Name |
|---|---|---|---|
| Fruits | Arecaceae | Euterpe edulis Martius L. | Juçara fruit |
| Arecaceae | Euterpe oleracea (L.) Mart | Acai berry | |
| Burseraceae | Canarium tramdenum Dai Yakoyl L. | Chinese black olive | |
| Ericaceae | Vaccinium fuscatum L. | Black highbush blueberry | |
| Ebenaceae | Diospyros nigra L. | Black sapote | |
| Fabaceae | Dialium guineense L. | Black velvet tamarind | |
| Grossulariaceae | Ribes nigrum L. | Black currants | |
| Grossulariaceae | Ribes lacustre L. | Black gooseberry | |
| Heaths | Vaccinium macrocarpon ’Early black’ L. | Cranberry ’Early black’ | |
| Moraceae | Morus nigra L. | Mulberries | |
| Moschatel | Sambucus L. | Black elderberry | |
| Myrtaceae | Syzygium cumini (L.) Skeels | Black plums/jamelão | |
| Oleaceae | Olea europaea L. | Black olives | |
| Punicaceae | Punica Granatum L. | Black pomegranate | |
| Rosaceae | Aristotelia chilensis L. | Maqui berry | |
| Rosaceae | Aronia melanocarpa L. | Black chokeberry | |
| Rosaceae | Rubus allegheniensis L. | Blackberries | |
| Rosaceae | Rubus occidentalis L. | Black raspberry | |
| Rosaceae | Prunus serotina L. | Black cherry | |
| Rose | Pyrus communis ’Black Worcester’ L. | Black Worcester pear | |
| Solanaceae | Lycium ruthenicum (L.) Murr. | Black Goji berries/wolfberry | |
| Vitaceae | Othello L. | Black grape | |
| Vitaceae | Vitis vinifera L. | Black grape | |
| Cereal grains and legumes | Buckwheats | Fagopyrum esculentum Moench L. | Black buckwheat |
| Poaceae | Oryza sativa L. | Black rice | |
| Poaceae | Sorghum bicolor L. | Black sorghum | |
| Poaceae | Avena strigosa (L.) Schreb. | Black oat | |
| Poaceae | Triticum Aestivum L. | Black wheat | |
| Poaceae | Zea mays L. | Black maize kernels | |
| Leguminosae | Lens culinaris (L.) Medik. | Black lentil | |
| Legumes | Glycine max (L.) Merr. | Black soybean | |
| Legumes | Cicer arietinum L. | Black chickpeas | |
| Legumes | Lathyrus niger L. | Black pea | |
| Legumes | Arachis hypogaea L. | Black peanut | |
| Fabaceae | Macrotyloma uniflorum L. | Black horse gram | |
| Fabaceae | Phaseolus vulgaris L. | Black bean | |
| Fabaceae | Phaseolus vulgaris L. | Black turtle bean | |
| Vegetables | Apiaceae | Daucus carota L. | Black carrot |
| Brassicaceae | Raphanus sativus (L.) var niger | Black radish | |
| Lauracea | Persea americana L. | Haas avocado skin | |
| Sargassaceae | Sargassum fusiforme L. | Hijiki seaweed | |
| Solanaceae | Solanum melongena L. | Black eggplant | |
| Solanaceae | Solanum lycopersicum L. | Sun black tomato | |
| Solanaceae | Petunia L. | Black Mamba Petunia/Crazytunia | |
| Solanaceae | Capsicum Annuum L. | Black Hungarian peppers | |
| Brassicaceae | Brassica oleracea L. | Black cabbage | |
| Daisy | Lactuca sativa L. | Lettuce ’Black Seeded Simpson’ | |
| Nightshade | Solanum tuberosum L. | Shetland black potato | |
| Bangiaceae | Pyropia abbottiae L. | Black seaweed | |
| Nut and seeds | Malvaceae | Theobroma cacao L. | Black Cocoa powder |
| Rubiaceae | Coffea L. | Coffee beans | |
| Asteraceae | Helianthus annuus L. | Black sunflower seeds | |
| Pedaliaceae | Sesamum indicum L. | Black sesame seeds | |
| Poppies | Papaver somniferum L. | Black opium poppy seeds | |
| Mints | Ocimum aspberry L. | Hoary basil seed | |
| Lamiaceae | Salvia hispanica L. | Black chia seeds | |
| Amaranthaceae | Chenopodium quinoa (L.) Willd. | Black quinoa | |
| Juglandaceae | Juglans nigra L. | Black walnuts | |
| Herb and Spices | Theaceae | Camellia sinensis L. | Black tea |
| Orchidaceae | Vanilla planifolia L. | Dried vanilla beans | |
| Piperaceae | Piper nigrum L. | Black pepper | |
| Ranunculaceae | Nigella sativa L. | Black cumin | |
| Ginger | Curcuma caesia L. | Black turmeric | |
| Ginger | Kaempferia parviflora L. | Black ginger | |
| Brassicaceae | Brassica nigra L. | Black mustard seeds | |
| - | Himalaya black salt | ||
| - | Activated charcoal | ||
| Meat and Seafoods | - | - | Bear black meat (Alaska Native foods) |
| Anoplopomatidae | Anoplopoma fimbria L. | Black cod | |
| Mytilidae | Choromytilus meridionalis L. | Black mussel | |
| Nephropidae. | Homarus Gammarus L. | Black lobster | |
| Ophichthidae | Callechelys catostoma L. | Black-striped snake eel | |
| Sepiidae | Sepia officinalis L. | Sepia ink | |
| Sepiolidae | Heteroteuthis dispar L. | Squid ink/cephalopod ink | |
| Plotosidae | Siluriformes L. | Catfish | |
| Portunidae | Scylla serrata L. | Black crab | |
| Amiidae | Amia calva L. | Bowfin black caviar | |
| Amphiumidae | Amphiuma means L. | Two-toed amphiuma/conger eel | |
| Amphiumidae | Amphiuma pholeter L. | One-toed amphiuma/conger eel | |
| Stichopodidae | Apostichopus japonicas L. | Sea cucumber | |
| Phasianidae | Ayam cemani L. | Black chicken | |
| Fungi | Auriculariaceae | Auricularia heimuer L. | Black wood ear mushroom |
| Cantharellaceae | Craterellus cornucopioides Cantharellales L. | Black trumpet mushroom | |
| Hymenochaetace | Ienonotus obliquus L. | Chaga mushroom | |
| Morchellaceae | Morchella elata L. | Black morel mushroom | |
| Pleurotaceae | Pleurotus ostreatus L. | Black oyster mushroom | |
| Polyporaceae | Fomes fomentarius L. | Tinder Fungus | |
| Psathyrellaceae | Coprinopsis atramentaria L. | Common ink cap mushroom | |
| Tuberaceae | Tuber melanosporum L. | Black truffle | |
| Xylariaceae | Xylaria polymorpha L. | Dead man’s fingers mushroom |

Table 2.
List of black processed foods and black dishes (gastronomy) with some production companies.
Table 2.
List of black processed foods and black dishes (gastronomy) with some production companies.
| Name | Description | Production Company/Origin | Ref. |
|---|---|---|---|
| Fermented Products | |||
| Black tea | Black tea is fully fermented green or white tea, which is consumed throughout the world with unique taste and flavor |
| [37] |
| Fermented Black garlic | Black garlic is made by fermenting fresh garlic for a certain time at high temperatures and humidity |
| [38] |
| Soy sauce | Soy sauce is a Japanese sauce, which is fermented from soybeans |
| [39] |
| Changsha stinky tofu | Changsha stinky tofu is made from fermented brine with bean curd |
| [40] |
| Black natto | Black natto is Japanese fermented black bean |
| [41] |
| Rice black vinegar | Black rice vinegar (kurozu) is traditional Japanese vinegar made from black rice |
| [42] |
| Century Eggs | Century Eggs or black eggs originated from China, created through an alkaline fermentation process with salt, ash, and lime |
| [43] |
| Black bean sauce (Douchi/Tochi) | Black bean sauce is a sauce made by fermenting salted black soybean, which is most popular in Chinese cuisine |
| - |
| Hoisin sauce (Tương Đen) | A Vietnamese traditional sauce made by mixing fermented black soybean with soy sauce, which is often used with Phở (Vietnamese national soup) |
| - |
| Beverages | |||
| Black Russian | The Black Russian is a cocktail of vodka and coffee liqueur |
| - |
| Coke | Coke is the most famous carbonated soft drink with a black color, which is produced by The Coca-Cola company with a secret recipe |
| - |
| Confectionery | |||
| Salty licorice | Salty licorice is a type of licorice made from the root extract of Glycyrrhiza glabra, adding ammonium chloride |
| - |
| Chinese mesona | Chinese mesona can turn into black jelly when cooking, which is the main ingredient of black grass jelly |
| [44] |
| Rice cakes with Ramie leaves | Ramie leaves are used to make rice cake, a traditional Vietnamese cake with the black color |
| [45] |
| Traditional Russian Black Bread | The most traditional Russian Black Bread is made from rye flour, sourdough starter, salt, and water |
| - |
| Black Forest cake | Black Forest cake is a German cherry-filled chocolate sponge cake with a rich cherry filling based on the German dessert Schwarzwälder Kirschtorte, literally "Black Forest Cherry-torte" |
| - |
| Black jelly eggs | A type of candy having a black color made from artificial colors including (red 40, blue 1, yellow 6, yellow 5) |
| - |
| Food ingredients | |||
| Black seed oil | Black seed oil is Indian traditional oil, made from Nigella sativa L. (black cumin) |
| [46] |
| Black garlic mayo | Black garlic mayo is a new product of the Heinz company, which was released on Halloween |
| - |
| Black sugar | Black sugar is unrefined sugar made by caramelizing sugarcane juice |
| [47] |
| Black truffle oil | Black truffle oil is mixed from extra virgin olive oil with truffle extracts forming a unique flavor with a black color |
| - |
| Black hot sauce | An extremely hot sauce made by mixing black pepper and soy sauce |
| - |
| Functional foods | |||
| Black currant seed oil | Black currant seed oil extracted from seed of black currant Ribes nigrum L. |
| - |
| Others | |||
| Black banana | - |
| - |
| Black apple | - |
| - |
| Aged black garlic | Aged black garlic made by the heat treatment of black garlic for a certain time and at an appropriate humidity |
| [48] |
| Black dishes | |||
| Black pudding | Black pudding is blood sausage made from pork or beef blood |
| [49] |
| Morcela de arroz | Morcela de arroz is a traditional Portuguese and Brazilian blood sausage made with rice |
| [50] |
| Jajangmyeon | Jajangmyeon is a type of Korean Chinese noodle made with Jajang sauce (a salty black soybean paste) |
| [51] |
| Black squid ink pasta | Black squid ink pasta is a traditional Italian dish, made by adding squid ink into pasta dough |
| - |
| Black bean soup | Black bean soup is made by cooking rice with black beans | - | - |
| Sweet black sesame soup | Sweet black sesame soup is made by cooking black sesame paste with sugar and rice. It is often served hot |
| - |
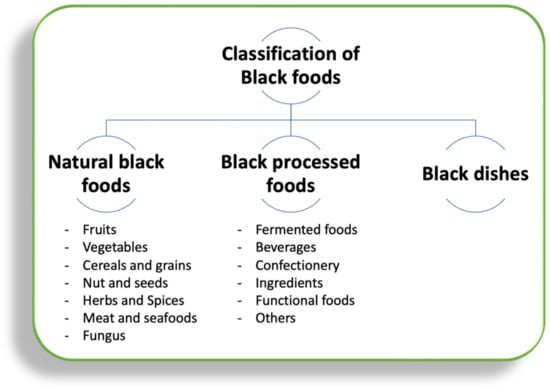
Figure 1.
