The Combination of Plant Diversity and Soil Microbial Diversity Directly and Actively Drives the Multifunctionality of Grassland Ecosystems in the Middle Part of the Northern Slopes of the Tian Shan under Grazing Disturbance
Abstract
1. Introduction
2. Materials and Methods
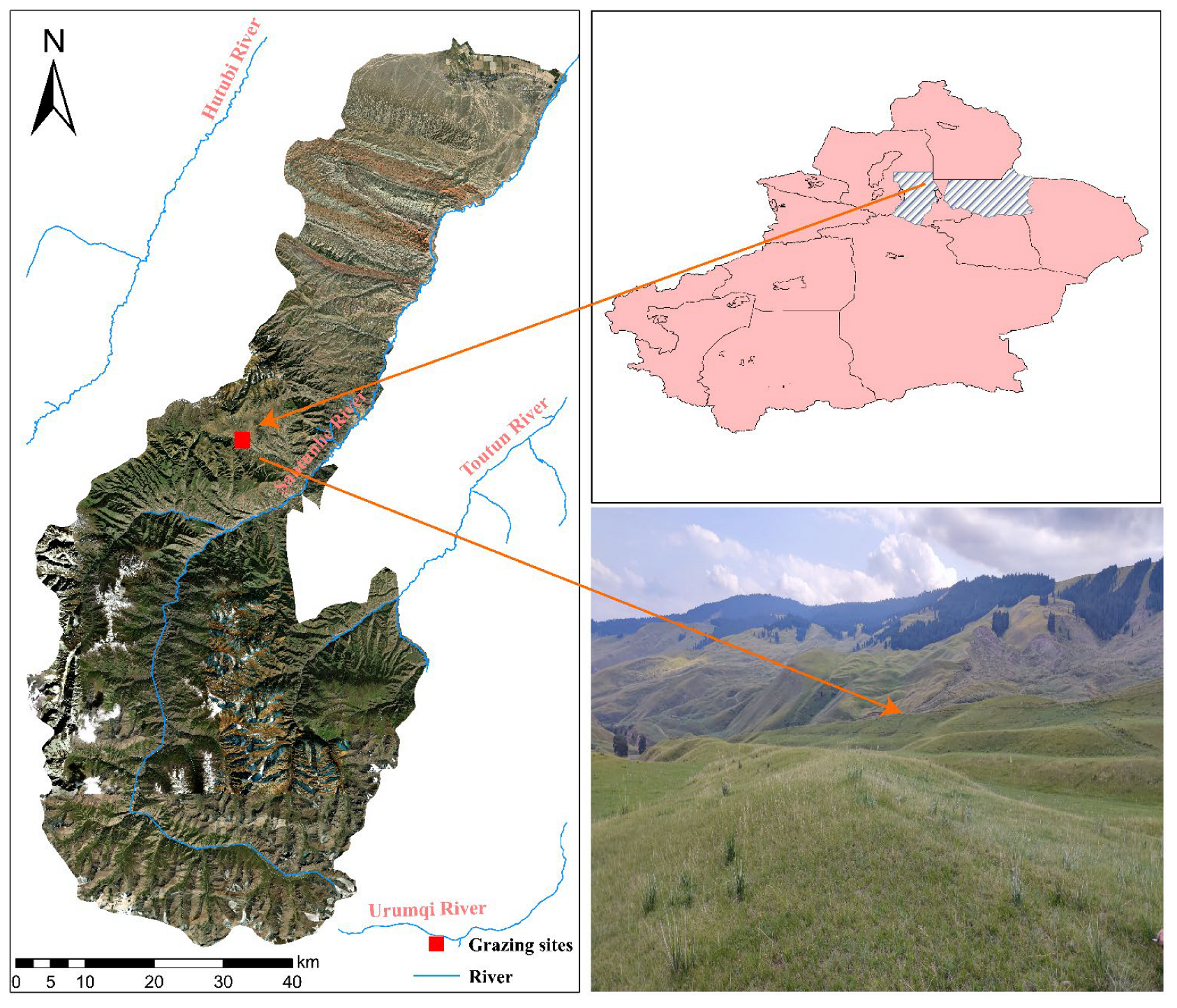
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Sample Collection and Index Determination
2.4. Determination of Soil Physical and Chemical Property Indicators
2.5. Soil Bacterial DNA Extraction and Sequencing
2.6. Quantification and Evaluation of Ecosystem Multifunctionality
2.7. Statistical Analyses
- (1)
- We calculated the plant diversity index based on the results of the plant community survey:
- (2)
- Processing of soil microbial data
- (3)
- Calculation of ecosystem multifunctionality index
- (4)
- Data analysis
3. Results
3.1. Effect of Different Grazing Intensity on Plant Diversity and Soil Microbial Index
3.2. Effect of Different Grazing Intensity on Soil Chemical Composition
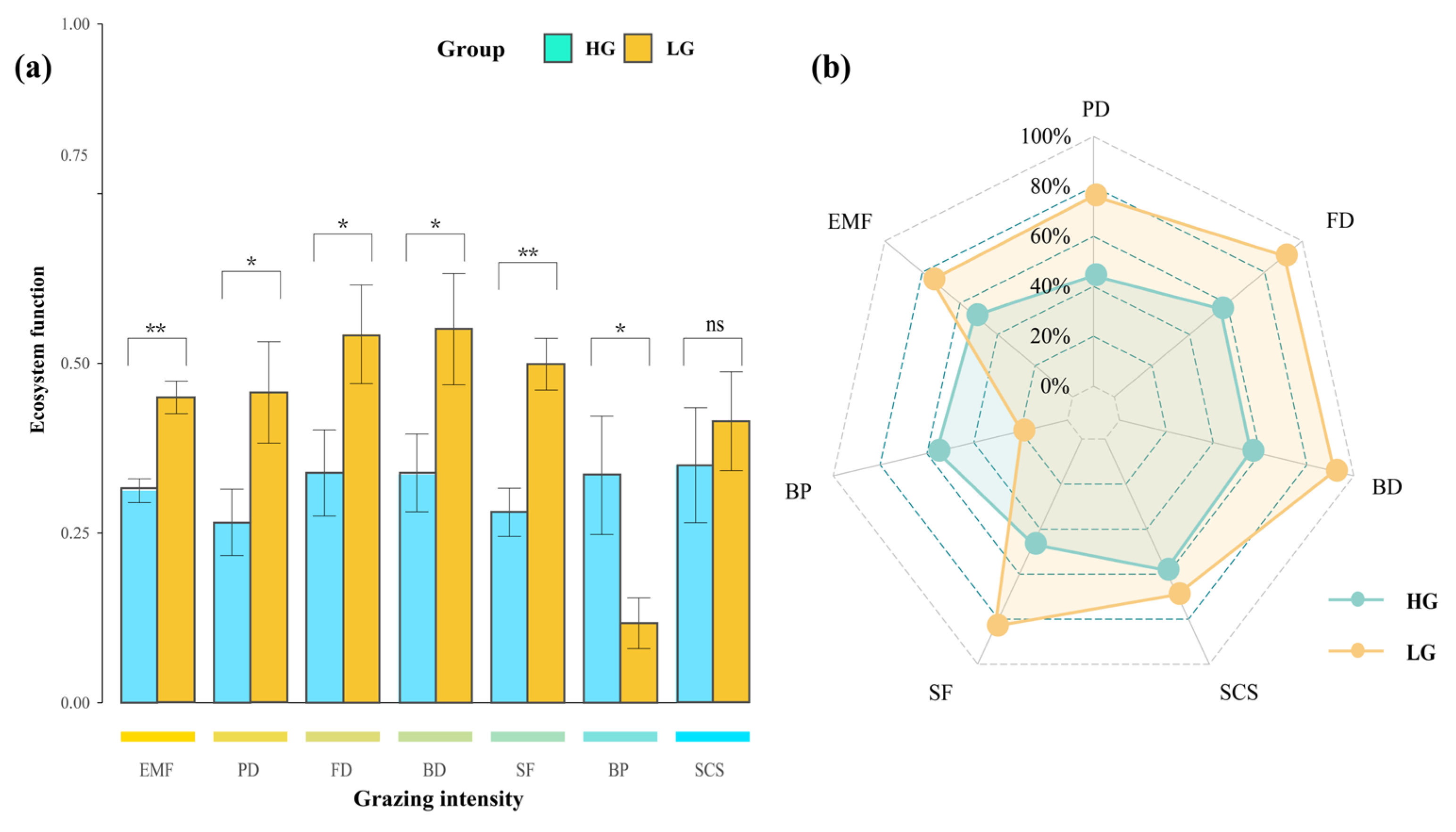
3.3. Effects of Different Grazing Intensities on Single Ecosystem Functions and Multifunctionality
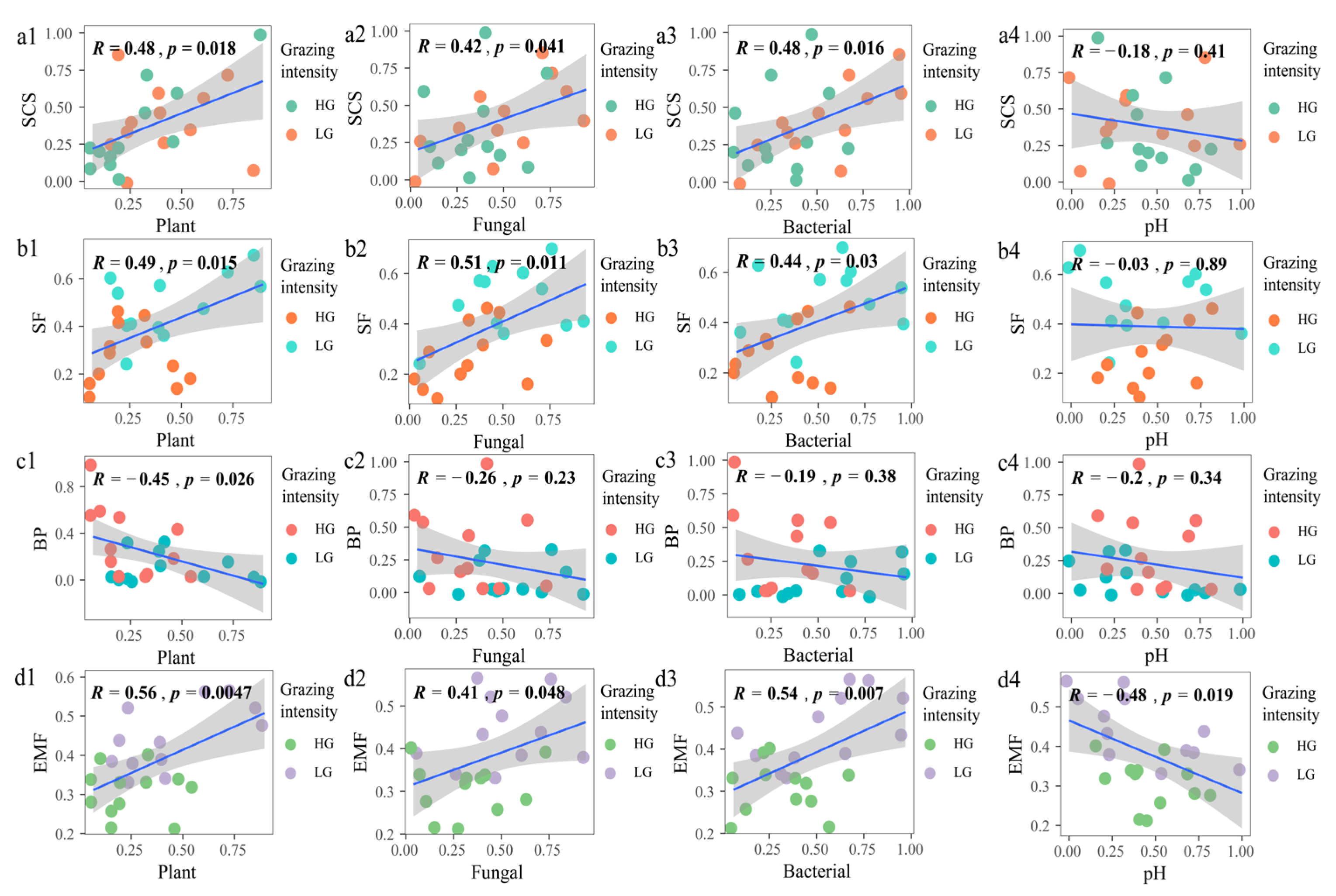
3.4. Relationship between Biodiversity and Ecosystem Function and Multifunctionality under Different Grazing Intensity
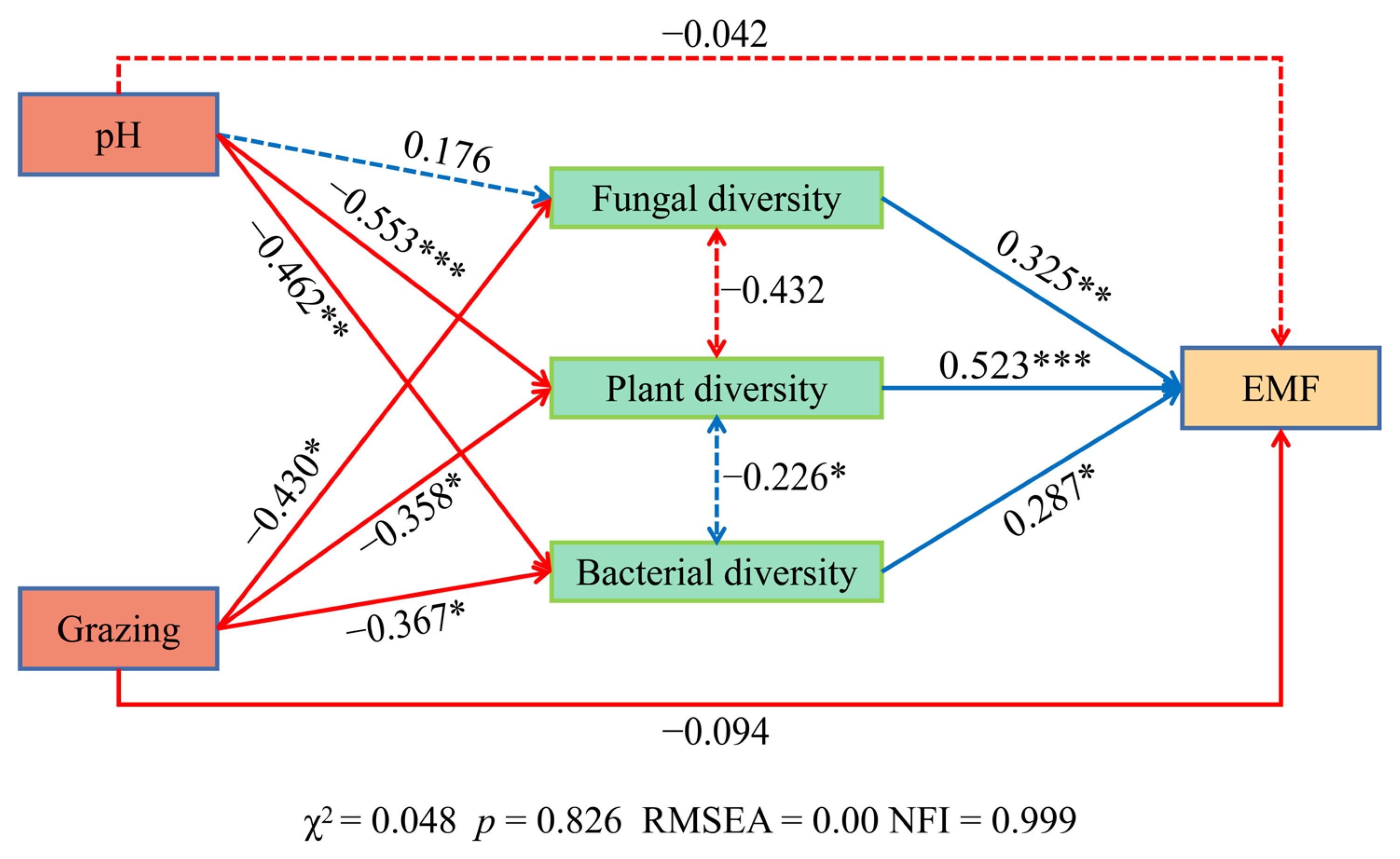
3.5. Effect of Biotic and Abiotic Factors on Ecosystem Multifunctionality under Different Grazing Intensity
4. Discussion
4.1. Effect of Biotic and Abiotic Factors on Ecosystem Multifunctionality under Different Grazing Intensities
4.2. Relationship between Biodiversity and Ecosystem Multifunctionality under Grazing Disturbance
4.3. Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, R.; Allen, V.; Waller, J.; Ohlenbusch, P. A Mobile Classroom Approach to Graduate Education in Forage and Range Sciences. J. Nat. Prod. 2004, 33, 117–120. [Google Scholar] [CrossRef]
- Wang, Y.; Wesche, K. Vegetation and soil responses to livestock grazing in Central Asian grasslands: A review of Chinese literature. Biodivers 2016, 25, 2401–2420. [Google Scholar] [CrossRef]
- Perrings, C.; Walker, B. Biodiversity, resilience and the control of ecological-economic systems: The case of fire-driven rangelands. Ecol. Econ. 1997, 22, 73–83. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cropper, A.; Reid, W. The millennium ecosystem assessment: What is it all about? Trends Ecol. Evol. 2004, 19, 221–224. [Google Scholar] [CrossRef]
- van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1220–1245. [Google Scholar] [CrossRef]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Skinner, R.H.; Barker, D.J.; Edwards, G.R.; Tracy, B.F.; Wedin, D.A. Plant Species Diversity and Management of Temperate Forage and Grazing Land Ecosystems. Crop. Sci. 2004, 44, 1132–1144. [Google Scholar] [CrossRef]
- Mori, A.S.; Lertzman, K.P.; Gustafsson, L. Biodiversity and ecosystem services in forest ecosystems: A research agenda for applied forest ecology. J. Appl. Ecol. 2016, 54, 12–27. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Tilman, D.; Wardle, D.A.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Mori, A.S. Biodiversity and ecosystem services in forests: Management and restoration founded on ecological theory. J. Appl. Ecol. 2017, 54, 7–11. [Google Scholar] [CrossRef]
