1. Introduction
Currently, permanent grassland ecosystem services are being increasingly appreciated in nature protection, in shaping the environment, landscape and culture [
1]. Therefore, various measures are being taken to assess their condition, protection and restoration. According to the European Environment Agency (EEA), in the 2013–2018 report, in most part of the European countries, the conservation status of many monitored sites of semi-natural grassland communities important for Europe is in an unfavourable state of preservation—from U1 (Unfavourable inadequate) to U2 (unfavourable bad) [
2]. Among them are the ones found in Poland, in the continental region, among others: Molinia meadows
Molinion (habitat code 6410),
Cnidion dubii meadows (6440),
Lowland hay meadows (habitat code 6510) and
Festuco-Bromatea (6210), the existence of which depends on extensive management. Unfortunately, many of these habitats and their species are now highly endangered. It has been shown that the most important threats to meadow and pasture communities in Europe include: abandonment of traditional utilisation (mowing and grazing), especially in the less-favoured areas (LFA)—particularly waterlogged, excessively moistened, marshes, in upland and mountain areas; and changing permanent grassland into arable land and temporary grassland, or afforestation [
3,
4]. Recently, due to the deepening climate change, environmental stresses have become a serious threat to these communities—mainly drought and high temperature, especially in the Pannonian and continental regions, as well as the spread of alien invasive plant species [
5]. The excessive drying of the habitats, especially on the organic soils, influences stopping the peat forming process, the intensification of the decomposition process, the mineralisation of organic matter and the decreasing in physico-aquatic properties of the soils [
6].
The impoverishment of floristic diversity is observed all over the world in various types of communities. This process has been progressing faster and faster since the second half of the last century. Individual species and even entire plant communities are disappearing, mainly due to habitat fragmentation, agricultural intensification, habitat degradation and climate change. The threat to the flora of Poland is growing rapidly, especially in highly urbanised regions or with intensive agriculture. The risk of extinction concerns mainly rare and endangered wild plant species. Currently, according to the World Conservation Union (IUCN), about 20,000 of the 300,000 vascular plant species are endangered. The current list of extinct and threatened plant species in Poland includes 765 plant species whose conservation status has been determined following the recommendations of the IUCN [
7]), which accounts for 30% of the Polish flora. In many regions of Poland, more than 30% of the native and domesticated vascular plant species are endangered.
Taking into account the problem of the disappearance of species and even entire plant communities in Europe and Poland, the restoration of multi-species meadow communities is an important task [
8,
9]. Various activities aimed at restoring ecosystems and protecting biodiversity for sustainable development have been carried out around the world, in different habitats, on a larger scale, for over twenty years. Restoration of meadow communities is a difficult task because there are many factors limiting the course of this process. Inadequate moisture conditions, nutrient content and soil pH can constitute a serious problem [
10]. The number of diaspores in the soil bank is greatly reduced [
11]; large arable fields or highways form barriers to their spread. In addition, in soil seed banks, annual dicotyledonous species, belonging to segetal weeds or ruderal plants, are often the most numerous [
12,
13].
There are many techniques for establishing and restoring environmentally valuable plant communities of grasslands excluded from use, and what is more, various methods of collecting seeds directly from semi-natural meadows have also been developed [
14]. Oversowings [
15] and direct drillings are performed using specialised seeders. Spreading hay with diaspores is applied [
16], often in combination with the removal of the top layer of soil [
17,
18]. Green hay spreading is used less frequently—it is possible only at small distances from donor stands [
19,
20]. Another method involves the production of seedlings, which is recommended especially when introducing rare and endangered species [
21]. This method is also used for species the dispersal of which is limited and species with low competitiveness and, as a result, low survival of seedlings and young plants in the presence of primary sward [
10]. The choice of method depends on the species, size of the area being restored and financial resources. In the current decade (2021–2030), known as the “Decade of ecosystem restoration” [
22], actions taken to restore degraded and damaged meadow ecosystems are of particular importance [
23].
