Potential for Sustainable Production of Natural Colorants in the Tropical Forest: A Biorefinery Case of Annatto Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Raw Material Characterization
2.2.1. Chemical Analysis
2.2.2. Proximate Analysis
2.2.3. Solid Analysis
2.2.4. Total Phenolic Content, Antioxidant Capacity, and Reducing Sugars
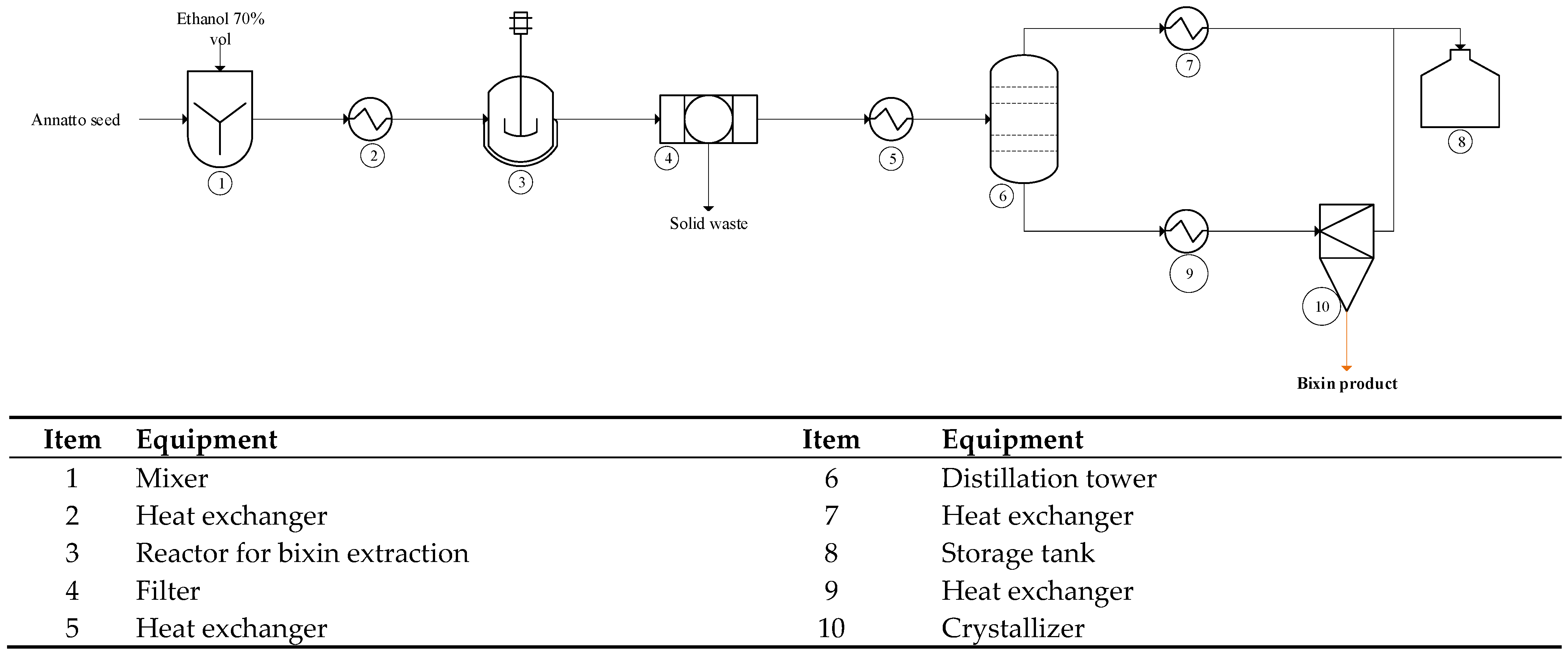
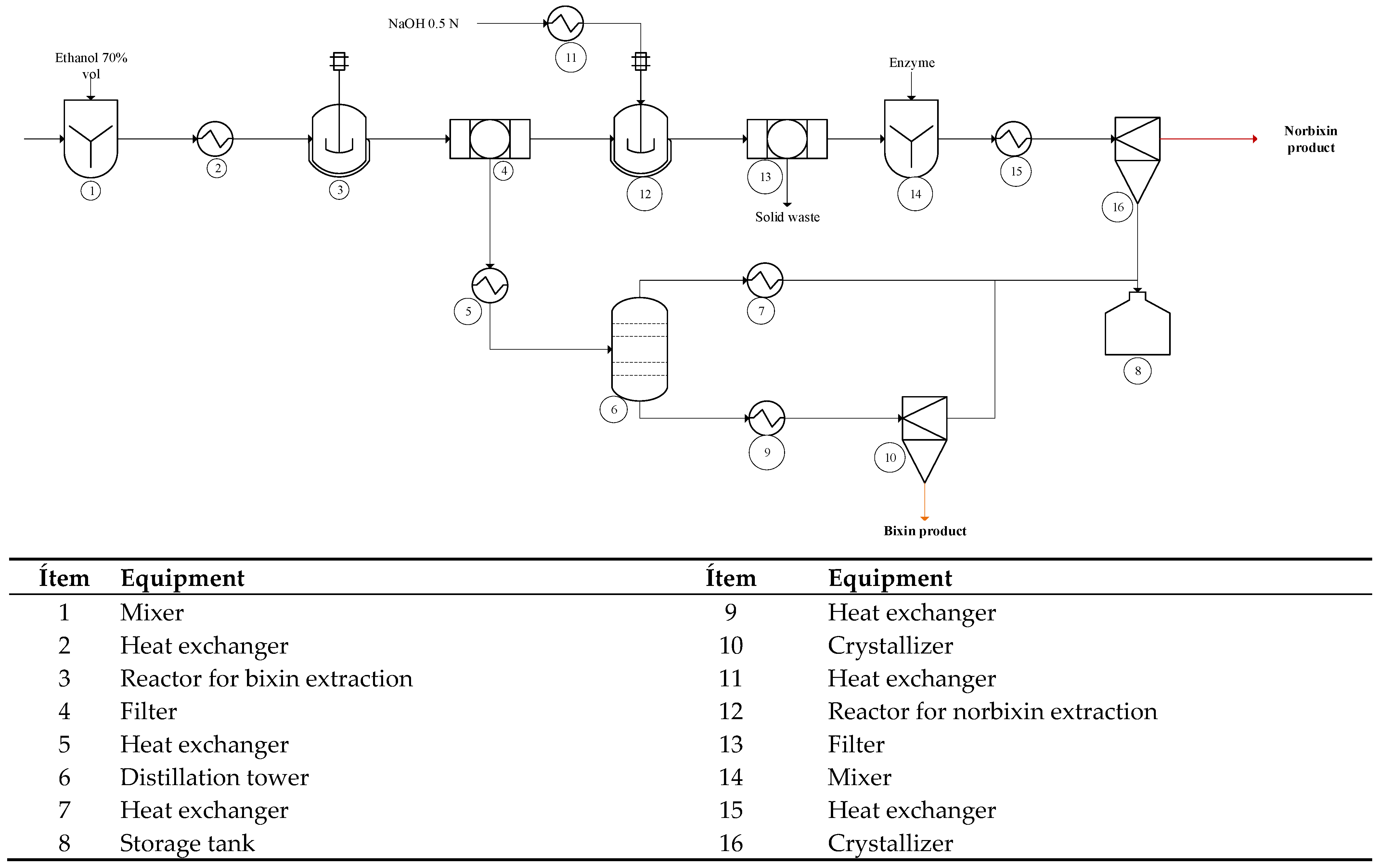
2.3. Bixin and Norbixin Extraction
2.4. Process Simulation
2.4.1. Process Description
Scenario 1
Scenario 2
2.5. Technical and Economic Assessment
2.6. Environmental Assessment
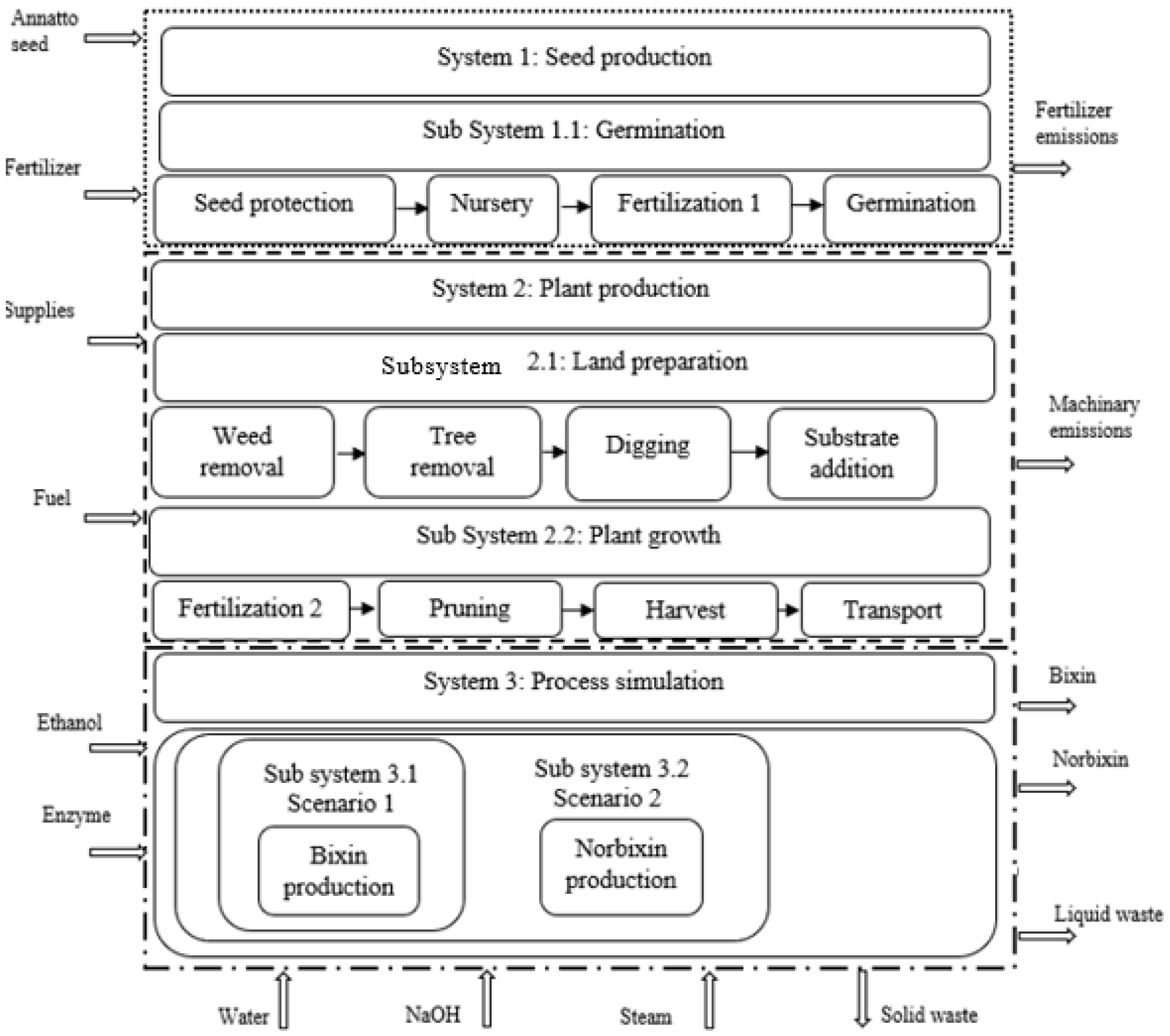
2.6.1. Goal and Scope Definition
2.6.2. System Boundary
2.6.3. Inventory Data Selection and Description
System 1
System 2
System 3
3. Results and Discussion
3.1. Raw Material Characterization
| Analysis | Component | Mass Composition (%) | ||
|---|---|---|---|---|
| This Work | Kumar et al. [50] | Valerio M et al. [53] | ||
| Chemical a | Initial moisture | 40 ± 0.25 | - | - |
| Cellulose | 18.81 ± 1.4 | 14.40 | - | |
| Hemicellulose | 11.34 ± 1.26 | 34.00 | - | |
| Lignin | 13.92 ± 1.6 | 10.86 | - | |
| Extractives | 28.46 ± 0.94 | - | - | |
| Fats | 2.76 ± 2.12 | - | 2.23 | |
| Protein | 8.71 ± 1.41 | 14.35 | 11.50 | |
| Pectin | 16.00 ± 2.56 | - | - | |
| Proximate analysis | Ash | 5.39 ± 1.63 | 5.23 | - |
| Fixed carbon | 8.63 ± 1.5 | - | - | |
| Volatile matter | 72.78 ± 2.16 | - | - | |
| Moisture | 13.2 ± 1.5 | - | - | |
| HHV (MJ/kg) | 16.14 | - | 10.98 | |
| Solid content b | Total solid | 87.29 ± 2.67 | - | - |
| Volatile solid | 82.26 ± 2.51 | - | - | |
| Extract analysis | TPC (mg Gallic Acid/g sample) | 5.45 ± 3.12 | - | - |
| AC IC50 (ug/mL sample) | 111.04 ± 2.58 | - | - | |