- Hector, A.; Hooper, R. Ecology. Darwin and the first ecological experiment. Science 2002, 295, 639–640. [Google Scholar] [CrossRef]
- Suter, M.; Huguenin-Elie, O.; Lüscher, A. Multispecies for multifunctions: Combining four complementary species enhances multifunctionality of sown grassland. Sci. Rep. 2021, 11, 3835. [Google Scholar] [CrossRef]
- Villnäs, A.; Norkko, J.; Hietanen, S.; Josefson, A.B.; Lukkari, K.; Norkko, A. The role of recurrent disturbances for ecosystem multifunctionality. Ecology 2013, 94, 2275–2287. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.; Gross, N.; Quero, J.L.; Carmona, C.P.; Ochoa, V.; Gozalo, B.; Delgado-Baquerizo, M.; Dumack, K.; Hamonts, K.; Singh, B.K.; et al. Cascading effects from plants to soil microorganisms explain how plant species richness and simulated climate change affect soil multifunctionality. Glob. Chang. Biol. 2018, 24, 5642–5654. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Delgado-Baquerizo, M.; Trivedi, P.; He, J.; Wang, J.; Singh, B.K. Identity of biocrust species and microbial communities drive the response of soil multifunctionality to simulated global change. Soil Biol. Biochem. 2017, 107, 208–217. [Google Scholar] [CrossRef]
- Luo, G.; Rensing, C.; Chen, H.; Liu, M.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 2018, 32, 1103–1116. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Xie, H.; Zhao, N.; Gao, Y. Heterogeneous microcommunities and ecosystem multifunctionality in seminatural grasslands under three management modes. Ecol. Evol. 2017, 7, 14–25. [Google Scholar] [CrossRef]
- Gross, N.; Le Bagousse-Pinguet, Y.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W.H. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef]
- Fry, E.L.; Savage, J.; Hall, A.L.; Oakley, S.; Pritchard, W.J.; Ostle, N.J.; Pywell, R.F.; Bullock, J.M.; Bardgett, R.D. Soil multifunctionality and drought resistance are determined by plant structural traits in restoring grassland. Ecology 2018, 99, 2260–2271. [Google Scholar] [CrossRef]
- Xie, H.T.; Wang, G.G.; Yu, M.K. Ecosystem multifunctionality is highly related to the shelterbelt structure and plant species diversity in mixed shelterbelts of eastern China. Glob. Ecol. Conserv. 2018, 16, e00470. [Google Scholar] [CrossRef]
- Jing, X.; Prager, C.M.; Classen, A.T.; Maestre, F.T.; He, J.S.; Sanders, N.J. Variation in the methods leads to variation in the interpretation of biodiversity-ecosystem multifunctionality relationships. J. Plant Ecol. 2020, 13, 431–441. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Jiang, K.; Li, L.; Wang, Y.; Tuerxunnayi, R. Effects of Different Grazing Intensities on Soil Bacterial Community Characteristics in Mountain Meadow. Chin. J. Grassl. 2021, 43, 37–44. (In Chinese) [Google Scholar] [CrossRef]
- Li, B. The rangeland degradation in north china and its preventive strategy. Zhongguo Nong Ye Ke Xue 1997, 30, 2–10. (In Chinese) [Google Scholar]
