Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solvent Extraction
2.2.2. Supercritical CO2 Extraction
2.2.3. Fatty Acid Composition Analyses
2.2.4. Analyses of Triglycerides (TAGs)
2.2.5. Total Phenolic Contents
2.2.6. Total Flavonoids Contents
2.2.7. Antioxidant Activity
DPPH Radical-Scavenging Method
ABTS Radical-Scavenging Assay
3. Results and Discussion
3.1. Composition of SC-CO2 Extracts: Oil Composition Analyses
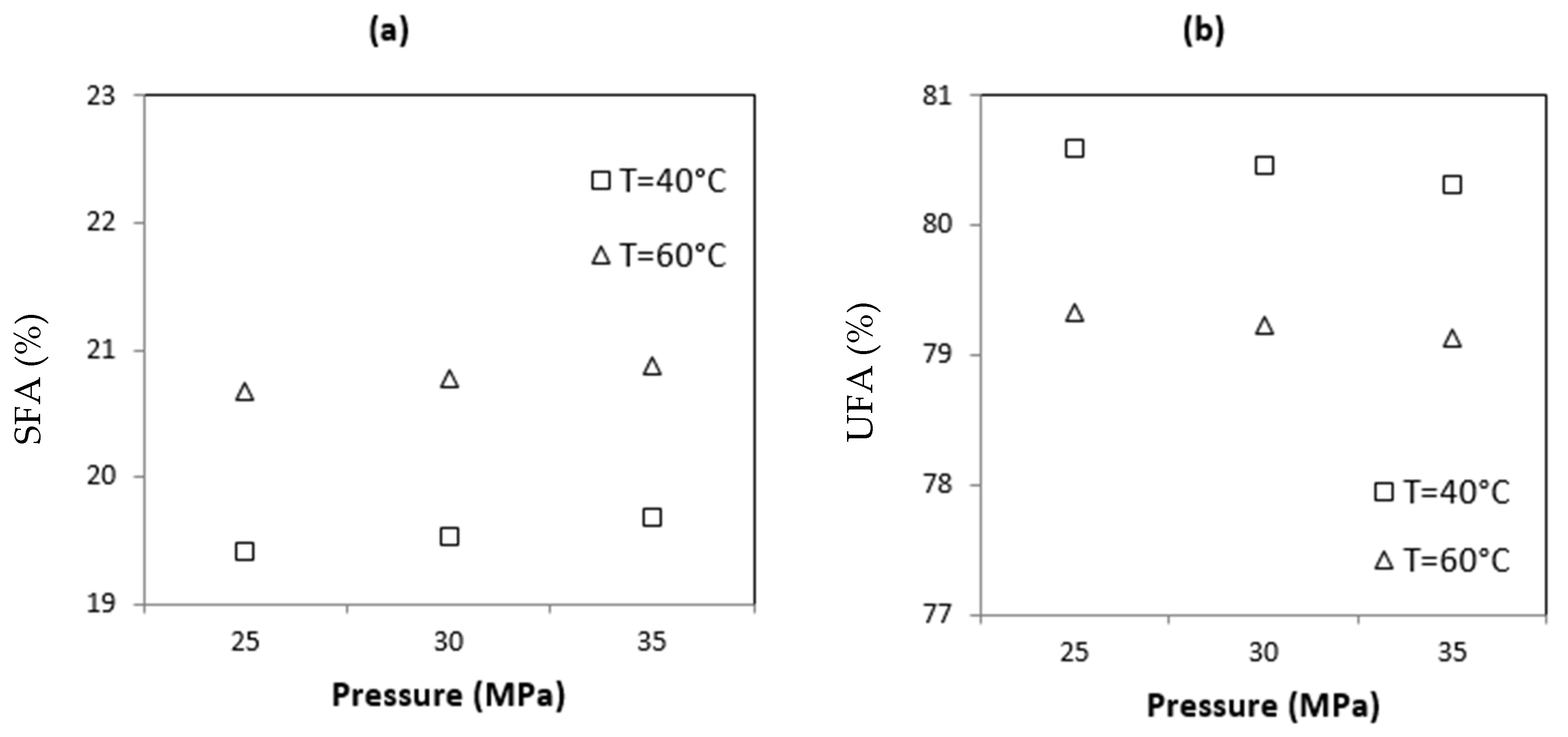
3.1.1. Fatty Acids
3.1.2. Triglycerides (TAGs)
3.2. Polar Fraction: Analysis of Lyophilized OMW Residue after the SFE
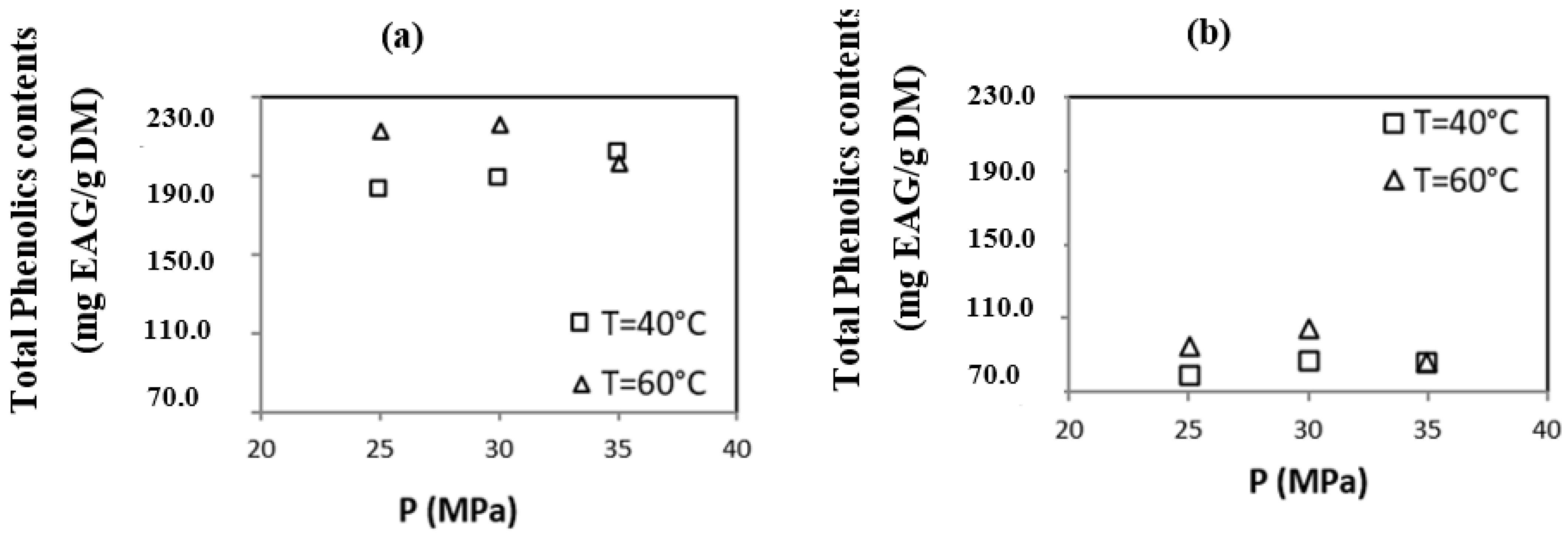
3.2.1. Total Phenolic Content
3.2.2. Total Flavonoids
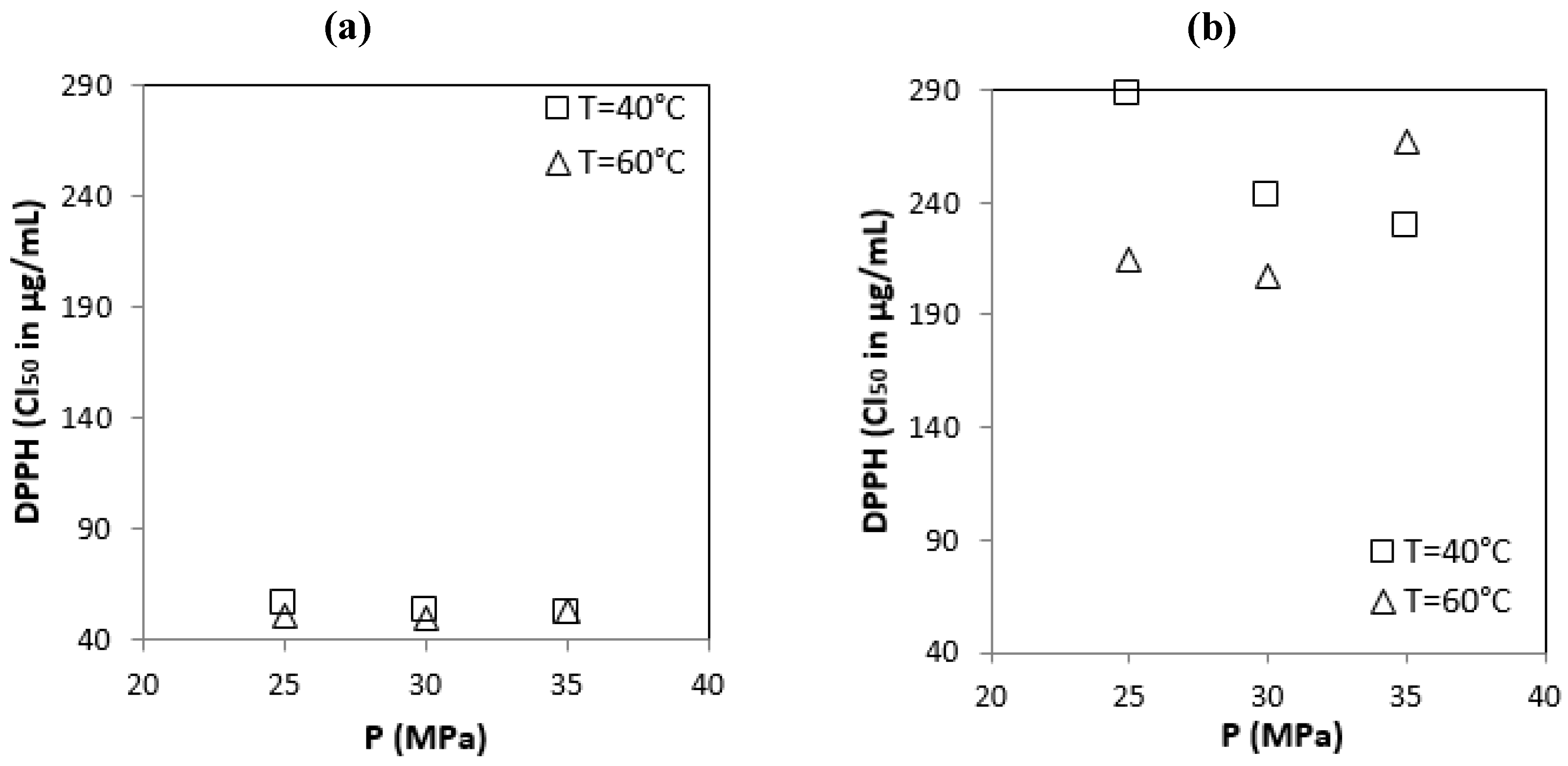
3.2.3. Free Radical-Scavenging Ability by the Use of a Stable DPPH Radical
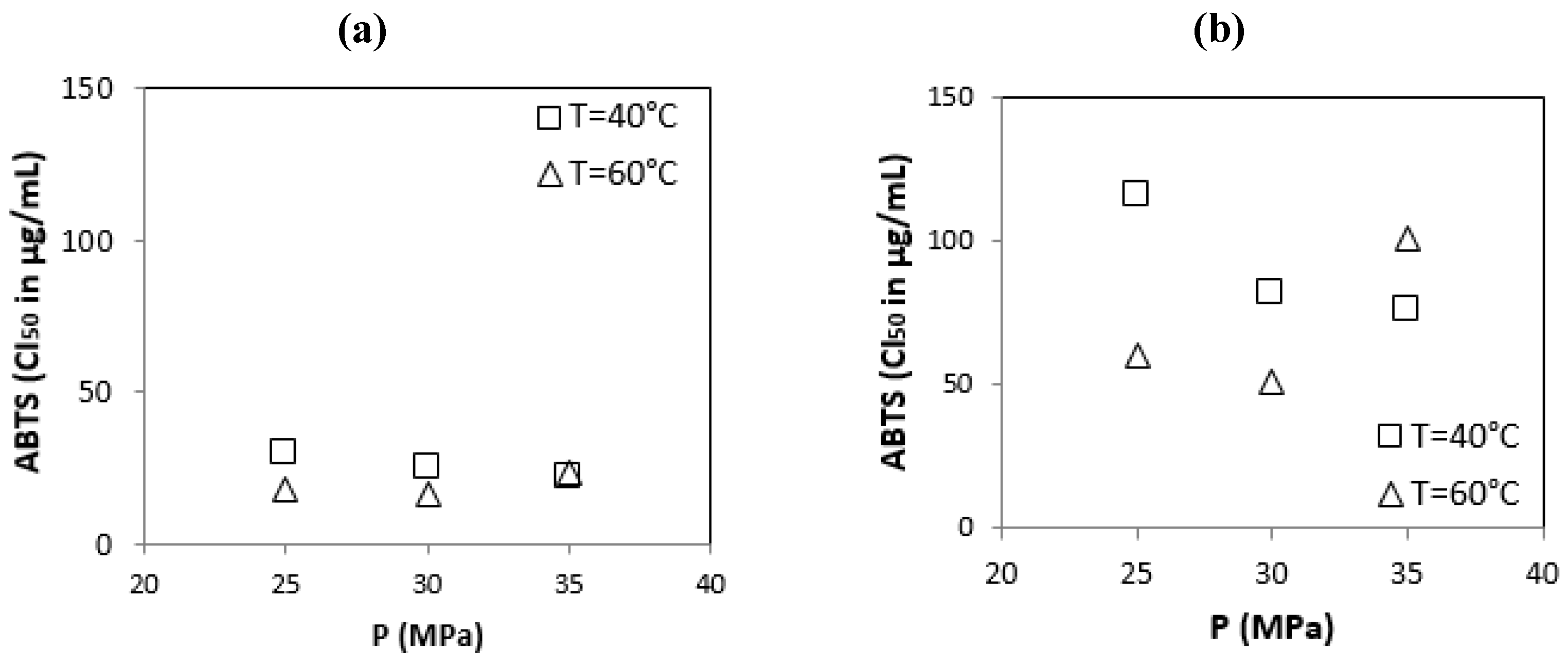
3.2.4. ABTS Free Radical-Scavenging Activity
3.3. Composition of the Oil Extracted by the Conventional Solvent Extraction
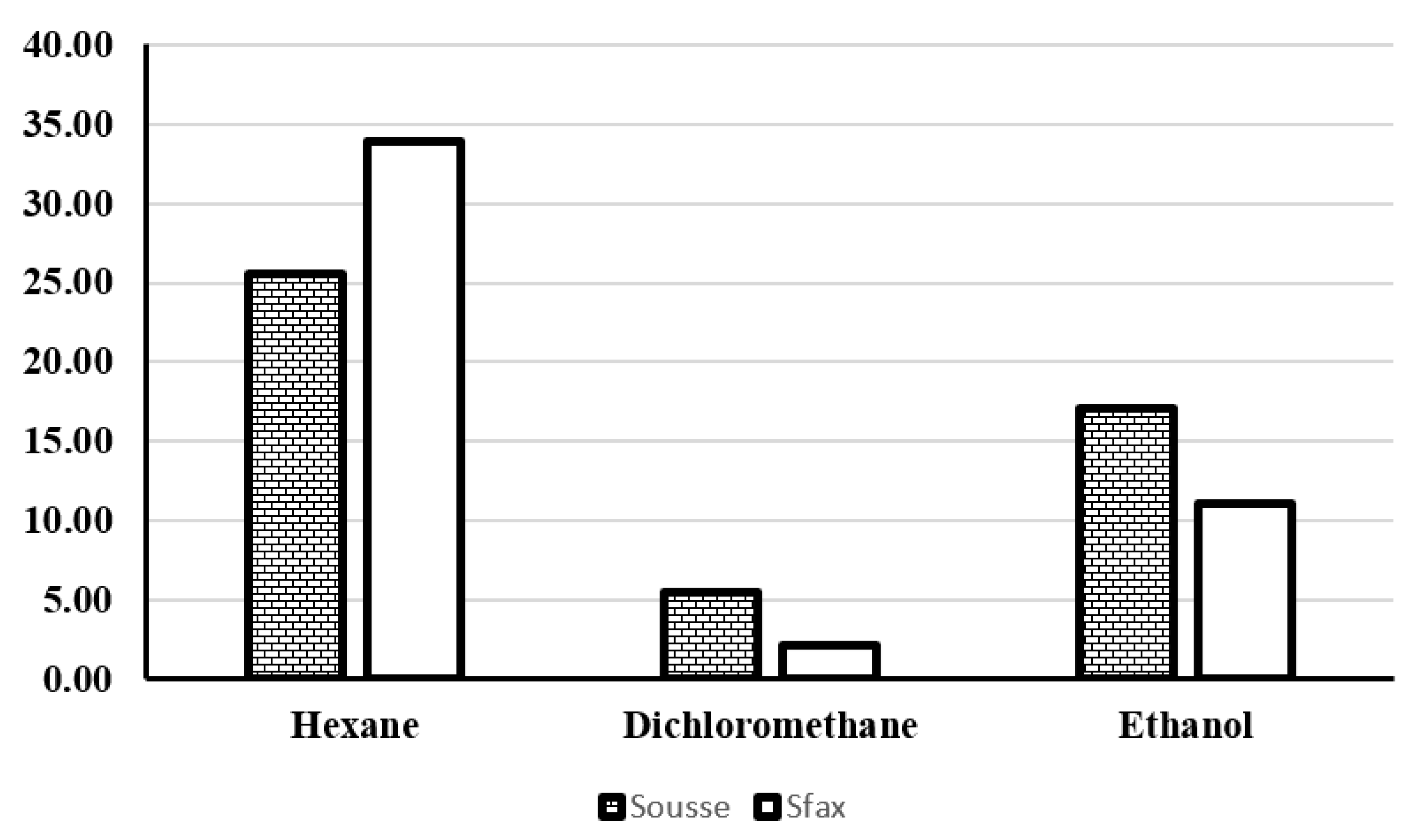
3.3.1. Evaluation of the Extracted Oil Yield
3.3.2. Analysis of Hexane Extracts
Fatty Acids
Triglycerides
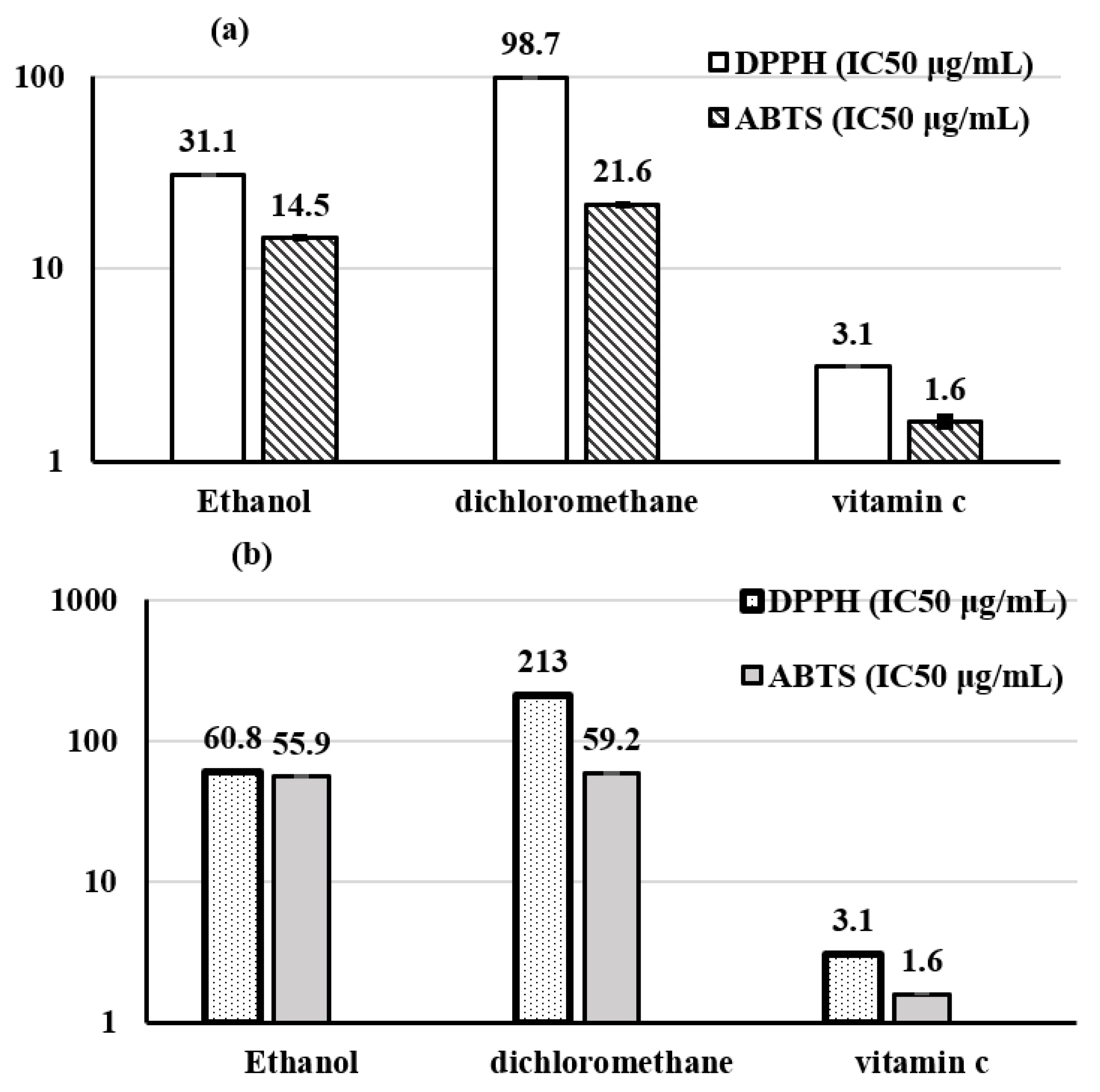
3.3.3. Polar Fraction: Analyses of Ethanol and Dichloromethane Extracts
Total Phenolic Content
Total Flavonoids
Free Radical-Scavenging Ability by the Use of a Stable DPPH Radical
ABTS Free Radical-Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamza, F.; Hanane, S. The Effect of Microhabitat Features, Anthropogenic Pressure and Spatial Structure on Bird Diversity in Southern Tunisian Agroecosystems. Ann. Appl. Biol. 2021, 179, 195–206. [Google Scholar] [CrossRef]
- Dakhli, R.; Gosh, A.; Wali, A.; Chandra, M.M.; Khatteli, H. Agricultural valorization of olive mill wastewater in arid regions of Tunisia: Short-term impact on soil biochemical properties and faba bean growth. Pol. J. Environ. Stud. 2021, 30, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.O.J.; Hazaimeh, S.A. Olive Mill Wastewater Treatment and Valorization by Extraction/Concentration of Hydroxytyrosol and Other Natural Phenols. Process Saf. Environ. Prot. 2021, 148, 495–523. [Google Scholar] [CrossRef]