| RS g Glucose/L sample | 1.27 ± 3.14 | - | - | |
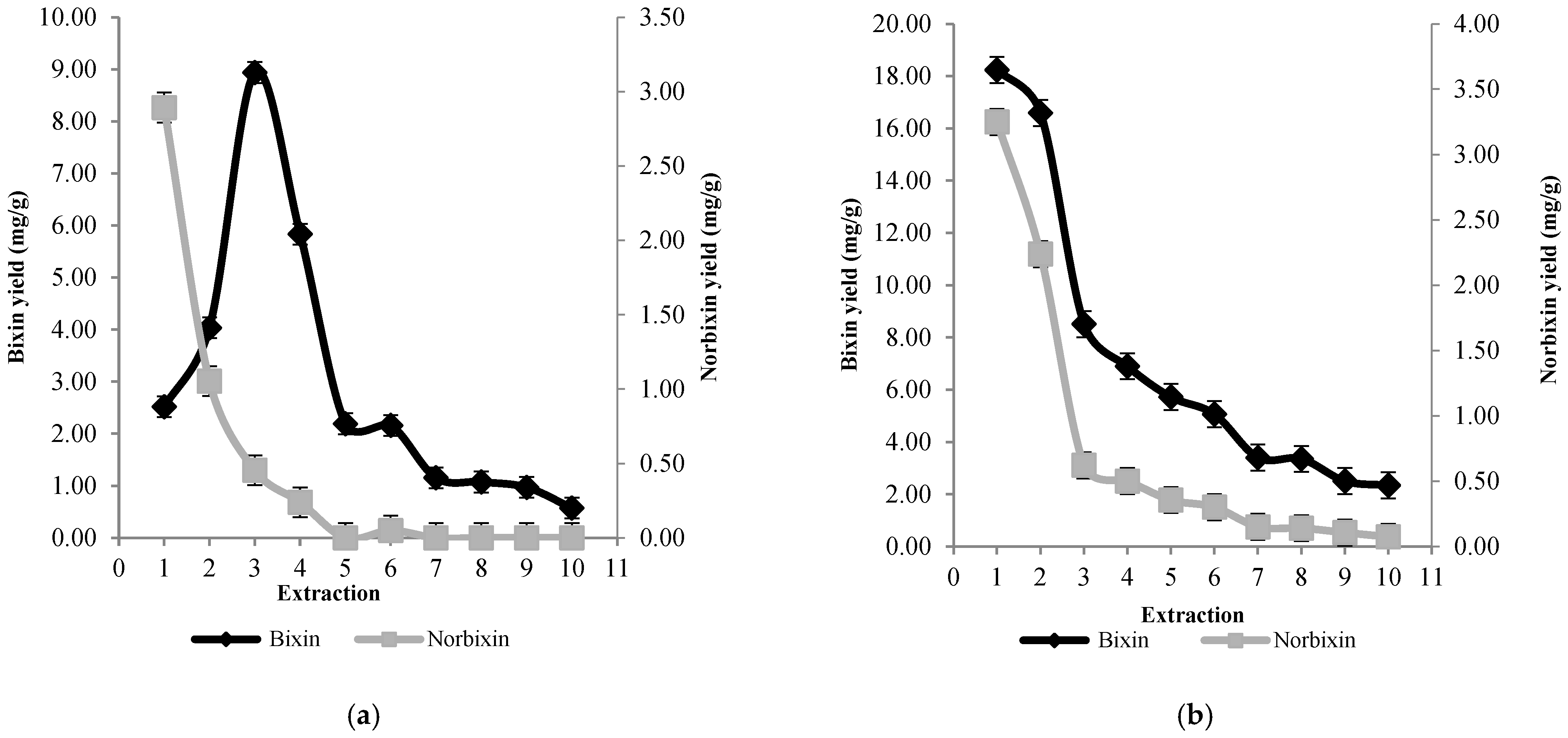
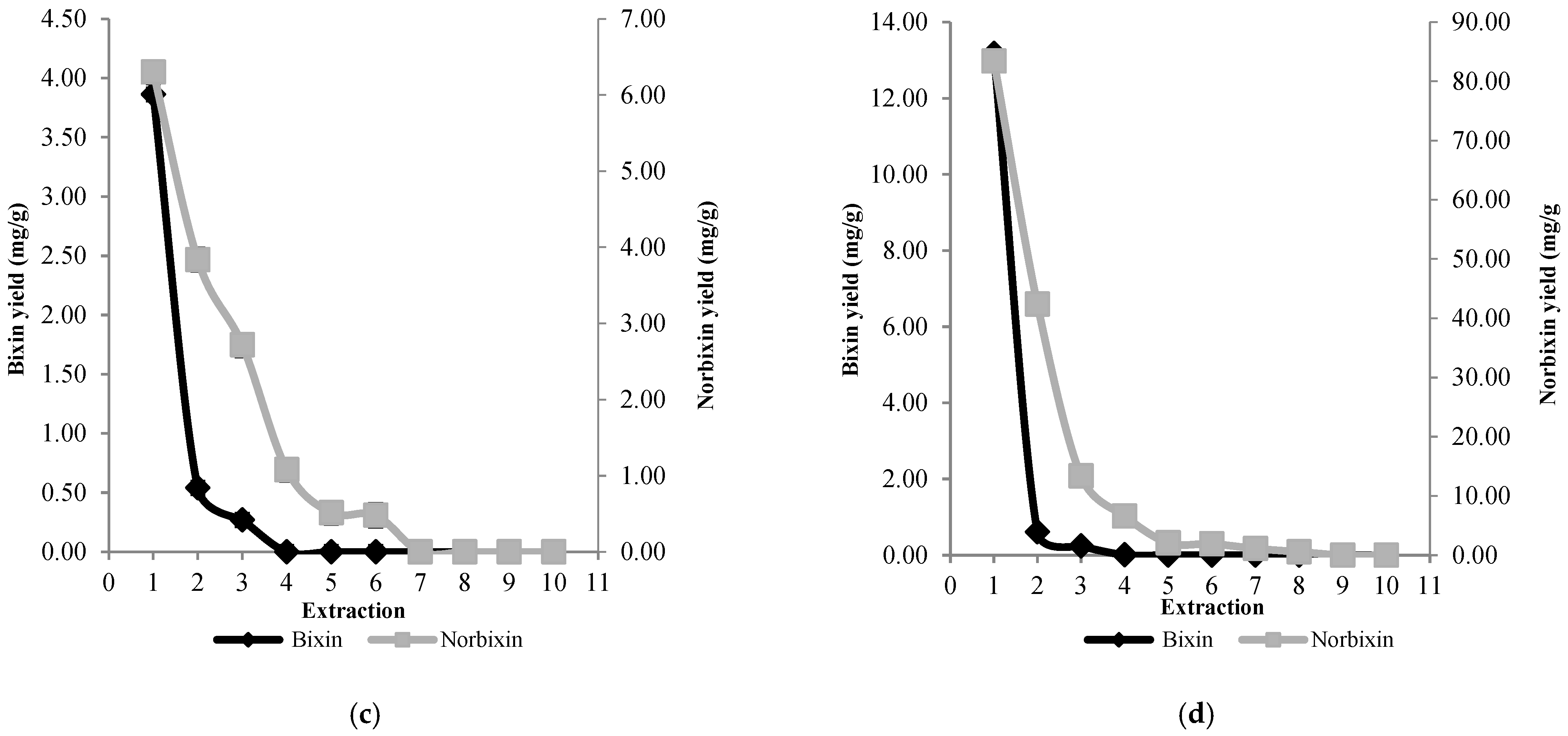
3.2. Extraction and Quantification of Bixin and Norbixin
3.3. Process Simulation
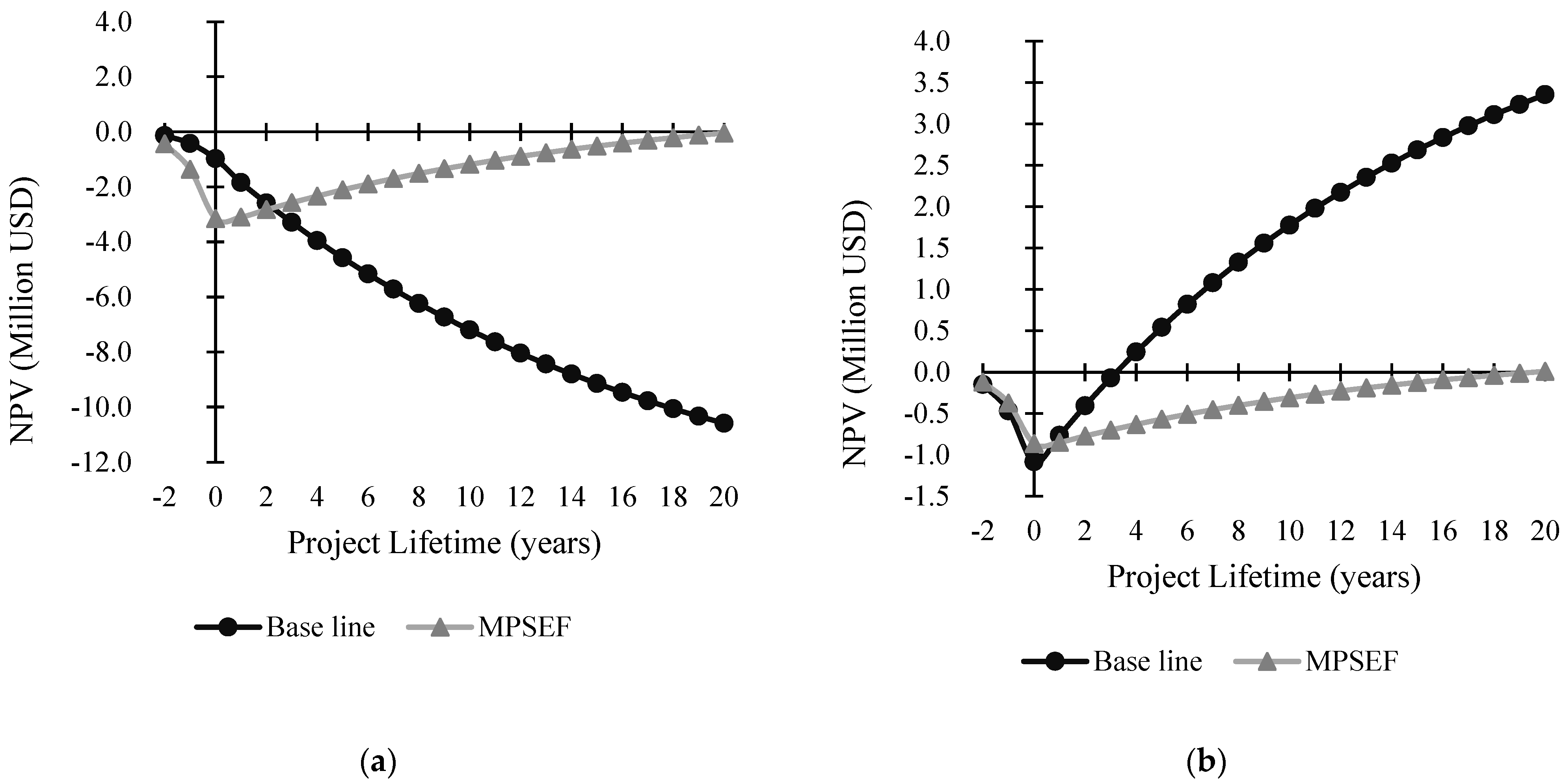
3.3.1. Technical and Economic Assessment
3.3.2. Economic Assessment
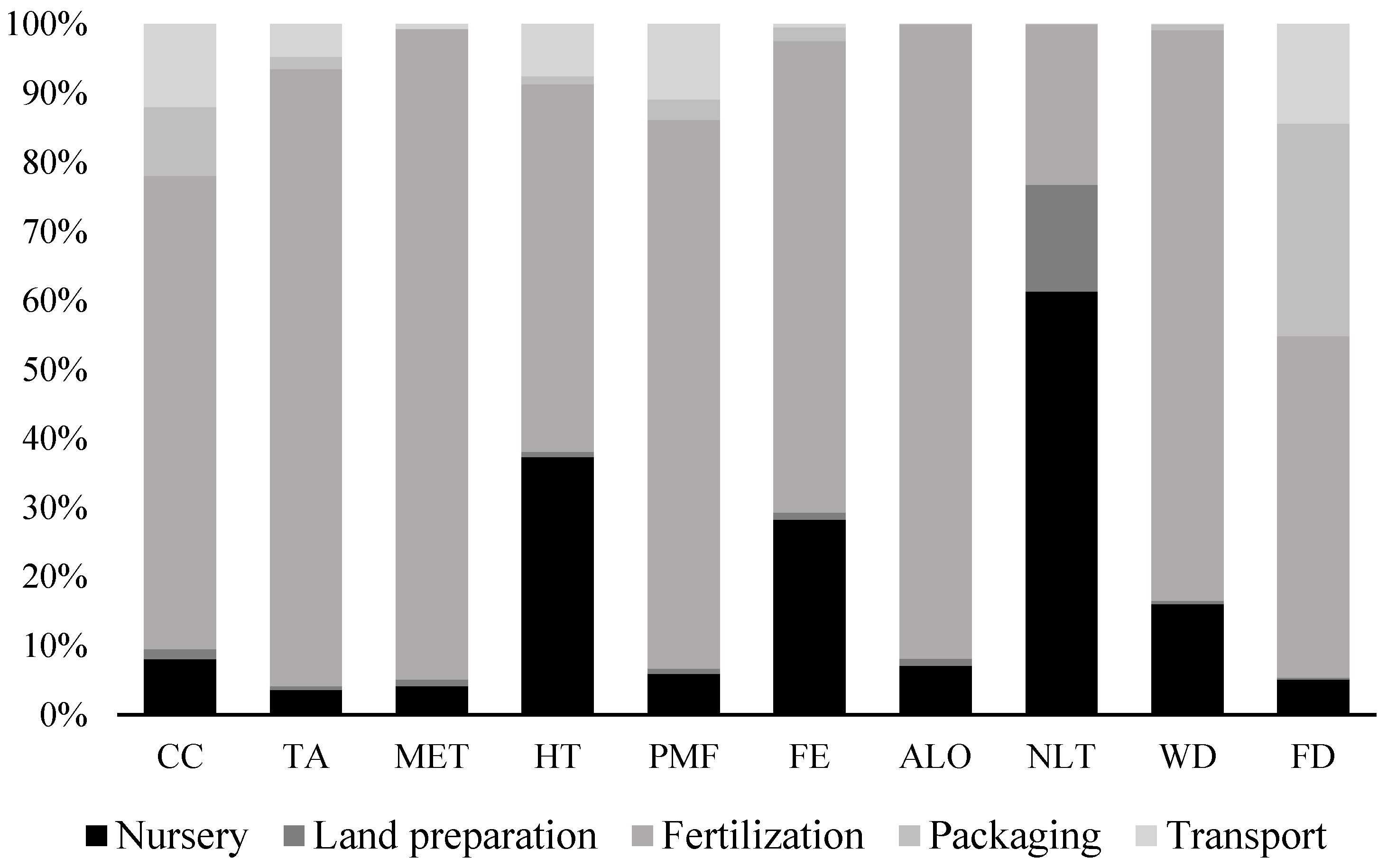
3.3.3. Environmental Assessment
Environmental Assessment of the Annatto Crop in Tropical Rainforests (Bio Productive-Chocó Region)
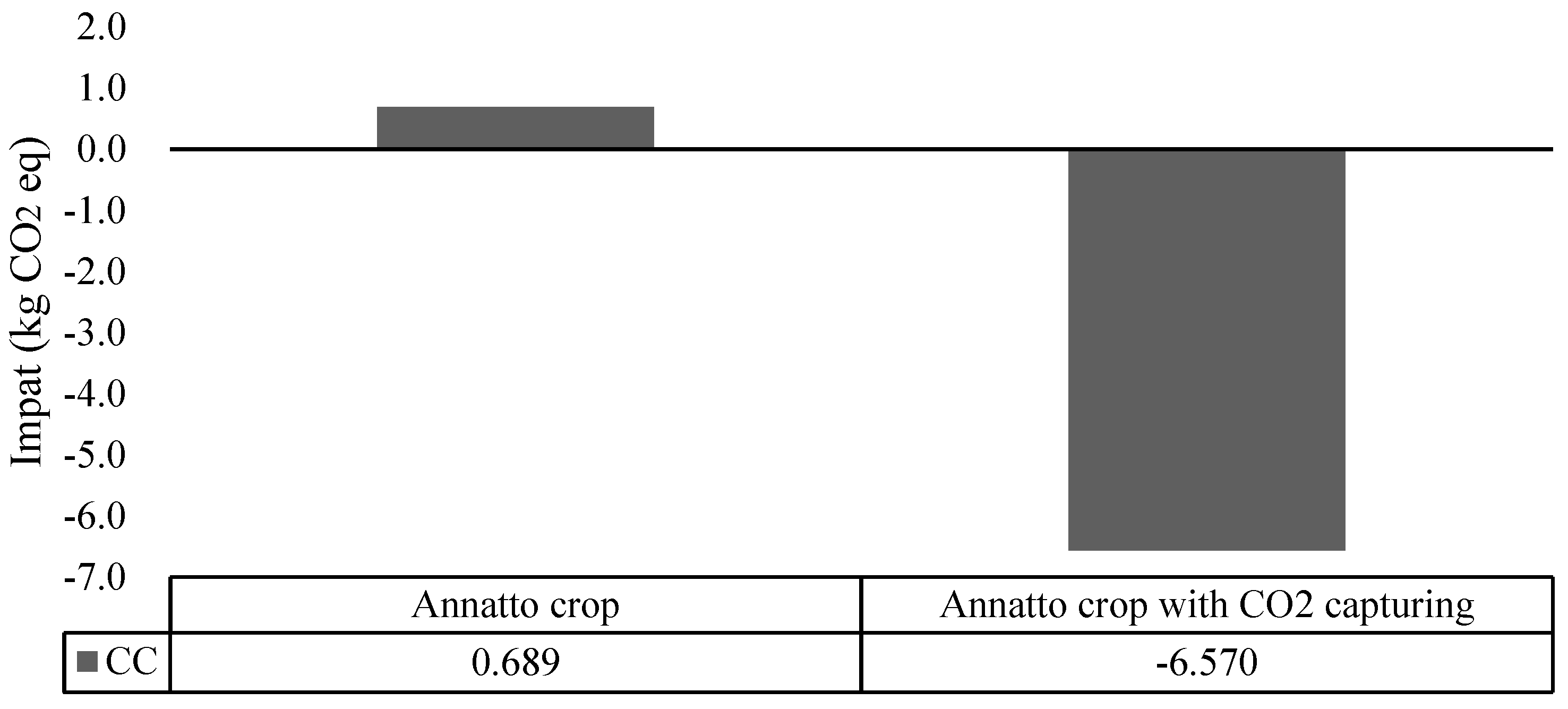
Environmental Assessment of the Annatto Crop in Tropical Forests Considering Carbon Sequestration
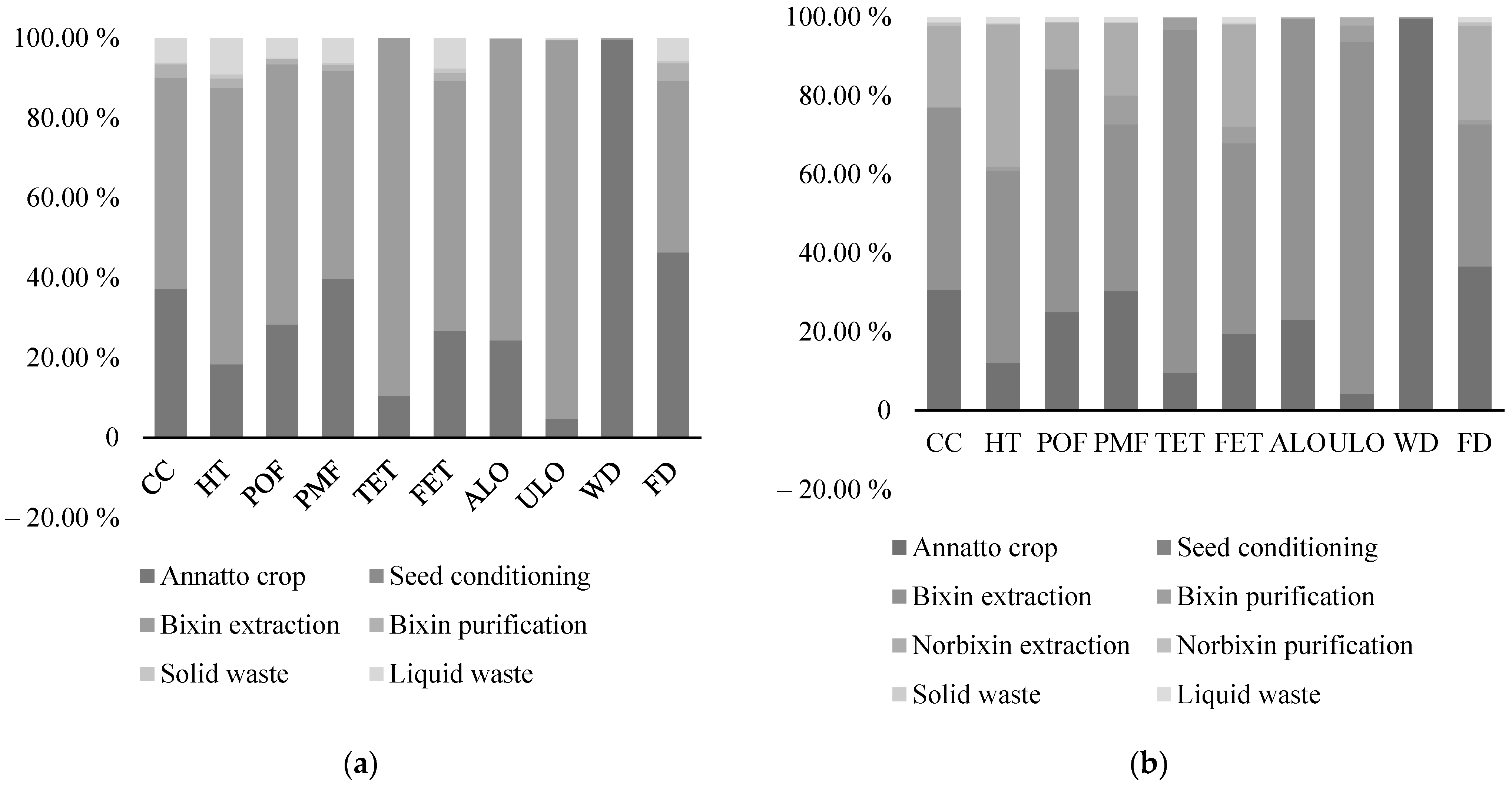
Environmental Assessment of the Biorefinery Schemes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehorn, P.R.; Navarro, L.M.; Schröter, M.; Fernandez, M.; Rotllan-Puig, X.; Marques, A. Mainstreaming biodiversity: A review of national strategies. Biol. Conserv. 2019, 235, 157–163. [Google Scholar] [CrossRef]
- Harrison, P.; Berry, P.; Simpson, G.; Haslett, J.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Karlsson-Vinkhuyzen, S.; Boelee, E.; Cools, J.; van Hoof, L.; Hospes, O.; Kok, M.; Peerlings, J.; van Tatenhove, J.; Termeer, C.J.; Visseren-Hamakers, I.J. Identifying barriers and levers of biodiversity mainstreaming in four cases of transnational governance of land and water. Environ. Sci. Policy 2018, 85, 132–140. [Google Scholar] [CrossRef]
- Marques, A.; Pereira, H.M.; Krug, C.; Leadley, P.W.; Visconti, P.; Januchowski-Hartley, S.R.; Krug, R.M.; Alkemade, R.; Bellard, C.; Cheung, W.W.; et al. A framework to identify enabling and urgent actions for the 2020 Aichi Targets. Basic Appl. Ecol. 2014, 15, 633–638. [Google Scholar] [CrossRef]