The overall classification of existing black foods, based on the classification of the European Commission [35], Food Data Central of United States Department of Agriculture (USDA) [36], with appropriate modifications.
4.1. Natural Black Foods
With the definition of black foods, there are over 90 foods screened having a black color from their origins, divided into seven main groups of foods including fruits, cereals and grains, vegetables, nut and seeds, herb and spices, meat and seafood, and fungi (Table 1). Black pigments are present in nature due to several reasons, which vary from animals to plants. Black pigments synthesized in fruits and flowers aim to attract the pollinator in winter when snow covers everything. This could be the main reason why most black fruits are found in berry groups that often grow in cold temperatures. Black fruit is the largest group with 21 commodities, which are listed in Table 1, mostly belonging to blackberries, including 13 families. Five representative images of black fruits are shown in Figure 2. Rosaceae, a rose family, is the largest family with five kinds of fruits including the maqui berry (Aristotelia chilensis L), black chokeberry (Aronia melanocarpa L.), blackberry (Rubus allegheniensis L.), black raspberry (Rubus occidentalis L.), and black cherry (Prunus serotina). However, there are some fruits with a black color that grow in warm temperatures, such as the black velvet tamarind (Dialium guineense L.), a native plant in Africa, or the Chinese black olive (Canarium tramdenum Dai Yakovl L.) found in Indochina. In some cases, fruits that are not fully black are still considered black fruits, such as the black sapote with green peel but full of black pulp as well as the Chinese black olive with a hard black skin and brown-black pulp. Referring to Nath et al. [52], a black color in fruits is a sign of maturity and results from the fruit ripening process. The biochemical and molecular changes that occur in the fruit during this time, including changes in hormone levels, gene expression, and metabolic pathways, can result in the accumulation of pigments, including the development of a black color [53]. However, research on the mechanism of black color formation in fruits is still limited by variations among fruit species and the influence of multiple factors from both the environment and genetics.
Contrarily, black formation in leaves and seeds is one way they protect themselves from animals and insects [54]. Black cereal is the second largest group of black foods, with 20 items (Table 1). The Poaceae and Legume families are the two main groups of this kind. The former consists of five commodities including black rice (Oryza sativa L.), black sorghum (Sorghum bicolor L.), black oat (Avena strigosa (L.) Schreb), black wheat (Triticum Aestivum L.), and black maize kernels (Zea mays L.) while the latter includes black soybean (Glycine max (L.) Merr.), black chickpea (Cicer arietinum), black pea (Lathyrus niger), and black peanut (Arachis hypogaea). Some black cereals are the result of breeding from other colored varieties. For example, black wheat is a hybrid between purple and blue wheat [55]. Black cereals with pictures are illustrated in Figure 3. Besides, several herbs and spices can be found with a black color, which are listed in Table 1 and illustrated in Figure 4. Several black herbs and spices are well-known food ingredients that have been used worldwide for decades, such as vanilla (Vanilla planifolia L.) or black cocoa powder (Theobroma cacao L.), black pepper (Piper nigrum L.), coffee (Coffea L.), and black tea (Camellia sinensis L.). However, black mustard seeds (Brassica nigra) are traditional spices in India. Kala Namak (Himalayan black salt) is a rock salt, mined from the Himalayan ranges in the India–Asia collision [56]. In addition, there are two black spices found in the ginger family including black turmeric (Curcuma caesia) and black ginger (Kaempferia parviflora).
In vegetables, 13 food items were found in 10 different families (Table 1). Black vegetables with pictures are illustrated in Figure 5. Many roots own a black hue, such as the black carrot (Daucus carota L.), black radish (Raphanus sativus (L.) var niger), or Shetland black potato (Solanum tuberosum). The Solanaceae family contains the largest number of black vegetables, including the black eggplant (Solanum melongena L.), sun black tomato (Solanum lycopersicum L.), black velvet petunia (Petunia), and black Hungarian peppers (Capsicum Annuum L.). The process of black coloration in black animals is complex, mostly due to genes. However, it can be completely different in marine animals and mammals. Several kinds of black seafood are listed in Table 1, including black lobster (Homarus Gammarus L.); black crab (Scylla serrata L.); black-striped snake eel (Callechelys catostoma L); two-toed amphiuma/conger eel (Amphiuma means L.); one-toed amphiuma/conger eel (Amphiuma pholeter L.); black cod; and ink from Sepia officinalis L. and Heteroteuthis dispar L. Black chicken (Ayam cemani L.) is the only animal listed as a black food, due to its blackness from skin to bond [5]. Fungi are a large group of species that range in color from red and orange to even black. Black pigments from fungi have been extracted to use in textile industries for many years. Nine edible black mushrooms are found in the list in Table 1. Black fungi with pictures are illustrated in Figure 6. While the black wood ear mushroom (Auricularia heimuer L.) is widely found and used in Asia, the black trumpet mushroom (Craterellus cornucopioides Cantharellales L.) and common ink cap mushroom (Coprinopsis atramentaria L.) are found in Europe and North America. As a functional food, the Chaga mushroom (Inonotus obliquus) is used as a traditional medicine in Siberia to treat various gastrointestinal diseases [57]. Black truffle (Tuber melanosporum L.) is the most famous expensive mushroom due to its unique flavor caused by its lipophilic volatile organism [58].
Recently, the potential of black food has been increasingly recognized through the introduction of many new varieties of black crops to meet the needs of customers around the world. More than 200 black rice varieties are recognized worldwide [59]. Four main varieties of black eggplant, Black Beauty’, ‘Black Bell’, ‘Florida Market’, and ‘Long Black’, have been developed [30]. Furthermore, black foods are reported to have greater sources of minerals and dietary fiber than foods with other colors. Black rice has minerals (Ca, P, Fe, and Zn) and dietary fiber, which are higher than brown and white rice [60]. In some cases, black foods are reported to contain a stronger flavor and aroma than the other-colored foods. In comparison with white sesame seeds, black sesame seeds are reported with stronger flavors and aromas due to the higher contents of fatty acids, proteins, and lignans [61]. Additionally, black foods are an abundant source of bioactive compounds. For instance, black cherries are reported to be a greater source of phenolic compounds (7017 mg/kg FW) compared to European bird cherries (124.14 mg/kg FW) while black cherries have been detected with lower amounts of cyanogenic glycosides—toxic compounds—than wild cherries [62]. Another study of Liu et al. [63] compares the phenolic compounds between three quinoa seed varieties (white, red, and black Chenopodium quinoa seeds). Black quinoa seed was detected with the highest total phenolic contents compared to red and white quinoa seeds [63].
In conclusion, 90 black foods found in nature have been divided into seven groups, from fruit to cereals to aquatic animals. The recent recognition of the potential of natural black foods has led to the introduction of many new black crop varieties to meet global customer demand. Black foods have been found to contain high levels of minerals and dietary fiber, as well as a distinct flavor and aroma and high concentrations of bioactive compounds, such as phenolic compounds. These findings highlight the potential health benefits of consuming black foods and the importance of further research in this area.
4.2. Black Processed Foods
Black color formation does not only come from natural biological processes but is also formed from food processing. A list of black processed foods is presented in Table 2 along with many black dishes (gastronomy) that are made from black foods all over the world. The heat-treatment process can affect the development of black color. After aging at a certain temperature and humidity, black garlic is produced owning a unique black color and unique flavors [38]. In another example, Chinese mesona (Mesona chinensis L.) is green at first, but it turns black when making jelly. Chinese mesona is the main ingredient of black grass jelly, a famous dessert in Asia [44]. The special heat treatment of glucose, called caramelization, can also form a black color in foods. For instance, black sugar is unrefined sugar produced from caramelizing sugarcane juice [47]. Furthermore, fermentation can also involve creating a black color and developing taste and smell. For example, a century egg was made through alkaline fermentation with salt, ash, and lime [43] with the real picture in Figure 7. In the case of black tea, fermentation forms its unique black color [37] and generates polyphenolic compounds such as theaflavins (TF), thearubigins (TR), and catechins in comparison with others tea, providing potential health benefits when consumed [64]. Soy sauce is made as a product of the fermentation process of soybean, which creates the natural black-brown color of soy sauce [39] and evokes a unique flavor profile with umami as a dominant taste [65]. Black processed foods can be created from black ingredients such as black seed oil from black cumin (Nigella sativa L.) [46], or rice black vinegar from black rice [42].
In conclusion, black processed food can be made using different types of processes, including fermentation, heat treatment, and the addition of black ingredients. Although black processed foods have a unique flavor and distinctive color, the number of products available is still limited. Therefore, optimizing production processes and diversifying products should be the focus of future studies. Additionally, further research is needed to explore both the positive and negative effects of these foods on human health, as well as the generation of flavor.
4.3. Black Dishes (Gastronomy)
Moreover, black dishes can be cooked from black materials including black natto from black soybean [41]; Jajangmyeon from black soybean paste [51]; black squid ink pasta from squid ink; or many different commercial products with black color due to adding activated charcoal. Black pudding and Morcela de arroz are special dishes cooked with pork or beef blood, which can turn a black color when well-cooked [50,66]. Blood is a rich source of iron and proteins and is often used as a high-protein ingredient in many traditional dishes [67]. Microorganisms are involved in the process of making Changsha stinky tofu, causing the black color [40]. Some black foods are made from the extracts of plant parts, for example, rice cakes with Ramie leaves created from the extracts of Boehmeria Nivea L. leaves with rice [45], or black licorice and salty licorice made from the extracts of roots of the licorice plant Glycyrrhiza glabra. The color extracted from black-colored foods has been used in several dishes over the world. Currently, squid ink (black color) is used to make black pasta, which is famous in Italian cuisine. In Asian countries such as Vietnam, Indonesia, and China, black rice has been used as a food colorant in bread, ice cream, and several other food products [68]. Black rice is also used to make yogurts [69], and Thai pork sausage [32]. Black sweet soup in Chinese culture is made from black sesame seeds. In 2022, black garlic was used to make black mayo in the Halloween campaign of the Kraft Heinz Foods Company (Pennsylvania, United States). Moreover, the fruit peel powder of Jamelão (Syzygium cumini (L.) Skeels) is studied for use as a natural colorant [70].

Figure 2.
Representative images showing black fruits: (A) black plums, (B) black grape, (C) Chinese black olive, (D) black blueberry. Photos by Duyen H.H. Nguyen.

Figure 3.
Representative images showing black cereals: (A) black horse gram, (B) black bean, (C) black chia seeds, (D) black sunflower seeds, (E) black rice, and (F) hoary basil seeds. Photos by Duyen H.H. Nguyen.

Figure 4.
Representative images showing black herbs and spices: (A) black coffee, (B) black mustard seeds, (C) Himalayan black salt, (D) black cumin, (E) black tea, (F) black pepper. Photos by Duyen H.H. Nguyen.

Figure 5.
Representative images showing black vegetables: (A) black radish, (B) black seaweed, (C) black eggplant, and (D) Chinese mesona. Photos by Duyen H.H. Nguyen.

Figure 6.
Representative images showing black fungi: (A) black trumpet mushroom and (B) black wood ear mushroom. Photos by Duyen H.H. Nguyen.

Figure 7.
Representative images showing black processed foods: (A) century eggs, (B) black garlic, (C) black banana, (D) Hoisin sauce (Tương Đen), (E) dark chocolate, (F) black sugar, (G) black pudding, and (H) black apple. Photos by Duyen H.H. Nguyen.