- Meksi, N.; Haddar, W.; Hammami, S.; Mhenni, M.F. Olive mill wastewater: A potential source of natural dyes for textile dyeing. Ind. Crops Products 2012, 40, 103–109. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Comegna, A.; Dragonetti, G.; Kodesova, R.; Coppola, A. Impact of olive mill wastewater (OMW) on the soil hydraulic and solute transport properties. Int. J. Environ. Sci. Technol. 2022, 19, 7079–7092. [Google Scholar] [CrossRef]
- Rharrabti, Y.; EI Yamani, M. Olive Mill Wastewater: Treatment and Valorization Technologies. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Sehar, S. Wastewater treatment of food industries through constructed wetland: A review. Int. J. Environ. Sci. Technol. 2019, 16, 6453–6472. [Google Scholar] [CrossRef]
- Al Bawab, A.; Abu-Dalo, M.; Khalaf, A.; Abu-Dalo, D. Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media. Catalysts 2022, 12, 539. [Google Scholar] [CrossRef]
- Mancuso, A.; Morante, N.; De Carluccio, M.; Sacco, O.; Rizzo, L.; Fontana, M.; Esposito, S.; Vaiano, V.; Sannino, D. Solar driven photocatalysis using iron and chromium doped TiO2 coupled to moving bed biofilm process for olive mill wastewater treatment. Chem. Eng. J. 2022, 450, 138107. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Solomakou, N.; Goula, A.M. Treatment of Olive Mill Wastewater by Adsorption of Phenolic Compounds. Rev. Environ. Sci. Biotechnol. 2021, 20, 839–863. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total Phenolic Content and Antioxidant Capacity of Agri-Food Waste and by-Products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.; Fernando, L. Antioxidant Properties of Natural Plant Extracts Containing Polyphenolic Compounds in Cooked Ground Beef. J. Food Sci. 2002, 67, 1364–1369. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The Health Effects of Dietary Unsaturated Fatty Acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Lucas, A.M.H.; Bento, A.F.M.L.; Vargas, R.M.F.; Scheffel, T.B.; Rockenbach, L.; Diz, F.M.; Capellari, A.R.; Morrone, F.B.; Cassel, E. Use of Supercritical CO2 to Obtain Baccharis Uncinella Extracts with Antioxidant and Antitumor Activity. J. CO2 Util. 2021, 49, 101563. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of Bio-Oils from Algae with Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Martins, D.; Martins, R.C.; Braga, M.E.M. Biocompounds Recovery from Olive Mill Wastewater by Liquid-Liquid Extraction and Integration with Fenton ’ s Process for Water Reuse. Environ. Sci. Pollut. Res. 2021, 28, 29521–29534. [Google Scholar] [CrossRef]
- Dali, I.; Aydi, A.; Stamenic, M.; Kolsi, L.; Ghachem, K.; Zizovic, I.; Manef, A.; Delgado, D.R. Extraction of Lyophilized Olive Mill Wastewater Using Supercritical CO2 Processes. Alex. Eng. J. 2022, 61, 237–246. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.B.; Park, K.A.; Hong, I.K. Characterization of Extraction and Separation of Rice Bran Oil Rich in EFA Using SFE Process. Sep. Purif. Technol. 1999, 15, 1–8. [Google Scholar] [CrossRef]
- International Olive Council. Determination of Fatty Acid Methyl Esters by Gas Chromatography; International Olive Council: Madrid, Spain, 2015; pp. 1–15. [Google Scholar]
- International Olive Council. Method of Analysis Determination of the Differences Betweenactual and Theoretical Content of Triacyglycerols with ECN 42; International Olive Council: Madrid, Spain, 2010; pp. 1–23. [Google Scholar]
- Wabaidur, S.M.; Obbed, M.S.; Alothman, Z.A.; Alfaris, N.A.; Badjah-Hadj-ahmed, A.Y.; Siddiqui, M.R.; Altamimi, J.Z.; Aldayel, T.S. Total Phenolic Acids and Flavonoid Contents Determination in Yemeni Honey of Various Floral Sources: Folin-Ciocalteu and Spectrophotometric Approach. Food Sci. Technol. 2020, 40, 647–652. [Google Scholar] [CrossRef]
- Samseny, R.; Mengome, L.; Angone, S. Evaluation of Anti-Inflammatory and Antioxidant Activities from Strychnos Icaja Baillon (Loganiaceae). J. Complement. Med. Res. 2021, 12, 235. [Google Scholar] [CrossRef]