- Persson, Å.; Runhaar, H.; Karlsson-Vinkhuyzen, S.; Mullally, G.; Russel, D.; Widmer, A. Editorial: Environmental Policy Integration: Taking stock of policy practice in different contexts. Environ. Sci. Policy 2018, 85, 113–115. [Google Scholar] [CrossRef]
- González-Orozco, C.E. Biogeographical regionalisation of Colombia: A revised area taxonomy. Phytotaxa 2021, 484, 247–260. [Google Scholar] [CrossRef]
- Copete, J.C.; Sanchez, M.; Cámara-Leret, R.; Balslev, H. Diversity of palm communities in the biogeographic Chocó and its relation with precipitation. Caldasia 2019, 41, 358–369. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Lucas, E.; Jaramillo, C.; Monro, A.; Morris, S.K.; Bogarín, D.; Greer, D.; Dodsworth, S.; Aguilar-Cano, J.; Meseguer, A.S.; et al. The Origin and Diversification of the Hyperdiverse Flora in the Chocó Biogeographic Region. Front. Plant Sci. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Moreira, P.A.; Lins, J.; Dequigiovanni, G.; Veasey, E.; Clement, C. The Domestication of Annatto (Bixa orellana) from Bixa urucurana in Amazonia. Econ. Bot. 2013, 69, 127–135. [Google Scholar] [CrossRef]
- Areeg, A.-E. Comparative Study of Some Natural and Artificial Food Coloring Agents on Hyperactivity, Learning and Memory Performance in Weanling Rats. Int. J. Sci. Basic Appl. Res. 2015, 21, 309–324. [Google Scholar]
- United States Agency for International Development. Plan de negocios achiote (Brixa orellana). 2015, Reporte de Consultoría 0019. Programa BIOREDD+; USAID: Washington, DC, USA, 2015.
- Rahmalia, W.; Fabre, J.-F.; Mouloungui, Z. Effects of Cyclohexane/Acetone Ratio on Bixin Extraction Yield by Accelerated Solvent Extraction Method. Procedia Chem. 2015, 14, 455–464. [Google Scholar] [CrossRef]