- Wen, L.; Dong, S.; Li, Y.; Wang, X.; Li, X.; Shi, J.; Dong, Q. The impact of land degradation on the C pools in alpine grasslands of the Qinghai-Tibet Plateau. Plant Soil 2013, 368, 329–340. [Google Scholar] [CrossRef]
- Yi, X.; Li, G.; Yin, Y. The impacts of grassland vegetation degradation on soil hydrological and ecological effects in the source region of the Yellow River—A case study in Junmuchang region of Maqin Country. Procedia Environ. Sci. 2012, 13, 967–981. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.S. The links between ecosystem multifunctionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Wang, L.; Delgado-Baquerizo, M.; Wang, D.; Isbell, F.; Liu, J.; Feng, C.; Liu, J.; Zhong, Z.; Zhu, H.; Yuan, X.; et al. Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 6187–6192. [Google Scholar] [CrossRef]
- Xiong, D.; Zhao, G.; Wu, J.; Shi, P.; Zhang, X. The relationship between species diversity and ecosystem multifunctionality in alpine grasslands on the Tibetan Changtang Plateau. Sheng Tai Xue Bao 2016, 36, 3362–3371. (In Chinese) [Google Scholar]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; García-Velázquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef]
- Zavaleta, E.S.; Pasari, J.R.; Hulvey, K.B.; Tilman, G.D. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl. Acad. Sci. USA 2010, 107, 1443–1446. [Google Scholar] [CrossRef]
- Dooley, Á.; Isbell, F.; Kirwan, L.; Connolly, J.; Finn, J.A.; Brophy, C. Testing the effects of diversity on ecosystem multifunctionality using a multivariate model. Ecol. Lett. 2015, 18, 1242–1251. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; Garcia-Gomez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.E.K.; Gamfeldt, L.; Isbell, F.; Lefcheck, J.S.; Griffin, J.N.; Hector, A.; Cardinale, B.J.; Hooper, D.U.; Dee, L.E.; Emmett, D.J. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 2014, 5, 111–124. [Google Scholar] [CrossRef]
- Maestre, F.T.; Castillo-Monroy, A.P.; Bowker, M.A.; Ochoa-Hueso, R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J. Ecol. 2012, 100, 317–330. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Niu, S. Toward a sustainable grazing management based on biodiversity and ecosystem multifunctionality in drylands. Curr. Opin. Environ. Sustain. 2021, 48, 36–43. [Google Scholar] [CrossRef]
- Pan, F.; Yan, R.; Zhao, J.; Li, L.; Hu, Y.; Jiang, Y.; Shen, J.; Neil, B.; McLaughlin, N.B.; Zhao, D.; et al. Effects of grazing intensity on soil nematode community structure and function in different soil layers in a meadow steppe. Plant Soil 2021, 471, 33–46. [Google Scholar] [CrossRef]
- Liang, M.; Smith, N.G.; Chen, J.; Wu, Y.; Guo, Z.; Gornish, E.S.; Liang, C. Shifts in plant composition mediate grazing effects on carbon cycling in grasslands. J. Appl. Ecol. 2020, 58, 518–527. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Jin, K.; Struik, P.C.; Sun, S.; Ji, B.; Zhang, Y.; Li, X. Soil bacterial and fungal communities are linked with plant functional types and soil properties under different grazing intensities. Eur. J. Soil Sci. 2022, 73, e13195. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Li, B.; Sun, F.; Zhao, X.; Wang, Y.; Lu, Z.; Zhang, D.; Fang, J. Fungal communities are more sensitive to nitrogen fertilization than bacteria in different spatial structures of silage maize under short-term nitrogen fertilization. Appl. Soil Ecol. 2022, 170, 104275. [Google Scholar] [CrossRef]