- Maaroufi, Z.; Cojean, S.; Loiseau, P.M.; Yahyaoui, M.; Agnely, F.; Abderraba, M.; Mekhloufi, G. In Vitro Antileishmanial Potentialities of Essential Oils from Citrus Limon and Pistacia Lentiscus Harvested in Tunisia. Parasitol. Res. 2021, 120, 1455–1469. [Google Scholar] [CrossRef]
- Revelou, P.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric Study of Fatty Acid Composition of Virgin Olive Oil from Four Widespread Greek Cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef]
- Skiada, V.; Agriopoulou, S.; Tsarouhas, P.; Katsaris, P.; Stamatelopoulou, E.; Varzakas, T. Evaluation and Origin Discrimination of Two Monocultivar Extra Virgin Olive Oils, Cultivated in the Coastline Part of North-Western Greece. Appl. Sci. 2020, 10, 6733. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Barrek, S.; Skanji, T.; Ghrabi, Z.G.; Zarrouk, H. Composition Chimique de l’huile de Graines d’ Onopordon Nervosum Subsp. Platylepis Murb (ASTÉRACÉES). J. La Société Chim. Tunis. 2007, 9, 23–28. [Google Scholar]
- Demirag, O.; Konuskan, D.B. Quality Properties, Fatty Acid and Sterol Compositions of East Mediterranean Region Olive Oils. J. Oleo Sci. 2021, 70, 51–58. [Google Scholar] [CrossRef]
- Melgosa, R.; Teresa Sanz, M.; Benito-Román, Ó.; Esther Illera, A.; Beltrán, S. Title: Supercritical Assisted Synthesis and Concentration of Monoacylglycerides Rich in Omega-3 Polyunsaturated Fatty Acids. J. CO2 Util. 2019, 31, 65–74. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Nagaz, K. Extraction and Quantification of Polyphenols of Olive Oil Mill Wastewater from the Cold Extraction of Olive Oil in the Region of Khenchela-Algeria. Gabj 2021, 5, 116–122. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Olive Mill Wastewater Treatment. Util. By-Prod. Treat. Waste Food Ind. 2004, 133–157. [Google Scholar] [CrossRef]
