Abstract
In this study, we aimed to investigate the presence of an alteration in the feeding ecology of fish after the deployment of anti-trawling reefs at the Punta Tramontana site (Sardinia, Italy). To achieve this aim, we examined prey in the stomach contents of two target species of fish: Diplodus annularis and Mullus surmuletus. The samples were obtained from fishing activities carried out over one year from June 2017 to October 2018 at two impact sites, selected by the presence of artificial reefs, and two control sites. The results showed that installing these artificial devices increased the food spectrum availability of the target species and induced two different ecological feeding behaviors, probably derived from their different ecologies. D. annularis changed its alimentary strategy from generalist at control sites to specialist at impact sites, focusing its diet on the crustacean Gnathia maxillaris. Mullus surmuletus was not affected by the presence of the barriers, which constitute an additional site where the fish feed during their foraging activities. In conclusion, these anti-trawling reefs, in addition to the purpose for which they are designed (in this case, avoiding illegal trawling), did not produce any negative environmental impact on surrounding marine biota.
1. Introduction
Widespread human activities in marine ecosystems are leading to a multitude of threats, ranging from habitat destruction and overharvesting to pollution and climate change, provoking a significant habitat loss for many marine species and decreasing the quality of the remaining habitats [1,2].
In particular, in the Mediterranean context, trawling is a widespread habit realized to satisfy the increasing demand for seafood [3], and it is frequently practiced illegally near the coast (even if it is banned at depths less than 50 m in accordance with article 111 of the Regulations for the Implementation of Law 963/1965 on the discipline of sea fishing D.P.R. No. 1639/1968) [4]. In addition, this unlawful activity jeopardizes sensitive habitats [1,5], which, in the context of the Mediterranean, consist of autochthonous species such as Posidonia oceanica (L.) Delile, 1813, meadows, or important spawning and nursery areas for a multitude of marine species [6].
To prevent trawling activities, anti-trawling reefs are increasingly being deployed in the Mediterranean Sea [4,7,8,9,10,11]. As a result, these devices interact with the surrounding environment, increasing habitat diversity and heterogeneity [4].
Anti-trawling structures can easily simulate the “habitat complexity” of natural ecosystems, such as rocky substrate environments, sustaining the richness and diversity assemblages of a great variety of marine species [1,2,12].
Differently to reefs employed in the enhancement of fish populations and the improvement of fisheries, which are built with rough surfaces, holes, and grooves to increase the interaction with marine organisms, anti-trawling reefs are built as simple and linear structures [10].
Nevertheless, the deployment of these devices can lead to several different scenarios, increasing, decreasing, or totally changing the richness and diversity of macrozoobenthic species (colonizing these devices) and of other larger species present in the environment (such as fish) [9].
The presence of new macrozoobenthic communities may also alter the ecological relationships of various species of fish, such as their feeding habits and their behavior [4].
Fish that feed on the artificial reefs can show an alteration in their alimentary strategy with a different composition in prey compared to the diet of species present in other natural habitats [4]. Additionally, these artificial structures can also serve as important sites for a variety of other marine organisms, acting as nursery habitats, as a refuge from predators, and as a restoring area in which to feed [13].
In this context, monitoring activities represent a fundamental tool for observing how artificial reefs affect marine biota. To date, there is scant published information describing the extent, purpose, and monitoring results of artificial reef projects, and studies investigating the effects of artificial reefs on marine biota are fairly dated [9].
The few published studies examined the changes in fish assemblages associated with the deployment of artificial reefs [14,15,16] and the development of the epibenthic communities [13,15,17,18].
On the other hand, few studies focus their attention on investigating the alteration that these reefs can cause to the feeding habits of the many species of fish present in the environment [19,20].
Based on this preliminary evidence, this study aims to examine possible changes in the feeding habits and alimentary strategies of fishes after the deployment of several anti-trawling reefs, selecting two species of fish—the annular seabream, Diplodus annularis (Linnaeus, 1758), and the striped red mullet, Mullus surmuletus (Linnaeus, 1758)—through the analysis of their stomach contents. We wanted to investigate whether the diet of the target species caught near the artificial reefs differs from the diet of the target species caught in natural habitats, and therefore if their feeding ecology has been altered by the presence of these artificial devices.
D. annularis and M. surmuletus were selected as target species for their abundance and representativeness of the area. Another fundamental aspect for which these species were chosen is their different ecological niches, which allow us to study the possible effects of barriers at different ecological levels. Indeed, M. surmuletus is a predominantly benthic species linked to rocky substrates, and D. annularis is a demersal species linked mostly to environments of prairies and sandy bottoms [21,22].
2. Materials and Methods
2.1. Study Site
This study was conducted in the Asinara Gulf (NW Sardinia coast, Italy) (Figure 1) where anti-trawling artifacts were placed in April 2017 at the Punta Tramontana (75 reef sets) and Fiume Santo (413 reef sets) sites.
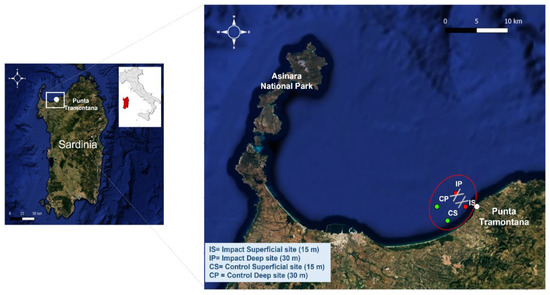
Figure 1.