- Natividad, L.R.; Rafael, R.R. Carotenoid Analyses and Antibacterial Assay of Annato (Bixa orèsllana L.), Carrot (Daucus carota L.), Corn (Zea mays L.) and Tomato (Solanum lycopersicum L.) Extracts. Res. J. Recent Sci. 2014, 3, 40–45. [Google Scholar]
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation Food Research International Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent ex. Food Res. Int. 2017, 99, 393–402. [Google Scholar] [CrossRef]
- Sánchez, S.S. Extracción de Bixina de très Variedades de Achiote (Bixa orellana L.) Utilizando très Solventes. Bachelor´s Thesis, Universidad Nacional de San Martin, Buenos Aires, Argentina, 2019. [Google Scholar]
- Isaza Jiménez, I.; Jaramillo Valencia, T.; Posada Uribe, L.F.; González Palacio, G.L. Evaluación del proceso de extracción alcalina de bixina de semillas de achiote (Bixa orellana). Bachelor´s Thesis, Universidad EAFIT, Medellín, Colombia, 2019. [Google Scholar]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Pagès, P.-B.; Le Pimpec-Barthes, F.; Bernard, A. Chirurgie des métastases pulmonaires des cancers colorectaux: Facteurs prédictifs de survie. Rev. Mal. Respir. 2016, 33, 838–852. [Google Scholar] [CrossRef]
- Machrafi, H. Zero-Carbon Energy Kioto. In Green Energy and Technology; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Yu, L.; Reitmeier, C.; Love, M. Strawberry Texture and Pectin Content as Affected by Electron Beam Irradiation. J. Food Sci. 1996, 61, 844–846. [Google Scholar] [CrossRef]
- Abbaszadeh, A.H. Pectin and galacturonic acid from citrus wastes. Master’s Thesis, University of Borås, Borås, Sweden, 2008. [Google Scholar]