- Kiritsakis, A.K.; Kiritsakis, K.A.; Tsitsipas, C.K. A Review of the Evolution in the Research of Antioxidants in Olives and Olive Oil during the Last Four Decades. Int. Soc. Nutraceuticals Funct. Foods 2020, 11, 31–56. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Nagaz, K.; Addad, D.; Secrafi, M.; Yahya, L.B. Phenolic Compounds and Biological Activities of Phenolic Extract of Olive Oil Mill Wastewater Issue from the Cold Extraction of Olive Oil from Khenchela (Algeria). Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental Effects Caused by Olive Mill Wastewaters: Toxicity Comparison of Low-Molecular-Weight Phenol Components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.Z.; Corke, H.; Sun, M. Systematic Evaluation of Natural Phenolic Antioxidants from 133 Indian Medicinal Plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Solana, M.; Rizza, C.S.; Bertucco, A. Exploiting Microalgae as a Source of Essential Fatty Acids by Supercritical Fluid Extraction of Lipids: Comparison between Scenedesmus Obliquus, Chlorella Protothecoides and Nannochloropsis Salina. J. Supercrit. Fluids 2014, 92, 311–318. [Google Scholar] [CrossRef]
- Martínez, J.; Carolina De Aguiar, A. Extraction of Triacylglycerols and Fatty Acids Using Supercritical Fluids -Review. Curr. Anal. Chem. 2014, 10, 67–77. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M.; Bruno, A.D.; Romeo, R.; Fedele, F.L.; Sicari, A.; et al. Antioxidant Activity Shown by Olive Pomace Extracts. J. Environ. Sci. Health Part B 2018, 1–8. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Polyphenolic Content in Olive Oil Waste Waters and Related Olive Samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, C. Antioxidant and Other Biological Activities of Olive Mill Waste Waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and Antioxidant Potential of Olive Oil Mill Wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]


| Parameters | Temperature | Pressure | SC-CO2 Flow Rate |
|---|---|---|---|
| Supercritical CO2 extraction | [40–60 °C] | [25–35 MPa] | 3.39–5.99 g/min |
| Sousse | Sfax | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | F1 | F2 | F3 | F4 | F5 | F6 | t-Test | Significance (0.05) | |