- Zhang, H.R.; Fu, G. Responses of plant, soil bacterial and fungal communities to grazing vary with grazing seasons and grassland types, Northern Tibet. Land Degra. Dev. 2020, 32, 1821–1832. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Vilonen, L.; Qu, Y.; Fu, X.; Shi, B.; Cui, H.; Gao, W.; Cai, H.; Sun, W. Grazing and nitrogen addition restructure the spatial heterogeneity of soil microbial community structure and enzymatic activities. Funct. Ecol. 2021, 35, 2763–2777. [Google Scholar] [CrossRef]
- Fan, J.L.; Zhang, C.H.; Jin, H.; Zhang, J.; Han, G.D. Grazing accelerates labile and recalcitrant soil carbon loss driving by rare microbial taxa in a desert steppe. Land Degra. Dev. 2021, 32, 4241–4253. [Google Scholar] [CrossRef]
- Romero-Ruiz, A.; Monaghan, R.; Milne, A.E.; Coleman, K.; Cardenas, L.M.; Segura-Quirante, C.; Whitmore, A.P. Modelling changes in soil structure caused by livestock treading. Geoderma 2023, 431, 116331. [Google Scholar] [CrossRef]
- Khomutova, T.E.; Fornasier, F.; Yeltsov, M.V.; Chernysheva, E.V.; Borisov, A.V. Influence of grazing on the structure and biological activity of dry steppe soils of the southern Russian Plain. Land Degrad. Dev. 2021, 32, 4832–4844. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Hao, X.; Alexander, T.W.; Shi, X.; Jin, L.; Thomas, B.W. Heavy grazing over 64 years reduced soil bacterial diversity in the foothills of the Rocky Mountains, Canada. Appl. Soil Ecol. 2020, 147, 103361. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.S.; Zhou, Q.W.; Zhao, Y.N.; Zhao, B.Y. Damage and control of major poisonous plants in the western grasslands of China? A review. Rangel. J. 2012, 34, 329. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Chen, R.; Li, Z.; Lin, X.; Feng, Y. Trade-off between microbial ecophysiological features regulated by soil fertility governs plant residue decomposition. Soil Tillage Res. 2023, 229, 105679. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Cheng, J.; Wei, G.; Lin, Y. Above- and belowground biodiversity drives soil multifunctionality along a long-term grassland restoration chronosequence. Sci. Total Environ. 2021, 772, 145010. [Google Scholar] [CrossRef]
- Viana, J.L.; Dalling, J.W. Soil fertility and water availability effects on trait dispersion and phylogenetic relatedness of tropical terrestrial ferns. Oecologia 2022, 198, 733–748. [Google Scholar] [CrossRef]