There are three main techniques for the conservation of rare and endangered plant species: in situ, ex situ and reintroduction. Planting (introduction or reintroduction) is the creation of new populations by intentionally establishing individuals of a species in a habitat/area where that species existed but disappeared. Plant individuals or their underground parts can be planted (e.g., rhizomes, bulbs—this applies to species with good vegetative reproduction and establishment abilities) [
24]. Planting of plant individuals is a relatively expensive but effective method of population restoration [
25] and improvement of species richness of plant communities, as it allows the omission of particularly sensitive periods, i.e., germination and early development phases, which take place in optimal conditions in the vegetation halls, which limits competition. Once seedlings are planted, they will encounter new abiotic and biotic conditions to which they are not well adapted and may be subject to strong selection as a result. Therefore, it seems that the key to successful introductions is minimising differences in habitats and mimicking the original conditions [
21].
The authors of numerous articles have been wondering what factors affect the success of reintroduction. What is more important: species characteristics, area characteristics or reintroduction technique? Kaye [
26] points out that all three groups of factors interact with each other. It has been found that reintroductions are most successful in habitats ecologically similar to those in which the introduced species are the most common. In addition, the methods used to introduce plants (sowing seeds vs. planting transplants) and the choice of appropriate microsites impact the results of reintroduction [
26]. Research on various methods of restoring meadow ecosystems showed that the most effective method was green hay transfer, resulting in the restored community being the most similar to the donor meadow [
27]. Schaumberger et al. [
20] emphasise that the choice of restoration method ultimately depends on cost and various circumstances, it is important that a large proportion of the transferred species can become established.
Reintroduction and introduction of native plant species becomes more and more important in the restoration of plant communities. This is a standard technique used in conservation and restoration of rare and endangered plant species populations. Moreover, according to Török et al. [
28], this technique is often used to improve species richness or propagule availability in seed sowing or hay-transfer restored fields. This accelerates the success of the restoration of planted species population, and at the same time the entire plant community. So far, there are few studies predicting the success of the development and maintenance of plant species after introduction on the basis of their condition and morphological features. The measure of the final fate of the introduction is the ability of the planted seedlings to flower and set fruit [
29], which indicates that the population is self-sufficient through the development of successive generations. Godefroid et al. [
30] found that survival, flowering and fruiting rates of reintroduced plant species were generally quite low (on average 52%, 19% and 16%, respectively). In addition, they showed a decrease in the success rate of introductions over time. Planting seedlings (compared to sowing seeds), increasing the number of planted seedlings, using grafts from stable source populations and careful site preparation, including the use of fences, had a positive effect on the results of introduction [
30].
Lowland hay meadows (
Arrhenatherion, habitat code 6510-1) are becoming increasingly rare across the Polish landscape, located beyond the reach of river flooding, in eutrophic and mesotrophic habitats, fresh, i.e., not too wet and not too dry [
31]. Extensively used, floristically rich, they usually occur in the form of small patches. Particularly valuable are the wetter fragments of these meadows with a large number of orchids. The main threat to their habitat is conversion to arable land or vegetable cultivation, as well as overseeding and intensive use.