Study area Punta Tramontana (Sardinia) and the four sampling sites: two impact sites characterized by the presence of anti-trawling reefs, Impact Superficial site (IS) and Impact Deep site (IP), located at different bathymetry (15 m and 30 m, respectively); and two control sites, Control Superficial site (CS) and Control Deep site (CP), selected because of the absence of anti-trawling reefs and selected at the same bathymetry as the impact sites (15 m and 30 m, respectively). The figure was generated by the QGIS sofware (ver. 3.16.7-Hannover, https://www.qgis.org/it/site/index.html (accessed on 10 June 2020)).
The deployment of these devices was performed in order to protect from any potential damage deriving from illegal trawling and the submarine cables of the Sardinia–Italian Mainland (SA.PE.I.) Electrical Project realized by Terna SpA [23] to provide the electrical connection between the Sardinian coast and the Latium coast across the Tyrrhenian Sea.
The artificial devices (tripods) are cross-shaped modules made of concrete and they are arranged in an attempt to inhibit the spaces necessary for the trajectory maneuvers of trawl nets and, at the same time, to determine the low impact on the seabed (support surface on the seabed is 40 × 40 cm per rod), especially on the P. oceanica biocenosis of Fiume Santo. To avoid any negative impact on the prairies of P. oceanica of the Fiume Santo area resulting from fishing activities, Punta Tramontana was the only area considered for fishing samplings because it is predominantly composed of sandy seabed alternating with a dead matte of P. oceanica seagrass (www.geoviewer.nnb.isprambiente.it (accessed on 1 May 2020)).
The samples were collected from two impact sites characterized by the presence of the artificial reefs and from two control sites selected 5 km away, exempt from the tripods and presenting the same environmental characteristics of the impact sites (similar sediments, depth, and oceanographic conditions). Both the impact and control sites were selected at two different depths: two surface stations at 15 m, named Impact Superficial site (IS) and Control Superficial site (CS), and two deep stations at 30 m named Impact Deep site (IP) and Control Deep site (CP).
2.2. Sampling Data
2.2.1. Dietary Analysis
We carried out fish sampling over a year in 5 seasonal campaigns (June 2017, October 2017, February 2018, June 2018, and October 2018) with the use of experimental barracuda-type gillnets (consisting of three different types of mesh, 15, 20, and 30 mm), which were lowered into the water at dusk (the timetable was variable according to the season) and hauled 4 h later. In the laboratory, individuals of the target species, M. surmuletus and D. annularis, were dissected for the analysis of their stomach contents. Stomachs were removed by excising the esophagus behind the oral cavity and the intestine just posterior to the pyloric caeca and preserved in a freezer. The number of empty tracts was recorded. Gut contents were sorted under a stereo microscope 150× and binocular microscope 1000×, prey were identified to the lowest possible taxonomic level, and their numbers were recorded for statistical analysis (Figure 2).
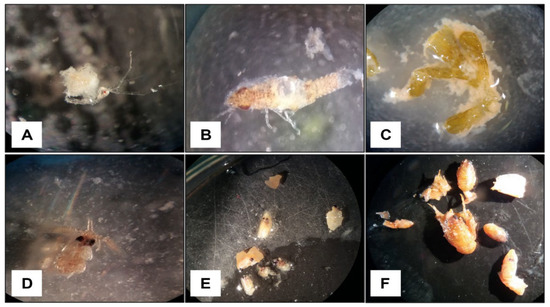
Figure 2.
Residues of prey items found in the stomach contents of D. annularis (A–C) and M. surmuletus (D–F), analyzed under a stereoscope. (A) Specimen of the Caprellidae family; (B) specimen of Gnathia maxillaris; (C) vegetal item; (D) specimen of Apanthura corsica; (E) specimens of 6 prey of Crustacea Decapoda, two belonging to the infraorder of Brachyura, four belonging to species of Athanas nitescens; (F) specimens of Galathea bolivari.
Macrobenthic on the artificial reefs and on the sediment near artificial reefs were sampled with the same frequency as the sampling of fish species. Benthic on the artificial reefs were sampled by removing the whole biological materials covering a 20 × 20 cm area by using scraping tools and the aspiration of the material with a compressed air sorbent (mesh of 1 mm). As regards invertebrates on the sediment near artificial reefs, a corer of 10 cm in diameter was used at a depth of 15 m of the seabottom. The sampled invertebrates were placed in special containers and filled with a 4% buffered formalin fixative solution. The identification of the invertebrates species at the lowest taxonomic level was performed in the laboratory.
2.2.2. Data Analysis
In order to study the feeding ecology of M. surmuletus and D. annularis and to detect the importance of each prey in the diet of the target species, a set of common indices in the stomach content analyses was used [24,25]. In particular, the Vacuity Coefficient (VI%) was used to identify the fraction of empty stomachs compared to the total sampled stomachs; the Abundance Index (N%) was calculated to define the number of each prey item in all non-empty stomachs in a sample; the Frequency of Occurrence (F%) of a given prey type was used to compute the number of stomachs in which that prey occurred.
Afterward, N% and F% were used to display through a Costello plot [26] the eventual alteration in the alimentary strategies of both D. annularis and M. surmuletus after the deployment of the artificial devices in the four examined sites (IS, IP, CS, CP) during the considered year.
Additionally, data regarding the composition of prey observed in the stomach contents of the target species were used to realize univariate and multivariate analyses.
As regards univariate analysis, the species richness index (Margalef), diversity index (Shannon–Weiner), and evenness index (Pielou) were computed to detect eventual changes in the predated items of gut contents of D. annularis and M. surmuletus across the examined year. The three ecological indices were performed with respect to the principal influencing factors: sites (IS, IP, CS, CP) and seasons (June 2017, October 2017, June 2018, October 2018).
Then, multivariate analysis was performed to detect similarity components in the composition of prey between the gut contents of the impact sites and control sites of the target species, the macrozoobenthos sampled on the artificial reefs, and the macroinvertebrates sampled on the sandy seabottom near artificial reefs [27]. Specifically, the similarities in the composition of considered items were calculated using the Bray–Curtis similarity coefficient [28], widely used in ecology analysis [27].