- Dostál, P. The temporal development of plant-soil feedback is contingent on competition and nutrient availability contexts. Oecologia 2021, 196, 185–194. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, H.Y.H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’Halloran, L.R.; Grace, J.B.; Anderson, T.M. Productivity is a poor predictor of plant species richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.H.; Pither, J.; Jentsch, A.; Sternberg, M.; Zobel, M.; Askarizadeh, D.; Bartha, S.; Beierkuhnlein, C.; Bennett, J.A.; Bittel, A.; et al. Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science 2015, 349, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.W. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.J.; Zhao, S.; Lu, W.X.; Ni, L.K.; Yan, Z.G.; Jiang, T.Y. Grazing altered the plant diversity-productivity relationship in the Jianghan plain of the Yangtze River basin. For. Ecol. Manag. 2023, 531, 120767. [Google Scholar] [CrossRef]
- Zaret, M.M.; Kuhs, M.A.; Anderson, J.C.; Seabloom, E.W.; Borer, E.T.; Kinkel, L.L. Seasonal shifts from plant diversity to consumer control of grassland productivity. Ecol. Lett. 2022, 25, 1215–1224. [Google Scholar] [CrossRef]
- Shao, J.J.; Zhou, X.H.; Groenigen, K.J.; Zhou, G.Y.; Zhou, H.M.; Zhou, L.Y.; Lu, M.; Xia, J.Y.; Jiang, L.; Hungate, B.A.; et al. Warming effects on grassland productivity depend on plant diversity. Glob. Ecol. Biogeogr. 2021, 31, 588–598. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.P.; Shen, R.H.; Gong, Y.; Wang, C.; Hong, P.B.; Reuman, D.C. Biodiversity stabilizes plant communities through statistical-averaging effects rather than compensatory dynamics. Nat. Commun. 2022, 13, 7804. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.Y.; Wang, Y.; Liu, X.; Cheng, J.; Zhang, J.; Baoyin, T.; Bardgett, R. High ecosystem multifunctionality under moderate grazing is associated with high plant but low bacterial diversity in a semi-arid steppe grassland. Plant Soil 2020, 448, 265–276. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef]
- Gao, X.L.; Lv, S.H.; Diao, Z.Y.; Wang, D.W.; Li, D.K.; Zheng, Z.R. Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China. Sustainability 2023, 15, 3434. [Google Scholar] [CrossRef]
- Peng, F.; Xue, X.; You, Q.; Sun, J.; Zhou, J.; Wang, T.; Tsunekawa, A. Change in the trade-off between above- and belowground biomass of alpine grassland: Implications for the land degradation process. Land Degrad Dev. 2020, 31, 105–117. [Google Scholar] [CrossRef]
- Navarro-Perea, M.; Pueyo, Y.; Moret, D.; Valverde, A.; Igual, J.M.; Alados, C.L. Plant-soil interactions in response to grazing intensity in a semi-arid ecosystem from NE Spain. Arid. Land Res. Manag. 2023, 37, 184–196. [Google Scholar] [CrossRef]
- Casenave, C.; Bisson, A.; Boudsocq, S.; Daufresne, T. Impact of biological nitrogen fixation and livestock management on the manure transfer from grazing land in mixed farming systems. J. Theor. Biol. 2022, 545, 111136. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.R.; Pato, R.L.; Dias, S.; Santos, D. Effects of Grazing Indigenous Laying Hens on Soil Properties: Benefits and Challenges to Achieving Soil Fertility. Sustainability 2022, 14, 3407. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing alters ecosystem functioning and C: N: P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Borer, E.T.; Harpole, W.S.; Adler, P.B.; Arnillas, C.A.; Bugalho, M.N.; Cadotte, M.W.; Caldeira, M.C.; Campana, S.; Dickman, C.R.; Dickson, T.L.; et al. Nutrients cause grassland biomass to outpace herbivory. Nat. Commun. 2020, 11, 6036. [Google Scholar] [CrossRef]