| 25/40 | 30/40 | 35/40 | 25/60 | 30/60 | 35/60 | 25/40 | 30/40 | 35/40 | 25/60 | 30/60 | 35/60 | |||
| C16:0 | 18.60 | 18.75 | 19.11 | 19.56 | 19.73 | 19.79 | 17.10 | 17.21 | 17.35 | 18.32 | 18.43 | 18.53 | 4.168 | 1.92 × 10−3 * |
| C16:1 | 2.35 | 2.37 | 2.39 | 2.45 | 2.48 | 2.51 | 2.60 | 2.63 | 2.65 | 2.71 | 2.73 | 2.77 | −6.857 | 4.4 × 10−5 * |
| C17:0 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | --- | Ns |
| C17:1 | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 2.236 | 0.049 * |
| C18:0 | 2.67 | 2.67 | 2.68 | 2.69 | 2.70 | 2.72 | 2.23 | 2.24 | 2.25 | 2.27 | 2.26 | 2.26 | 43.911 | 9.01 × 10−13 * |
| C18:1 | 61.08 | 61.01 | 60.97 | 60.54 | 60.50 | 60.45 | 59.10 | 59.02 | 58.95 | 58.84 | 58.8 | 58.77 | 14.186 | 5.96 × 10−8 * |
| C18:2 | 13.93 | 13.87 | 13.62 | 13.44 | 13.30 | 13.26 | 17.43 | 17.36 | 17.30 | 16.36 | 16.26 | 16.19 | −11.924 | 3.10 × 10−7 * |
| C18:3 | 0.54 | 0.52 | 0.51 | 0.48 | 0.46 | 0.45 | 0.82 | 0.80 | 0.78 | 0.75 | 0.77 | 0.76 | −15.915 | 1.97 × 10−8 * |
| C20:0 | 0.45 | 0.43 | 0.42 | 0.43 | 0.43 | 0.42 | 0.33 | 0.35 | 0.33 | 0.35 | 0.35 | 0.32 | 13.036 | 1.33 × 10−7 * |
| C20:1 | 0.24 | 0.24 | 0.25 | 0.26 | 0.25 | 0.25 | 0.24 | 0.24 | 0.24 | 0.25 | 0.25 | 0.25 | 0.877 | 0.40 ns |
| FA | 21.34 | 21.49 | 21.86 | 22.32 | 22.50 | 22.58 | 19.41 | 19.53 | 19.68 | 20.67 | 20.77 | 20.87 | 5.276 | 3.6 × 10−4 * |
| MUFA | 64.19 | 64.12 | 64.10 | 63.76 | 63.74 | 63.71 | 62.34 | 62.31 | 62.24 | 62.22 | 62.20 | 62.18 | 17.639 | 6.20 × 10−9 * |
| PUFA | 14.47 | 14.39 | 14.13 | 13.92 | 13.76 | 13.71 | 18.25 | 18.16 | 18.08 | 17.11 | 17.03 | 16.95 | −12.319 | 2.2813 × 10−7 * |
| UFA | 78.66 | 78.51 | 78.23 | 77.68 | 77.50 | 77.42 | 80.59 | 80.47 | 80.32 | 79.33 | 79.23 | 79.13 | −5.209 | 3.6 × 10−4 * |
| Sousse | Sfax | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | F1 | F2 | F3 | F4 | F5 | F6 | t-Test | Significance (0.05) | |
| 25/40 | 30/40 | 35/40 | 25/60 | 30/60 | 35/60 | 25/40 | 30/40 | 35/40 | 25/60 | 30/60 | 35/60 | |||