Subsequently, the similarity results were analyzed through the multi-dimensional scaling (nMDS) graphic representation, which consists of arranging the data on a two-dimensional plane, and from which the value of similarity can be analyzed.
The above-listed ecological indices and multivariate analysis were performed using the statistical package PRIMER6 [29].
3. Results
3.1. Alimentary Strategy in D. annularis
A total of 294 samples of D. annularis were caught. Of the total number of examined stomachs, 249 were empty (VI = 85%), whilst 45 (FI = 15%) had full and analyzable stomachs (Table 1).

Table 1.
Empty and full stomachs and Vacuity Index (VI%) of D. annularis at the Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), and Control Deep site (CP).
A broad diversity of prey items was found in the stomachs of this target species (Figure 3 and Table S1). An analysis based on the Abundance Index (N%) showed that Isopoda (33%) and Ostracoda taxa (31%) constituted the most abundantly observed groups, which with other crustacean groups make up N% = 86% of the diet, followed by Mollusca (N% = 8) and Polychaeta taxa (N% = 6).
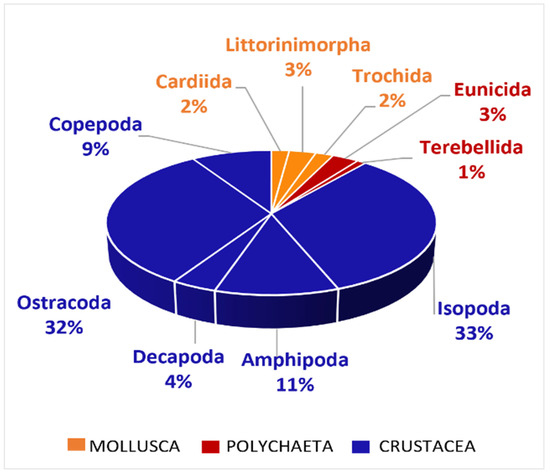
Figure 3.
Prey items found in the stomach contents of D. annularis, grouped into main taxa. Prey belonging to the Mollusca taxon are represented in orange, prey belonging to the Polychaeta taxon are represented in red, and prey belonging to the Crustacea taxon are represented in blue.
Figure 4 presents the alimentary strategy of D. annularis analyzed through a Costello plot at impact sites (IS, IP) and at control sites (CS, CP) during the five sampling campaigns.
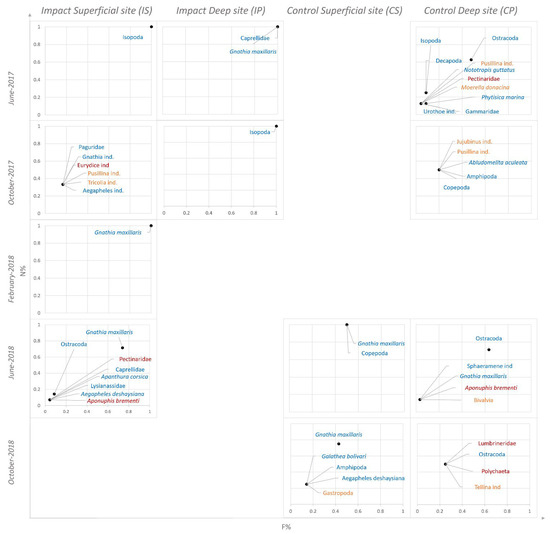
Figure 4.
Costello plot illustration (1990) to observe how the alimentary strategy of D. annularis changes at the impact sites (IS, IP) and control sites (CS, CP) during the five seasonal campaigns. Prey belonging to the Polychaeta taxon are represented in red, prey belonging to the Mollusca taxon are represented in yellow, prey belonging to the Crustacea taxon are represented in blue, and prey belonging to the Echinodermata taxon are represented in green.
Crustaceans, particularly the Caprellidae family, Isopoda order, and G. maxillaris species, were the dominant prey for all individuals of D. annularis at impact sites (IS, IP) during all the sampled periods (Figure 4).
On the contrary, D. annularis fed on a great range of prey belonging to other taxa, which have been found occasionally and sporadically in terms of abundance at IS in the campaigns of October 2017 and June 2018 (Figure 4).
Lastly, many preys were fed on rarely and occasionally by D. annularis at control sites (CS, CP) all over the period, and only a few individuals specialized in feeding on the Ostracoda taxon (Figure 4).
The ecological indices of Margalef and Shannon–Weiner (Figure 5) show that the richness and diversity of prey have low values at impact sites (both in IS and IP), except for a few stomach samples, which at the IS present a wide range of prey in the October 2017 and June 2018 campaigns.
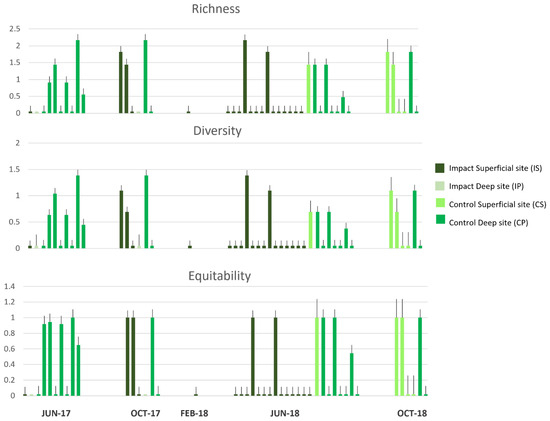
Figure 5.
Ecological indices of the richness (Margalef), diversity (Shannon-Weiner), and equitability (Pielou) of gut contents of D. annularis through the five sampling campaigns at the Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), Control Deep site (CP).