5. Chemical Perspectives on Pigments of Black Foods
Due to the complexity of natural pigments, black color from many foods is still not clearly defined yet. Black color can be formed from one or the combination of many pigments, which is illustrated in Figure 8. Table 3 lists the reported pigments forming black color in black foods. There are several pigments that are involved in black formation; however, melanins and anthocyanins are mostly presented in studied black foods. Furthermore, black color can be attributed to other pigments including chlorophylls, carotenoids, and tannins. While melanins are heat-stable pigments and mostly have a black to dark brown color [71], anthocyanins, chlorophylls, tannins, and carotenoids can form black color in high concentration [10,72]. Based on the distribution, chemical properties, and application of these components, they can be categorized into three main groups: (1) anthocyanins, (2) melanins, and (3) other pigments such as tannins, chlorophyll, and carotenoids. Furthermore, black formation can be different in terms of processed foods. Two non-enzymatic browning reactions, the Maillard reaction and caramelization, can be involved in creating black. The black color of some black processed foods and black dishes can be formed due to adding activated charcoal as a natural black colorant.
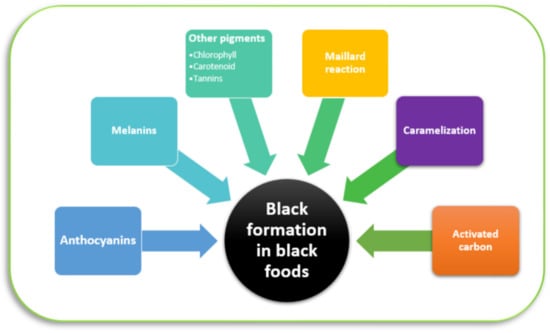
Figure 8.
The illustration of the source of black color in natural black foods and processed black foods.

Table 3.
Pigment compounds found in black foods.
5.1. Melanins in Black Foods and Their Correlation with Black Pigment
5.1.1. Properties and Classification of Melanins
Melanins have been researched over several decades due to their presence very early in most living kingdoms, from the times of the dinosaurs and first primitive creatures [115]. Melanin has drawn the growing interest of the science community due to its unique features applicable to biomedicines and industries, such as ultraviolet-visible (UV-Vis) light, and its stable free radical state [116]. Furthermore, melanin can be used as the potential source of natural black colorants because of its resistance ability to environmental factors such as extreme changing temperatures, high humidity, and radio-protectivity [117]. Melanin has been observed for many years; however, researchers are still not completely familiar with its chemical structures and role in biological systems [118]. There are many factors causing the difficulty in characterizing melanin: (1) melanins are insoluble in a broad range of solvents and pH so that it is hard to purify melanins from natural sources; (2) there is the lack of an accurate method for determining the ratio of melanin- structured units; (3) melanin has a complex molecular structure and organization.
In nature, melanins are divided into three main groups including eumelanin, pheomelanin, and allomelanin [3,4]. These groups are different in their monomer units and synthesis pathways. Eumelanin or nitrogen-containing melanin is commonly found in animals, or microorganisms, and is synthesized by the oxidative polymerization of tyrosine or phenylalanine [4]. Eumelanin and pheomelanin are biopolymers composed of different monomer units from the same precursor dopaquinone, an intermediate for the formation of both eumelanin and pheomelanin [111]. Sulfur-containing units are the biggest difference between pheomelanin and eumelanin, which are found in red hair, feathers, and fowls [111]. Meanwhile, allomelanin or nitrogen-free melanin is one of the pigment compounds with high molecular weight formed by the oxidation and polymerization of phenols with precursors including dihydro-folate, homogentisic acid, and catechol [3,4]. Most melanins are made up of dark brown to black piments (eumelanin and allomelanin), while only pheomelanin forms a yellowish to reddish color [119]. The presence of melanins is critical for animals, plants, and fungi. Eumelanins are needed for metabolism and synthesis in animals, while allomelanin forms to protect seeds from natural enemies.
5.1.2. Animal Melanins in Black Foods
Eumelanins can be easily found in natural biological systems. However, they are only present in certain organs such as the eyes, hair, or skin and are rarely observed throughout the entire animal. Black chromatophores appear in spotting on the skin of several animals such as the butterfly. Melanocytes produce melanosomes that move into the surrounding skin cells, giving the skin a darker color. According to Nganvongpanit et al. [5], there is an exception for black chicken where melanins are found in 33 organs distributed in 10 systems such as the nervous system or skeletal system. Breeds of animals are the major reason causing pigment migration and formation in several organs; however, the melanin-forming pattern is not the same in all organs. Due to the similarity in the structure of eumelanins from different origins, all eumelanins have equivalent optical properties, which can absorb ultraviolet radiation to protect tissue from damage [120].
The presence of eumelanins in black animals is listed in Table 3 for the following species: catfish [111], black chicken [5], the crab Sesarma reticulatum [112], black fish skin [113] and Amphiuma liver [2]. The biochemistry of melanin formation in marine animals is quite similar to the process of plant melanins. L-tyrosine is catalyzed by the enzyme tyrosine into dihydroxyphenyl L-alanine (DOPA), and then DOPA is oxidized to melanin [121]. However, mammalian melanin is converted in a different way due to the lack of tyrosinase in tissue. Dihydroxyphenyl L-alanine oxidase, an enzyme present in the mammalian skin tissue, is involved in the oxidation process of DOPA instead of tyrosine [121]. Eumelanins and hormones in animals have a close relationship. For instance, melanophore-stimulating hormone takes the main role in stimulating melanin synthesis, which results in the black skin of fish [113].
5.1.3. Plant Melanins in Black Foods
Allomelanins in plants are found mostly in fungus, herbs, and some in cereals (Table 3). The synthesis of melanin in plants is correlated with the enzymatic browning reaction happening in plant tissues [3]. However, the chemical structure of plant melanins has not been fully studied in recent years [71]. To detect plant melanins in foods, alkaline and acid extraction are the two most common methods because melanins cannot dissolve in water and in most organic solvents [8]. Besides, allomelanin can lose its color when exposed to strong oxidative agents such as hydrogen peroxide, potassium permanganate, potassium dichromate, and sodium hypochlorite [8]. The melanic nature of black color in several herbs, cereals and seeds has been proved within the following species: black cumin [110], black oat [8], black mustard [3], black sesame seeds [94,95], and black sunflower seeds [2]. Melanins take a major role in the development of seeds. Firstly, the major aim of black formation in seeds is camouflage [54]. Due to the black pigmentation of most wild cereal hulls, black seeds can be invisible to birds and others natural enemies. Furthermore, the accumulation of melanic pigments on the surface of seeds can help seeds absorb more energy to mature and germinate [3]. A comparative study of barley landraces with black and white seeds demonstrated that the former tend to mature earlier than the latter. In comparison between colored barley seeds, the black ones tend to mature earlier than the white seeds [122].
Melanins are not only detected in black seeds but also in black tea (fermented green tea), and create the special black colors for this tea [109]. Melanins could be created through the fermentation process from the abundance of polyphenols and polyphenol oxidase (PPO) in fresh green tea leaves. Tea-melanin has been proved to have strong antioxidant and anticancer activities [109]. Allomelanins can be created by food processing, such as black garlic made from a heat treatment process [4]. A comparative study of melanins in black garlic and eumelanin in sepia ink demonstrated that the former could replace the latter in food additives, food packaging, and biomedical applications due to the similarities in antioxidant activity [4].
5.1.4. Fungal Melanins in Black Foods
Table 3 shows that melanins are found in mostly edible black fungus, while there are no melanins detected in any black fruits or black vegetables. Some mushrooms have been used as natural dye for several years in Europe, Asia, and North Africa [123]. Because of their exceptional properties, fungal melanins have been used widely in the textile, medical, and food industries. Edible fungal melanin can be used as a natural colorant in the food and medicinal industries. Moreover, fungal melanins are insoluble and heat-stable pigments that are only broken down by oxidation and alkaline solution [124].
The presence of melanins brings numerous functions for fungi. In severe environments, the survival ability of fungi could be increased by providing protection against environmental stress [124]. Fungal melanins can withstand harsh conditions, such as those found in Antarctica [125], and even in contaminated nuclear reactors [126]. In addition, fungi can resist UV light, oxidizing agents, and ionizing radiation due to melanins [117]. Melanic pigments in fungus are converted from tyrosine and hydroxylated via quinones by enzymatic browning reactions including oxidations and polymerizations [127]. For example, the melanins of Agaricus bisporus are converted from θ-glutaminyl-4-hydroxybenzene (GHB) by tyrosinase [128]. Therefore, melanins obtained from edible fungus not only work as natural colorants but also enrich functional products with great antioxidant and antimicrobial activity.
5.1.5. Health Benefits of Melanins in Black Foods and Their Application
In addition to their availability in various black foods, several health benefits of melanins have been reported. According to ElObeid et al. [129], melanins have numerous biological and pharmacological properties such as being photoprotective, immunological, antioxidant, anti-inflammatory. Melanins extracted from natural materials can protect against UV radiation and protect from bacteria [130]. Furthermore, recent research has also suggested that melanin-based therapies may have potential in treating cancer [131]. However, more studies are needed to fully understand the health benefits of melanins, how melanin may affect disease processes, and their potential use as a drug via different routes of administration.
In terms of application in food packaging, melanins have gained attention for developing active food packaging due to their strong UV protection, antioxidant, and antibacterial properties [132]. Various polymers have been used to make active packaging films that contain melanin. For example, melanin from watermelon seeds was mixed with whey protein concentrate to form active food packaging with several properties such as UV blocking, creating a water vapor barrier, mechanical properties and antioxidant activity [133]. Roy et al. [134] reported the combination between melanin and carrageenan forming food packaging with antioxidant, UV-barrier, thermal stability, and mechanical properties. Additionally, melanin has been utilized as a sustainable source for producing metallic nanoparticles and as a stabilizer [132].
5.2. Anthocyanins in Black Foods
Besides melanins, anthocyanins take a major role in forming black appearance in foods, especially in plants. Anthocyanins are accumulated in the outer mesocarp of purple and black fruits as well as in the barrier cells of black leaves. Consistent with the chlorophyll content of the respective genotypes, the chloroplast density is greater in the cells of the black fruit [72]. Anthocyanins are plant secondary metabolites that have been studied well regarding their structure and health-promoting effects [19,135]. Recently, anthocyanins in foods have drawn the significant attention of the science community as a promising natural pigment that can replace artificial colorants. Anthocyanins were detected in 31 black-colored foods along with over 70 listed anthocyanin compounds and highlighted major pigments (Table 3). Most black foods detected with available anthocyanins widely distributed were fruit, vegetable, herb, and spice groups.
5.2.1. Chemical Properties of Anthocyanins in Black Foods
The basic C6-C3-C6 anthocyanin structure is the combination of anthocyanidins (aglycones) linking with sugars, which can be separated into anthocyanidin sugar-free aglycones and anthocyanin glycosides depending on structure [10]. Anthocyanidins are formed from two aromatics that are separated by an oxygenated heterocycle [136]. The sugar moieties are often mono- or disaccharide units such as glucose, galactose, rhamnose, arabinose, rutinose, or xylose [137]. Glucose, galactose, rutinose, and arabinose are the sugars most frequently attached to anthocyanidins, as reported in the study of Bakowska-Barczak et al. [138]. Sugar moieties are typically attached to the hydroxyl group at position 3 of the anthocyanidin. Then, positions 5 and/or 7 are the second priorities [139].