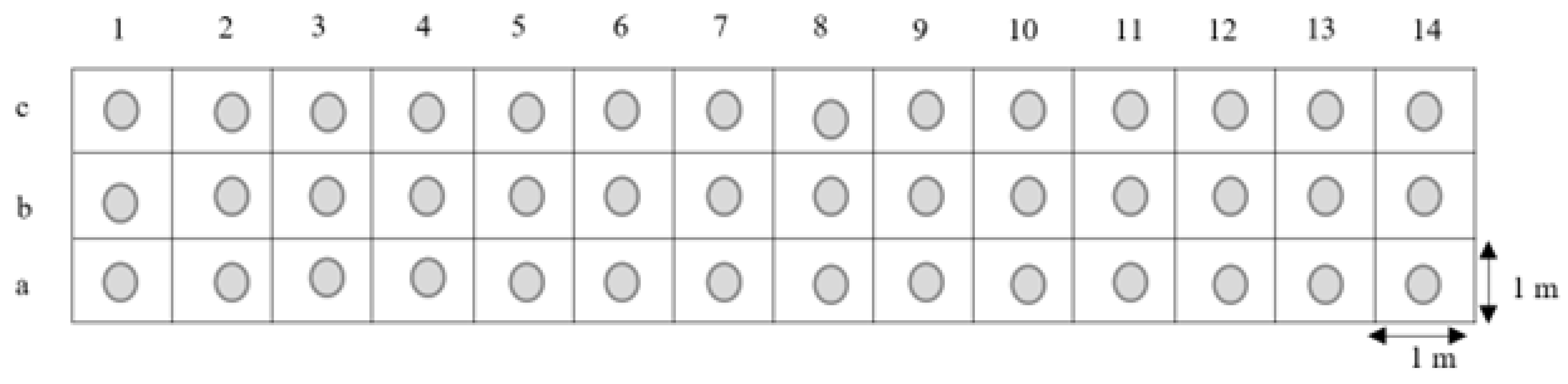
The aim of the study was to determine the possibility of predicting the effectiveness of the introduction of meadow plant species into impoverished patches of meadows dominated by Arrhenatherum elatius (30%) and Urtica dioica (40%) in a landscape nature reserve. The working hypothesis assumed that the parameters determining the condition, growth and development of plants after the introduction of species from the dicotyledonous class indicate their survival in the meadow sward and the improvement of the natural values of the “Ursynów Escarpment” landscape reserve.
4. Discussion
Due to the high diversity of meadow habitats, not all methods of restoring plant communities can be applied everywhere. With limited seed resources, better results are obtained after planting seedlings compared to sowing seeds. The success of the introduction may be determined by the number of introduced individuals (seedlings). The results of our field research (micro-plots) showed that a small (compared to that recommended in the literature) number of seedlings of species with greater potential allowed them to be successfully introduced. In the research literature, the suggested number of introduced seedlings should range from 500 [
51] to 5000 [
52]. However, in most experiments of this type less than 100 seedlings were used, which results from the high labour and cost of such research. At the same time, it should be noted that large range in the number of planted seedlings per species (from 5 to 48) could have determined the maintenance of the population of the tested species and the final success of the introduction, especially species with a low number of seedlings. This is in line with earlier research by Reed et al. [
53] who found that the greater the number of seedlings, the better their competitiveness in relation to primary sward species, the greater the survival rate, and the greater the ability of the population to adapt to new habitats as a result of adaptive genetic variation (genetic drift).