- Lindtner, P.; Gömöryová, E.; Gömöry, D.; Stašiov, S.; Kubovčík, V. Development of physico-chemical and biological soil properties on the European ground squirrel mounds. Geoderma 2019, 339, 85–93. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; He, X.Z.; Hou, F.J. Sheep Trampling Modifies Soil and Plant C: N:P Stoichiometry in a Typical Steppe of the Loess Plateau. Rangel. Ecol. Manag. 2021, 76, 100–108. [Google Scholar] [CrossRef]
- Evju, M.; Austrheim, G.; Halvorsen, R.; Mysterud, A. Grazing responses in herbs in relation to herbivore selectivity and plant traits in an alpine ecosystem. Oecologia 2009, 161, 77–85. [Google Scholar] [CrossRef]
- Roberts, A.J.; Johnson, N.C. Effects of Mob-Grazing on Soil and Range Quality Vary with Plant Species and Season in a Semiarid Grassland. Rangel. Ecol. Manag. 2021, 79, 139–149. [Google Scholar] [CrossRef]
- Felton, A.J.; Snyder, R.E.; Shriver, R.K.; Suding, K.N.; Adler, P.B. The influence of life-history strategy on ecosystem sensitivity to resource fluctuations. J. Ecol. 2021, 109, 4081–4091. [Google Scholar] [CrossRef]
- Norman, H.C.; Cocks, P.S.; Galwey, N.W. Populations of two annual clover species evolved in response to 13 years of grazing management and phosphate fertilizer application. Grass Forage Sci. 2020, 75, 64–75. [Google Scholar] [CrossRef]
- Liu, D.; Chang, P.S.; Power, S.A.; Bell, J.; Manning, P. Changes in plant species abundance alter the multifunctionality and functional space of heathland ecosystems. New Phytol. 2021, 232, 1238–1249. [Google Scholar] [CrossRef]
- Velmurugan, A.; Swarnam, T.P.; Jaisankar, I.; Swain, S.; Subramani, T. Vegetation–soil–microbial diversity influences ecosystem multifunctionality across different tropical coastal ecosystem types. Trop. Ecol. 2021, 63, 273–285. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, P.; Wang, F.H.; Han, W.X.; Qiao, M.; Dong, W.X.; Hu, C.S.; Zhu, D.; Chu, H.Y.; Zhu, Y.G. The ecological clusters of soil organisms drive the ecosystem multifunctionality under long-term fertilization. Environ. Int. 2022, 161, 107133. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, Q.H.; Xie, L.L.; Yin, C.Y. Interspecific plant-plant interactions increase the soil microbial network stability, shift keystone microbial taxa, and enhance their functions in mixed stands. For. Ecol. Manag. 2023, 533, 120851. [Google Scholar] [CrossRef]
- Van der Plas, F.; Manning, P.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Hector, A.; Ampoorter, E.; Baeten, L.; et al. Jack-of-all-trades effects drive biodiversity-ecosystem multifunctionality relationships in European forests. Nat. Commun. 2016, 7, 11109. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Lang, X.; Huang, X.; Su, J. Species richness, not abundance, drives ecosystem multifunctionality in a subtropical coniferous forest. Ecol. Indic. 2021, 120, 106911. [Google Scholar] [CrossRef]