Seventeen anthocyanins exist in nature with difference in the number and position of hydroxyl groups and/or methyl ether groups [140]. However, there are six major anthocyanindin skeletons, which form more than six hundred anthocyanins found in foods, including cyanidin, delphinidin, peonidin, pelargonidin, petunidin, and malvidin [10,12,13]. Chemically, the difference of these compounds comes from the hydroxylation and methoxylation patterns of ring B. There are a large variety of anthocyanins in black foods, which make up six major anthocyanidins including Cyanidin, Malvidin, Pelargonidin, Peonidin, Petunidin, Delphinidin, and sugar moieties including glucose, galactose, xylose, sambubiose, and disaccharide derivates. 3′-subtituted anthocyanins present the most in black foods. However, there are several black foods without any research about their pigments or anthocyanins contained.
5.2.2. Anthocyanins and Their Relation to Black Color in Black Foods
Anthocyanins are also known as water-soluble pigments that can create a great diversity of colors ranging from orange to dark colors. The purple to black color is formed due to the biosynthesis of anthocyanins in fruits [73]. The accumulation of anthocyanins at high concentration can form black color in plants such as in black fruits and black flowers [10]. This formation brings positive effects to plants in two ways: (1) the biosynthesis of black color can increase the internal temperature of plants to reduce the damage of cold weather; (2) pollination can happen easier due to the attractive color in winter. The process of forming black pigments in plants, including anthocyanins or melanins, is aimed to bring the best possible plant development.
In plants, anthocyanins can decide color due to their chemical structure and physico- chemical and environment conditions. The same anthocyanins can create different colors at different pH values. As pH indicators, anthocyanins turn to red at low pH, blue at high pH, or mostly colorless at a middle value. Colors can even be decided by the concentration and the quantity of anthocyanins in foods [141]. The high accumulation of cyanidin-based anthocyanins in plants can form a black color [14]. Black foods are richer in anthocyanins than other colored foods. For example, black wheat is found with 26 different anthocyanins, which is the highest number of anthocyanins compared to other kinds [101]. Furthermore, anthocyanins can turn into black pigments by changing environment conditions. Due to sunlight exposure, red sorghum with a high amount of 3-deoxyanthocyanidin can turn into black sorghum [142]. On the positive side, the presence of black anthocyanins in plants, especially fruits, can save them from damage by abiotic stress. For example, white Ranunculus glacialis flowers cannot make more seeds and are not warmer than dark flowers [10]. During winter, there are two main factors that lead to the formation of dark color in flowers: the increase in the internal temperature of flowers [143] and the attraction of pollinators [10]. Co-pigmentation mechanisms are reported as an interesting unique feature of the antho-cyanin family, where flavones can co-participate in the appearance of color with antho-cyanin by protecting anthocyanin molecules [139]. However, in some cases, this pathway does not work out. Because anthocyanin and flavone have the same biosynthetic pathway, it can be assumed that the absence of flavones contributes to high anthocyanin accumulation by eliminating this competition [14].
5.2.3. Anthocyanins in Black Foods
The total anthocyanin contents (TAC) of black foods were measured in many studies and are listed in Table 4, ranging from 7.01 to 1375 mg/100 g. The TACs of black foods were recorded as significantly higher than other colored foods of the same types. For example, the TAC of the black walnut was approximately 3 times higher than the TAC of the English walnut at 80.62 and 27.10 mg/100 g FW, respectively [102]. There are many varieties of the same black plant species so that the TAC of one black food could be in a range. Awika et al. [100] reported that the TAC of black sorghum (400–980 mg/100 g FW) was significantly higher than the TAC of other sorghums (20–100 mg/100 g FW) when observing five different varieties of black sorghum. Anthocyanins can come from a variety of sources in black foods. Some black foods are a source of one anthocyanidin such as cyanidin in the blackberry, fresh black olive, black chokeberry, and black sapote; some of delphinidin in the black lentil and black eggplant. Some black foods contain multi-anthocyanidins such as the mulberry (cyanidin and pelargonidin), black rice (cyanidin and peonidin), or the black bean (malvidin, petunidin, and delphinidin). Chemical structure of major anthocyanins in black foods was illustrated in Figure 9.
Cyanidin derivatives were detected in all reported black foods except black lentil, black eggplant, and black Hungarian peppers (Table 3). The black appearance was reported as related to the contents of cyanidin-based anthocyanins in plants, besides the absence of flavones and accumulation of total anthocyanins [14]. Veberic et al. [144] reported that cyanidin was most commonly found, identifying anthocyanidin in berry species. In Table 3, cyanidin glycosides are found as the predominant anthocyanidins in several black foods: mulberry, blackberry, acai berry, black currant, fresh black olive, black raspberry, black chokeberry, black cherry, black rice, and black soybean. Furthermore, cyanidin 3-glucoside, cyanidin 3-rutinose, cyanidin 3-sambubinoside, cyanidin 3-xylose, cyanidin 3- arabinoside, and cyanidin 3,5-diglucoside are predominant cyanidin-based anthocyanins in black foods (Table 3). Among the six major cyanidin derivatives detected, cyanidin 3-glucoside is found as the main agent in several black foods, with the largest percentages in the blackberry (86.5%) [75], mulberries (76.6%) [74], black cherry (53.2%) [62], black soybean (75.8%) [104] (Table 4). Cyanidin 3-Rutinoside showed the second highest numbers of appearance, following cyanidin 3-sambubinoside and cyanidin 3-xylose. In the case of black olives, there are two main anthocyanins detected in fresh black olives, cyanidin 3-rutinoside (50.88%) and cyanidin-3-glucoside (49.2%), which account for over 90% of total anthocyanins [83]. However, these compounds cannot be detected after the fermentation process, but the black color still remains [83]. Other pigments could have been synthesized during processing; however, those pigments have not been figured out yet.
After cyanidin, delphinidin glycosides are the second most commonly detected anthocyanin compounds in black foods. Delphinidin derivatives were identified in nine black foods including the maqui berry, black plum, black currant, black bean, black lentil, black soybean, black eggplant, black Hungarian pepper, wolfberry (Table 3). The high accumulation of delphinidin is involved in the black pigmentation of the fruits and leaves of selected genotypes along with chlorophyll and carotenoid pigments [72]. The black lentil is a potential source of delphinidin derivatives, with two anthocyanins identified: delphinidin 3-glucosylarabinosidelphinidin and delphinidin 3,5- diglucoside [91]. Delphinidin 3-glucoside, delphinidin 3,5-diglucoside, delphinidin 3- rutinoside, and delphinidin 3-sambubinoside are the four main delphinidin derivatives in black foods (Table 3). Delphinidin 3-glucoside accounts for the majority of total anthocyanin content of the Othello grape skin (40.6%) [145] while delphinidin 3,5-diglucoside is found as the largest percentage of TAC in black plums (45%) [82] and the maqui berry (12.4%) [76] (Table 4). The only anthocyanin found in the black Hungarian pepper, Capsicum annuum L. is delphinidin-3-p-coumaroylrutinoside-5-glucoside [72].
Glycosides of peonidin present in several black foods including black fruits (acai berry, black plum, black cherry, cranberry ’early black’), black cereals (black rice, black wheat, black maize kernels, and black soybean), and black vegetables such as the Shetland black potato (Table 3). However, they only present in trace amounts in most of the listed black foods. Peonidin 3-glucoside is noticed with the largest portion of total anthocyanin content, with 34.5% in black rice [98] (Table 4), while these pigments are found in small amounts in the maqui berry [76], acai berry [77], black cherry [62], black wheat [101], black maize kernels [103], black soybean [104]. Peonidin 3,5-diglucoside is detected in both black wheat [101] and black plum [78]. In terms of identified black foods, peonidin 3-galactoside and peonidin 3-arabinoside are only found in cranberry ’early black’ [92]. Pelargonidin glycosides present in seven black fruits including the black soybean, black maize kernels, black wheat, black elderberry, black raspberry, black grape, and mulberry, whereas pelargonidin 3-glucoside presents in all the listed foods except black raspberry (Table 4). Pelargonidin 3-sambubinoside is only found in black elderberry [93] and pelargonidin 3-rutinoside is detected in black raspberry [85] as the unique pelargonidin derivative
Petunidin derivatives were found as the major anthocyanins in the black soybean, black bean, black plum, wolfberry, while only a trace amount was detected in the Shetland black potato, black wheat, and ′Summer black′ grape (Table 3). In the black plum (Jamelao), petunidin 3,5- diglucoside accounted for the largest percentage of total anthocyanin content with 42%. Petunidin 3-glucoside was the predominant petunidin-based pigment in the black bean [99] and black soybean [104] while it presented in black wheat [101] and the black grape [79] in small amounts. Furthermore, in the wolfberry, petunidin was detected as the main anthocyanin with 11 derivatives including Petunidin-3-O-rhamnoside (p-coumaroyl)-5-O-glucoside, Petunidin-3-rutinoside (cis-p coumaroyl)-5-O-diglucoside, Petunidin-3-rutinoside (trans p- coumaroyl)-5-O-diglucoside, Petunidin-3-O-rutinoside (caffeoyl)-5-O-glucoside, Petunidin- 3-O-rutinoside (feruloyl)-5-O-glucoside (p-coumaroyl), Petunidin-3-O-rutinoside (cis p- coumaroyl)-5-O-glucoside, Petunidin-3-O-rutinoside (trans-p-coumaroyl)-5-Oglucoside, Petunidin-3-O-rutinoside (cis feruloyl)-5-O-galactoside, Petunidin-3-O-rutinoside (cis feruloyl)- 5-O-glucoside, Petunidin-3-O-rutinoside (trans-feruloyl)-5-O-galactoside, Petunidin-3-O- rutinoside (trans-feruloyl)-5-O-glucoside [87]. Glycosides of malvidin were only detected in five black foods: black wheat, black bean, black grape, wolfberry, and black plum. Malvidin 3-glucoside was detected as a major anthocyanin in the ′Summer black′ grape [79] and black bean [103], while malvidin 3,5-diglucose was identified in black plum [78], and black bean [103] in trace amounts. Furthermore, 3-deoxyanthocyanins were only detected in black sorghum (Sorghum bicolor L.); they are more stable than other anthocyanins, including epigeninidin, apigeninidin- 5-glucoside, 7-Omethyl apigeninidin, luteolinidin, luteolinidin-5-glucoside, and 5- methoxy-luteolinidin [104].
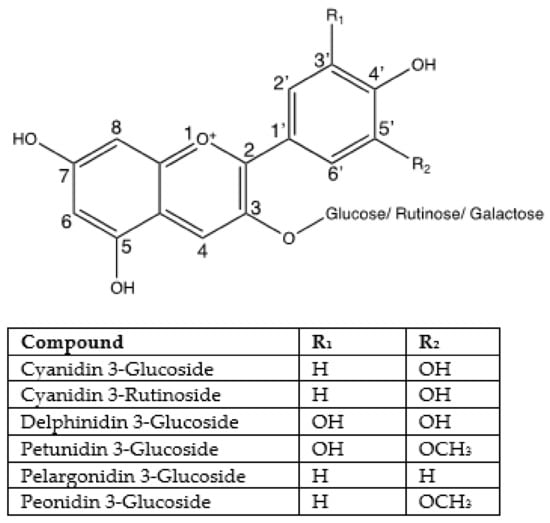
Figure 9.
Chemical structure of major anthocyanins in black foods.

Table 4.
Total anthocyanin content and percentages of main anthocyanin compounds reported in black foods.
Table 4.