On the contrary, gut contents present a great richness and diversity of prey at control sites (CS and CP) in all sampling campaigns (Figure 5).
The equitability index shows that the same stomach contents with high values in richness and diversity also have a good value of equitability; on the contrary, lower values in richness and diversity of prey correspond to minimal values of equitability (Figure 5).
The analysis of nMDS (Figure 6) shows a great dissimilarity between the composition of macrozoobenthos sampled from the sandy seabottom surrounding the artificial reefs and macroinvertebrates sampled on artificial reefs, indicating that the species present in these two habitats are composed of different species. On the contrary, prey species of the gut contents are slightly overlapped with macroinvertebrates sampled on artificial reefs, indicating that prey found in the stomach contents of D. annularis are the same as those sampled on artificial reefs. Nevertheless, the prey at impact sites (IS, IP) is represented more by the species of artificial reefs rather than the species of sandy seabottom.
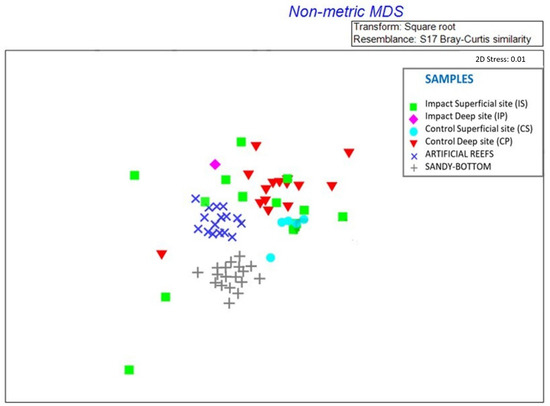
Figure 6.
Non-metric Multi-Dimensional Scaling analysis shows the similarity in the composition of prey at sampling sites: Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), and Control Deep site (CP). Prey are represented by different colors according to the different sampling sites.
3.2. Alimentary Strategy in M. surmuletus
As regards M. surmuletus, a total of 159 individuals were caught. Only 47 had full stomachs (FI% = 42%) and 112 had empty stomachs (VI% = 58) (Table 2).

Table 2.
Empty and full stomachs and the Vacuity Index (VI%) of M. surmuletus at the Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), and Control Deep site (CP).
The diet composition consisted of 4 major prey taxa: Crustacea, Mollusca, Polychaeta, and Echinodermata (Figure 7 and Table S2).
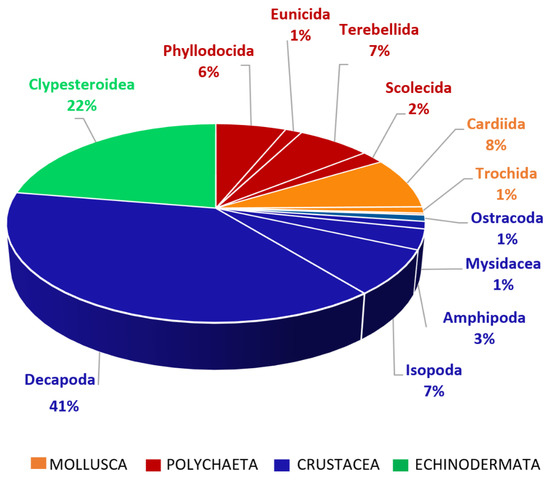
Figure 7.
Prey items found in the stomach contents of M. surmuletus, grouped into main taxa. Prey belonging to the Mollusca taxon are represented in orange, prey belonging to the Polychaeta taxon are represented in red, prey belonging to the Crustacea taxon are represented in blue, and prey belonging to the Echinodermata taxon are represented in green.
Crustaceans are the most predated group (N = 51%), among which the most abundant are species belonging to the Decapoda (n = 39%), Isopoda (n = 7%), and Amphipoda (n = 3%) taxa. Echinodermata, exclusively the Echinocyamus pusillus species, constituted the second position in the feeding habits of the striped red mullet (22%). Other taxa were less abundant (Polychaeta n = 16%, Mollusca n = 9%) (Figure 7). The alimentary strategy of M. surmuletus is extremely generalist (Figure 8). Both at impact sites (IS, IP) and control sites (CS, CP), there is a wide diversity in species belonging to different taxa, all predated sporadically and rarely (Figure 8). Only a few items represented at the top left of the graph are to be considered dominant in the feeding habits of a few individuals of M. surmuletus (Figure 8).
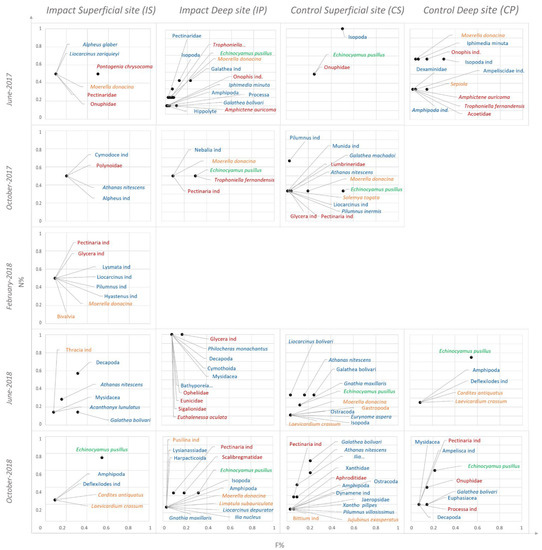
Figure 8.
Costello plot illustration (1990) to observe how the alimentary strategy of M. surmuletus changed at the impact sites (IS, IP) and control sites (CS, CP) during the five seasonal campaigns. Prey belonging to the Polychaeta taxon are represented in red, prey belonging to the Mollusca taxon are represented in yellow, prey belonging to the Crustacea taxon are represented in blue, and prey belonging to the Echinodermata taxon are represented in green.