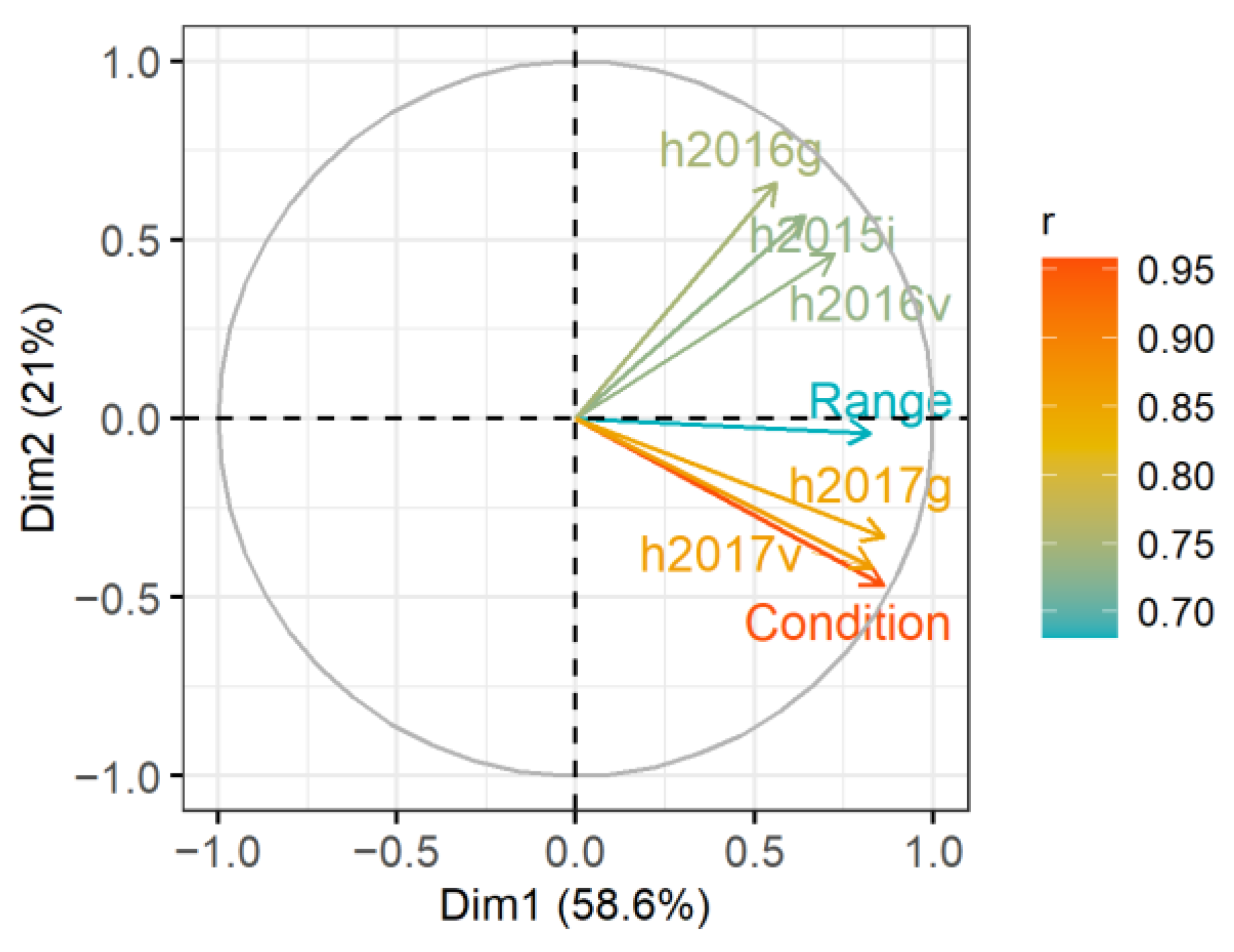
The results of discussed studies showed that species developing rhizomes easily spread and quickly increase the population range. Therefore, in assessing the success of the introduction, more emphasis should be placed on the biology of the species, which was also emphasised by Godefroid et al. [
30]. It is necessary to expand knowledge about the population dynamics of individual species, habitat selectivity and their response to stress factors, including meteorological factors.
Achillea millefolium and
Veronica longifolia easily occupied new localities. In the changing climatic conditions of Poland, these species have been characterised by an increase in the number of new localities in recent decades [
54].
The ability of species to produce seeds should also be assessed, because the persistence of perennial species introduced into a community is the result of vegetative and generative reproduction. During summer of the second year of study (first year after planting), the flowering of 9 out of 14 planted species was observed (
Figure 14).
Achillea millefolium,
Centaurea stoebe,
Dianthus deltoides,
Hypericum perforatum,
Scabiosa ochroleuca and
Veronica longifolia flowered profusely and produced seeds. This suggested the possibility of maintaining and further developing in a new place (place of introduction), because, as stated by Menges [
29], the measure of the final fate of the introduction is the ability of seedlings to flower and set fruit. Two low species,
Plantago lanceolata and
Potentilla erecta bloomed less profusely and were easily suppressed by the plants of the original sward. These species are better maintained in a low sward, more often mowed or grazed.