Total anthocyanin content and percentages of main anthocyanin compounds reported in black foods.
| Cy | De | Pn | ||||||
|---|---|---|---|---|---|---|---|---|
| Black Foods | TAC (mg/100 g FW) | 3-Glu | 3-Ru | 3-Ga | 3-Glu | 3,5-diGlu | 3-Glu | Refs. |
| Maqui berry | 137.6 | T | - | - | 12.4 | 17.2 | T | [76] |
| Acai berry | na | 25 | 67.4 | - | - | - | T | [146] |
| Othello grape skin | na | 17.8 | - | - | 40.6 | - | [145] | |
| Black currants | 476 | 7.1 | 40.2 | - | 14.0 | - | T | [81,147] |
| Juçara fruit | 290 | 42.7 | 53.1 | - | - | - | - | [78] |
| Black chokeberry | 195,876 (DW) | 2.2 | - | 65.5 | - | - | - | [148] |
| Black cherry | 402 | 53.2 | 44.3 | - | - | - | 1 | [62] |
| Black rice | na | 34 | 32.8 | - | - | 34.5 | [97,98] | |
| Black soybean | na | 75.8 | - | 1 | 8.8 | - | 1.1 | [104] |
Abbreviations: TAC, Total anthocyanin content; Cy, Cyanidin; Glu, Glucoside; Pg, Pelargonidin; Pn, Peonidin; Pt, Petunidin; Gal, Galactoside; De, Delphinidin; (-) Not detected, T: Trace amounts, less than two of total anthocyanin composition; na: Data currently not available; DW, dry weight; FW, fresh weight.
5.2.4. Health Benefits of Anthocyanins and Their Application
In recent years, anthocyanins have become famous because of their vast applications, potential health benefits and abundant natural sources. Anthocyanins are powerful antioxidants that help protect cells against damage from free radicals, which can contribute to the development of chronic diseases such as cancer, heart disease, and diabetes [149]. Li et al. [150] reported anthocyanins with anticancer, anti-inflammatory, anti-obesity and antidiabetic properties. Anthocyanins have also been studied for their potential neuroprotective effects. One study found that the consumption of blueberry anthocyanins improved cognitive function in older adults, suggesting a potential role for these compounds in preventing age-related cognitive decline [151].
In addition to their potential health benefits, anthocyanins also have numerous applications in the food and cosmetic industries. These pigments are used as natural colorants in a wide range of products, including beverages and confectionery [152]. Anthocyanins are also used as natural pigments in cosmetic products [153]. Some studies have suggested that anthocyanins may have potential as natural preservatives in food products, with research indicating that they may help extend the shelf life of certain foods [154].
5.3. Maillard Reaction, Caramelization and Their Relation to Black Color
Black color formation in black processed foods and black dishes could be contributed to by the Maillard reaction. The Maillard reaction is a famous chemical reaction that occurs in the presence of amino acids with reducing sugars at high temperature over a certain time. According to Murata et al. [155], the black and brown colors of the Maillard reaction are formed by melanoidins, the major Maillard reaction products (MRPs), as well as several kinds of low-molecular-weight pigments. However, due to the complexity of the melanin structure, it is not surprising that the structure of melanoidins is still not yet defined. Several low-molecular-weight pigments were listed as the precursors of melanins in the Maillard reaction [155]. Black garlic [20] as well as black coffee [21] were reported as containing MRPs.
Beside the Maillard reaction, caramelization also takes a role in forming the black color of black processed foods and black dishes. Both reactions are non-enzymatic browning reactions that create dark brown color at a high temperature. However, the Maillard reaction relates to the presence of amino acids, while caramelization is the pyrolysis of sugars. In the listed black foods (Table 3), black sugar [22] was recorded as a product of the caramelization process. Caramel is the largest “natural” colorant group that can be found [23]. According to MacDougall et al. [21], up until the present, caramel color cannot be classified into “synthetic “or “natural” food colorants due to its origins. However, caramel color or caramelization is used widely as an indispensable technique in Asian dishes because of its distinct color and taste. At present, the structure of caramelization products has not been elucidated, except for isosacchrosan, a pigment at the early stage of caramelization [21]. There are four main types of caramel that have been found, including ammonia caramel, sulfite caramel, sulfite ammonia caramel, and plain caramel. Ammonia caramel is often found in beer and bakery goods, while ammonia sulfite is found in the production of soft drinks such as coke [23]. Non-enzymatic browning reactions (the Maillard reaction and caramelization) take the main role in forming black color in foods, especially in cooking and processing, such as in black coffee [21] or black sugar [22]. However, the formation of black tea is totally different [8], and comes from an enzymatic reaction.
5.4. Tanins
Tannins are a class of polyphenolic compounds that are widely distributed in plants and are present in a number of natural foods [80]. Tannins are found in several black foods including blackberries [75,80], black currants [80], black grapes [80], black walnut [102], black tea [80,108], and black cumin [109]. They are known to impart an astringent taste and bitter aftertaste and might contribute to the black or dark color in these foods [80,108,156]. However, this is not the case with anthocyanins and melanins; black appearance does not reflect the presence of tannins in foods. According to Dykes et al. [157], some black sorghum varieties do not contain condensed tannins, while the presence of condensed tannins relates to genes. Tannins are involved in the browning reaction in foods, which should be considered in black food production [80].
5.5. Other Pigments in Black Foods
Carotenoid and chlorophyll are also present in black foods as pigments (Table 3). Along with the high accumulation of anthocyanins or melanins, the presence of these pigments can enhance the color intensity of black foods. Among several black foods, chlorophyll is only found in black Hungarian peppers [72]. The combination of anthocyanin, chlorophyll, and carotenoids to form black and purple colors was reported in the study of Lightbourn et al. [72], with different colors for Capsicum annuum L.
5.6. Activated Charcoal
Activated charcoal should be mentioned in this part because it has been used for several year as a food ingredient to create black color in processed food and restaurant foods. Activated carbon is a type of processed carbon owning internal porosity [158]. Due to the porous structure, activated carbon is easily soluble in liquid as a food ingredient. Until now, activated charcoal has been used as a food ingredient in confectionery and beverages. According to the European Commission [35], activated charcoal can be used in food at 1 g per quantified portion. Furthermore, activated charcoal can help to reduce excessive flatulence after consumption [35].
6. Health Benefits of Black Foods
Black foods deserve attention not only because of their distinctive color but also their abundance of bioactive phenolic compounds mentioned above, such as anthocyanins, chlorophylls, carotenoids, or melanins. Phenolic compounds are a group of substances with high antioxidant properties useful for preventing several diseases such as cancers and diabetes [159]. According to Middleton et al. [160], phenolic compounds when tested on an animal model could control tumor development in the initial stage of many types of cancers such liver and colon cancer, or leukemia. The health benefits of several black foods are reported in Table 5 with a study model. Most black foods contain a large number of anthocyanins and melanins, so that studied black foods were mostly reported with high antioxidant, anti-inflammatory, and anticancer activities (Table 5). In comparison with other fruits and vegetables, berries were stated to be the richest sources of antioxidant activity [161]. Black berries were reported with antioxidant and anti-inflammatory activities including the acai berry [162], black chokeberry [89,163], blackberry [164], black currant [11], maqui berry [165], black elderberry [166], and mulberry [167].
Furthermore, some black foods were recorded with different health benefits. For example, the black chokeberry (Aronia melanocarpa L.) was reported as having a supportive influence on the profiles of lipids, fasting plasma glucose, and blood pressure, which could prevent chronic diseases such as diabetes and cardiovascular disease [89]. Consuming a certain amount of black elderberry over a period of time might induce the positive effect of reducing total cholesterol content [163]. Moreover, the study of Kaume et al. [9] mentioned that consuming blackberries every day in certain amounts can prevent neurodegenerative diseases such as Alzheimer’s disease. The study of Muñoz-Falcón et al. [30] described developing black-colored eggplants and their benefits for human health. Black eggplants have been used widely in Europe and North America due to the good effects of black eggplant in terms of hypolipidemic and anti-angiogenic activities. The extract of black pomegranate can prevent the growth of the melanoma cell line and is antiangiogenic and antiproliferative [168]. Many black processed foods have been proved to have great benefits to human health. The study of Sato et al. [169] reported that fermented black garlic contained 10-fold higher antioxidant activity in comparison with fresh garlic in vitro. Black tea has been found to have antioxidant, anticancer, and anti-obesity properties through in vivo testing and clinical trials [170]. The review of Jayachandran et al. [171] comprehensively described the health benefits of soy sauce and black natto including anti-diabetic, anti-cancer, anti-inflammatory, and antioxidant activities.

Table 5.
Pharmacological and Medicinal Activities of Black foods.
Table 5.
Pharmacological and Medicinal Activities of Black foods.
| Black Foods | Study Model | Pharmacological and Medicinal Activities (Disease) | Ref. |
|---|---|---|---|
| Fruits | |||
| Acai Berry | na | Anti-cholinesterase, antioxidant capabilities (Alzheimer’s Disease) | [162] |
| Black chokeberry | In vivo/In vitro on human cells and animals | Reducing the total cholesterol content, preventing diabetes and cardiovascular disease | [89,163] |
| Blackberry | na | Anti-inflammatory, antiviral, anti-proliferant, and anticarcinogenic properties | [164] |
| Black currant | Clinical trials on humans | Therapeutic potential for hypertension and other cardiovascular diseases, cancer, diabetes, and eye-related disease. | [11] |
| Black plum | In vitro | Antibacterial, antifungal, antiviral, anti-genotoxic, anti-inflammatory, anti-ulcerogenic, cardioprotective, anti-allergic, anticancer, chemopreventive, radioprotective, free radical scavenging, antioxidant, hepatoprotective, anti-diarrheal, hypoglycemic, and antidiabetic effects | [172] |
| Maqui berry | Clinical trials on humans, animal/In vitro/In vivo | Antioxidant, anti-inflammatory, anti-atherogenic, anti-carcinogenic, cardio-protective effects | [165] |
| Black pomegranate | Clinical trials on humans, animal/In vitro/In vivo | Antioxidant, anti-inflammatory, prevent skin photoaging, prevent cardiovascular disease, prevent diabetes | [168] |
| Black olive | Clinical trials on humans/In vitro | Antioxidant, anti-inflammatory, reduce risk of stroke, type 2 diabetes alleviation, anticancer, improve digestive system, improve immune response | [63] |
| Black elderberry | Clinical trials on humans, animal/In vitro | Antioxidant, reduce oxidative stress, antiviral, antitumor | [166] |
| Mulberry | Clinical trials on animals /In vitro/In vivo | Antioxidant, antiatherosclerosis, immunomodulatory, antitumor, antihyperglycemic, hypolipidemic, neuroprotective activity | [167] |
| Black velvet tamarind | In vitro | Antioxidant | [173] |
| Black sapote | na | Antioxidant, anti-inflammatory, anticancer activity, reduce hypertension, improve antiallergenic effect, prevent Alzheimer’s | [106] |
| Jucara fruit | In vivo, animal | Antioxidant capacity, anti-inflammatory, prebiotic effect, reduction of cardiovascular risk | [174] |
| Vegetables | |||
| Black eggplants | na | Anti-angiogenic activities | [30] |
| Cereals | |||
| Black quinoa seeds | In vitro | Antioxidant, anti-inflammatory, and antitumor activities | [63] |
| Black bean | In vitro | Technological functionality and antidiabetic potential by inhibiting α-glucosidase, α-amylase, DPP-IV, and glucose uptake. | [175] |
| Black walnut | Clinical trial on humans | Antioxidant, anti-inflammatory, reduce cardiovascular disease | [102] |
| Black chia seed | Animal Lab | Antioxidant | |
| Fungus | |||
| Black truffle | na | Antioxidant, anti-inflammation, immunomodulatory, antitumor, anti-depressant | [176] |
| Black trumpet mushroom | na | Anti-inflammatory, antimugagenic, cytotoxic, hypoglycemic, antioxidant | [177] |
| Black oyster mushroom | na | Hepatoprotective, antiviral, anti-lipidemic, anti-aging, anti-neoplastic, anti-inflammatory, antitumor, immuno-modulatory, anti-mutagenic, hypotensive, antioxidant, anti-diabetic, anti-arthritic, hypocholesterolemic | [177] |
| Black processed foods | |||
| Black tea | Humans/In vitro/In vivo | Antioxidant, anticancer, anti-obesity | [170] |
| Fermented black garlic | Animals/In vitro | Antioxidant, anticancer, antiobesity, hepatoprotective, anti-inflammatory, antiallergic, alleviating dyslipidemia, a cardioprotective effect | [38,48] |
| Soy sauce | na | Antioxidant, anti-diabetic, anticancer, anti-inflammatory, anti-hyperlipidemic, blood pressure maintenance, immunostimulatory activity, neurostimulatory effect, bone health maintenance, prevents osteoporosis | [171] |
na: Data currently not available.