In Figure 9, the ecological indices report great values of richness and diversity of prey across all sampled sites in all sampling campaigns, and the highest values were recorded at the sites of IS in February 2018 and IP in June 2018.
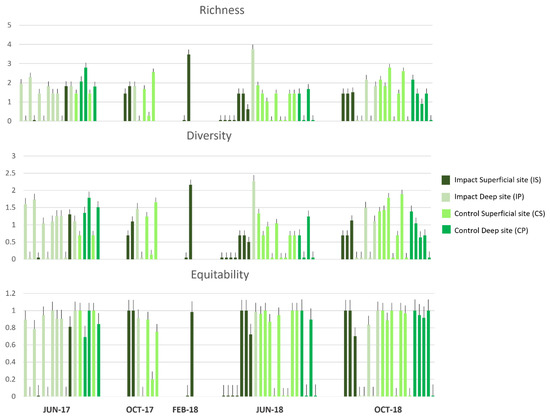
Figure 9.
Ecological indices of the richness (Margalef), diversity (Shannon–Weiner), and equitability (Pielou) of the gut contents of M. surmuletus through the five sampling campaigns at the Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), and Control Deep site (CP).
Equitability shows high values in all sampled sites. Therefore, the distribution of prey is homogeneous, with no preference for one item over another (Figure 9).
In Figure 10, nMDS shows a great dissimilarity between the composition of invertebrates sampled on the anti-trawling reefs and invertebrates sampled on the seabottom substrate surrounding the artificial reefs, indicating that the species present in these two habitats are composed of different species. As regards the prey of M. surmuletus, they show similarity both for species sampled on anti-trawling reefs and for species sampled on the seabottom, since in Figure 10 they are represented as overlapping.
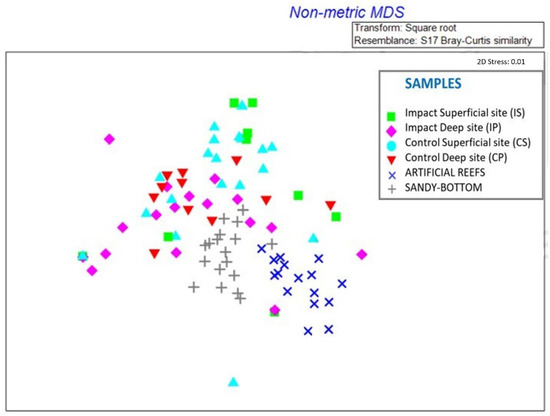
Figure 10.
Non-metric Multi-Dimensional Scaling analysis shows the similarity in the composition of prey at sampling sites: Impact Superficial site (IS), Impact Deep site (IP), Control Superficial site (CS), and Control Deep site (CP). Prey are represented with different colors according to the different sampling sites.
4. Discussion
In this paper, we firstly contributed and updated the pre-existing knowledge of the feeding ecology of D. annularis and M. surmuletus through their gut contents in the Mediterranean context, highlighting the predated items, which have often been taxonomically identified at the species level, thus obtaining a more precise view of the feeding habits of the target species.
Additionally, we examined an eventual alteration in the feeding habits of the studied species of fish after the deployment of the anti-trawling reefs at the Punta Tramontana site.
This aspect is fundamental to verify how artificial reefs, in addition to an anti-trawling function, interact with the species of the surrounding habitat.
We suggest three alternative hypotheses: (1) anti-trawling reefs actually contribute to increasing the availability of trophic resources of the fish attracted to these structures; (2) anti-trawling reefs modify the feeding habits of the fishes and therefore alter the ecology of the species; and (3) fish avoid anti-trawling reefs, so these devices decrease the substrate where the species can forage.
It must be prefaced that several constraints were encountered in the analysis of the stomach contents of the species.
First, many stomachs were empty. The high percentage of empty stomachs may reflect short periods of feeding followed by periods of rapid digestion [30].
Moreover, some gut contents were not analyzable as they were damaged and appeared completely white and dry, and other times they were completely black.
On the other hand, a difficulty concerning the analysis and identification of the stomach contents was the fragmentation of prey due to digestion in progress, and therefore the lack of diagnostic characteristics on which to center the taxonomic identification of prey.
4.1. Effects of Anti-Trawling Reefs on D. annularis
In the sampled areas, the diet of D. annularis consisted of the Crustacea taxon as the dominant prey (Figure 3), followed by the Mollusca and Polychaeta taxa in smaller quantities.
D. annularis is widely considered a generalist species [4,20,21,30,31], which adjusts its diet to the food items available in the surrounding habitat.
Indeed, while the same composition of diet was found in a study conducted in Portugal [32,33], where the target species fed mainly on small crustaceans, different species of prey, consisting of mollusks and vegetal items, were found in the stomach contents of annular seabream in the Gulf of Gabes area (Tunisia) [30].
As regards the alimentary strategy of annular seabream (Figure 4), significant differences were found between the two impact sites (IS, IP) and the two control sites (CS, CP).
Indeed, D. annularis showed a preference in its diet for the isopod G. maxillaris during the entire examined period, both at IS and IP, while at the two control sites they fed on prey belonging to different taxa and a few individuals fed on the Ostracoda taxon as their dominant prey. This result suggests that the diet of this species can change according to the habitat in which the individuals are caught. Evident changes in the feeding ecology of annular seabream, following the installation of artificial anti-trawling reefs, were already observed by [4], who studied this phenomenon in Alicante (Spain), deducing preferences for copepods at artificial reef sites and for appendicularians and doliolids at control sites, characterized by the presence of P. oceanica meadows.
