Abstract
Importation and translocation of seeds and live animals for aquaculture purposes are well-established practices in the European and Italian shellfish market. However, these routines may be responsible for alien species introduction and spread, representing a risk for aquaculture activities, human health, as well as for environmental and biodiversity conservation. To estimate and reduce the potential impact of alien and locally absent species on aquatic habitats due to aquaculture practices, Member States have adopted Council Regulation (EC) No 708/2007, which provides guidance for risk analysis and contingency measures for mitigation. Despite this legal framework, traceability data for shellfish movements are currently lacking and need to be improved in all EU Member States. The present work presents an updated literature summary of alien species associated with bivalve farming and trading. The information herein collected will be helpful to upgrade the traceability system of farmed bivalves in Italian marine waters with reference to non-target species, representing a knowledge baseline for setting bio security plans to reduce their risk of introduction and further spreading.
Keywords:
alien species; pathogens; parasites; associated species; shellfish; oysters; clams; mussels; Italy; biological invasions; NIS; aquaculture; hitchhikers; translocation; introductions; vectors 1. Introduction
Shellfish aquaculture represents a relevant agrifood resource for European countries, with a total production of 554,004 tons and a global economic value of more than 1 billion US dollars in 2020 alone [1]. Among all Aquatic Sciences and Fisheries Information System (ASFIS) species available from FAO Fishery and Aquaculture Statistics for 2020 [1], mussel production played the major role, accounting for 423,769 tons (76%), followed by oysters (99,791 tons, 18%), clams (28,741 tons, 5.2%) and other species such as scallops, cockles and murex (0.8% globally). It is noteworthy that the values for year 2020 should be considered in the framework of the global pandemic scenario that inevitably implied a decline in production; however, general trends showed fluctuations also in the previous years (2017–2020) for both volumes and values, with a constant decline in the last three years.
Concerning Italy, shellfish aquaculture production accounted for 74,972 tons in 2020 [1], primarily due to mussel production (50,338 tons, 67%), followed by clams (24,453 tons, 32%) and oysters (1%).
Based on the FAO statistics [1], Italian shellfish farming is based on four relevant species: Mytilus galloprovincialis (Lamarck, 1819), the native Mediterranean mussel; the alien Ruditapes philippinarum (Adams and Reeve, 1850), commonly known as Manila clam; the native grooved carpet shell Ruditapes decussatus (Linnaeus, 1758) and the alien Pacific oyster Crassostrea gigas (Thunberg, 1793). Small productions of alien blue mussel Mytilus edulis (Linnaeus, 1758) are also locally developed in Sardinia region.
It is worth mentioning that the nomenclature of the Pacific oyster is controversial. After the taxonomy revision based on ITS2 rRNA sequencing [2], C. gigas was renamed Magallana gigas (Thunberg, 1793). However, although the World Register of Marine Species (WoRMS) describes C. gigas as an “unaccepted name”, most aquaculture researchers and companies still use it [3]. Moreover, the European Union Reference Laboratory (EURL) for Molluscs Diseases refers to Pacific oyster as C. gigas. Therefore, in this paper C. gigas was selected as the common name used in aquaculture, although formally, both names were selected for bibliographic research (see Section 2).
Trends in Italian shellfish aquaculture (2017–2020) showed that mussel production declined each year, mainly due to environmental conditions, and Manila clam were subjected to fluctuations related to seed availability. Native flat oyster Ostrea edulis (Linnaeus 1758) production decreased in all Europe, as in Italy. Conversely, Pacific oyster production, which is based on hatchery-produced seed, has increased in the last three years (from 80 tons in 2018 to 182 in 2020), with a compound annual growth rate (CAGR) of 7.8% between 2017 and 2020 [1].
Mediterranean mussel cultivation in Italy develops along many coastal areas, from the Northern Adriatic Sea regions (Friuli Venezia Giulia, Veneto, Emilia-Romagna) to the Southern Adriatic ones (Apulia region), from the Ligurian Sea along the Tyrrhenian coast to the islands, especially in Sardinia [4]. Natural seeds are generally collected from local cultivated stocks and/or from natural populations. However, importation of live animals at different growth stages, generally from Spain, is a common practice: live mussels can be subjected to a short depuration process or cultivated for longer periods. It should be noted that, as stated in EU Regulation No 1379/2013, article 38 1c [5], after six months of cultivation, shellfish can be considered as produced in the country where they are finally harvested.
In Italy, Manila clam is cultivated in the coastal lagoons of the Northern Adriatic Sea, in licensed areas directly managed by farmer’s cooperatives. Farming is mainly based on seeds collected from natural spat [6], generally at the Po and Brenta River deltas and in the Venice Lagoon, then distributed to the licensed areas. Limited productions of native clam R. decussatus are also based in Sardinia and Sicily. A local hatchery is currently producing Manila clam and grooved carpet shell seeds in the Goro Lagoon (Po River Delta), but despite the efforts to improve hatchery technologies and seed production, clam farming in Italy, as in Europe, still mainly depends on natural seeds [7].
As for oysters, cultivation is carried out in Sardinia, Apulia and with small volumes also in the Marche region, due to the release of new state-owned concessions in marine coastal zones and the establishment of new production sites in areas with suitable environmental characteristics [4]. The production of native flat oyster in European aquaculture has drastically decreased from an average production of 9100 tons in the 1990s to approximately 3300 in 2015 [8] due to the consecutive occurrence of two parasitic diseases, marteiliosis and bonamiosis, the latter being the more serious [9,10,11,12]. For this reason, Pacific oyster is currently farmed with seeds generally imported from French and Dutch hatcheries, although limited seed production also occurs in Sardinia and in the Goro Lagoon.
As clearly inferable from the above-described scenario, importation and translocation of seeds and live animals for aquaculture purposes are well-established practices in the European and Italian market. However, both practices are responsible for the biological invasions of alien species, which increases the risks for aquaculture practices, food and consumers’ safety, as well as for environmental and biodiversity conservation ([13] and references within).
Introductions of alien species and translocations of locally absent ones for aquaculture purposes in the EC are regulated by Council regulation (EC) No 708/2007 [14], and subsequent amendments (EC regulations No 506/2008, No 535/2008 and No 304/2011). Act No 708/2007 sets as a future goal the need to optimize benefits associated with use of alien species and, at the same time, to avoid alterations to ecosystems, preventing negative biological interactions with indigenous populations and restricting the spread of non-target species and detrimental impacts on natural habitats. Moreover, the regulation suggests European countries develop their own framework to ensure adequate protection of aquatic habitats from the risks associated with the use of non-native species in aquaculture. Despite this legal framework, traceability data for shellfish movements are currently lacking and should be improved in all EU Member States.
The aim of the present work is to draft an updated summary of the literature data of alien species associated with bivalve farming and trading. The information herein collected will be helpful to upgrade the traceability system of farmed bivalves in Italian marine waters with reference to non-target species. Moreover, this review represents a knowledge baseline for setting biosecurity plans to reduce their risk of introduction and further spreading.
2. Materials and Methods
Article Collection
For the present review, the bibliographic search was conducted exploiting WoS (Web of Science), Scopus and Google Scholar. Although WoS and Scopus are the main sources for a scientific literature search, Google Scholar was also included to check for other additional publications that were eventually not indexed in WoS and Scopus.
The scientific research was restricted to the following time span: January 2000–May 2022. The topics and/or keywords used for the research were: “shellfish introduction”, “shellfish translocation”, “shellfish aquaculture”, “oyster”, “mussel”, “clam”, “Pacific oyster”, “Magallana gigas”, “Crassostrea gigas”, “Manila clam”, “Ruditapes philippinarum”, “blue mussel”, “Mytilus edulis”, combined with “alien species”, “hitchhikers”, “associated NIS”, “pathogens” and “virus”. Considering that the WoRMS (see details in the introduction section) adopted M. gigas as the species name back in 2016, we also decided to use it for bibliographic research.
Among the retrieved results, we only considered the papers referring to the bivalve species currently farmed in Europe.
From this first search, 48 scientific papers were obtained; bibliographic research was then consolidated by expert judgement, including all relevant references found in the selected papers to integrate the final database (Figure 1). This schematic flow chart illustrates the logical framework of the bibliographic research from the available literature, modified from [15,16]:
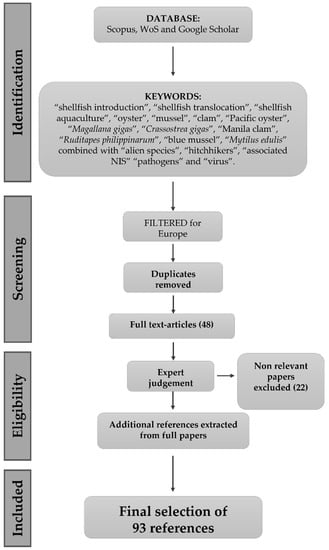
Figure 1.