7. Challenges with Black Food Production
Despite the numerous potential benefits of black processed foods, such as improved visual appeal, high antioxidant content, and the generation of unique flavors, there are also some limitations that must be considered when it comes to their production. One of these challenges is the potential release of harmful substances during the production process. The Maillard reactions that occur during black food production have the potential to produce harmful substances such as 5-hydroxymethylfurfural (HMF) and acrylamide, which have been shown through scientific studies to pose a risk to human health [178,179]. The effective control of these substances is crucial to ensuring the safety of our food supply. Furthermore, despite some research on the benefits of black foods, there is still a lack of complete understanding of the mechanism behind the formation of black pigments and the influence of environmental and genetic factors. While activated charcoal is often used in black dishes for its color, there are some concerns that need to be addressed. It is crucial to note that there is limited scientific evidence to support the potential health benefits of consuming activated charcoal in food. Additionally, consuming large amounts of activated charcoal regularly can interfere with nutrient and medication absorption, which can negatively impact health [180]. It is crucial to use activated charcoal in food in moderation, and it may be worthwhile to consider alternative methods of achieving black coloration. This approach can help to promote the consumption of healthy and nutritious foods while minimizing the risks associated with activated charcoal intake.
Therefore, it is important to further research to fully understand the production and safety of black foods associated with the developing of new production methods that minimize the release of harmful substances.
8. General Discussion
Black foods have not received much attention to date, although they have a lot of potential in their diverse varieties, massive number of natural pigments, and human health benefits (Figure 10). Recently, the potential of black food has been increasingly recognized through the introduction of many new varieties of black crops to meet the needs of customers around the world. More than 200 types of black rice are recognized worldwide [59]. Four main varieties of black eggplant are developed in Europe and North America for commerce [30]. Furthermore, black foods are reported with greater sources of minerals and dietary fiber than foods with other colors. Black rice has minerals (Ca, P, Fe, and Zn) and dietary fiber that are higher than in brown and white rice [60]. In some cases, black foods are reported to contain a stronger flavor and aroma than normal-colored foods. In comparison with white sesame seeds, black sesame seeds are reported with stronger flavors and aromas due to higher contents of fatty acids, proteins, and lignans [61]. In dry conditions, dark-colored flowers have more scent than light-colored blossoms [181]. Another study of Liu et al. [63] compares phenolic compounds between three quinoa seed varieties (white, red, and black Chenopodium quinoa seeds). Black quinoa seeds are detected with the highest total phenolic contents compared to red and white quinoa seeds. Around one-hundred and fifty black foods are listed in this review with representative pictures, and categorized into three main groups (natural black foods, processed black foods, and black dishes). Six sub-classes are listed as natural black foods (fruits, vegetables, cereals, herbs, meat, seafood, fungi), whereas six sub-classes include black processed foods (fermented foods, beverages, confectionary, food ingredients, functional foods, and heat-treatment products). Therefore, black food can be found in any type of food material and food product.

Figure 10.
A summary of black foods, and their potential for human health.
Black foods need to be discussed in terms of sources and safety for human health. Melanins and anthocyanins are major pigments found in black foods with high potential health benefits and antioxidant activities. While melanin is a brown to black heat-stable pigment, cyanidin derivatives are seen in close relation to black color accumulation. Animals and plants show different pathways to synthesizing black color, with the involvement of different pigments. In several countries, some black foods have been used as functional foods for health treatments or natural food colorants. In addition, black foods are also a promising source of phenolic compounds that could be used in food and the pharmaceutical and cosmetics industries. Besides, the Maillard reaction and caramelization involve the formation of black processed foods, which can create unique flavors and tastes. All the compounds that form black foods are safe and even good for health, while the Maillard reaction and caramelization are seen as the major chemical reactions applied in gastronomy. Due to the high concentration of black pigments, black foods are reported with several health benefits, for example, antioxidant, anti-inflammatory, and anti-cancer properties.
9. Conclusions and Future Perspectives
This review provides an overview of black foods and chemical perspectives of black food formation in general. Black foods can be found anywhere in nature, for example as fruits, vegetables, cereals, herbs, and even fungi, or produced through processing, for example black vinegar, or cooking as with black garlic or black pudding. Then, the chemical aspects of black color in black food are considered. In animals, black color mostly comes from melanin, while both melanin and anthocyanin are involved in the formation of black plants. Melanin, a heat-stable pigment, is the potential source of natural black colorants because of its resistance to environmental factors such as extreme changing temperatures, high humidity, and its radio-protectivity. Meanwhile, anthocyanins are water-soluble pigments that are phenolic compounds with high antioxidant activities. Both melanin and anthocyanins in black foods could be used as functional foods or natural food colorants in the food, biomedical, and textile industries. However, there are still many unsolved questions and aspects that need to be clarified. Currently, many black foods have not been noticed and studied, especially in terms of the chemical compounds and pigment compounds contained in black foods. The relevance of other pigments in the intensity of black color has still not been figured out yet. In conclusion, black foods have garnered attention for their unique visual appeal; however, it is imperative to engage in further research to determine the potential effects of their consumption on human health. This includes considering both the composition and processing of black foods in order to make informed and evidence-based decisions in promoting a nutritious and well-balanced diet.
Author Contributions
Conceptualization, J.P. and D.H.H.N.; methodology, D.H.H.N.; validation, X.L., G.T. and P.H.; writing—original draft preparation, X.L., G.T., P.H., D.H.H.N.; writing-discussion and conclusion, H.E.-R., D.H.H.N., visualization, D.H.H.N., supervision, J.P., H.E.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful for the support of the 2020-1.1.2-PIACI-KFI-2020-00100 Project “Development of innovative food raw materials based on Maillard reaction by functional transformation of traditional and exotic mushrooms for food and medicinal purposes”, which funded by National Research, Development and Innovation Office, Hungary. The research was also supported by the Stipendium Hungaricum Scholarship Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clydesdale, F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, R.A.; Piattelli, M.; Fattorusso, E. The structure of melanins and melanogenesis—IV. Tetrahedron 1964, 20, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Rhim, J.-W. Isolation and characterization of melanin from black garlic and sepia ink. LWT Food Sci. Technol. 2019, 99, 17–23. [Google Scholar] [CrossRef]
- Nganvongpanit, K.; Kaewkumpai, P.; Kochagul, V.; Pringproa, K.; Punyapornwithaya, V.; Mekchay, S. Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus). Animals 2020, 10, 777. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, X.; Wang, X.; Yang, R.; Mao, J.; Wang, X.; Wang, X.; Wang, X.; Zhang, Q.; et al. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef]
- Varga, M.; Berkesi, O.; Bányai, I.; Darula, Z.; May, N.V.; Palágyi, A. Structural characterization of allomelanin from black oat. Phytochemistry 2016, 130, 313–320. [Google Scholar] [CrossRef]
- Sava, V.M.; Yang, S.-M.; Hong, M.-Y.; Yang, P.-C.; Huang, G.S. Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem. 2001, 73, 177–184. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Ahmad, S.; Chen, J.; Chen, G.; Huang, J.; Zhou, Y.; Zhao, K.; Lan, S.; Liu, Z.; Peng, D. Why Black Flowers? An Extreme Environment and Molecular Perspective of Black Color Accumulation in the Ornamental and Food Crops. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC–MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.a.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Deguchi, A.; Ohno, S.; Hosokawa, M.; Tatsuzawa, F.; Doi, M. Endogenous post-transcriptional gene silencing of flavone synthase resulting in high accumulation of anthocyanins in black dahlia cultivars. Planta 2013, 237, 1325–1335. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and Characterization of Anthocyanins by High-Performance Liquid Chromatography−Electrospray Ionization−Tandem Mass Spectrometry in Common Foods in the United States: Vegetables, Nuts, and Grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef]
- Fan-Chiang, H.-J.; Wrolstad, R.E.; Wrolstad, R.E. Anthocyanin Pigment Composition of Blackberries. J. Food Sci. 2006, 70, 22. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Algarra, M.; Fernandes, A.; Mateus, N.; de Freitas, V.; Esteves da Silva, J.C.G.; Casado, J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Compos. Anal. 2014, 33, 71–76. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef]
- MacDougall, D. Colour in Food: Improving Quality; Woodhead Publishing: Sawston, UK, 2002. [Google Scholar]
- Samaras, T.S.; Camburn, P.A.; Chandra, S.X.; Gordon, M.H.; Ames, J.M. Antioxidant Properties of Kilned and Roasted Malts. J. Agric. Food Chem. 2005, 53, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Todaro, A.; Cimino, F.; Rapisarda, P.; Catalano, A.E.; Barbagallo, R.N.; Spagna, G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009, 114, 434–439. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Complement. Med. Res. 2016, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wang, S.Y.; Lin, H.-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Colak, N.; Kurt-Celebi, A.; Gruz, J.; Strnad, M.; Hayirlioglu-Ayaz, S.; Choung, M.-G.; Esatbeyoglu, T.; Ayaz, F.A. The Phenolics and Antioxidant Properties of Black and Purple versus White Eggplant Cultivars. Molecules 2022, 27, 2410. [Google Scholar] [CrossRef]
- Muñoz-Falcón, J.E.; Prohens, J.; Vilanova, S.; Nuez, F. Diversity in commercial varieties and landraces of black eggplants and implications for broadening the breeders’ gene pool. Ann. Appl. Biol. 2009, 154, 453–465. [Google Scholar] [CrossRef]
- Arai, S. Global view on functional foods: Asian perspectives. Br. J. Nutr. 2002, 88, S139–S143. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Naksawat, S. Application of natural colorant from black rice bran for fermented Thai pork sausage-Sai Krok Isan. Int. Food Res. J. 2017, 24, 1529. [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color: The Fifteen Causes of Color; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Chu, J. MIT Engineers Develop ‘Blackest Black’Material to Date. MIT News. 2019. Available online: https://news.mit.edu/2019/blackest-black-material-cnt-0913#:~:text=The%20material%20is%20made%20from,the%20blackest%20material%20on%20record (accessed on 12 September 2019).