The change in the feeding habits of D. annularis may depend on several factors, such as the presence of different compositions and abundances of macroinvertebrate species associated with rocky substrates—in this case, simulated by artificial reefs [4].
Furthermore, this could also depend on the ecology of the annular seabream, which tends to be a sedentary species that settle at the same site and suffer more from the presence of barriers [19,20,34].
The changes in the diet of the target species analyzed through Costello plots are also supported by the values obtained from the ecological indices (Figure 5). Specialist behavior, indicated by the low values in richness and diversity indices, was observed at impact sites where annular seabream focused its diet on crustacean species, while a generalist strategy, indicated by the high values of the same indices, was deduced at both control sites.
Evidence showing that D. annularis fed near the artificial reefs was obtained with nMDS analysis, which shows an overlap between the prey in the stomach contents of D. annularis caught at the impact sites and the macroinvertebrate species that come from the recruitment on the reefs.
Moreover, [10] observed that artificial structures represent a further source of food for the studied species, demonstrating that prey items were exclusively or chiefly found on the artificial barriers. A similar trophic linkage between the annular seabream and the benthic communities associated with artificial substrates was also shown along the Mediterranean French coast [15] and in the Ligurian Sea [20]. On the contrary, [4,7] reported that the preferred feeding sites of D. annularis were the sandy bottoms around the reef and not the reef itself in the Central Tyrrhenian Sea.
4.2. Effects of Anti-Trawling Reefs on M. surmuletus
As regards M. surmuletus, its diet consists of crustaceans as the preferential taxon, according to [35,36,37,38,39,40]; other items belonging to the Echinodermata, Mollusca, and Polychaeta taxa are present less frequently (Figure 7).
No alterations were observed in the feeding strategy of the striped red mullet between the impact sites and control sites. Indeed, the studied species showed a generalist behavior in its diet over the examined period (Figure 8).
This aspect is evident also in the ecological indices (Figure 9), which present high values of richness and diversity at all sites over the whole period, highlighting the generalist feeding strategy of the striped red mullet.
The results agree with those of [16], who concluded that this species is not stressed by the presence of protective structures, and of [34], who observed that mobile species expand their food spectrum with artificial-reef-colonizing invertebrates, but show less definite alteration in feeding habits than sedentary fish species.
This explains why the MDS analysis (Figure 10) represents such dispersed data, and there is no defined similarity between samples of the impact sites’—artificial reefs and samples of the control sites—sandy seabottom.
Thus, M. surmuletus has expanded its trophic niche with the macrozoobenthos of the barriers without modifying its feeding behavior.
According to the obtained results, few studies were found discussing the consequences of the alimentary strategy of M. surmuletus after the installation of artificial devices.
Among these studies, [14,16,34] reported that the striped red mullet suffers no negative effects from the presence of anti-trawling artificial barriers. On the contrary, these structures can be of support for feeding or even increase the foraging activity of the species.
5. Conclusions
Anti-trawling reefs may serve as a useful management tool in coastal areas to avoid trawling. Once deployed, these structures provide several other services representing important habitats for macrozoobenthic communities [13,17,18], protecting seagrass beds from illegal trawling [8,41], and providing new habitats for fish species, increasing trophic resources [7,19,42]. On the other hand, artificial reefs can alter the ecology of several marine species [4,43,44], such as the feeding habits of fish in the area [9].
In this study, D. annularis, being a more sedentary species, suffers more from the presence of the barriers, changing its alimentary strategy from generalist at control sites to specialist at impact sites, and focusing its diet on G. maxillaris species.
On the contrary, M. surmuletus, due to the fact that it constantly moves to look for prey available in the area, is not affected at all by the presence of barriers, which constitute an additional source of food when it passes near these artificial devices.
Since the two studied species showed two different behaviors following the installation of the barriers, monitoring plans remain fundamental steps that should always be considered after the deployment of these devices to monitor how the fauna and flora communities of the surrounding area are influenced and altered.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15010011/s1, Table S1. List of prey found in the stomach contents of D. annularis and percentage values of the corresponding Indices of Abundance (N%), Frequency (F%) and Specific Abundance (PSA). Table S2. List of prey found in the stomach contents of M. surmuletus and percentage values of the corresponding Indices of Abundance (N%), Frequency (F%) and Specific Abundance (PSA).
Author Contributions
Conceptualization, S.S., M.S., S.L., O.N., A.A. and P.T.; data curation, S.S.; formal analysis, S.S.; investigation, S.S.; methodology, S.S.; writing—original draft, S.S.; writing—review and editing, S.S., M.S., S.L., O.N., A.A. and P.T.; supervision, M.S. and P.T.; validation, M.S. and P.T.; visualization, M.S. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was supported by funds from the Ministry of Education, University, and Research for the base research individual activities, and by the Grant of Excellence Departments, MIUR-Italy (Article 1, Paragraphs 314-337, Law 232/2016).
Institutional Review Board Statement
The studied species of fish were selected from the catch realized with the collaboration of expert fishermen. The dissection and analysis of fish were performed on non-living specimens in the laboratory.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to the fact that the monitoring study is not yet completed. Data are, however, available from the corresponding author on reasonable request.
Acknowledgments
Thanks to the the fishermen for their availability and for the fundamental help they provided us during the sampling activities.
Conflicts of Interest
The authors declare no financial interests. The authors declare that they have no conflict of interest.