Schematic flow diagram presenting the pipeline used for the literature revision and reference selection (1961–2022).
At the final stage, 93 references [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] were selected for data collection (Table S1).
All the scientific names were carefully checked, validated and eventually amended referring to WoRMS (https://www.marinespecies.org/, accessed on 1 July 2022). WoRMS was used even to report the original range of species distribution.
3. Results
Pathogens, parasites and other associated species already introduced through shellfish aquaculture, or likely suitable for introduction/translocation by commercial species of oysters, clams and mussels are listed in Table S1. The data for 150 taxa (genus or species) were collected for Bacteria, Chromista, Plantae and Animalia, plus four viruses or variants.
The data retrieved from literature included generic vectors indicated as “shellfish transfer” (ST), or bivalve groups such as “oysters” (O), “clams” (C), “mussels” (M) or eventually “natural dispersal” (ND); whenever available, vector species were specified: the literature data refer to six commercial species: Crassostrea gigas, Ruditapes philippinarum, Mytilus edulis, Crassostrea virginica (Gmelin, 1791), Mercenaria mercenaria (Linnaeus, 1758) and Mya arenaria Linnaeus, 1758 (Table S1). For each item, the vector of introduction, the taxonomic classification, the current distribution in Europe, the first record in Europe, the original range of distribution and the references are indicated (Table S1) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109].
The overall data on main vector groups and associated organisms are shown in Figure 2a and Figure 2b, respectively.
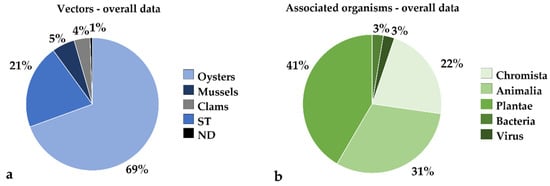
Figure 2.
(a) Pie chart presenting the overall data on main vectors involved in alien species introduction and spread: oysters, mussels; clams; ST = shellfish transfer; ND = natural dispersal. (b) Pie chart presenting the overall data on alien species associated with bivalve aquaculture, divided into five main groups: Chromista; Animalia; Plantae; Bacteria and Viruses. Data are expressed as percentages.
Associated organisms for each vector group are also shown in Figure 3.
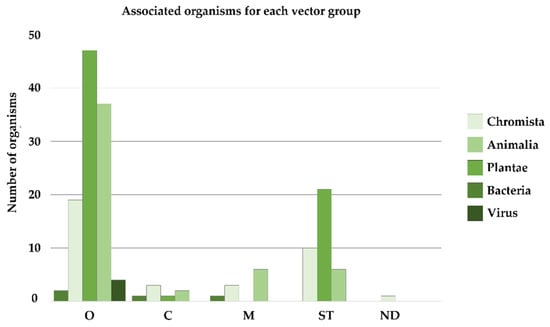
Figure 3.
Number of alien species associated with bivalve aquaculture, indicated for each vector group (O = oysters; C = clams; M = mussels; ST = shellfish transfer; ND = natural dispersal).
Detailed results are described in the subsections below for each bivalve group when details on the vectors were available.
3.1. Oysters
The literature data herein analyzed refer to the general category of “oysters” (O) but also to three species where specified: the introduced Pacific oyster Crassostrea gigas (the majority of the data), the Atlantic oyster Crassostrea virginica and also the native European oyster Ostrea edulis.
Generally speaking, virus infections originated from new introductions and/or oyster translocations, namely, concerning Ostreid herpesvirus (OsHV-1) [20,21,22], Ostreid irido-like virus [21] and Whispovirus, the agent of White Spot Syndrome [18,110], a typical infectious agent of cultivated and wild shrimp, also found in Pacific oysters. Moreover, oyster introductions/translocations are responsible for the transmission of bacteria species such as Vibrio aestuarianus [33], Nocardia crassostreae [25] and of Ciliophora such as Ancistrocoma sp. (Chatton and Lwoff, 1926); Trichodina sp. (Ehrenberg, 1830); Cercozoa such as Bonamia exitiosa (Hine, Cochennac and Berthe, 2001) and Bonamia ostreae (Pichot, Comps, Tigé, Grizel and Rabouin, 1979); Haplosporidium costale. (Wood and Andrews, 1962); Haplosporidium nelsoni (Haskin, Stauber and Mackin, 1966); Haplosporidium tapetis; Mikrocytos mimicus [44]; Marteilia refringens (Grizel, Comps, Bonami, Cousserans, Duthoit and Le Pennec, 1974) (see Table S1 for specific references).
The Dinoflagellatae Alexandrium catenella ((Whedon and Kofoid) Balech, 1985) diffusion was also associated with oyster introduction/translocations [21].
Many red and green algae (Rhodophyta, Chlorophyta) and invertebrate species belonging to different taxonomic groups (Polychaeta, Amphipoda, Cirripedia, Copepoda, Isopoda, Malacostraca, Gymnolaemata, Ascidiacea, Anthozoa, Bivalvia, Platyhelminthes and Porifera), have been introduced/translocated through oyster farming around Europe, including both parasites and non-parasites species (Table S1).
3.2. Clams
The literature data herein refer to the general category of “clams” (C) or specifically to Manila clam Ruditapes philippinarum, hard clam Mercenaria mercenaria and softshell clam Mya arenaria (Table S1).
As for the Manila clam, it is nowadays widely accepted that its introduction and following translocations among European countries are responsible for the diffusion in Europe of the Myzozoa Perkinsus olsenii (Lester and Davis), both in Portugal and Spain, as in the Atlantic and Mediterranean coast of France ([20,48,51] and references within). Moreover, the spread of Vibrio tapetis along the European Atlantic coast is associated with Manila clam aquaculture practices [23,24]. Manila clam populations can also be affected by Cercozoa Haplosporidium tapetis [34] that can be widespread though the translocations of clams to other European countries.
As for associated species, R. philippinarum was suggested as the vector of the first introduction for the Pacific isopod Paranthura japonica (Richardson, 1909), in Arcachon Bay (France) during the 1970s [86] and then to its secondary spread to further Mediterranean marinas [76,83,87]. The Manila clam was also suggested as one of the possible vectors of introduction of another Pacific isopod, Ianiropsis serricaudis, in the Venice Lagoon (Gurjanova, 1936) [81].
Hard clam M. mercenaria is assumed to have been the vector that introduced the red algae Aglaothamnion halliae ((Collins) Aponte, D.L. Ballantine and J.N. Norris, 1997) in the Venice Lagoon [63].
Finally, the soft shell clam M. arenaria was suggested as the possible vector of introduction of Perkinsus chesapeaki (McLaughlin, Tall, Shaheen, El Sayed and Faisal, 2000) from the USA to Europe, where it then infected other clam species such as R. philippinarum and Ruditapes decussatus [48,49,50].
3.3. Mussels
Bacteria Francisella halioticida was associated with Mytilus spp. cultivation [26] and with consequent mass mortality events [31]; moreover, mussel farming (M) was identified as the possible vector of introduction for Pacific Bryozoa Tricellaria inopinata (d’Hondt and Occhipinti Ambrogi, 1985) ([91] and references within) and one of the vectors of introduction and spread of African Ascidiacea Aplidium accarense (Millar, 1953) [92] into Atlantic and Mediterranean European waters.
Mussel farming was also suggested as responsible for the introduction of Amphipoda Caprella scaura (Templeton, 1836) from the Indian Ocean [53,77] and of Indo-Pacific Porifera Paraleucilla magna (Klautau, Monteiro and Borojevic, 2004) in the Mediterranean [76]. The very first record (preceding its formal description) was reported in year 2001 in the Mar Piccolo of Taranto (Ionian Sea) [109], although according to local mussel farmers, the species had been present as long as 20–30 years earlier [111]. Aquaculture, together with shipping traffic, was identified as the most probable vector for P. magna’s recent expansion along the Western Mediterranean coast [76,111], as also specifically suggested for the Ligurian Sea [106,107,108].