- European Commission. EU Register of nutrition and health claims made on foods. Off. J. Eur. Union 2012, 432, 40. [Google Scholar]
- Martin, C.L.; Montville, J.B.; Steinfeldt, L.C.; Omolewa-Tomobi, G.; Heendeniya, K.Y.; Adler, M.E.; Moshfegh, A.J. USDA Food and Nutrient Database for Dietary Studies 2011–2012; US Department of Agriculture, Agricultural Research Service, Food Surveys Research Group: Washington, DC, USA, 2014. [Google Scholar]
- Muthumani, T.; Kumar, R.S.S. Influence of fermentation time on the development of compounds responsible for quality in black tea. Food Chem. 2007, 101, 98–102. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef]
- Yokotsuka, T. Soy sauce biochemistry. Adv. Food Res. 1986, 30, 195–329. [Google Scholar]
- Tang, H.; Li, P.; Chen, L.; Ma, J.-K.; Guo, H.-H.; Huang, X.-C.; Zhong, R.-M.; Jing, S.-Q.; Jiang, L.-W. The formation mechanisms of key flavor substances in stinky tofu brine based on metabolism of aromatic amino acids. Food Chem. 2022, 392, 133253. [Google Scholar] [CrossRef]
- Hu, Y.; Ge, C.; Yuan, W.; Zhu, R.; Zhang, W.; Du, L.; Xue, J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J. Sci. Food Agric. 2010, 90, 1194–1202. [Google Scholar] [CrossRef]
- Hashimoto, M.; Obara, K.; Ozono, M.; Furuyashiki, M.; Ikeda, T.; Suda, Y.; Fukase, K.; Fujimoto, Y.; Shigehisa, H. Separation and characterization of the immunostimulatory components in unpolished rice black vinegar (kurozu). J. Biosci. Bioeng. 2013, 116, 688–696. [Google Scholar] [CrossRef]
- Teng, F.; Bito, T.; Takenaka, S.; Yabuta, Y.; Watanabe, F. Yolk of the century egg (pidan) contains a readily digestible form of free vitamin B12. J. Nutr. Sci. Vitaminol. 2016, 62, 366–371. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, Y.; Huang, N.; Fu, C.; Tang, F.; Jiang, Q. The research advances on Mesona chinensis Benth in China. J. South. Agric. 2011, 42, 657–660. [Google Scholar]
- Nguyen, D.H.D.; Tran, P.L.; Ha, H.S.; Lee, J.S.; Hong, W.S.; Le, Q.T.; Oh, B.C.; Park, S.H. Presence of β-amylase in ramie leaf and its anti-staling effect on rice cake. Food Sci. Biotechnol. 2015, 24, 37–40. [Google Scholar] [CrossRef]
- Michelitsch, A.; Rittmannsberger, A.; Hüfner, A.; Rückert, U.; Likussar, W. Determination of isopropylmethylphenols in black seed oil by differential pulse voltammetry. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, W.R. Nutritional and functional components of non centrifugal cane sugar: A compilation of the data from the analytical literature. J. Food Compos. Anal. 2015, 43, 194–202. [Google Scholar] [CrossRef]
- Czompa, A.; Szoke, K.; Prokisch, J.; Gyongyosi, A.; Bak, I.; Balla, G.; Tosaki, A.; Lekli, I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. Int. J. Mol. Sci. 2018, 19, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Lee, M.H.; Yong, H.I.; Kim, T.-K.; Choi, H.-J.; Kim, M.-R.; Choi, Y.-S. Effects of added cereal fibers on the quality characteristics of black pudding prepared with duck blood. Poult. Sci. 2022, 101, 101694. [Google Scholar] [CrossRef]
- Pereira, J.A.; Dionísio, L.; Patarata, L.; Matos, T.J.S. Effect of packaging technology on microbiological and sensory quality of a cooked blood sausage, Morcela de Arroz, from Monchique region of Portugal. Meat Sci. 2015, 101, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Imm, B.-Y.; Lee, J.-H.; Yeo, I.-H. Capability analysis of sensory quality of Jajang sauce. Food Sci. Biotechnol. 2009, 18, 745–748. [Google Scholar]
- Nath, P.; Bouzayen, M.; Mattoo, A.K.; Pech, J.C. Fruit Ripening: Physiology, Signalling and Genomics; Bouzayen, M., Ed.; CABI: Wallingford, UK, 2014. [Google Scholar]
- Seymour, G.B.; Taylor, J.E.; Tucker, G.A. Biochemistry of Fruit Ripening; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Zhu, B.-F.; Si, L.; Wang, Z.; Zhou, Y.; Zhu, J.; Shangguan, Y.; Lu, D.; Fan, D.; Li, C.; Lin, H.; et al. Genetic control of a transition from black to straw-white seed hull in rice domestication. Plant Physiol. 2011, 155, 1301–1311. [Google Scholar] [CrossRef]
- Dhua, S.; Kumar, K.; Kumar, K.; Kumar, Y.; Kumar, Y.; Singh, L.; Sharanagat, V.S. Composition, characteristics and health promising prospects of black wheat: A review. Trends Food Sci. Technol. 2021, 112, 780–794. [Google Scholar] [CrossRef]
- Chander, V.; Tewari, D.; Negi, V.; Singh, R.; Upadhyaya, K.; Aleya, L. Structural characterization of Himalayan black rock salt by SEM, XRD and in-vitro antioxidant activity. Sci. Total Environ. 2020, 748, 141269. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus obliquus—From folk medicine to clinical use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef]
- De Angelis, F.; Arcadi, A.; Marinelli, F.; Paci, M.; Botti, D.; Pacioni, G.; Miranda, M. Partial structures of truffle melanins. Phytochemistry 1996, 43, 1103–1106. [Google Scholar] [CrossRef]
- Roy, S.C.; Shil, P. Black Rice Developed through Interspecific Hybridization (O. sativa x O. rufipogon): Origin of Black Rice Gene from Indian Wild Rice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rahim, M.A.; Umar, M.; Habib, A.; Imran, M.; Khalid, W.; Lima, C.M.G.; Shoukat, A.; Itrat, N.; Nazir, A.; Ejaz, A.; et al. Photochemistry, Functional Properties, Food Applications, and Health Prospective of Black Rice. J. Chem. 2022, 2022, 2755084. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, Y.; Li, J.; Sun, W. Black Sesame Seeds Ethanol Extract Ameliorates Hepatic Lipid Accumulation, Oxidative Stress, and Insulin Resistance in Fructose-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2018, 66, 10458–10469. [Google Scholar] [CrossRef]
- Brozdowski, J.; Waliszewska, B.; Loffler, J.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. Composition of Phenolic Compounds, Cyanogenic Glycosides, Organic Acids and Sugars in Fruits of Black Cherry (Prunus serotina Ehrh.). Forests 2021, 12, 762. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Jolvis Pou, K.R. Fermentation: The Key Step in the Processing of Black Tea. J. Biosyst. Eng. 2016, 41, 85–92. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and sensory characteristics of soy sauce: A review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Cachaldora, A.; García, G.; Lorenzo, J.M.; García-Fontán, M.C. Effect of modified atmosphere and vacuum packaging on some quality characteristics and the shelf-life of “morcilla”, a typical cooked blood sausage. Meat Sci. 2013, 93, 220–225. [Google Scholar] [CrossRef]
- Ofori, J.A.; Hsieh, Y.-H.P. The Use of Blood and Derived Products as Food Additives; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Murali, R.; Kumar, N. Black Rice: A novel ingredient in food processing. J. Nutr. Food Sci. 2020, 10, 771. [Google Scholar]
- Nontasan, S.; Moongngarm, A.; Deeseenthum, S. Application of functional colorant prepared from black rice bran in yogurt. Apcbee Procedia 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Santiago, M.C.P.A.; Gouvêa, A.C.M.S.; Peixoto, F.M.; Borguini, R.G.; Godoy, R.L.O.; Pacheco, S.; Nascimento, L.S.M.; Nogueira, R.I. Characterization of jamelão ( Syzygium cumini (L.) Skeels) fruit peel powder for use as natural colorant. Fruits 2016, 71, 3–8. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Lightbourn, G.J.; Griesbach, R.J.; Novotny, J.A.; Clevidence, B.A.; Rao, D.D.; Stommel, J.R. Effects of Anthocyanin and Carotenoid Combinations on Foliage and Immature Fruit Color of Capsicum annuum L. J. Hered. 2008, 99, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Gerardi, C.; et al. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef]
- Jin, Q.; Jin, Q.; Yang, J.; Ma, L.; Cai, J.; Li, J. Comparison of Polyphenol Profile and Inhibitory Activities Against Oxidation and α-Glucosidase in Mulberry (Genus Morus) Cultivars from China. J. Food Sci. 2015, 80, C2440–C2451. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T.; Tamura, H.; Tamura, H.; Harden, L.A. Delphinidin 3-O-(2-O-beta-D-Glucopyranosyl-alpha-l-arabinopyranoside): A novel anthocyanin identified in Beluga black lentils. J. Agric. Food Chem. 2005, 53, 4932–4937. [Google Scholar] [CrossRef]
- Duan, C.-Q.; Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Ferreira, I.C.F.R.; Santos-Buelga, C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Hawken, P.; Talcott, S.T. Phytochemical, antioxidant and pigment stability of acai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res. Int. 2007, 40, 620–628. [Google Scholar] [CrossRef]
- De Brito, E.S.; Araújo, M.C.P.d.; de Araujo, M.C.P.; Alves, R.E.; Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanins present in selected tropical fruits: Acerola, jambolão, jussara, and guajiru. J. Agric. Food Chem. 2007, 55, 9389–9394. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, H.; Fang, J. Changes of Anthocyanin Component Biosynthesis in ‘Summer Black’ Grape Berries after the Red Flesh Mutation Occurred. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Brito, B.d.N.d.C.; da Silva Pena, R.; Lopes, A.S.; Chisté, R.C. Anthocyanins of Jambolão (Syzygium cumini): Extraction and pH-Dependent Color Changes. J. Food Sci. 2017, 82, 2286–2290. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Pinna, I.; Agabbio, M. Anthocyanin and colour evolution in naturally black table olives during anaerobic processing. LWT Food Sci. Technol. 2005, 38, 425–429. [Google Scholar] [CrossRef]
- Harborne, J.B.; Hall, E. Plant polyphenols-XIII. Phytochemistry 1964, 3, 453–463. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Urinary Excretion of Black Raspberry (Rubus occidentalis) Anthocyanins and Their Metabolites. J. Agric. Food Chem. 2006, 54, 1467–1472. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Characterization of a new anthocyanin in black raspberries (Rubus occidentalis) by liquid chromatography electrospray ionization tandem mass spectrometry. Food Chem. 2006, 94, 465–468. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, W.; Chen, J.; Pan, H.; Xu, E.; Chen, S.; Ye, X.; Chen, J. Establishment of anthocyanin fingerprint in black wolfberry fruit for quality and geographical origin identification. LWT 2022, 157, 113080. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Black Goji Berry Anthocyanins: Extraction, Stability, Health Benefits, and Applications. ACS Food Sci. Technol. 2021, 1, 1360–1370. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in Chokeberry (Aronia melanocarpa L.) Polyphenols during Juice Processing and Storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Anthocyanins: Chemistry, Processing & Bioactivity; Springer Nature: Berlin, Germany, 2022. [Google Scholar]
- Zapsalis, C.; Francis, F.J. Cranberry Anthocyanins. J. Food Sci. 1965, 30, 396–399. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of Anthocyanin Profile of Four Elderberry Species and Interspecific Hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black sesame pigment: DPPH assay-guided purification, antioxidant/antinitrosating properties, and identification of a degradative structural marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Yin, P.; Lu, M.; Kong, Q.; Rong, R.; Liu, G. Structure characterization of melanin in black sesame by GC/MS. Se Pu=Chin. J. Chromatogr. 2001, 19, 268–269. [Google Scholar]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Takeoka, G.R.; Dao, L.T.; Full, G.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthocyanins from black sorghum and their antioxidant properties. Food Chem. 2005, 90, 293–301. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Câmara, C.R.S.; Schlegel, V. A Review on the Potential Human Health Benefits of the Black Walnut: A Comparison with the English Walnuts and Other Tree Nuts. Int. J. Food Prop. 2016, 19, 2175–2189. [Google Scholar] [CrossRef]