- Liu, M.; He, W.; Zhang, Z.; Sun, J.; Cong, N.; Nie, X.; Wang, Y.; Zhang, L.; Yang, B.; Chen, Y.; et al. Mutual feedback between above- and below-ground controls the restoration of alpine ecosystem multifunctionality in long-term grazing exclusion. J. Clean. Prod. 2022, 333, 130184. [Google Scholar] [CrossRef]
- Shu, Y.; Jiang, L.; Liu, F.; Lv, G. Effects of plant diversity and abiotic factors on the multifunctionality of an arid desert ecosystem. PLoS ONE 2022, 17, e0266320. [Google Scholar] [CrossRef] [PubMed]
- Teague, R.; Dowhower, S.L. Links of microbial and vegetation communities with soil physical and chemical factors for a broad range of management of tallgrass prairie. Ecol. Indic. 2022, 142, 109280. [Google Scholar] [CrossRef]
- Wang, D.; Du, J.; Zhang, B.; Ba, L.; Hodgkinson, K.C. Grazing Intensity and Phenotypic Plasticity in the Clonal Grass Leymus chinensis. Rangel Ecol. Manag. 2017, 70, 740–747. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Gao, Q.; Li, X.; Zhang, Y.; Ding, Y.; Ouyang, S.; Yurtaev, A.; Kuzyakov, Y. Moderate grazing increases newly assimilated carbon allocation belowground. Rhizosphere 2022, 22, 100547. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, J.; Xu, Z. Analysis of the tradeoffs between provisioning and regulating services from the perspective of varied share of net primary production in an alpine grassland ecosystem. Ecol. Complex. 2014, 17, 79–86. [Google Scholar] [CrossRef]
- Zander, S.V.; Heidi, J.H.; Michael, D.C. Does defoliation frequency and severity influence plant productivity? The role of grazing management and soil nutrients. Afr. J. Range Forage Sci. 2020, 38, 1–16. [Google Scholar] [CrossRef]
- Siegwart, L.; Jourdan, C.; Piton, G.; Sugihara, S.; Van den, M.K.; Bertrand, I. Root distribution and properties of a young alley-cropping system: Effects on soil carbon storage and microbial activity. Plant Soil 2022, 482, 601–625. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Bai, B.; Qi, R.; Ploch, S.; Breider, F.; Thines, M. Nutrient Availability Does Not Affect Community Assembly in Root-Associated Fungi but Determines Fungal Effects on Plant Growth. mSystems 2022, 7, e0030422. [Google Scholar] [CrossRef]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Spitzer, C.M.; Wardle, D.A.; Lindahl, B.D.; Sundqvist, M.K.; Gundale, M.J.; Fanin, N.; Kardol, P. Root traits and soil micro-organisms as drivers of plant–soil feedbacks within the sub-arctic tundra meadow. J. Ecol. 2021, 110, 466–478. [Google Scholar] [CrossRef]
- Beck, J.J. Variation in plant–soil interactions among temperate forest herbs. Plant Ecol. 2021, 222, 1225–1238. [Google Scholar] [CrossRef]

| Type | Ecosystem Functioning | Ecosystem Function Indicators |
|---|---|---|
| Supply function | Grassland productivity (GP) | Above-ground biomass |
| Regulating function | Soil fungal diversity (SFD) | Fungal Shannon index; Fungal Simpson index; Fungal ACE index; Fungal Chaol index |
| Soil bacterial diversity (SBD) | Bacterial Shannon index; Bacterial Simpson index; Bacterial ACE index; Bacterial Chaol index | |