It is worth noticing that mussel farming was also suggested as the vector of introduction of at least two Copepod parasite species, such as Mytilicola intestinalis (Steuer, 1902) and Pseudomyicola spinosus spinosus (Raffaele and Monticelli, 1885), from the Mediterranean to the Atlantic coasts of France [39]. The recent data [79] suggest that the species may have entered the Mediterranean following the Lessepsian migration.
Regarding the blue mussel Mytilus edulis, concerns are arising since infections by Myzozoa Toxoplasma gondii (Nicolle and Manceaux, 1908), Cryptosporidium parvum and Dinoflagellata Giardia duodenalis were described in European populations ([47] and reference within). In detail, C. parvum and G. duodenalis were already known to infect both the Mediterranean mussel and blue mussel ([47] and reference within), while T. gondii was detected for the first time in blue mussel in Europe and in Sardinian mussel farms [47].
4. Discussion
Alien species introduced with bivalve aquaculture activities may represent a possible risk in almost three different ways: (i) they can directly affect the cultivated/wild spats of commercial species, with huge economic impacts; (ii) affect the quality of the environment (e.g., potentially toxic phytoplanktonic species); (iii) affect the biodiversity and the ecosystem services of invaded habitats. Bivalve mollusks filter large volumes of water (10 to 15 L/h), and pathogens such as bacteria, viruses and protozoans can thereby become concentrated in their organs and tissues [112].
Among all commercial bivalves herein described, oyster farming is recognized as the major source of introduction for many alien species, particularly for Pacific oyster Crassostrea gigas, followed by mussels and clams. Although Pacific oyster farming currently represents only 1% of the Italian production, it is noteworthy that it has been increasing since 2018 [1]. It represents a promising sector of national shellfish aquaculture [4], therefore, it should be carefully monitored and managed to prevent new introductions of associated alien species.
In terms of economic impacts, ostreid herpesvirus 1 microvariants (OsHV-1 μvar) are associated with the recurrent mass mortality events in Pacific oysters, which have affected the oyster industry in several regions across the world. OsHV-1 μvar appeared in 2008 in France, then spread throughout Europe from the Mediterranean Sea to Scandinavia ([113] and references within). Moreover, by 2010, the disease emerged on a global scale, causing mass mortality also in Australia and New Zealand [113]. In France, the fourth-largest producer of Pacific oysters in the world and the largest in Europe, mortality reached 80–100% in 2008, and in 2015, it was reported to be 50–60%. The production decreased by 25%: from 107,390 tons in 2001 to 79,220 tons in 2012; consequently, the price of oysters increased from €4.93/kg in 2001 to €7.78/kg in 2012 [113]. Moreover, previous studies have demonstrated the presence of other viruses, such as White Spot Syndrome Virus (WSSV) in European culture facilities affecting penaeid shrimp [17] and potentially C. gigas [18], thus, increasing the possibility of oyster contamination with possible damages on oyster cultivation.
Another interesting case regards Nocardia crassostreae, a bacterium that causes nocardiosis in Pacific oyster [25]. As suggested by [114], this bacterium might have originated from an environmental source and may occur wherever the Pacific oyster is cultured. Routine surveillance of shellfish diseases through histological analysis showed nocardiosis in European flat oysters, with pathological lesions similar to those found in N. crassostreae-infected Pacific oysters [25]. This example clearly shows how pathogens can be transferred from an alien cultured species to a native one.
What emerged from the literature review process were the critical issues of emerging diseases. For instance, a novel pathogen found in C. gigas was received for investigation by the Centre for Environment, Fisheries and Aquaculture Science on the North Norfolk coast (Brancaster), UK, which described the very first infection by Mykrocytos spp. in Pacific oyster cultured in Europe that represented a new potential threat to the mollusk industry. The pathogen was identified as Mykrocytos mimicus, and its presence was associated with 90% mortality in May near Schiermonnikoog, an island in the Dutch Wadden Sea [45].
Similarly, new emerging pathogens such as fungi have been described in Mercenaria mercenaria and a parasite similar to Mikrocytos mackini has been detected in cockles Cerastoderma edule (Linnaeus 1758); these new pathogens can affect other bivalve species of commercial and conservation interest.
As for clams, the main risks are related to the diffusion of Vibrio tapetis and Perkinsus olsenii ([48] and references within). P. olsenii was introduced into the Northeastern Atlantic and Mediterranean Sea through the transfer of Ruditapes philippinarum from Asia, and only subsequently has it proven able to infect also native Ruditapes decussatus. Consequently, in Italy, an active monitoring of P. olsenii was put in place. As a result, P. olsenii was confirmed to have appeared not only in Italy but even in Spain, Turkey and Portugal, where it was associated with mortality [115].
In the case of mussels, an interesting case refers to the emergence of an unknown pathogen in bivalves in 2014. At that time, along the Atlantic coast of France, the bacteria Francisella halioticida, associated with mass mortalities in abalones and scallops, was detected within inflammatory granulomas in mussels that also experienced high mortality [30]. This parasite, actually present in the Atlantic waters of Europe, may have been introduced in the Mediterranean waters through shellfish translocations.
The vulnerability of mussels to pathogens might differ through different life stages due to genetic factors and environmental conditions. For example, Mytilus galloprovincialis and Mytilus edulis × M. galloprovincialis hybrids showed less susceptibility to mortality than pure M. edulis [116]; at vulnerable stages, such as spawning, pathogenic effects might become prevalent [117].
Introduction and translocations of bivalves may favor the diffusion of pathogens at a larger scale and at different taxonomic levels. Bivalve mollusks (M. galloprovincialis, R. philippinarum and C. gigas) are also hosts of betanodaviruses that could be transmitted to finfish, considering that viruses in finfish and in bivalve mollusks are genetically similar [118]. The release of these viruses from bivalve mollusks may represent a dangerous source of viruses also for finfish [119]. Risks for humans have also increased with the consumption of bivalves, e.g., a relationship between shellfish consumption and infection by Cryptosporidium spp. was suspected and then recently documented ([47] and references within).
In addition to the impacts described so far, some pathogens included in Table S1 can cause some of the diseases listed in Annex II of Regulation (EU) 2016/429 [120] and subsequent amendments on transmissible animal diseases, repealing certain acts in the area of animal health (‘Animal Health Law’). Among them, infections caused by Bonamia exitiosa, Bonamia ostreae, Marteilia refringens and Whispovirus (White Spot Syndrome) are included. All these infections are classified as C, D and E, meaning that they are relevant diseases to some Member States, for which measures need to be taken to prevent their spreading to other Member States officially declared as free of disease or that have eradication programs for the listed pathogens.
Above parasites and pathogens, some alien species may feed directly on cultivated bivalves, affecting the commercial stocks. This is the case of gastropod Rapana venosa (especially feeding on Mediterranean mussel) and their associated communities, as well as for the Atlantic oyster drill Urosalpinx cinerea, which consumes oysters, mussels and clams, representing a serious pest to the commercial oyster industry [121].
Regarding the risks of (ii) introducing potentially toxic phytoplanktonic species, the literature data herein collected showed that at least one species of Dinoflagellate Alexandrium spp. (namely, A. catenella) (see Table S1) was introduced from the Northwest Pacific Ocean to Mediterranean France, following C. gigas cultivation [21]. A. catenella is among the group of Alexandrium species that produce toxins causing paralytic shellfish poisoning [122], the so-called PSP syndrome.
Therefore, shellfish translocations are a serious risk for the introduction and diffusion of phytoplanktonic species, not only alien but also native and locally distributed, which potentially produce toxic compounds. In fact, the intervalvular water of live animals and the water used to collect and transport the seeds may constitute a suitable environment for potentially toxic phytoplankton. This issue should be carefully assessed since the frequency and impact of harmful algal blooms and the toxin occurrence is a concrete and relevant problem for Italian shellfish production [123,124,125].
Another example of non-target species regards the diatom Coscinodiscus wailesii, which produces mucilage able to aggregate, sink and cover the seabed, favoring anoxic conditions, reducing light penetration [121] and causing extensive clogging of fishing nets, aquaculture cages and other equipment [126,127].
Finally, non-target species introduced with aquaculture practices may also impact (iii) the biodiversity and the ecosystem services of receiving habitats. Ref. [121] proposed an updated inventory of 87 marine species in Europe, representing 13 phyla, which have a documented high impact on ecosystem services or biodiversity. Almost 30% of the assessed species had an impact on entire ecosystem processes or on wider ecosystem functioning, more often in a negative way.