References
- Di Franco, E.; Di Franco, A.; Calò, A.; Di Lorenzo, M.; Mangialajo, L.; Bussotti, S.; Bianchi, C.N.; Guidetti, P. Inconsistent Relationships among Protection, Benthic Assemblage, Habitat Complexity and Fish Biomass in Mediterranean Temperate Rocky Reefs. Ecol. Indic. 2021, 128, 107850. [Google Scholar] [CrossRef]
- Carminatto, A.A.; Rotundo, M.M.; Butturi-Gomes, D.; Barrella, W.; Petrere Junior, M. Effects of Habitat Complexity and Temporal Variation in Rocky Reef Fish Communities in the Santos Estuary (SP), Brazil. Ecol. Indic. 2020, 108, 105728. [Google Scholar] [CrossRef]
- Worm, B. Averting a Global Fisheries Disaster. Proc. Natl. Acad. Sci. USA 2016, 113, 4895–4897. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jerez, P.; Gillanders, B.M.; Rodríguez-Ruiz, S.; Ramos-Esplá, A.A. Effect of an Artificial Reef in Posidonia Meadows on Fish Assemblage and Diet of Diplodus annularis. ICES J. Mar. Sci. 2002, 59, S59–S68. [Google Scholar] [CrossRef][Green Version]
- Costello, C.; Ovando, D.; Clavelle, T.; Kent Strauss, C.; Hilborn, R.; Melnychuk, M.C.; Branch, T.A.; Gaines, S.D.; Szuwalski, C.S.; Cabral, R.B.; et al. Global Fishery Prospects under Contrasting Management Regimes. Proc. Natl. Acad. Sci. USA 2016, 113, 5125–5129. [Google Scholar] [CrossRef] [PubMed]
- Leitão, F.; Santos, M.N.; Monteiro, C.C. Contribution of Artificial Reefs to the Diet of the White Sea Bream (Diplodus sargus). ICES J. Mar. Sci. 2007, 64, 473–478. [Google Scholar] [CrossRef][Green Version]
- Ardizzone, G.; Somaschini, A.; Belluscio, A. Prediction of Benthic and Fish Colonization on the Fregene and Other Mediterranean Artificial Reefs. In Artificial Reefs in European Seas; Springer: Dordrecht, The Netherlands, 2000; pp. 113–128. [Google Scholar] [CrossRef]
- González-Correa, J.M.; Bayle, J.T.; Sánchez-Lizaso, J.L.; Valle, C.; Sánchez-Jerez, P.; Ruiz, J.M. Recovery of Deep Posidonia Oceanica Meadows Degraded by Trawling. J. Exp. Mar. Biol. Ecol. 2005, 320, 65–76. [Google Scholar] [CrossRef]
- Feary, D.A.; Burt, J.A.; Bartholomew, A. Artificial Marine Habitats in the Arabian Gulf: Review of Current Use, Benefits and Management Implications. Ocean Coast. Manag. 2011, 54, 742–749. [Google Scholar] [CrossRef]
- Fabi, G.; Spagnolo, A. Artificial Reefs in the Management of Mediterranean Sea Fisheries; CRC Press: Boca Raton, FL, USA, 2011; pp. 167–186. [Google Scholar] [CrossRef]
- Baine, M. Safety Effectiveness of Highway Design Features: Cross Sections. Volume III. Ocean Coast. Manag. 2001, 44, 19. [Google Scholar]
- Hensel, E.; Allgeier, J.E.; Layman, C.A. Effects of Predator Presence and Habitat Complexity on Reef Fish Communities in The Bahamas. Mar. Biol. 2019, 166, 136. [Google Scholar] [CrossRef]
- Perkol-Finkel, S.; Shashar, N.; Benayahu, Y. Can Artificial Reefs Mimic Natural Reef Communities? The Roles of Structural Features and Age. Mar. Environ. Res. 2006, 61, 121–135. [Google Scholar] [CrossRef]
- Iannibelli, M.; Musmarra, D. Effects of Anti-Trawling Artificial Reefs on Fish Assemblages: The Case of Salerno Bay (Mediterranean Sea). Ital. J. Zool. 2008, 75, 385–394. [Google Scholar] [CrossRef]
- Ruitton, S.; Francour, P.; Boudouresque, C.F. Relationships between Algae, Benthic Herbivorous Invertebrates and Fishes in Rocky Sublittoral Communities of a Temperate Sea (Mediterranean). Estuar. Coast. Shelf Sci. 2000, 50, 217–230. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Jerez, P.; Ramos-Esplá, A. Changes in Fish Assemblages Associated with the Deployment of an Antitrawling Reef in Seagrass Meadows. Trans. Am. Fish. Soc. 2000, 129, 1150–1159. [Google Scholar] [CrossRef]
- Ardizzone, G.D.; Gravina, M.F.; Belluscio, A. Temporal Development of Epibenthic Communities. Bull. Mar. Sci. 1989, 44, 592–608. [Google Scholar]
- Thanner, S.E.; McIntosh, T.L.; Blair, S.M. Development of Benthic and Fish Assemblages on Artificial Reef Materials Compared to Adjacent Natural Reef Assemblages in Miami-Dade County, Florida. Bull. Mar. Sci. 2006, 78, 57–70. [Google Scholar]
- Fabi, G.; Manoukian, S.; Spagnolo, A. Feeding Behavior of Three Common Fishes at an Artificial Reef in the Northern Adriatic Sea. Bull. Mar. Sci. 2006, 78, 39–56. [Google Scholar]
- Relini, G.; Relini, M.; Torchia, G.; De Angelis, G. Trophic Relationships between Fishes and an Artificial Reef. ICES J. Mar. Sci. 2002, 59, S36–S42. [Google Scholar] [CrossRef][Green Version]
- Matić-Skoko, S.; Antolić, B.; Kraljević, M. Ontogenetic and Seasonal Feeding Habits of the Annular Seabream (Diplodus annularis L.) in Zostera Sp. Beds, Eastern Adriatic Sea. J. Appl. Ichthyol. 2004, 20, 376–381. [Google Scholar] [CrossRef]