- Salinas Moreno, Y.; Sanchez, G.S.; Hernandez, D.R.; Lobato, N.R. Characterization of Anthocyanin Extracts from Maize Kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, N.S.; Shin, S.-O.; Shin, S.-H.; Lim, S.-G.; Suh, D.-Y.; Baek, I.-Y.; Park, K.-Y.; Ha, T.J. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009, 112, 226–231. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Zhan, Y.; Zhan, K.; Chang, X.; Yang, H.; Li, Z. Characterization and purification of anthocyanins from black peanut (Arachis hypogaea L.) skin by combined column chromatography. J. Chromatogr. A 2017, 1519, 74–82. [Google Scholar] [CrossRef]
- Jiménez-González, O.; Guerrero-Beltrán, J.Á. Diospyros digyna (black sapote), an Undervalued Fruit: A Review. ACS Food Sci. Technol. 2021, 1, 3–11. [Google Scholar] [CrossRef]
- Eichhorn, S.; Winterhalter, P. Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Res. Int. 2005, 38, 943–948. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.-i.; Nishioka, I. Tannins and related compounds. CXIV. Structure of novel fermentation products, theogallinin, theaflavonin and desgalloyl theaflavonin from black tea, and changes of tea leaf polyphenols during fermentation. Chem. Pharm. Bull. 1992, 40, 1383–1389. [Google Scholar] [CrossRef]
- Mallik, S.; Sharangi, A.; Sarkar, T. Phytochemicals of coriander, cumin, fenugreek, fennel and black cumin: A preliminary study. Natl. Acad. Sci. Lett. 2020, 43, 477–480. [Google Scholar] [CrossRef]
- Al-Mufarrej, S.I.; Hassib, A.M.; Hussein, M.F. Effect of Melanin Extract from Black Cumin Seeds (Nigella sativa L.) on Humoral Antibody Response to Sheep Red Blood to Cells in Albino Rats. J. Appl. Anim. Res. 2006, 29, 37–41. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Kumar, R.; Kumar, R.; Joy, K.P. Melanins as biomarkers of ovarian follicular atresia in the catfish Heteropneustes fossilis: Biochemical and histochemical characterization, seasonal variation and hormone effects. Fish Physiol. Biochem. 2015, 41, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Fingerman, M.; Nagabhushanam, R.; Philpott, L. Physiology of the melanophores in the crab sesarma reticulatum. Biol. Bull. 1961, 120, 337–347. [Google Scholar] [CrossRef]
- Vissio, P.G.; Darias, M.J.; Di Yorio, M.P.; Pérez Sirkin, D.I.; Delgadin, T.H. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. Gen. Comp. Endocrinol. 2021, 301, 113662. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Díaz-Godínez, G. Mushroom Pigments and Their Applications. In Biomolecules from Natural Sources, 1st ed.; Gupta, V.K., Sarker, S.D., Sharma, M., Pirovani, M.E., Usmani, Z., Jayabaskaran, C., Eds.; Wiley: New York, NY, USA, 2022; pp. 82–100. [Google Scholar]
- Wogelius, R.A.; Manning, P.L.; Barden, H.; Edwards, N.; Webb, S.; Sellers, W.; Taylor, K.; Larson, P.; Dodson, P.; You, H. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 2011, 333, 1622–1626. [Google Scholar] [CrossRef]
- Di Mauro, E.; Xu, R.; Soliveri, G.; Santato, C. Natural melanin pigments and their interfaces with metal ions and oxides: Emerging concepts and technologies. MRS Commun. 2017, 7, 141–151. [Google Scholar] [CrossRef]
- Pagano, M.C.; Dhar, P.P. Fungal pigments: An overview. In Fungal Biomolecules: Sources, Applications and Recent Developments; Wiley: New York, NY, USA, 2015; pp. 173–181. [Google Scholar]
- Mbonyiryivuze, A.; Mbonyiryivuze, A.; Mwakikunga, B.W.; Dhlamini, S.M.; Maaza, M. Fourier Transform Infrared Spectroscopy for Sepia Melanin. Phys. Mater. Chem. 2015, 3, 25–29. [Google Scholar] [CrossRef]
- Prota, G. Melanin pigmentation in mammals. Endeavour 1976, 35, 32–38. [Google Scholar] [CrossRef]
- Nofsinger, J.B.; Weinert, E.E.; Simon, J.D. Establishing structure-function relationships for eumelanin. Biopolymers 2002, 67, 302–305. [Google Scholar] [CrossRef]
- Lerner, A.B.; Fitzpatrick, T.B. Biochemistry of melanin formation. Physiol. Rev. 1950, 30, 91–126. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Van Leur, J. Genetic diversity in barley landraces from Syria and Jordan. Euphytica 1987, 36, 389–405. [Google Scholar] [CrossRef]
- Phipps, E. Natural Dyes: Sources, Tradition, Technology and Science; Archetype: London, UK, 2008. [Google Scholar]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Rosa, L.H.; Almeida Vieira, M.d.L.; Santiago, I.F.; Rosa, C.A. Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl.(Caryophyllaceae) in Antarctica. FEMS Microbiol. Ecol. 2010, 73, 178–189. [Google Scholar] [CrossRef]
- Zhdanova, N.N.; Zakharchenko, V.A.; Vember, V.V.; Nakonechnaya, L.T. Fungi from Chernobyl: Mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol. Res. 2000, 104, 1421–1426. [Google Scholar] [CrossRef]
- Velíšek, J.; Cejpek, K. Pigments of higher fungi-a review. Czech J. Food Sci. 2011, 29, 87–102. [Google Scholar] [CrossRef]
- Musso, H. The pigments of fly agaric, Amanita muscaria. Tetrahedron 1979, 35, 2843–2853. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Zhou, J. Selective antiviral activity of synthetic soluble L-tyrosine and L-dopa melanins against human immunodeficiency virus in vitro. Antivir. Res. 1991, 15, 11–25. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Carpentier, A.F. Applications of melanin and melanin-like nanoparticles in cancer therapy: A review of recent advances. Cancers 2021, 13, 1463. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 4629–4655. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.; Bartkowiak, A.; Sobolewski, P. Whey protein concentrate/isolate biofunctional films modified with melanin from watermelon (Citrullus lanatus) seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of carrageenan-based functional nanocomposite films incorporated with melanin nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2006. [Google Scholar]
- Noda, Y.; Kneyuki, T.; Igarashi, K.; Mori, A.; Packer, L. Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology 2000, 148, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J.; Markakis, P.C. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Bąkowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Pol. J. Food Nutr. Sci. 2005, 14, 55. [Google Scholar]
- Delgado-Vargas, F.; Jiménez, A.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Harborne, J.B.; Grayer, R.J. The anthocyanins. In The Flavonoids; Springer: New York, NY, USA, 1988; pp. 1–20. [Google Scholar]
- Markakis, P.; Jurd, L. Anthocyanins and their stability in foods. CRC Crit. Rev. Food Technol. 1974, 4, 437–456. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-deoxyanthocyanins from sorghum. J. Agric. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef]
- Tikhomirov, B.; Shamurin, V.; Shtepa, V. The temperature of arctic plants. Russian. Acad. Sci. USSR Biol. Ser. 1960, 3, 429–442. [Google Scholar]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Paun, N.; Botoran, O.R.; Botoran, O.R.; Niculescu, V.-C.; Niculescu, V. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Appl. Sci. 2022, 12, 936. [Google Scholar] [CrossRef]
- Earling, M.; Beadle, T.; Niemeyer, E.D. Açai Berry (Euterpe oleracea) Dietary Supplements: Variations in Anthocyanin and Flavonoid Concentrations, Phenolic Contents, and Antioxidant Properties. Plant Foods Hum. Nutr. 2019, 74, 421–429. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of berry anthocyanins on cognitive performance, vascular function and cardiometabolic risk markers: A systematic review of randomized placebo-controlled intervention studies in humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. Lwt 2022, 153, 112527. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N.F.; Gameiro, P.; Coelho, P. Anthocyanin-related pigments: Natural allies for skin health maintenance and protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 128, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.M. Activated Carbon Applications in the Food and Pharmaceutical Industries; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Middleton, M.E., Jr. Biological properties of plant flavonoids: An overview. Int. J. Pharmacogn. 1996, 34, 344–348. [Google Scholar] [CrossRef]
- Soto-Hernández, M.; Tenango, M.P.; García-Mateos, R. Phenolic Compounds: Biological Activity; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Guerrero, J.; Ciampi, L.; Castilla, A.; Medel, F.; Schalchli, H.; Hormazabal, E.; Bensch, E.; Alberdi, M. Antioxidant capacity, anthocyanins, and total phenols of wild and cultivated berries in Chile. Chil. J. Agric. Res. 2010, 70, 537–544. [Google Scholar] [CrossRef]
- ALNasser, M.N.; Mellor, I.R.; Carter, W.G. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R. Flavonoids from berries and grapes. Funct. Foods Biochem. Process. Asp. 2002, 2, 71–133. [Google Scholar]
- Masoodi, H.; Villaño, D.; Zafrilla, P. A comprehensive review on fruit Aristotelia chilensis (Maqui) for modern health: Towards a better understanding. Food Funct. 2019, 10, 3057–3067. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Dana, N.; Javanmard, S.H.; Rafiee, L. Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res. Pharm. Sci. 2015, 10, 117. [Google Scholar]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Baliga, M.S.; Bhat, H.P.; Baliga, B.R.V.; Wilson, R.; Palatty, P.L. Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): A review. Food Res. Int. 2011, 44, 1776–1789. [Google Scholar] [CrossRef]
- Ololade Zacchaeus, S.; Anuoluwa Iyadunni, A.; Adejuyitan Johnson, A.; Uyaboerigha Daubotei, I. Black Velvet Tamarind: Phytochemical Analysis, Antiradical and Antimicrobial Properties of the Seed Extract for Human Therapeutic and Health Benefits. J. Phytopharm. 2021, 10, 249–255. [Google Scholar]
- Schulz, M.; Borges, G.d.S.C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Juçara fruit (Euterpe edulis Mart.): Sustainable exploitation of a source of bioactive compounds. Food Res. Int. 2016, 89, 14–26. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Mojica, L.; Berhow, M.A.; de Mejia, E.G. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential health benefits of natural products derived from truffles: A review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Radović, J.; Leković, A.; Tačić, A.; Dodevska, M.; Stanojković, T.; Marinković, T.; Jelić, Č.; Kundakovic-Vasović, T.; Spórna-Kucab, A.; Tekieli, A. Black Trumpet, Craterellus cornucopioides (L.) Pers.: Culinary Mushroom with Angiotensin Converting Enzyme Inhibitory and Cytotoxic Activity. Pol. J. Food Nutr. Sci. 2022, 72, 171–181. [Google Scholar] [CrossRef]
- Fiore, A.; Troise, A.D.; Ataç Mogol, B.e.; Roullier, V.; Gourdon, A.; El Mafadi Jian, S.; Hamzalıoğlu, B.A.l.; Gökmen, V.; Fogliano, V. Controlling the Maillard reaction by reactant encapsulation: Sodium chloride in cookies. J. Agric. Food Chem. 2012, 60, 10808–10814. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Activated charcoal for acute poisoning: One toxicologist’s journey. Journal of medical toxicology 2010, 6, 190–198. [Google Scholar] [CrossRef]
- Sullivan, C.N.; Koski, M.H. The effects of climate change on floral anthocyanin polymorphisms. Proc. R. Soc. B 2021, 288, 20202693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).