Among them, 17 species are also listed in Table S1, confirming that shellfish aquaculture may represent a serious risk for the introduction of non-target species, eventually becoming highly invasive. As an example, many macroalgae species, such as Asparagopsis armata, Bonnemaisonia hamifera, Codium fragile subsp. fragile, Agarophyton vermiculophyllum, Grateloupia turuturu, Sargassum muticum (N), Undaria pinnatifida and Womersleyella setacea (E, N), may dominate algal assemblages affecting native macroalgae and sessile invertebrates, creating monospecific stands and homogenized microhabitats [121,128,129,130]. Moreover, A. armata and B. hamifera can grow on other primary producers with their filaments, reducing the light availability [121].
Concerning invertebrate animal species, the bivalve Arcuatula senhousia, for example, is responsible for the deposition of large amounts of organic matter, altering the nutritional quality of the sediment, leading to the shallowing of the redox potential discontinuity layer, and making the environment within or under byssal mats unsuitable for adults or larvae of other species [131].
Sessile invertebrates such as the gastropod Crepidula fornicata (E, N), the ascidian Styela clava and the bryozoan Tricellaria inopinata were reported to dominate benthic communities, outcompeting other sessile species for space or food, e.g., [132,133].
The results herein presented also showed that although oyster, clam and mussel farming activities may have played a relevant role in alien species introduction, their subsequent spread may also have been facilitated by other vectors.
As an example, the Pacific isopod Paranthura japonica represents one of the most widespread marine aliens in the Mediterranean Sea [76]. While the initial findings of P. japonica suggested an association with aquaculture transfers (oysters and clams), more recent data on its distribution suggest that it is a polyvectic species, since its rapid expansion and recent records in ports and harbors are associated with a combination of vectors and pathways, not easily identified [76].
The African ascidian Aplidium accarense also represents a potential risk for human activities and natural biodiversity, since it can easily be moved from one place to another through shipping, farming facilities and mussel commercialization.
Concerning the impact on biodiversity, a paradigmatic example is related to Pinna nobilis (Linnaeus 1758) mass mortalities. A haplosporidian parasite, Haplosporidium pinnae, is considered the likely cause of the fan mussel mass mortalities across the South-Western Mediterranean Sea [134]. This parasite is phylogenetically close to a haplosporidian parasite of shrimp Penaeus vannamei that caused mass mortality in penaeid shrimp [134]. This case, although not directly connected to bivalve cultivation, underlines the high risks of possible pathogen evolution and transmission between commercial species and endemic ones, such as P. nobilis listed as “Critically Endangered” by the IUCN Red List of Threatened Species and protected under the EU Habitats Directive (European Council Directive 92/43/EEC).
To summarize, the data retrieved from the literature have highlighted the role of shellfish farming practices as vectors for introduction and spread of non-target associated alien species; therefore, main efforts should be placed to control these phenomena, such as the implementation of cautionary measures to reduce the impacts of these species, both on farming activities and on marine ecosystems. For instance, few inventories of pathogens and their hosts present on national territories are available, and current surveillance and management measures remain insufficient [135].
This review paper provides a baseline of scientific knowledge that should encourage Italy to implement the traceability of commercial bivalve introductions and translocations, in the framework of Council Regulation (EC) No 708/2007. Specific biosecurity plans on the introduction and translocation traceability should be implemented, following the positive examples of Ireland, Australia and New Zealand. These countries have already enforced a valuable Code of Practices to promote procedures and protocols aiming to prevent the introduction and spread of invasive species.
As an example, taking into account the Irish Marine Aquaculture Code of Practice (2009), the main key points of a successful biosecurity plan for shellfish farming may be summarized as follows: (i) remove plant and animal material from equipment or the hull of boats; (ii) introduce a specific risk assessment system to help identify risks, action points and procedures; (iii) control the biofouling on aquaculture equipment; (iv) prevent fouling of vessels and mooring lines; (v) remove fouling prior to long distance journeys; (vi) remove unused equipment and stock; (vii) raise awareness of the issue by engaging different stakeholders in marine aquaculture activities.
Specifically, the risk assessment protocol (2009) requires that organizations audit their activities, recognize all stages in the process, trace the potential risk points in relation to invasive species, identify where measures can be put in place to prevent the spread of invasive species, both by individual farmers or by co-operation of different operators, in collaboration with local inspectors.
Of note, the monitoring and control of alien species in the marine environment are also regulated by the Marine Strategy Framework Directive (MSFD) No. 56/2008. In particular, Descriptor no. 2 (D2) focuses the attention on alien species introduced by human activities in order to reduce their appearance and impact; this may prevent these species from adversely altering the marine ecosystems.
The results presented in this paper could, therefore, be useful to support the monitoring activities referred to as D2, namely, as a scientific baseline to early detect non-target species in harbors and shellfish farming areas (Target 2.1 of MSFD) where aquaculture practices are carried out without efficient control measures.
5. Conclusions
Shellfish farming represents a valuable productive sector for European and Italian aquaculture, mainly focused on mussels, clams and oysters. However, the updated literature data herein collected—consolidated by expert judgement—clearly showed that many alien non-target species (pathogens, parasites and associated species) can be introduced and may spread through shellfish farming and commercialization, with possible risks arising for the industry, food safety, human health and biodiversity conservation.
This review may contribute to fill knowledge gaps about non-target species, providing a scientific baseline for implementing the traceability system of farmed bivalves. In the framework of Council Regulation (EC) No 708/2007 as amended and supplemented, Italy and other Member States should implement procedures for risk analysis and contingency plans for mitigation of non-target species. Plans already developed in Ireland, Australia and New Zealand could be considered as valuable examples.
Moreover, monitoring activities related to Descriptor 2 of MSFD (“Non-indigenous species introduced by human activities are at levels that do not adversely alter the ecosystem”) could benefit from the data provided in this paper on non-target species associated with shellfish aquaculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15043536/s1, Table S1. Pathogens, parasites and other associated species already introduced through shellfish farming, or potentially suitable for introduction/translocation by commercial species of oysters, clams and mussels. For each item, the vector of introduction, the taxonomic classification, the current distribution in Europe, the first record in Europe, the original range of distribution and the references have been indicated.
Author Contributions
Conceptualization, L.D.B., S.C. and G.M.; methodology, L.D.B., S.C. and G.M.; software, L.D.B. and S.C.; validation L.D.B., S.C., G.A. and G.M.; formal analysis, L.D.B., S.C., V.D. and G.A.; resources, G.M.; data curation, L.D.B. and S.C.; writing—original draft preparation, L.D.B., S.C., G.A. and G.M.; writing—review and editing, all Authors; visualization, L.D.B., S.C. and V.D.; supervision, G.M.; project administration, S.C. and G.M., funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Research was found by ISPRA. Laura Di Blasio received a research grant financed by MASAF and ISPRA within the technical assistance “Scientific and technical support in fulfilling EU and national obligations for the use of exotic and locally absent species in aquaculture under EC Reg. No 708/2007” (Project L00AMC01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the four anonymous referees for their appreciation of the work and all useful suggestions to improve the manuscript. Moreover, the authors would like to thank the professional native English speaker who carefully revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. Fishery and Aquaculture Statistics. Global aquaculture production 1950-2020 (FishStatJ). In FAO Fisheries and Aquaculture Division; FAO: Rome, Italy, 2022; Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 2 May 2022).
- Salvi, D.; Mariottini, P. Molecular taxonomy in 2D: A novel ITS2 rRNA sequence-structure approach guides the description of the oysters’ subfamily Saccostreinae and the genus Magallana (Bivalvia: Ostreidae). Zool. J. Linn. Soc.-Lond 2017, 179, 263–276. [Google Scholar] [CrossRef]
- Bayne, B.; Anglès d’Auriac, M.; Backeljau, T.; Beningerd, P.; Boudrye, P.; Carnegief, R.; Davisg, J.; Guoh, X.; Hedgecocki, D.; Krausej, M.; et al. A scientific name for Pacific oysters. Aquaculture 2019, 499, 373. [Google Scholar] [CrossRef]
- Marino, G.; Petochi, T.; Cardia, F. Assegnazione di Zone Marine per l’Acquacoltura (AZA). Guida Tecnica; Documenti Tecnici; ISPRA: Roma, Italy, 2020; 214p. [Google Scholar]
- EC Regulation 1379/2013 of 11 December 2013 on the Common Organisation of the Markets in Fishery and Aquaculture Products, Amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and Repealing Council Regulation (EC) No 104/2000. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:354:0001:0021:EN:PDF (accessed on 16 June 2022).