| ECN42 | 3.62 | 3.50 | 3.41 | 3.13 | 3.07 | 2.95 | 1.40 | 1.33 | 1.25 | 1.13 | 1.08 | 1 | 10 | 1.36 × 10−8 * |
| ECN44 | 8.92 | 8.90 | 8.92 | 8.92 | 8.91 | 8.92 | 10.77 | 10.75 | 10.77 | 10.77 | 10.76 | 10.77 | 10 | 3.62 × 10−22 * |
| ECN46 | 31.23 | 31.33 | 31.39 | 31.69 | 31.8 | 31.91 | 34.71 | 34.83 | 34.87 | 35.09 | 35.22 | 35.28 | 10 | 4.72 × 10−10 * |
| ECN48 | 51.10 | 51.24 | 51.31 | 51.55 | 51.62 | 51.71 | 48.48 | 48.55 | 48.61 | 48.74 | 48.82 | 48.90 | 10 | 5.20 × 10−10 * |
| ECN50 | 5.13 | 5.03 | 4.97 | 4.71 | 4.60 | 4.51 | 4.64 | 4.54 | 4.50 | 4.27 | 4.12 | 4.05 | 10 | 7.94 × 10−3 * |
| Origin of OMWs | Operating Condition [P (MPa)/T (°C)] | Total Phenolic Content mg GAE/g | Content Flavonoïdes mg EQ/g | DPPH IC50 μg/mL. | ABTS IC50 μg/mL. |
|---|---|---|---|---|---|
| Sousse | S1 (25/40) | 181.70 ± 1.60 | 104.00 ± 0.90 | 55.90 ± 0.10 | 29.60 ± 1.00 |
| S2 (30/40) | 188.20 ± 2.70 | 108.40 ± 1.40 | 53.40 ± 0.10 | 25.60 ± 0.00 | |
| S3 (35/40) | 201.50 ± 0.90 | 112.90 ± 0.80 | 52.10 ± 0.10 | 22.10 ± 0.30 | |
| S4 (25/60) | 211.70 ± 1.00 | 112.50 ± 1.10 | 50.50 ± 0.30 | 18.20 ± 0.10 | |
| S5 (30/60) | 216.10 ± 1.90 | 121.50 ± 1.60 | 50.10 ± 0.00 | 16.90 ± 0.10 | |
| S6 (35/60) | 196.00 ± 0.90 | 103.20 ± 1.00 | 52.70 ± 0.20 | 23.30 ± 0.40 | |
| Sfax | F1 (25/40) | 79.10 ± 1.00 | 25.60 ± 0.30 | 287.50 ± 2.70 | 116.20 ± 0.10 |
| F2 (30/40) | 87.30 ± 2.70 | 27.00 ± 0.10 | 243.30 ± 0.20 | 82.20 ± 0.40 | |
| F3 (35/40) | 85.80 ± 1.70 | 33.70 ± 0.90 | 229.40 ± 0.00 | 75.60 ± 0.50 | |
| F4 (25/60) | 94.80 ± 1.50 | 38.10 ± 1.50 | 214.20 ± 0.10 | 60.40 ± 0.20 | |
| F5 (30/60) | 104.20 ± 0.50 | 43.90 ± 0.50 | 207.10 ± 3.10 | 51.40 ± 0.30 | |
| F6 (35/60) | 85.90 ± 2.00 | 21.00 ± 1.00 | 266.90 ± 0.20 | 100.90 ± 0.00 | |
| t-test | 16.868 | 17.663 | −14.843 | −5.777 | |
| Significance (0.05) | 1.13 × 10−8 * | 7.20 × 10−9 * | 3.86 × 10−8 * | 1.79 × 10−4 * | |
| FA (%) | Sousse | Sfax |
|---|---|---|
| C16:0 | 18.64 | 14.37 |
| C16:1 | 2.32 | 2.42 |
| C17:0 | 0.03 | 0.22 |
| C17:1 | 0.13 | 0.09 |
| C18:0 | 2.76 | 2.44 |
| C18:1 | 61.62 | 61.20 |
| C18:2 | 13.36 | 17.75 |