- Mazzola, A.; Lopiano, L.; La Rosa, T.; Sarà, G. Diel Feeding Habits of Juveniles of Mullus surmuletus (Linneo, 1758) in the Lagoon of the Stagnone Di Marsala (Western Sicily, Italy). J. Appl. Ichthyol. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- Bacci, T.; Rende, S.F.; Nonnis, O.; Maggi, C.; Izzi, A.; Gabellini, M.; Massara, F.; Di Tullio, L. Effects of Laying Power Cables on a Posidonia oceanica (L.) Delile Prairie: The Study Case of Fiume Santo (NW Sardinia, Italy). J. Coast. Res. 2013, 65, 868–873. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach Contents Analysis—A Review of Methods and Their Application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A New Approach to Graphical Analysis of Feeding Strategy from Stomach Contents Data—Modification of the Costello (1990) Method. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Costello, M.J. Predator Feeding Strategy and Prey Importance: A New Graphical Analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional Dissimilarity as a Robust Measure of Ecological Distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar]
- Clarke, K.R.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Chaouch, H.; Ben Abdallah-Ben Hadj Hamida, O.; Ghorbel, M.; Jarboui, O. Feeding Habits of the Annular Seabream, Diplodus annularis (Linnaeus, 1758) (Pisces: Sparidae), in the Gulf of Gabes (Central Mediterranean). Cah. Biol. Mar. 2014, 55, 13–19. [Google Scholar]
- Pallaoro, A.; Šantić, M.; Jardas, I. Feeding Habits of the Saddled Bream, Oblada Melanura (Sparidae), in the Adriatic Sea. Cybium 2003, 27, 261–268. [Google Scholar]
- Pita, C.; Gamito, S.; Erzini, K. Feeding Habits of the Gilthead Seabream (Sparus aurata) from the Ria Formosa (Southern Portugal) as Compared to the Back Seabream (Spondyliosoma Cantharus) and the Annular Seabream (Diplodus annularis). J. Appl. Ichthyol. 2002, 18, 81–86. [Google Scholar] [CrossRef]
- Gamito, S.; Pires, A.; Pita, C.; Erzini, K. Food Availability and the Feeding Ecology of Ichthyofauna of a Ria Formosa (South Portugal) Water Reservoir. Estuaries 2003, 26, 938–948. [Google Scholar] [CrossRef]
- Cenci, E.; Pizzolon, M.; Chimento, N.; Mazzoldi, C. The Influence of a New Artificial Structure on Fish Assemblages of Adjacent Hard Substrata. Estuar. Coast. Shelf Sci. 2011, 91, 133–149. [Google Scholar] [CrossRef]
- Bautista-Vega, A.A.; Letourneur, Y.; Harmelin-Vivien, M.; Salen-Picard, C. Difference in Diet and Size-Related Trophic Level in Two Sympatric Fish Species, the Red Mullets Mullus Barbatus and Mullus Surmuletus, in the Gulf of Lions (North-West Mediterranean Sea). J. Fish Biol. 2008, 73, 2402–2420. [Google Scholar] [CrossRef]
- Golani, D. Niche Separation between Colonizing and Indigenous Goatfishes (Mullidae) of the Mediterranean Coast of Israel. J. Fish Biol. 1994, 45, 503–513. [Google Scholar] [CrossRef]
- Golani, D.; Galil, B. Trophic Relationships of Colonizing and Indigenous Goatfishes (Mullidae) in the Eastern Mediterranean with Special Emphasis on Decapod Crustaceans. Hydrobiologia 1991, 218, 27–33. [Google Scholar] [CrossRef]
- Labropoulou, M.; Machias, A.; Tsimenides, N.; Eleftheriou, A. Feeding Habits and Ontogenetic Diet Shift of the Striped Red Mullet, Mullus Surmuletus Linnaeus, 1758. Fish. Res. 1997, 31, 257–267. [Google Scholar] [CrossRef]
- Labropoulou, M.; Papadopoulou-Smith, K.-N. Foraging Behaviour Patterns of Four Sympatric Demersal Fishes. Estuar. Coast. Shelf Sci. 1999, 49, 99–108. [Google Scholar] [CrossRef]
- Pavičić, M.; Šiljić, J.; Brajčić Jurica, D.; Matić-Skoko, S. Feeding Habits of the Striped Red Mullet, Mullus Surmuletus in the Eastern Adriatic Sea. Acta Adriat. 2018, 59, 123–136. [Google Scholar] [CrossRef]
- Sánchez-Jerez, P.; Ramos-Esplá, A.A. Detection of Environmental Impacts by Bottom Trawling on Posidonia oceanica (L.) Delile Meadows: Sensitivity of Fish and Macroinvertebrate Communities. J. Aquat. Ecosyst. Health 1996, 5, 239–253. [Google Scholar] [CrossRef]
- Walker, B.K.; Henderson, B.; Spieler, R.E. Fish Assemblages Associated with Artificial Reefs of Concrete Aggregates or Quarry Stone Offshore Miami Beach, Florida, USA. Aquat. Living Resour. 2002, 15, 95–105. [Google Scholar] [CrossRef]
- Becker, A.; Taylor, M.D.; Lowry, M.B. Monitoring of Reef Associated and Pelagic Fish Communities on Australia’s First Purpose Built Offshore Artificial Reef. ICES J. Mar. Sci. 2017, 74, 277–285. [Google Scholar] [CrossRef]
- Burt, J.; Bartholomew, A.; Usseglio, P.; Bauman, A.; Sale, P.F. Are Artificial Reefs Surrogates of Natural Habitats for Corals and Fish in Dubai, United Arab Emirates? Coral Reefs 2009, 28, 663–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).