- Chiesa, S.; Lucentini, L.; Freitas, R.; Nonnis Marzano, F.; Breda, S.; Figueira, E.; Caill-Milly, N.; Herbert, R.; Soares, A.M.V.M.; Argese, E. A history of invasion: COI phylogeny of Manila clam Ruditapes philippinarum in Europe. Fish Res. 2017, 186, 25–35. [Google Scholar] [CrossRef]
- Robert, R.; Sánchez, J.L.; Pérez-Parallé, L.; Ponis, E.; Kamermans, P.; O’Mahoney, M. A glimpse on the mollusc industry in Europe. Aquac. Eur. 2013, 38, 5–11. [Google Scholar]
- Šegvić-Bubić, T.; Žužul, I.; Talijančić, I.; Ugrin, N.; Lepen Pleić, I.; Žuvić, L.; Stagličić, N.; Grubišić, L. Translocation and Aquaculture Impact on Genetic Diversity and Composition of Wild Self-Sustainable Ostrea edulis Populations in the Adriatic Sea. Front. Mar. Sci. 2020, 7, 84. [Google Scholar] [CrossRef]
- Grizel, H.; Comps, M.; Bonami, J.R.; Cousserans, F.; Duthoit, J.L.; Le Pennec, M.A. Recherche de l’agent de la maladie de la glande digestive de Ostrea edulis Linné. Bull. Inst. Pêches Marit. 1974, 240, 7–30. [Google Scholar]
- Grizel, H.; Mialhe, E.; Chagot, D.; Boulo, V.; Bachère, E. Bonamiosis: A model study of diseases in marine molluscs. Am. Fish. Soc. Spec. Publ. 1988, 18, 1–4. [Google Scholar]
- Hudson, E.B.; Hill, B.J. Impact and spread of bonamiosis in the UK. Aquaculture 1991, 93, 279–285. [Google Scholar] [CrossRef]
- Robert, R.; Borel, M.; Pichot, Y.; Gilles, T. Growth and mortality of the European oyster Ostrea edulis in the Bay of Arcachón (France). Aquat. Living Res. 1991, 4, 265–274. [Google Scholar] [CrossRef]
- Sicuro, B.; Tarantola, M.; Valle, E. Italian aquaculture and the diffusion of alien species: Costs and benefits. Aquac. Res. 2016, 47, 3718–3728. [Google Scholar] [CrossRef]
- EC Regulation No 708/2007 of 11 June 2007 Concerning Use of Alien and Locally Absent Species in Aquaculture. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007R0708&from=EN (accessed on 22 September 2022).
- Chiesa, S.; Azzurro, E.; Bernardi, G. The genetics and genomics of marine fish invasions: A global review. Rev. Fish Biol. Fish. 2019, 29, 837–859. [Google Scholar] [CrossRef]
- Trapasso, G.; Chiesa, S.; Freitas, R.; Pereira, E. What do we know about the ecotoxicological implications of the rare earth element gadolinium in aquatic ecosystems? Sci. Total. Environ. 2021, 781, 146273. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Lightner, D.V. Cases of White Spot Disease (WSD) in European shrimp farms. Aquaculture 2011, 319, 302–306. [Google Scholar] [CrossRef]
- Vazquez-Boucard, C.; Alvarez-Ruiz, P.; Escobedo-Fregoso, C.; Anguiano-Vega, G.; Duran-Avelar, M.d.J.; Pinto, V.S.; Escobedo-Bonilla, C.M. Detection of white spot syndrome virus (WSSV) in the Pacific oyster Crassostrea gigas. J. Invertebr. Pathol. 2010, 104, 245–247. [Google Scholar] [CrossRef] [PubMed]
- OIE. Infection with white spot syndrome virus. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2021. [Google Scholar]
- Rodgers, C.J.; Carnegie, R.B.; Chávez-Sánchez, M.C.; Martínez-Chávez, C.C.; Furones Nozal, M.D.; Hine, P.M. Legislative and regulatory aspects of molluscan health management. J. Invertebr. Pathol. 2015, 131, 242–255. [Google Scholar] [CrossRef]
- Mineur, F.; Le Roux, A.; Maggs, C.A.; Verlaque, M. Positive feedback loop between introductions of non-native species and cultivation of oysters in Europe. Conserv. Biol. 2014, 28, 1667–1676. [Google Scholar] [CrossRef]
- Arzul, I. Situation of European mollusc production regarding diseases. B Eur. Assoc. Fish Pat 2018, 38, 130–139. [Google Scholar]
- Paillard, C.; Maes, P. Origine pathogène de l’“anneau brun” chez Tapes philippinarum (Mollusque, bivalve). C.R. Acad. Sci. 1989, 309, 235–241. [Google Scholar]
- Flassch, J.P.; Barret, J.; Mazurie, J.; Maes, P.; Nicolas, J.L.; Noel, T.; Paillard, C.; Le Pennec, M. L’élevage de la palourde, programme national de recherche sur la maladie de l’anneau brun. In Les Mollusques Marins, Biologie et Aquaculture, Lfremer; Actes de Colloque: Brest, France, 1992; Volume 14, pp. 127–140. [Google Scholar]
- Engelsma, M.Y.; Roozenburg, I.; Joly, J.-P. First isolation of Nocardia crassostreae from Pacific oyster Crassostrea gigas in Europe. Dis. Aquat. Org. 2008, 80, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Béchemin, C.; Soletchnik, P.; Polsenaere, P.; Le Moine, O.; Pernet, F.; Protat, M.; Fuhrman, M.; Quéré, C.; Goulitquer, S.; Corporeau, C.; et al. Surmortalités de la moule bleue Mytilus edulis dans les Pertuis Charentais. Rapport d’expertise. Ifremer, mars 2014. Available online: http://archimer.ifremer.fr/doc/00229/34022/32387.pdf (accessed on 1 June 2022).
- Allain, G.; Bernard, I. Les mortalités de moules en 2014 et 2015 vues par les professionnels. In Compte-Rendu de la Phase 1: Synthèse sur L’émergence, la Propagation et L’installation des Mortalités—Rapport Technique; CRC Bretagne Nord: Morlaix, France, 2016. [Google Scholar]
- Travers, M.A.; Pépin, J.F.; Soletchnik, P.; Guesdon, S.; Le Moine, O. Mortalités de Moules Bleues Dans les Pertuis Charentais—MORBLEU. Ifremer, 2016. Available online: http://archimer.ifremer.fr/doc/00324/43539/ (accessed on 1 June 2022).