Verbascum thapsus did not finish flowering, the plants in the flowering phase were knocked over by animals.
Lysimachia vulgaris did not bloom because its shoots were bitten by animals. This fact confirms previous reports that the area where introductions (reintroductions) are carried out should be fenced [
30]. The other three species (
Artemisia campestris,
Eryngium planum,
Inula britannica) did not bloom either. The biology of flowering and seed setting of many “wild” species in meadow habitats is still poorly understood. Godefroid et al. [
30] found that, in most cases, introduced seedlings only occasionally set seeds or did not set seeds at all, indicating that most introductions are not successful in the long term.
A significant decrease in the success of the introduction has already been observed in the third year of the study (second year after the introduction). It was probably related to unfavourable weather conditions. Due to progressive climate change, the frequency of years with unfavourable climatic conditions increases, which we observed during the research period—droughts in 2015 and 2016, and excessive rainfall and, consequently, high groundwater levels in 2017. The high groundwater level reduced the oxygen supply to the roots and caused the death of well-developed plants of
Centaurea stoebe, Dianthus deltoides and
Scabiosa ochroleuca, which are drought-tolerant species. The influence of environmental factors, such as drought, day–night variation, shedding, soil disturbance and irrigation on the functional traits of meadow species was emphasised by, among others, Isselstein et al. [
55]. In central Poland, weather extremes (more often prolonged droughts) are becoming more frequent and more prominent. Therefore, in subsequent years, the range of species on muck soils may be affected by the groundwater level (both very low and high). In favourable hydrological systems, they can develop and maintain in the sward, provided that the vegetation is systematically mown and thus limiting the competitiveness of the old sward. It is difficult to predict the fate of planted species, as it requires a much longer research period than three years. Godefroid et al. [
30] found that often too optimistic assessment of the success of introductions is based on the short-term results, monitoring of the development of the introduced species usually ceases after 4 years.
In addition, the ecological awareness of the society should be constantly raised through various types of educational activities. They make it possible to stimulate the local community to management in accordance with the requirements of valuable grassland communities. In addition, in order to support farmers managing areas of special habitat protection (SACs), appropriate subsidies are necessary, e.g., under climate, agricultural and environmental programs, which will allow to preserve valuable habitats. Activities in this area should be prioritised on the basis of the EU Restoration Plan 2030, which aims to stop deterioration rate of protected habitats and species by 2030.
5. Conclusions
The results of our study indicated that, among 14 dicotyledonous species introduced in the habitat of post-bog soils, a landscape nature reserve, three species Achillea millefolium, Hypericum perforatum, Veronica longifolia have the best introduction efficiency. This group of species was distinguished by the good condition and the presence of generative shoots in the second year after the introduction. Tragopogon pratensis and Verbascum thapsus are plants with the two-year life cycle; their seeds have the ability to persist in the form of a soil seed bank and can sprout in subsequent years. Most planted species have low introduction success, mainly due to the poor condition and low height. However, it should be emphasised that the studies are too brief to unequivocally judge about the success or failure of reintroduction, especially in the case of biennial species.