| C18:3 | 0.40 | 0.46 |
| C20:0 | 0.53 | 0.83 |
| C20:1 | 0.21 | 0.22 |
| SFA | 21.43 | 17.03 |
| MUFA | 64.81 | 64.76 |
| PUFA | 13.76 | 18.21 |
| UFA | 78.57 | 82.97 |
| Sousse | Sfax | |
|---|---|---|
| ECN42 | 3.73 | 1.61 |
| ECN44 | 8.85 | 10.64 |
| ECN46 | 30.04 | 34.13 |
| ECN48 | 53.14 | 49.72 |
| ECN50 | 4.24 | 3.90 |
| Extract | Origin of OMW | Total Phenolic Content mg GAE/g | Flavonoids Content mg EQ/g | DPPH CI50 μg/mL. | ABTS CI50 μg/mL. |
|---|---|---|---|---|---|
| Ethanol | Sousse | 170.60 ± 0.90 | 160.30 ± 1.70 | 31.10 ± 0.10 | 14.50 ± 0.10 |
| Sfax | 67.60 ± 0.70 | 52.20 ± 0.90 | 60.80 ± 0.30 | 55.90 ± 0.80 | |
| dichloromethane | Sousse | 124.20 ± 0.50 | 113.50 ± 2.40 | 98.70 ± 0.30 | 21.60 ± 0.30 |
| Sfax | 61.60 ± 0.20 | 48.50 ± 1.10 | 213.00 ± 0.00 | 59.20 ± 0.70 | |
| t-test | 3.539 | 3.687 | 0.748 | 101.81 | |
| Significance (0.05) | 0.071 (ns) | 0.066 (ns) | 0.478 (ns) | 0.01 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dali, I.; Abdelwahab, A.T.; Aydi, A.; Fares, N.; Eladeb, A.; Hamzaoui, M.; Abderrabba, M.; Abdelfattah, M.A.; Guetat, A. Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches. Sustainability 2023, 15, 3360. https://doi.org/10.3390/su15043360
Dali I, Abdelwahab AT, Aydi A, Fares N, Eladeb A, Hamzaoui M, Abderrabba M, Abdelfattah MA, Guetat A. Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches. Sustainability. 2023; 15(4):3360. https://doi.org/10.3390/su15043360
Chicago/Turabian StyleDali, Imen, Abdelrahman T. Abdelwahab, Abdelkarim Aydi, Nouha Fares, Aboulbaba Eladeb, Mondher Hamzaoui, Manef Abderrabba, Marwa A. Abdelfattah, and Arbi Guetat. 2023. "Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches" Sustainability 15, no. 4: 3360. https://doi.org/10.3390/su15043360
APA StyleDali, I., Abdelwahab, A. T., Aydi, A., Fares, N., Eladeb, A., Hamzaoui, M., Abderrabba, M., Abdelfattah, M. A., & Guetat, A. (2023). Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches. Sustainability, 15(4), 3360. https://doi.org/10.3390/su15043360