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. British Standards Institution: London, UK, 2013; Volume 3, pp. 2013–2015. [CrossRef]
- Feng, F.; Liu, W.Y.; Chen, Y.S.; Guo, Q.L.; You, Q.D. Study on derivatives of gambogic acid. J. China Pharm. Univ. 2005, 36, 302–305. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Sci. Hortic. 2016, 213, 281–286. [Google Scholar]
- Marinova, G.; Batchvarov, V. Methods DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Piedrahita-Rodríguez, S.; Solarte-Toro, J.C.; Piñeres, P.P.; Ortiz-Sánchez, M.; Pérez-Cordero, A.; Cardona-Alzate, C.A. Analysis of a biorefinery with multiple raw materials in the context of post-conflict zones in Colombia: Plantain and avocado integration in the Montes de María region. Biomass-Convers. Biorefinery 2022, 12, 4531–4548. [Google Scholar] [CrossRef]
- Cardarelli, C.R.; Benassi, M.D.T.; Mercadante, A.Z. Characterization of different annatto extracts based on antioxidant and colour properties. LWT-Food Sci. Technol. 2008, 41, 1689–1693. [Google Scholar] [CrossRef]
- Noppe, H.; Martinez, S.A.; Verheyden, K.; Van Loco, J.; Beltran, R.C.; De Brabander, H. Determination of bixin and norbixin in meat using liquid chromatography and photodiode array detection. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 17–24. [Google Scholar] [CrossRef]
- Gosetti, F.; Frascarolo, P.; Mazzucco, E.; Gianotti, V.; Bottaro, M.; Gennaro, M. Photodegradation of E110 and E122 dyes in a commercial aperitif: A high performance liquid chromatography–diode array–tandem mass spectrometry study. J. Chromatogr. A 2008, 1202, 58–63. [Google Scholar] [CrossRef]
- Agronet. Reporte:Área, Producción y Rendimiento Nacional por Cultivo. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 30 June 2022).