- Bernard, I.; Allain, G. Mortalités des Moules en Bretagne Nord: Bilan des Connaissances—Rapport Technique; CRC Bretagne Nord: Morlaix, France, 2017. [Google Scholar]
- Charles, M.; Villalba, A.; Meyer, G.; Trancart, S.; Lagy, C.; Bernard, I.; Houssin, M. First detection of Francisella halioticida in mussels Mytilus spp. experiencing mortalities in France. Dis. Aquat. Org. 2020, 140, 203–208. [Google Scholar] [CrossRef]
- Capelle, J.J.; Garcia, A.B.; Kamermans, P.; Engelsma, M.Y.; Jansen, H.M. Observations on recent mass mortality events of marine mussels in the Oosterschelde, the Netherlands. Aquac. Int. 2021, 29, 1737–1751. [Google Scholar] [CrossRef]
- Lupo, C.; Travers, M.A.; Tourbiez, D.; Barthélémy, C.F.; Beaunée, G.; Ezanno, P. Modeling the Transmission of Vibrio aestuarianus in Pacific Oysters Using Experimental Infection Data. Front. Vet. Sci. 2019, 6, 142. [Google Scholar] [CrossRef]
- Axén, C.; Carnegie, R.; Cheslett, D.; Eriksson-Kallio, A.M.; Grade, A.; Haenen, O.; Kristmundsson, A.; Kvamme, B.O.; Levsen, A.; Lillehaug, A.; et al. Working Group on Pathology and Diseases of Marine Organisms (WGPDMO). ICES Sci. Rep. 2020, 2, 41. [Google Scholar] [CrossRef]
- Pires, D.; Grade, A.; Ruano, F.; Afonso, F. Histopathologic Lesions in Bivalve Mollusks Found in Portugal: Etiology and Risk Factors. J. Mar. Sci. Eng. 2022, 10, 133. [Google Scholar] [CrossRef]
- Carrasco, N.; Villalba, A.; Andree, K.B.; Engelsma, M.Y.; Lacuesta, B.; Ramilo, A.; Gairín, I.; Furones, M.D. Bonamia exitiosa (Haplosporidia) observed infecting the European flat oyster Ostrea edulis cultured on the Spanish Mediterranean coast. J. Invertebr. Pathol. 2012, 110, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Abollo, E.; Ramilo, A.; Cao, A.; Culloty, S.C.; Villalba, A. Observations raise the question if the Pacific oyster, Crassostrea gigas, can act as either a carrier or a reservoir for Bonamia ostreae or Bonamia exitiosa. Parasitology 2010, 137, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Haydar, D.; Wolff, W. Predicting invasion patterns in coastal ecosystems: Relationship between vector strength and vector tempo. Mar. Ecol. Prog. Ser. 2011, 431, 1–10. [Google Scholar] [CrossRef]
- Wolff, W.J. Non-indigenous marine and estuarine species in the Netherlands. Zool. Meded. 2005, 79, 1–116. [Google Scholar]
- Goulletquer, P.; Bachelet, G.; Sauriau, P.G.; Noel, P. Open Atlantic coast of Europe—A century of introduced species into French waters. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 276–290. [Google Scholar] [CrossRef]
- Rodgers, C.J.; Mohan, C.V.; Peeler, E.J. The spread of pathogens through trade in aquatic animals and their products. Rev. Sci. Tech. Off. Int. Epiz. 2011, 30, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Comps, M.; Tige, G.; Grizel, H. Etudes ultrastructurale d’un protiste parasite de l’huitre Ostrae edulis L. Acad. Sci. Paris 1980, 2, 383–384. [Google Scholar]
- Renault, T. Appearance and spread of diseases among bivalve molluscs in the northern hemisphere in relation to international trade. Rev. Sci. Tech. Oie 1996, 15, 551–561. Available online: https://archimer.ifremer.fr/doc/00000/2913/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Renault, T.; Stokes, N.A.; Chollet, B.; Cochennec, N.; Berthe, F.; Gérard, A.; Burreson, E.M. Haplosporidiosis in the Pacific oyster Crassostrea gigas from the French Atlantic coast. Dis. Aquat. Organ. 2000, 42, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H.; Stentiford, G.D.; Bateman, K.S.; Berney, C.; Feist, S.W.; Longshaw, M.; Okamura, B.; Stone, D.; Ward, G.; Wood, C.; et al. Mikrocytids are a broadly distributed and divergent radiation of parasites in aquatic invertebrates. Curr. Biol. 2014, 24, 807–812. [Google Scholar] [CrossRef] [PubMed]
- ICES. Report of the Working Group on Pathology and Diseases of Marine Organisms (WGPDMO); ICES: Riga, Latvia, 2018. [Google Scholar]
- Arzul, I.; Lupo, C.; Engelsma, M.; Garcia, C. Do parasites of the genus Mikrocytos present a risk for the production of shellfish in Europe? National Shellfisheries Association. In Proceedings of the Program and Abstracts of the 110th Annual Meeting, Seattle, WA, USA, 18–22 March 2018. [Google Scholar]
- Tedde, T.; Marangi, M.; Papini, R.; Salza, S.; Normanno, G.; Virgilio, S.; Giangaspero, A. Toxoplasma gondii and Other Zoonotic Protozoans in Mediterranean Mussel (Mytilus galloprovincialis) and Blue Mussel (Mytilus edulis): A Food Safety Concern? J. Food Protect. 2019, 82, 535–542. [Google Scholar] [CrossRef]
- Ruano, F.; Batista, F.M.; Arcangeli, G. Perkinsosis in the clams Ruditapes decussatus and R. philippinarum in the Northeastern Atlantic and Mediterranean Sea: A review. J. Invertebr. Pathol. 2015, 131, 58–67. [Google Scholar] [CrossRef]
- Arzul, I.; Chollet, B.; Michel, J.; Robert, M.; Garcia, C.; Joly, J.P.; François, C.; Miossec, L. One Perkinsus species may hide another: Characterization of Perkinsus species present in clam production areas of France. Parasitology 2012, 139, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Ramilo, A.; Pintado, J.; Darriba, S.; Ruiz, P.; Villalba, A.; Abollo, E. Perkinsus spp. in clams Ruditapes decussatus, Ruditapes philippinarum, Venerupis senegalensis and Tapes rhomboides in Galicia (NW Spain). First detection of Perkinsus chesapeaki in Spain by DGGE. J. Shellfish Res. 2012, 31, 336. [Google Scholar]
- Cigarría, J.; Rodríguez, C.; Fernández, J.M. Impact of Perkinsus sp. on Manila clam Ruditapes philippinarum beds. Dis. Aquat. Organ. 1997, 29, 117–120. [Google Scholar] [CrossRef]
- Verlaque, M. Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot for marine species introduction in Europe. Oceanol. Acta 2001, 24, 29–49. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Belmonte, M.; Portacci, G.; Rubino, F. Activities and vectors responsible for the biological pollution in the Taranto Seas (Mediterranean Sea, southern Italy): A review. Environ. Sci. Pollut. Res. 2016, 23, 12797–12810. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E. Biodiversity and mollusc transfer: Need of observance of the laws to avoid alien seaweeds introduction. Biol. Mar. Mediterr. 2010, 17, 175–176. [Google Scholar]
- Verlaque, M.; Boudouresque, C.F.; Mineur, F. Oyster tranfers as a vector for marine species introductions: A realistic approach based on the macrophytes. In Impact of Mariculture on Coastal Ecosystems; Briand, F., Ed.; CIESM Workshop Monographs: Monaco-Ville, Monaco, 2007; pp. 39–47. [Google Scholar]
- Carlton, J.T. (Maritime Studies Program, Williams College—Mystic Seaport, Mystic, CT, USA). Personal communication.
- Gollasch, S.; Haydar, D.; Minchin, D.; Reise, K.; Wolff, W.J. Introduced aquatic species of the North Sea coasts. In Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives; Rilov, G., Crooks, J.A., Eds.; Springer: Berlin, Germany, 2008; pp. 507–528. [Google Scholar]
- Grizel, H.; Héral, M. Introduction into France of the Japanese oyster (Crassostrea gigas). ICES J. Mar. Sci. 1991, 47, 399–403. [Google Scholar] [CrossRef]
- Wolff, W.J.; Reise, K. Oyster imports as a vector for the introduction of alien species into northern and western European coastal waters. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 193–205. [Google Scholar] [CrossRef]
- Critchley, A.T.; Dijkema, R. On the presence of the introduced brown alga Sargassum muticum, attached to commercially imported Ostrea edulis in the S.W. Netherlands. Bot. Mar. 1984, 27, 211–216. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Saracino, O.D. Undaria pinnatifida (Fucophyceae, Laminariales) spread in the central Mediterranean: Its occurrence in the Mar Piccolo of Taranto (Ionian Sea, southern Italy). Cryptogam. Algol. 2000, 21, 305–309. [Google Scholar] [CrossRef]
- Perrone, C.; Cecere, E. Two Solieriacean algae new to the Mediterranean: Agardhiella subulata and Solieria filiformis (Rhodophyta, Gigartinales). J. Phycol. 1994, 30, 98–108. [Google Scholar] [CrossRef]
- Wolf, M.A.; Buosi, A.; Juhmani, A.-S.F.; Sfriso, A. Shellfish import and hull fouling as vectors for new red algal introductions in the Venice Lagoon. Estuar. Coast. Shelf S. 2018, 215, 30–38. [Google Scholar] [CrossRef]
- Guiry, M.D.; AlgaeBase, G.G.M. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 12 February 2010).