| Plant diversity (PD) | Plant Shannon index; Plant Simpson index; Plant Margalef index; Plant Pielou index | |
| Support function | Soil carbon storage (SCS) | Soil organic carbon; |
| Soil fertility (SF) | Soil total nitrogen; Soil available nitrogen; Soil total phosphorus; Soil available phosphorus; Soil total potassium; Soil available potassium |
| Treatment | LG | HG |
|---|---|---|
| Margalef index | 0.87 ± 0.33 a | 0.5 ± 0.16 b |
| Simpson index | 0.49 ± 0.18 a | 0.36 ± 0.11 b |
| Shannon index | 0.97 ± 0.40 a | 0.63 ± 0.17 b |
| Pielou index | 0.67 ± 0.15 a | 0.49 ± 0.12 b |
| Bacterial Chao1 index | 2375.67 ± 162.80 a | 2256.71 ± 98.99 b |
| Bacterial Ace index | 2391.24 ± 164.43 a | 2261.94 ± 98.72 b |
| Bacterial Shannon index | 7.67 ± 1.62 a | 7.73 ± 1.59 a |
| Bacterial Simpson index | 0.5 ± 0.51 a | 0.5 ± 0.52 a |
| Fungal Chao1 index | 814 ± 140.32 a | 657.98 ± 186.70 b |
| Fungal Ace index | 801.76 ± 126.17 a | 656.14 ± 174.98 b |
| Fungal Shannon index | 5.55 ± 0.95 a | 4.86 ± 0.61 b |
| Fungal Simpson index | 0.64 ± 0.45 a | 0.32 ± 0.43 a |
| Treatment | LG | HG |
|---|---|---|
| Soil organic carbon (g/kg) | 11.52 ± 3.09 a | 9.69 ± 2.98 b |
| Total nitrogen (g/kg) | 1.48 ± 0.42 a | 0.73 ± 0.14 b |
| Total phosphorus (g/kg) | 0.86 ± 0.03 a | 0.78 ± 0.02 b |
| Total potassium (g/kg) | 23.76 ± 0.66 a | 21.45 ± 1.06 b |
| Available nitrogen (g/kg) | 201.32 ± 67.03 a | 79.69 ± 15.38 b |
| Available Phosphorus (g/kg) | 17.39 ± 2.53 a | 12.25 ± 1.54 b |
| Available potassium (g/kg) | 653.35 ± 142.64 a | 453.11 ± 57.69 b |
| pH | 8.86 ± 0.52 a | 8.95 ± 0.33 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Zhang, Q.; Wang, Y.; Li, H.; Yang, Y.; Reyimu, T. The Combination of Plant Diversity and Soil Microbial Diversity Directly and Actively Drives the Multifunctionality of Grassland Ecosystems in the Middle Part of the Northern Slopes of the Tian Shan under Grazing Disturbance. Sustainability 2023, 15, 5673. https://doi.org/10.3390/su15075673
Jiang K, Zhang Q, Wang Y, Li H, Yang Y, Reyimu T. The Combination of Plant Diversity and Soil Microbial Diversity Directly and Actively Drives the Multifunctionality of Grassland Ecosystems in the Middle Part of the Northern Slopes of the Tian Shan under Grazing Disturbance. Sustainability. 2023; 15(7):5673. https://doi.org/10.3390/su15075673
Chicago/Turabian StyleJiang, Kangwei, Qingqing Zhang, Yafei Wang, Hong Li, Yongqiang Yang, and Tursunnay Reyimu. 2023. "The Combination of Plant Diversity and Soil Microbial Diversity Directly and Actively Drives the Multifunctionality of Grassland Ecosystems in the Middle Part of the Northern Slopes of the Tian Shan under Grazing Disturbance" Sustainability 15, no. 7: 5673. https://doi.org/10.3390/su15075673
APA StyleJiang, K., Zhang, Q., Wang, Y., Li, H., Yang, Y., & Reyimu, T. (2023). The Combination of Plant Diversity and Soil Microbial Diversity Directly and Actively Drives the Multifunctionality of Grassland Ecosystems in the Middle Part of the Northern Slopes of the Tian Shan under Grazing Disturbance. Sustainability, 15(7), 5673. https://doi.org/10.3390/su15075673