- Jahuey, M.V. Aplicación de recubrimiento comestible para reducir la absorción de aceite durante el proceso de freído en los alimentos. Tesis Universidad Autónoma Agraria. Tesis Ingeniero en Ciencia y Tecnología de Alimentos. 2005. Available online: https://1library.co/document/yr3e1lp7-universidad-aut%C3%B3noma-agraria-antonio-divisi%C3%B3n-departamento-tecnolog%C3%ADa-alimentos.html (accessed on 15 December 2022).
- Salvá Ruiz, B.; Campos Gutierrez, D. Utilizacion de enzimas en la extraccion de colorante a partir de semillas de achiote (Bixa orellana L.). Anales Cientificos UNALM. pp. 2–17. Available online: https://dokumen.tips/documents/bixina-con-enzimas.html?page=1 (accessed on 15 December 2022).
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Mercado Libre. Sodio Hidroxido En Lentejas, Para Analisis. Available online: https://articulo.mercadolibre.com.co/MCO-807274649-sodio-hidroxido-en-lentejas-para-analisis-_JM#position=6&search_layout=stack&type=item&tracking_id=091b3a06-53df-4e91-8c23-065d534f4a86 (accessed on 15 December 2022).
- Alibaba. Celluclast Price. Available online: https://spanish.alibaba.com/g/cellulase-enzyme-price.html (accessed on 15 December 2022).
- MercadoLibre. Etanol. Available online: https://listado.mercadolibre.com.co/etanol (accessed on 15 December 2022).
- Albarelli, J.Q.; Santos, D.T.; Cocero, M.J.; Meireles, M.A.A. Economic Analysis of an Integrated Annatto Seeds-Sugarcane Biorefinery Using Supercritical CO2 Extraction as a First Step. Materials 2016, 9, 494. [Google Scholar] [CrossRef]
- Alibaba. Norbixin Price. Available online: https://www.alibaba.com/showroom/norbixin.html (accessed on 15 December 2022).
- ISO14040; Environmental management—Life cycle assessment—Principles and framework Management. International Organization for Standardization: Geneva, Switzerland, 2018; Volume 2018, p. 60.
- Yepes, M.F.; Navarrete, A.P.; Duque, D.A.; Phillips, A.J.; Cabrera, J.F.; Álvarez, K.R.; García, E.; Ordoñez, M.C. Protocolo para la Estimación Nacional y Subnacional de Biomasa—Carbono en Colombia; Instituto de Hidrología, Meteorología, y Estudios Ambientales-IDEAM: Bogotá, Colombia, 2011; Volume 59.
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- MacDicken, K.G. A Guide to Monitoring Carbon Storage in Forestry and Agroforestry Projects; Winrock International Institute for Agriculture Development: Arlington, TX, USA, 1997. [Google Scholar]
- Aristizábal-Marulanda, V.; García-Velásquez, C.A.; Alzate, C.A.C. Environmental assessment of energy-driven biorefineries: The case of the coffee cut-stems (CCS) in Colombia. Int. J. Life Cycle Assess. 2021, 26, 290–310. [Google Scholar] [CrossRef]
- Meñaca, E.V.; Del Valle, U.; Restrepo, J.; Colmenares, A.J. Antioxidant activity of the inclusion complex of Bixa orellana seeds extract in ß-cyclodextrin obtained by supercritical co2. Vitae 2018, 25, 83–91. [Google Scholar] [CrossRef]
- Rojas, A.F.; Flórez, C.; López, D.F. Use prospects of some agroindustrial waste. Rev. Cuba. Química 2018, 31, 31–52. [Google Scholar]
- Özyuğuran, A.; Yaman, S. Prediction of Calorific Value of Biomass from Proximate Analysis. Energy Procedia 2017, 107, 130–136. [Google Scholar] [CrossRef]
- Kumar, P.S.; Reddy, Y.R.; Ramesh, S.; Gobinath, S.; Ramana, D.B.V. Evaluation of Pigment Extracted Annatto Seed (Bixa Orellana) By Chemical, in-Vitro and in-Sacco Techniques in Buffaloes. Buffalo Bull. 2007, 26, 5–9. Available online: http://ibic.lib.ku.ac.th/e-bulletin/2007-5.htm (accessed on 15 December 2022).
- Stringheta, P.C.; Silva, P.I.; Costa, A.G. Annatto/Urucum—Bixa orellana. Exot. Fruits 2018, 23–30. [Google Scholar] [CrossRef]
- Mantovani, N.C.; Grando, M.F.; Xavier, A.; Otoni, W.C. Evaluation of annato (Bixa orellana L.) genotypes through the morphological characteristics of fruits, seeds productivity and bixin content. Cienc. Florest. 2013, 23, 355–362. [Google Scholar]
- Valério, M.A.; Ramos, M.I.L.; Neto, J.A.B.; Macedo, M.L. Annatto seed residue (Bixa orellana L.): Nutritional quality. Food Sci. Technol. 2015, 35, 326–330. [Google Scholar] [CrossRef]
- Raddatz-Mota, D.; (Iztapalapa), U.A.M.; Pérez-Flores, L.J.; Carrari, F.; Incani, M.; Asis, R.; Mendoza-Espinoza, J.; León-Sánchez, F.D.; Rivera-Cabrera, F. Chemical characterization and quantification of the pigment extraction yield of seven mexican accessions of Bixa orellana. Rev. Mex. De Ing. Quim. 2016, 15, 727–740. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; Lima, J.L.F.D.C.; Bragagnolo, N. In vitro scavenging capacity of annatto seed extracts against reactive oxygen and nitrogen species. Food Chem. 2011, 127, 419–426. [Google Scholar] [CrossRef]
- Giridhar, P.; Venugopalan, A.; Parimalan, R. A review on annatto dye extraction, analysis and processing—A Food Technology Perspective. J. Sci. Res. Rep. 2014, 3, 327–348. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Poveda-Giraldo, J.A.; Alzate, C.A.C. Comparison of furfural and biogas production using pentoses as platform. Sci. Total. Environ. 2020, 728, 138841. [Google Scholar] [CrossRef]
- Segura, M.A.; Andrade, H.J. Huella de carbono en cadenas productivas de cafe (Coffea arabica L.) con diferentes estandares de certificación en Costa Rica. Luna Azúl 2012, 60–77. Available online: http://www.scielo.org.co/pdf/luaz/n35/n35a05.pdf (accessed on 15 December 2022).