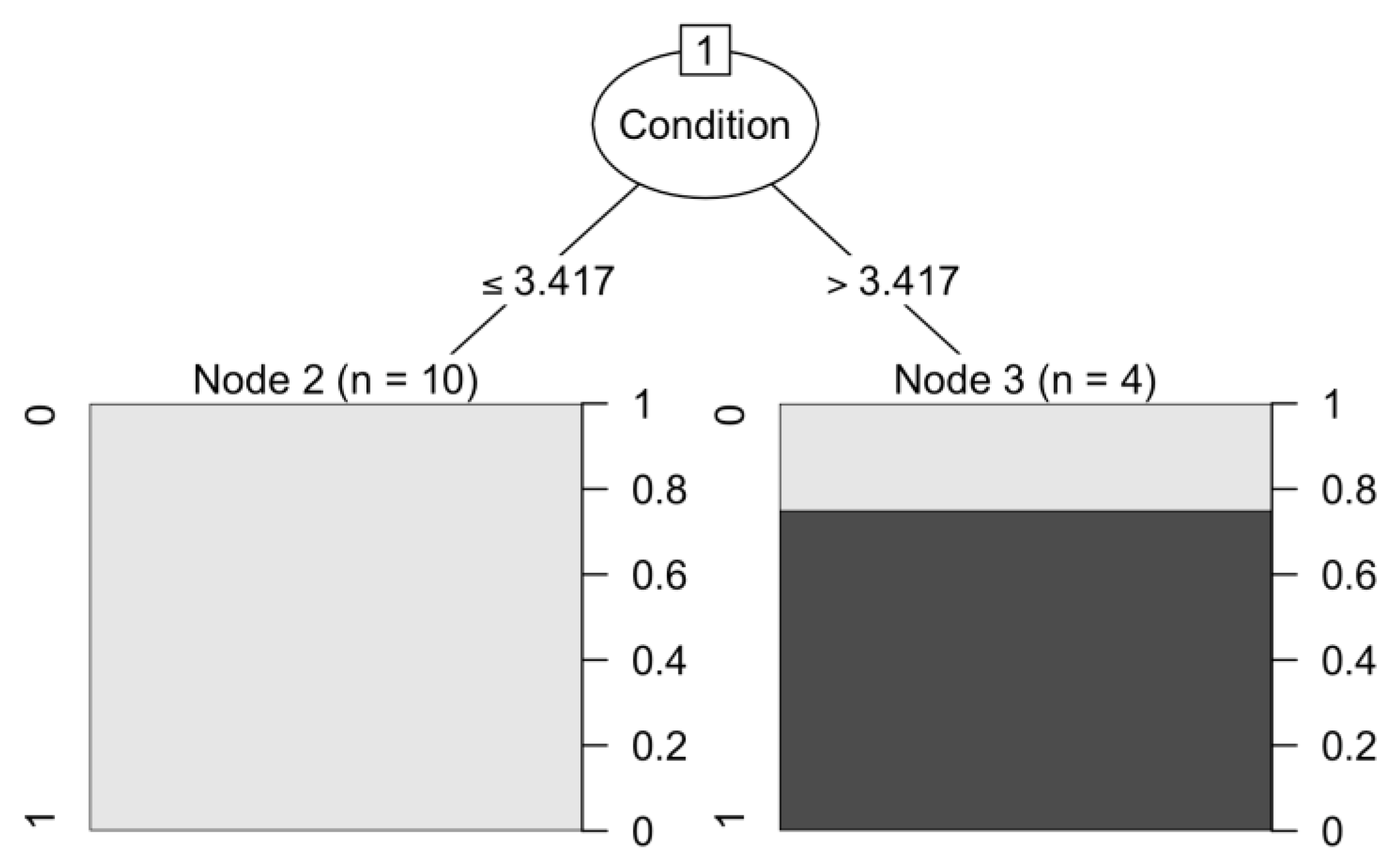
The decision tree model used showed that the condition is the main variable enabling the classification of species population survival. The cut-off value for survival was 3.417 on the 1–5 scale. Plant condition values higher than this threshold indicate the potential success of the introduction of species from the group distinguished in the analysis of hierarchical clusters (Achillea millefolium, Hypericum perforatum, Veronica longifolia). It has been suggested to include the assessment of the condition of plants in the systematic monitoring of restored plant communities.
In assessing the effectiveness of the species introduction, more attention should be paid to: (1) the biology of the species, (2) the critical development phases of the planted species against the background of weather conditions and the competitiveness of the old sward, and (3) the condition of the population should be monitored for a long time. These issues should constitute further directions of research in this field.