- Maggs, C.A.; Stegenga, H. Red algal exotics on North Sea coasts. Helgol. Meeresun. 1999, 52, 243–258. [Google Scholar] [CrossRef]
- Eno, N.C.; Clark, R.A.; Sanderson, W.G. Non-Native Marine Species in British Waters: A Review and Directory; Joint Nature Conservation Committee: Peterborough, UK, 1997. [Google Scholar]
- Cecere, E.; Petrocelli, A.; Portacci, G.; Mineur, F. Grateloupia minima (Rhodophyta, Gigartinales) in the Thau Lagoon and in the Mar Piccolo of Taranto: First report for the Mediterranean Sea. Boll. Mus. Ist. Biol. Univ. Genova 2011, 73, 78. [Google Scholar]
- Cecere, E.; Moro, I.; Wolf, M.A.; Petrocelli, A. The introduced seaweed Grateloupia turuturu (Rhodophyta, Halymeniales) in two Mediterranean transitional water systems. Bot. Mar. 2011, 54, 23–33. [Google Scholar] [CrossRef]
- Petrocelli, A. (Institute for theMarine Coastal Environment (IAMC), UOS Taranto CNR, Taranto, Italy). 2016; Unpublished data. [Google Scholar]
- Cecere, E.; Petrocelli, A.; Verlaque, M. Morphology and vegetative reproduction of the introduced species Hypnea cornuta (Rhodophyta, Gigartinales) in the Mar Piccolo of Taranto (Italy), Mediterranean Sea. Bot. Mar. 2004, 47, 381–388. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Bárbara, I. Seaweeds from sandcovered rocks of the atlantic Iberian peninsula. Part 1. The rhodomelaceae (Ceramiales, rhodophyta). Cryptogam. Algol. 2013, 34, 325–422. [Google Scholar] [CrossRef]
- McIvor, L.; Maggs, C.A.; Provan, J.; Stanhope, M.J. rbcL sequences reveal multiple cryptic introductions of the Japanese red alga Polysiphonia harveyi. 2001, 10, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Petrocelli, A.; Cecere, E.; Verlaque, M. Alien marine macrophytes in transitional water systems: New entries and reappearances in a Mediterranean coastal basin. Bioinvasions Rec. 2013, 2, 177–184. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A. Floristic and biogeographic considerations about the benthic macroalgal flora in the Gulf of Taranto. Biogeographia 2004, 25, 7–18. [Google Scholar] [CrossRef]
- Korringa, P. The shell of Ostrea edulis as a habitat. Arch. Neerl. Zool. 1951, 10, 32–152. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhpinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5, e3954. [Google Scholar] [CrossRef]
- Eleftheriou, A.; Anagnostopoulou-Visilia, E.; Anastasopoulou, E.; Ateş, S.A. New Mediterranean biodiversity records. Mediterr. Mar. Sci. 2011, 12, 491–508. [Google Scholar] [CrossRef]
- Zullo, V. Balanus trigonus Darwin (Cirripedia, Balaninae) in the Atlantic basin: An Introduced Species? B Mar. Sci. 1992, 50, 66–74. [Google Scholar]
- Feis, M.E.; Goedknegt, M.A.; Arzul, I.; Chenuil, A.; den Boon, O.; Gottschalck, L.; Kondo, Y.; Ohtsuka, S.; Shama, L.N.S.; Thieltges, D. Global invasion genetics of two parasitic copepods infecting marine bivalves. Sci. Rep. 2019, 9, 12730. [Google Scholar] [CrossRef]
- Karuza, A.; Caroppo, C.; Monti, M. ‘End to end’ planktonic trophic web and its implications for the mussel farms in the Mar Piccolo of Taranto (Ionian Sea, Italy). Environ. Sci. Pollut. R 2016, 23, 12707–12724. [Google Scholar] [CrossRef]
- Marchini, A.; Ferrario, J.; Occhipinti-Ambrogi, A. Confirming predictions: The invasive isopod Ianiropsis serricaudis Gurjanova, 1936 (Crustacea: Peracarida) is abundant in the Lagoon of Venice (Italy). Acta Adriat. 2016, 57, 331–336. [Google Scholar]
- Marchini, A.; Ferrario, J.; Occhipinti-Ambrogi, A. The Relative Importance of Aquaculture and Shipping as Vectors of Introduction of Marine Alien Species: The Case of Olbia (Sardinia); CIESM: Kiel, Germany, 2016. [Google Scholar]
- Martínez-Laiz, G.; Ros, M.; Guerra-García, J.M. Marine exotic isopods from the Iberian Peninsula and nearby waters. PeerJ 2018, 6, e4408. [Google Scholar] [CrossRef]
- Forniz, C.; Maggiore, F. New records of Sphaeromatidae from the Mediterranean Sea (Crustacea, Isopoda). Oebalia 1985, 11, 779–783. [Google Scholar]
- Lorenti, M.; Keppel, E.; Petrocelli, A.; Sigovini, M.; Tagliapietra, D. The non-indigenous Paranthura japonica Richardson, 1909 (Isopoda: Anthuroidea: Paranthuridae) from the Mar Piccolo lagoon, Taranto (Italy, Mediterranean Sea). Environ. Sci. Pollut. R 2016, 23, 12791–12796. [Google Scholar] [CrossRef] [PubMed]
- Lavesque, N.; Sorbe, J.C.; Bachelet, G.; Gouillieux, B.; De Montaudouin, X.; Bonifacio, P.; Blanchet, H.; Dubois, S. Recent discovery of Paranthura japonica Richardson, 1909 (Crustacea: Isopoda: Paranthuridae) in European marine waters (Arcachon Bay, Bay of Biscay). Bioinvasions Rec. 2013, 2, 215–219. [Google Scholar] [CrossRef]
- Marchini, A.; Sorbe, J.; Torelli, F.; Lodola, A.; Occhipinti-Ambrogi, A. The non-indigenous Paranthura japonica Richardson, 1909 in the Mediterranean Sea: Travelling with shellfish? Mediterr. Mar. Sci. 2014, 15, 545–553. [Google Scholar] [CrossRef]
- Gollasch, S. The Asian decapod Hemigrapsus penicillatus (de Haan, 1935) (Grapsidae, Decapoda) introduced in European waters: Status quo and future perspective. Helgol. Meeresun 1998, 52, 359–366. [Google Scholar] [CrossRef]
- Loxton, J.; Wood, C.A.; Bishop, J.D.D.; Porter, J.S.; Spencer Jones, M.; Nall, C.R. Distribution of the invasive bryozoan Schizoporella japonica in Great Britain and Ireland and a review of its European distribution. Biol. Invasions 2017, 19, 2225–2235. [Google Scholar] [CrossRef]
- de Blauwe, H.; Faasse, M. Smittoidea prolifica Osburn, 1952 (Bryozoa, Cheilostomatida), a Pacific bryozoan introduced to the Netherlands (Northeast Atlantic). Bull. Inst. R. Sci. Nat. Belg. 2004, 74, 33–39. [Google Scholar]
- Lodola, A.; Savini, D.; Occhipinti-Ambrogi, A. First record of Tricellaria inopinata (Bryozoa: Candidae) in the harbours of La Spezia and Olbia, Western Mediterranean Sea (Italy). Mar. Biodivers. Rec. 2012, 5, e41. [Google Scholar] [CrossRef]
- Montesanto, F.; Chimienti, G.; Gissi, C.; Mastrototaro, F. Spread of the non-indigenous ascidian Aplidium accarense (Millar, 1953) in the Eastern Mediterranean Sea: Morphological and molecular tools for an accurate identification. Mediterr. Mar. Sci. 2021, 22, 246–254. [Google Scholar] [CrossRef]
- Lützen, J. Styela clava Herdman (Urochordata, Ascidiacea), a successful immigrant to North West Europe: Ecology, propagation and chronology of spread. Helgol. Meeresun 1999, 52, 383–391. [Google Scholar] [CrossRef]
- Carlton, J.T. History, Biogeography, and Ecology of the Introduced Marine and Estuarine Invertebrates of the Pacific Coast of North America. Ph.D. Thesis, University of California, Davis, CA, USA, 1979. [Google Scholar]
- Gollasch, S.; Riemann-Zürneck, K. Transoceanic dispersal of benthic macrofauna: Haliplanella luciae (Verrill, 1898) (Anthozoa, Actinaria) found on a ship’s hull in a shipyard dock in Hamburg harbour, Germany. Helgol. Meeresun 1996, 50, 253–258. [Google Scholar] [CrossRef]
- Sciberras, M.; Schembri, P.J. A critical review of records of alien marine species from the Maltese Islands and surrounding waters (Central Mediterranean). Mediterr. Mar. Sci. 2007, 8, 41–66. [Google Scholar] [CrossRef]
- Cachia, C. Notes on some uncommon species of molluscs from the Maltese Islands. Boll. Malac. 1981, 17, 291–294. [Google Scholar]
- Schembri, P.J. On the occurrence of Gibbula (Steromphala) cineraria (L.) (Trochidae) in the Maltese Islands. Boll. Mal. 1979, 15, 319–320. [Google Scholar]
- Crocetta, F. Marine alien Mollusca in Italy: A critical review and state of the knowledge. J. Mar. Biol. Assoc. UK 2012, 92, 1357–1365. [Google Scholar] [CrossRef]