- Ortiz, S.-M.; Alzate, C. Comparative environmental life cycle assessment of orange peel waste in present productive chains. J. Clean. Prod. 2021, 322, 128814. [Google Scholar] [CrossRef]
- Kitamura, R.; Sugiyama, C.; Yasuda, K.; Nagatake, A.; Yuan, Y.; Du, J.; Yamaki, N.; Taira, K.; Kawai, M.; Hatano, R. Effects of Three Types of Organic Fertilizers on Greenhouse Gas Emissions in a Grassland on Andosol in Southern Hokkaido, Japan. Front. Sustain. Food Syst. 2021, 5, 649613. [Google Scholar] [CrossRef]
- Na Talang, R.P.; Sirivithayapakorn, S. Environmental impacts and economic benefits of different wastewater management schemes for molasses-based ethanol production: A case study of Thailand. J. Clean. Prod. 2020, 247, 119141. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.-M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Saer, A.; Lansing, S.; Davitt, N.H.; Graves, R.E. Life cycle assessment of a food waste composting system: Environmental impact hotspots. J. Clean. Prod. 2013, 52, 234–244. [Google Scholar] [CrossRef]
- Acevedo, L.D.V.; De Colombia, U.N.; Henao, J.A.V.; Grisales, N.M. Assessment of the environmental impact of three types of fertilizers on the cultivation of coffee at the Las Delicias indigenous reservation (Cauca) starting from the life cycle assessment. Rev. Fac. Ing. 2016, 93–101. [Google Scholar] [CrossRef]
- Higuera, J.Z.P. Estudio comparado sobre la huella de carbono en cultivos de banano en los paises de Colombia, República Dominicana, Ecuador y Guatemala. J. Food Sci. 2017, 43. [Google Scholar] [CrossRef]
- Arboleda, J.A.U. Huella de carbono en los sistemas de producción agrícola dominantes en el municipio de Falán. Tolima 2012, 33, 348–352. [Google Scholar]
- Gerbal, L. Assessing the Carbon Footprint and Climate Risk of Most Consumed Food Products in Cali, Colombia: Methodological Development of a Decision Support Tool; International Center for Tropical Agriculture: Palmira, Colombia, 2019. [Google Scholar]
- Barrera-Ramírez, J.; Prado, V.; Solheim, H. Life cycle assessment and socioeconomic evaluation of the illicit crop substitution policy in Colombia. J. Ind. Ecol. 2019, 23, 1237–1252. [Google Scholar] [CrossRef]
- Levasseur, A. Climate Change. In LCA Compendium—The Complete World of Life Cycle Assessment; Springer: Berlin/Heidelberg, Germany, 2015; pp. 139–162. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Preciado-Barragán, J.; Hurtado-Vásquez, C. Estimation of stored carbon in a productive forest: Upper watershed of the river Domingodó. Visión Electrón. 2019, 2, 222–233. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=8077412 (accessed on 15 December 2022).
- Amézquita, M.C. Captura De Carbono En Sistemas De Pasturas Y Silvopastoriles En Cuatro Ecosistemas De América Tropical Vulnerables Al Cambio Climático. Nac. Ambient. 2008, 27, 2–12. [Google Scholar]
- Machado, K.S.; Seleme, R.; Maceno, M.M.; Zattar, I.C. Carbon footprint in the ethanol feedstocks cultivation—Agricultural CO2 emission assessment. Agric. Syst. 2017, 157, 140–145. [Google Scholar] [CrossRef]
- Thannimalay, L.; Yusoff, S.; Zawawi, N.Z. Life Cycle Assessment of Sodium Hydroxide. Aust. J. Basic Appl. Sci. 2013, 7, 421–431. [Google Scholar]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life cycle analysis of β-carotene extraction techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
| Item | Units | Value | Reference |
|---|---|---|---|
| Low-pressure steam | USD ton−1 | 1.57 | [36] |
| Medium-pressure steam | USD ton−1 | 8.18 | [36] |
| High-pressure steam | USD ton−1 | 9.86 | [36] |
| Electricity | kWh | 0.1 | [36] |
| Water | USD m−3 | 0.74 | [36] |
| NaOH | USD kg−1 | 0.6 | [37] |
| Celluclast | USD kg−1 | 3.7 | [38] |
| Ethanol | USD gallon−1 | 2.05 | [39] |
| Bixin | USD kg−1 | 15.75 | [40] |
| Norbixin | USD kg−1 | 18.00 | [41] |
| Item | Equation | Variables | Ref |
|---|---|---|---|
| Aerial biomass | [43] | ||
| Belowground biomass | [44] | ||
| Soil organic carbon | [45] |
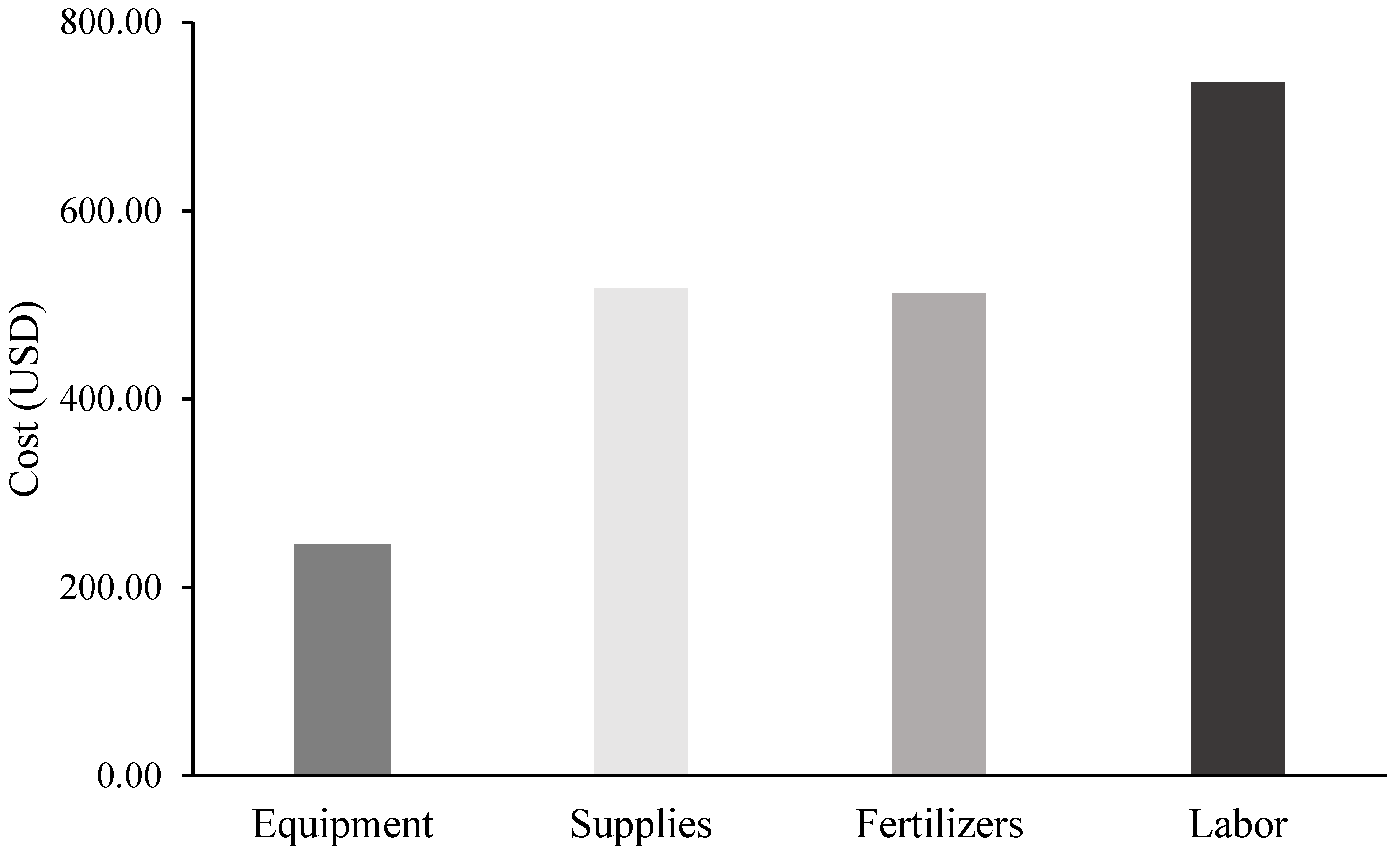
| Input Compounds | Machinery/Supplies Inputs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage | Subsystem | Activity | Name | Time (Month) | Name | Value | Unit | Name | Value | Unit | Fuel Consumption L h−1 | Type of Machinery |
| 1 | Seed protection | Seed protection | Ash | 0.2 | Ash | 637.5 | kg | - | - | - | - | Manual |
| 2 | Nursery | Nursery construction | Wood | 1 | - | - | - | Wood supports 1 | 53.3 | kg ha−1 | - | Manual |
| Sunshade greenhouse | - | - | - | Polyethylene | 4.2 | kg ha−1 | - | Manual | ||||
| 3 | Germinator | Bed construction | Wood bed | 3 | - | - | - | Wood bed | 144 | kg ha−1 | - | Manual |
| Wood supports | - | - | - | Wood supports 2 | 12.96 | kg ha−1 | - | Manual | ||||
| Substrate | Sand | Sand | 4060.26 | kg | - | - | - | - | Manual | |||
| Compost | Compost | 523.9 | kg | - | - | - | - | Manual | ||||
| Substrate sterilization | Water | Water | 3056.11 | kg | - | - | - | - | Manual | |||
| Bags | Bags | - | - | - | Polyethylene | 19.76 | kg ha−1 | - | Manual | |||
| First fertilization ** | Super Magro | Super Magro | 17.42 | L | - | - | - | - | ||||
| Cattle manure | 5.807 | kg | - | - | - | - | ||||||