- Martel, C.; Viard, F.; Bourguet, D.; Garcia-Meunier, P. Invasion by the marine gastropod Ocinebrellus inornatus in France. II. Expansion along the Atlantic coast. Mar. Ecol. Prog. Ser. 2004, 273, 163–172. [Google Scholar] [CrossRef]
- Faasse, M.; Ligthart, M. American (Urosalpinx cinerea) and Japanese oyster drill (Ocinebrellus inornatus) (Gastropoda: Muricidae) flourish near shellfish culture plots in the Netherlands. Aquat. Invasions 2009, 4, 321–326. [Google Scholar] [CrossRef]
- Mann, R.; Harding, J.M. Invasion of the North American Atlantic coast by a large predatory Asian mollusc. Biol. Invasions 2000, 2, 7–22. [Google Scholar] [CrossRef]
- Kerckhof, F.; Vink, R.J.; Nieweg, D.C.; Post, J.N.J. The veined whelk Rapana venosa has reached the North Sea. Aquat. Invasions 2006, 1, 35–37. [Google Scholar] [CrossRef]
- Faasse, M.; Ligthart, M. The American oyster drill, Urosalpinx cinerea (Say, 1822), introduced to the Netherlands increased risks after ban on TBT? Aquat. Invasions 2007, 2, 402–406. [Google Scholar] [CrossRef]
- Carlton, J.T. Molluscan invasions in marine and estuarine communities. Malacologia 1999, 41, 439–454. [Google Scholar]
- Tamburini, M.; Keppel, E.; Marchini, A.; Repetto, M.F.; Ruiz, G.M.; Ferrario, J.; Occhipinti-Ambrogi, A. Monitoring Non-indigenous Species in Port Habitats: First Application of a Standardized North American Protocol in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 700–730. [Google Scholar] [CrossRef]
- Bertolino, M.; Longo, C.; Corriero, G.; Pansini, M.; Bavestrello, G. Prima segnalazione della specie tropicale Paraleucilla magna Klautau et al. 2004 (Porifera, Calcarea) nel Mar Ligure. In Proceedings of the 74th National Congress of the Italian Union of Zoology, Modena, Italy, 11–13 September 2019. [Google Scholar]
- Longobardi, L.; Bavestrello, G.; Betti, F.; Cattaneo-Vietti, R. Long-term changes in a Ligurian infralittoral community (Mediterranean Sea): A warning signal? Reg. Stud. Mar. Sci. 2017, 14, 15–26. [Google Scholar] [CrossRef]
- Longo, C.; Liaci, L.; Manuel, M.; Correiro, G. Note sui poriferi del Mar Grande e del Mar Piccolo do Taranto (Mar Ionio). Biol. Mar. Mediterr. 2004, 11, 440–443. [Google Scholar]
- WOAH. Manual of Diagnostic Tests for Aquatic Animals. 2022. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 19 December 2022).
- Longo, C.; Mastrototaro, F.; Corriero, G. Occurrence of Paraleucilla magna (Porifera: Calcarea) in the Mediterranean Sea. J. Mar. Biol. Assoc. 2007, 87, 1749–1755. [Google Scholar] [CrossRef]
- Robertson, L.J. The potential for marine bivalve shellfish to act as transmission vehicles for outbreaks of protozoan infections in humans: A review. Int. J. Food Microbiol. 2007, 120, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, M.; Castinel, A.; Cheslett, D.; Furones Nozal, D.; Whittington, R.J. The impacts of ostreid herpesvirus 1 microvariants on Pacific oyster aquaculture in the Northern and Southern Hemispheres since 2008. Rev. Sci. Tech. Off. Int. Epiz. 2019, 38, 491–509. [Google Scholar] [CrossRef]
- Elston, R.A. Infectious diseases of the Pacific oyster Crassostrea gigas. Annu. Rev. Fish Dis. 1993, 3, 259–276. [Google Scholar] [CrossRef]
- Arcangeli, G. Diagnostic, epidemiological and regulatory updates in animal health in aquaculture. In Proceedings of the IIZZSS Annual Meeting 2022, Organized by NRL for Fish, Mollusc and Crustacean Diseases, Legnaro, Italy, 20–21 October 2022. [Google Scholar]
- Benabdelmouna, A.; Garcia, C.; Ledu, C.; Lamy, P.; Maurouard, E.; Dégremont, L. Mortality investigation of Mytilus edulis and Mytilus galloprovincialis in France: An experimental survey under laboratory conditions. Aquaculture 2018, 495, 831–841. [Google Scholar] [CrossRef]
- Charles, M.; Trancart, S.; Oden, E.; Houssin, M. Experimental infection of Mytilus edulis by two Vibrio splendidus-related strains: Determination of pathogenicity level of strains and influence of the origin and annual cycle of mussels on their sensitivity. J. Fish Dis. 2019, 43, 9–21. [Google Scholar] [CrossRef]
- Panzarin, V.; Fusaro, A.; Monne, I.; Cappellozza, E.; Patarnello, P.; Bovo, G.; Capua, I.; Holmes, E.C.; Cattoli, G. Molecular epidemiology and evolutionary dynamics of betanodavirus in southern Europe. Infect. Genet. Evol. 2012, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Grodzki, M.; Panzarin, V.; Guercio, A.; Purpari, G.; Serratore, P.; Ciulli, S. Detection and molecular characterization of betanodaviruses retrieved from bivalve molluscs. J. Fish Dis. 2018, 41, 603–611. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0429&from=IT (accessed on 16 November 2022).
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- MacKenzie, A.L. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. N. Z. J. Mar. Fresh 2014, 48, 430–465. [Google Scholar] [CrossRef]
- Costa, A.; Alio, V.; Sciortino, S.; Nicastro, L.; Cangini, M.; Pino, F.; Servadei, I.; La Vignera, A.; Fortino, G.; Monaco, S.; et al. Algal blooms of Alexandrium spp. and Paralytic Shellfish Poisoning toxicity events in mussels farmed in Sicily. Ital. J. Food Saf. 2021, 10, 9062. [Google Scholar] [CrossRef] [PubMed]
- Rubini, S.; Albonetti, S.; Menotta, S.; Cervo, A.; Callegari, E.; Cangini, M.; Dall’Ara, S.; Baldini, E.; Vertuani, S.; Manfredini, S. New Trends in the Occurrence of Yessotoxins in the Northwestern Adriatic Sea. Toxins 2021, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Boalch, G.T.; Harbour, D.S. Unusual diatom off the coast of south-west England and its effect on fishing. Nature 1977, 269, 687–688. [Google Scholar] [CrossRef]
- Boalch, G.T. Algal blooms and their effects on fishing in the English Channel. Hydrobiologia 1984, 116/117, 449–452. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Ceccherelli, G.; Cinelli, F. Interactive effect of sedimentation and Caulerpa racemosa var. cylindracea invasion on macroalgal assemblages in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2005, 64, 467–474. [Google Scholar] [CrossRef]
- Cebrian, E.; Linares, C.; Marschal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol. Invasions 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Svensson, J.R.; Nylund, G.M.; Cervin, G.; Toth, G.B.; Pavia, H. Novel chemical weapon of an exotic macroalga inhibits recruitment of native competitors in the invaded range. J. Ecol. 2013, 101, 140–148. [Google Scholar] [CrossRef]
- Mistri, M.; Rossi, R.; Fano, E.A. The spread of an alien bivalve (Musculista senhousia) in the Sacca di Goro Lagoon (Adriatic Sea, Italy). J. Mollus. Stud. 2004, 70, 257–261. [Google Scholar] [CrossRef]
- Safriel, U.N.; Sasson-Frosting, Z. Can colonizing mussel outcompete indigenous mussel? J. Exp. Mar. Biol. Ecol. 1988, 117, 211–226. [Google Scholar] [CrossRef]
- Kochmann, J.; Buschbaum, C.; Volkenborn, N.; Reise, K. Shift from native mussels to alien oysters: Differential effects of ecosystem engineers. J. Exp. Mar. Biol. Ecol. 2008, 364, 1–10. [Google Scholar] [CrossRef]
- Catanese, G.; Graua, A.; Valencia, J.M.; Garcia-Marchc, J.R.; Vázquez-Luisd, M.; Alvarezd, E.; Deuderod, S.; Darribae, S.; Carballalf, M.J.; Villalbaf, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Patholi. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- AAC. Recommendation—Risks of Bivalve Mollusc Pathogen Emergence in Connection with Climate Change. 2022. Available online: https://aac-europe.org/images/jdownloads/Recommendations/EN/19.AAC_Recommendation_-_Risks_of_bivalve_pathogen_emergence_in_connection_with_climate_change_2022_19_.pdf (accessed on 24 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).