| Molasses | 5.807 | kg | - | - | - | - | ||||||
| Water | 330.97 | kg | - | - | - | - | Manual | |||||
| Aspersion | - | - | - | - | - | - | - | Manual | ||||
| Irrigation | - | - | - | - | - | - | - | - | ||||
| 4 | Ground preparation | Weed removal | Machete | 1 | - | - | - | - | - | - | - | Manual |
| Tree removal | Hatchet | - | - | - | - | - | - | - | Manual | |||
| Herbicides | - | - | - | - | - | - | - | - | Manual | |||
| Digging | Palin | - | - | - | - | - | - | - | Manual | |||
| Substrate addition | Substrate 1 | Sand | 416.67 | kg | - | - | - | - | Manual | |||
| Compost | 208.33 | kg | - | - | - | - | Manual | |||||
| Irrigation | - | - | - | - | - | - | - | - | - | |||
| 5 | Plant growth | Second fertilization ** | SuperMagro | 18 | SuperMagro | 18.75 | L | - | - | - | - | Manual |
| Cattle manure | 6.25 | kg | - | - | - | - | ||||||
| Molasses | 6.25 | kg | - | - | - | - | ||||||
| Water | 356.25 | kg | - | - | - | - | Manual | |||||
| Aspersion | - | - | - | - | - | - | - | Manual | ||||
| Third fertilization ** | SuperMagro | SuperMagro | 937.5 | L | - | - | - | - | Manual | |||
| Cattle manure | 312.5 | kg | - | - | - | - | ||||||
| Molasses | 312.5 | kg | - | - | - | - | ||||||
| Water | 17,812.5 | - | - | - | - | |||||||
| Aspersion | - | - | - | - | - | - | - | Manual | ||||
| Pruning | Pruning shears | - | - | - | - | - | - | - | Manual | |||
| 6 | Production and harvest | Harvest | Pruning shears | 0.5 | - | - | - | - | - | - | - | Manual |
| Gather | Bags | - | - | - | Fique * | 34.28 | kg ha−1 | - | Manual | |||
| Transport | Transport | - | - | - | Transport | - | - | 1.84 | Truck | |||
| Scenario | Process | Input | Input Rate * | Output | Output Rate * |
|---|---|---|---|---|---|
| Scenario 1 | Bixin extraction | Annatto seed | 1.00 | - | - |
| Ethanol (70%v/v) | 11.23 | - | - | ||
| Water | 4.81 | - | - | ||
| Bixin filtration | - | - | Solid waste | 0.59 | |
| Bixin purification | - | - | Liquid waste | 16.04 | |
| - | - | Bixin | 0.05 | ||
| Scenario 2 | Bixin extraction | Annatto seed | 1.00 | - | - |
| Ethanol (70%v/v) | 11.23 | - | - | ||
| Water | 4.81 | - | - | ||
| Bixin purification | Liquid waste | 16.04 | |||
| Bixin | 0.05 | ||||
| Norbixin extraction | Water | 14.51 | - | - | |
| NaOH | 0.29 | - | - | ||
| Norbixin purification | Enzyme | 0.003 | Solid waste | 0.45 | |
| Norbixin separation | - | - | Liquid waste | 14.78 | |
| - | - | Norbixin | 0.17 |
| Scenario | Process | Energy Demand (MJ kg−1) | |
|---|---|---|---|
| Cooling Water | Steam | ||
| Scenario 1 | Colorant extraction | - | 130.07 |
| Colorant purification | 333.79 | 475.29 | |
| Total | 333.79 | 605.36 | |
| Scenario 2 | Colorant extraction | - | 380.12 |
| Colorant purification | 364.44 | 644.46 | |
| Total | 364.44 | 1024.58 | |
| Scheme Type | CapEx (M-USD kg−1) | OpEx (M-USD/year) | Product | Sale Cost (USD kg−1) | Production Cost (USD kg−1) | Revenue (USD kg−1) |
|---|---|---|---|---|---|---|
| Scenario 1 | 0.74 | 4.99 | Bixin | 15.75 | 20.17 | −4.42 |
| Scenario 2 | 0.83 | 13.48 | Bixin | 15.75 | 15.52 | 0.23 |
| Norbixin | 18 | 17.36 | 0.64 | |||
| Total | 33.75 | 32.88 | 0.87 | |||
| Base case (study region) * | - | Annatto seed | 3.40 | 2.9 | 0.50 |
| Crop | Climate Change (kg CO2 eq/kg Product/Year) | Reference |
|---|---|---|
| Annatto | 0.48 | This work |
| Banana | 1.3 | [65] |
| Cocoa | 2.6 | [66] |
| Rice | 1.46 | [67] |
| Illicit crops (coca) | 590 | [68] |
| Impact Category | Unit | Scenario 1 | Scenario 2 |
|---|---|---|---|
| CC | kg CO2 eq | 5.57 | 6.54 |
| HT | kg 1,4-DB eq | 1.168 | 1.724 |
| POF | kg NMVOC | 0.034 | 0.038 |
| PMF | kg PM10 eq | 0.021 | 0.025 |
| TET | kg 1,4-DB eq | 0.061 | 0.064 |
| FET | kg 1,4-DB eq | 0.036 | 0.047 |
| ALO | m2a | 4.014 | 4.563 |
| ULO | m2a | 0.441 | 0.471 |
| WD | m3 | 3.164 | 3.012 |
| FD | kg oil eq | 1.366 | 1.681 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agudelo Patiño, T.; Poveda-Giraldo, J.A.; Salas Moreno, M.H.; Rengifo Mosquera, G.; Cardona Alzate, C.A. Potential for Sustainable Production of Natural Colorants in the Tropical Forest: A Biorefinery Case of Annatto Seeds. Sustainability 2023, 15, 3079. https://doi.org/10.3390/su15043079
Agudelo Patiño T, Poveda-Giraldo JA, Salas Moreno MH, Rengifo Mosquera G, Cardona Alzate CA. Potential for Sustainable Production of Natural Colorants in the Tropical Forest: A Biorefinery Case of Annatto Seeds. Sustainability. 2023; 15(4):3079. https://doi.org/10.3390/su15043079
Chicago/Turabian StyleAgudelo Patiño, Tatiana, Jhonny Alejandro Poveda-Giraldo, Manuel Haminton Salas Moreno, Gysela Rengifo Mosquera, and Carlos Ariel Cardona Alzate. 2023. "Potential for Sustainable Production of Natural Colorants in the Tropical Forest: A Biorefinery Case of Annatto Seeds" Sustainability 15, no. 4: 3079. https://doi.org/10.3390/su15043079
APA StyleAgudelo Patiño, T., Poveda-Giraldo, J. A., Salas Moreno, M. H., Rengifo Mosquera, G., & Cardona Alzate, C. A. (2023). Potential for Sustainable Production of Natural Colorants in the Tropical Forest: A Biorefinery Case of Annatto Seeds. Sustainability, 15(4), 3079. https://doi.org/10.3390/su15043079





