Three-Dimensional Simulation on the Effects of Different Parameters and Pt Loading on the Long-Term Performance of Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Numerical Model
2.1. Model Assumptions [42,43]
- The cell operating conditions are at steady state.
- The inlet gas is an incompressible ideal gas.
- Flow is incompressible and laminar in PEMFCs.
- Isotropic media include porous layers such as GDL, MPL, and CL.
- The thermophysical characteristics and operating temperature of the cell are both constant.
2.2. Control Equations
2.3. Boundary Conditions
2.3.1. Inlet Flow Channel
2.3.2. Outlet Flow Channel
2.3.3. Wall
2.4. Pt Degradation Model
2.5. Current Cycle Model
2.6. Model Validation
3. Results and Discussion
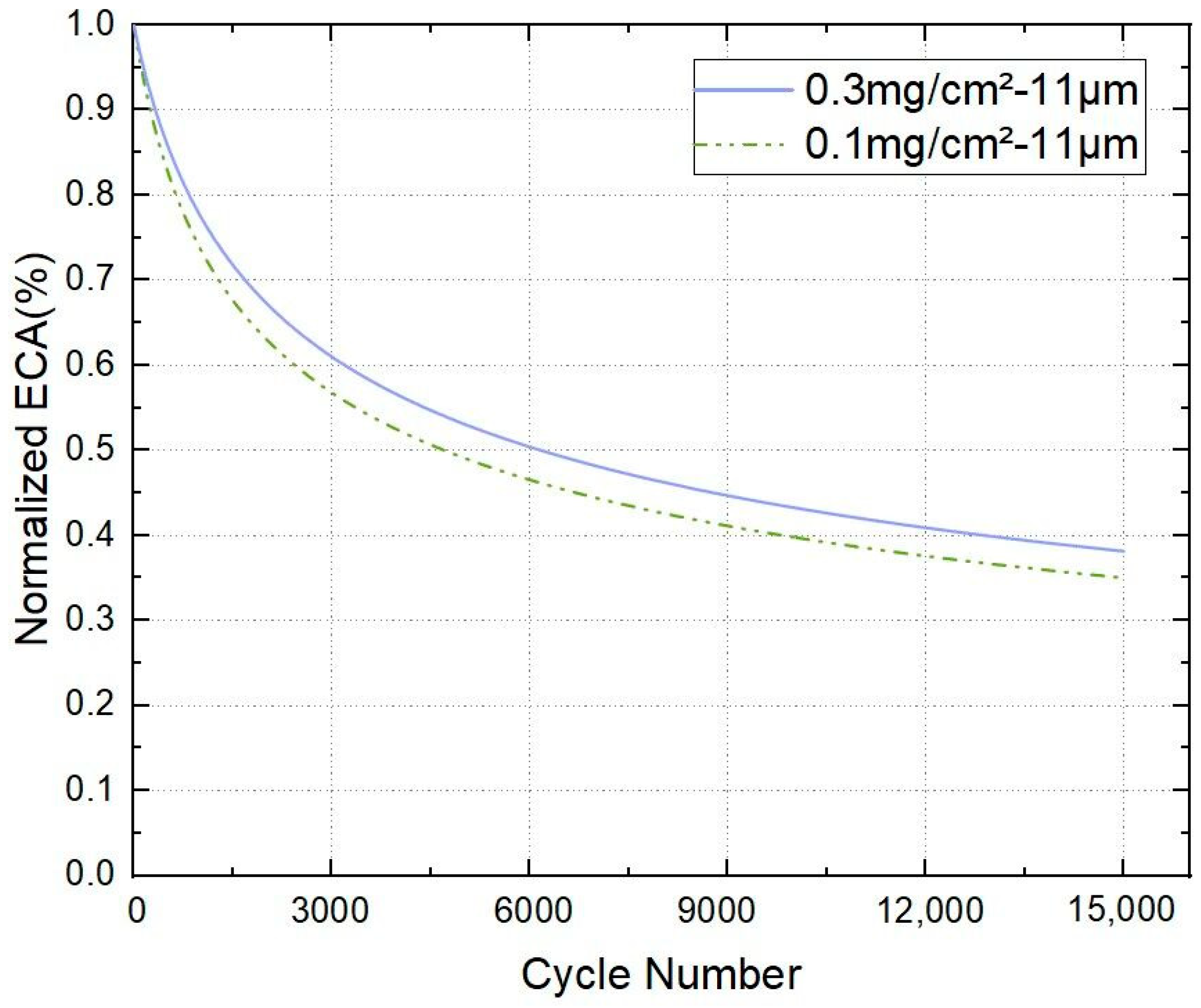
3.1. Pt Degradation Leads to Performance Loss of PEMFC
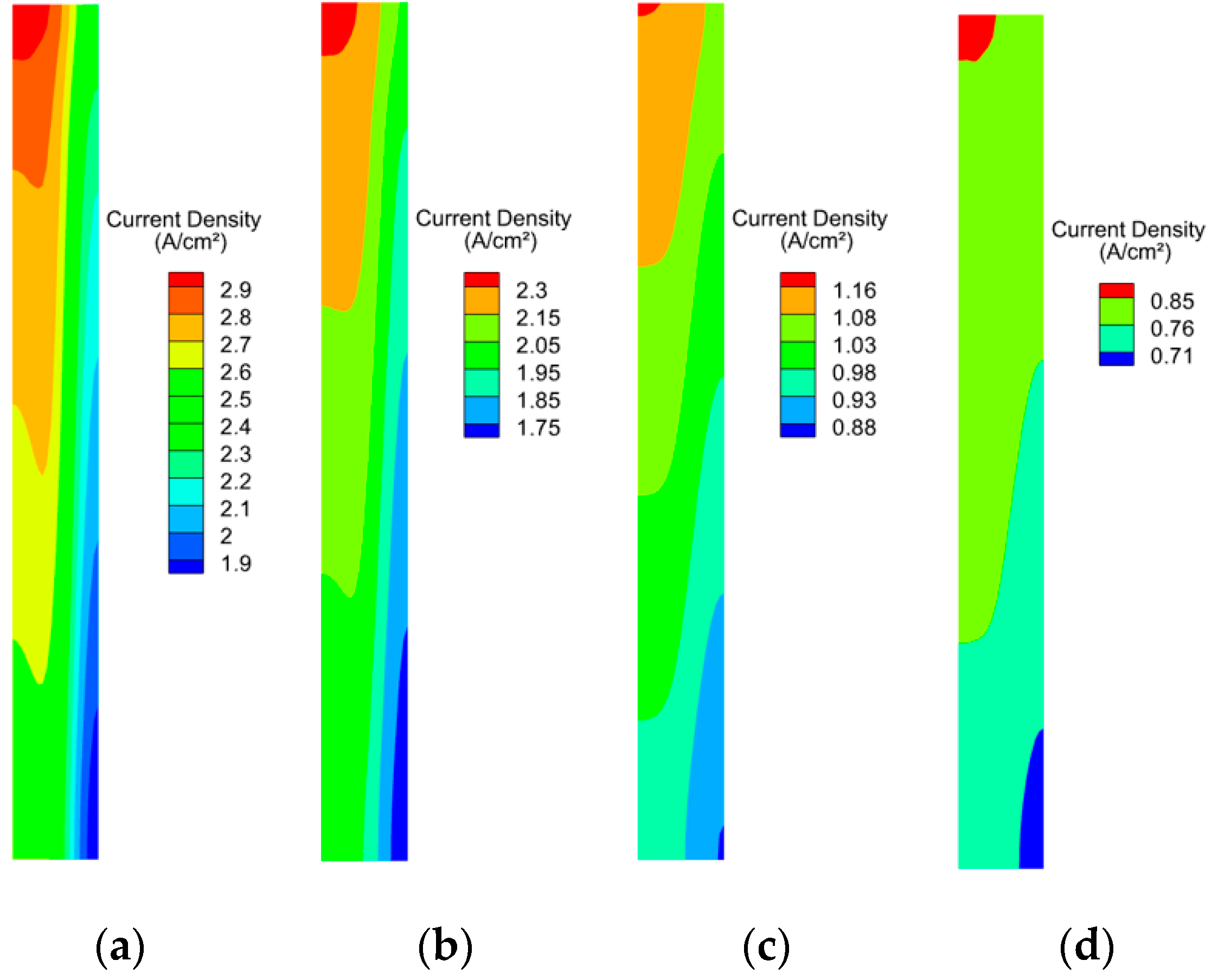
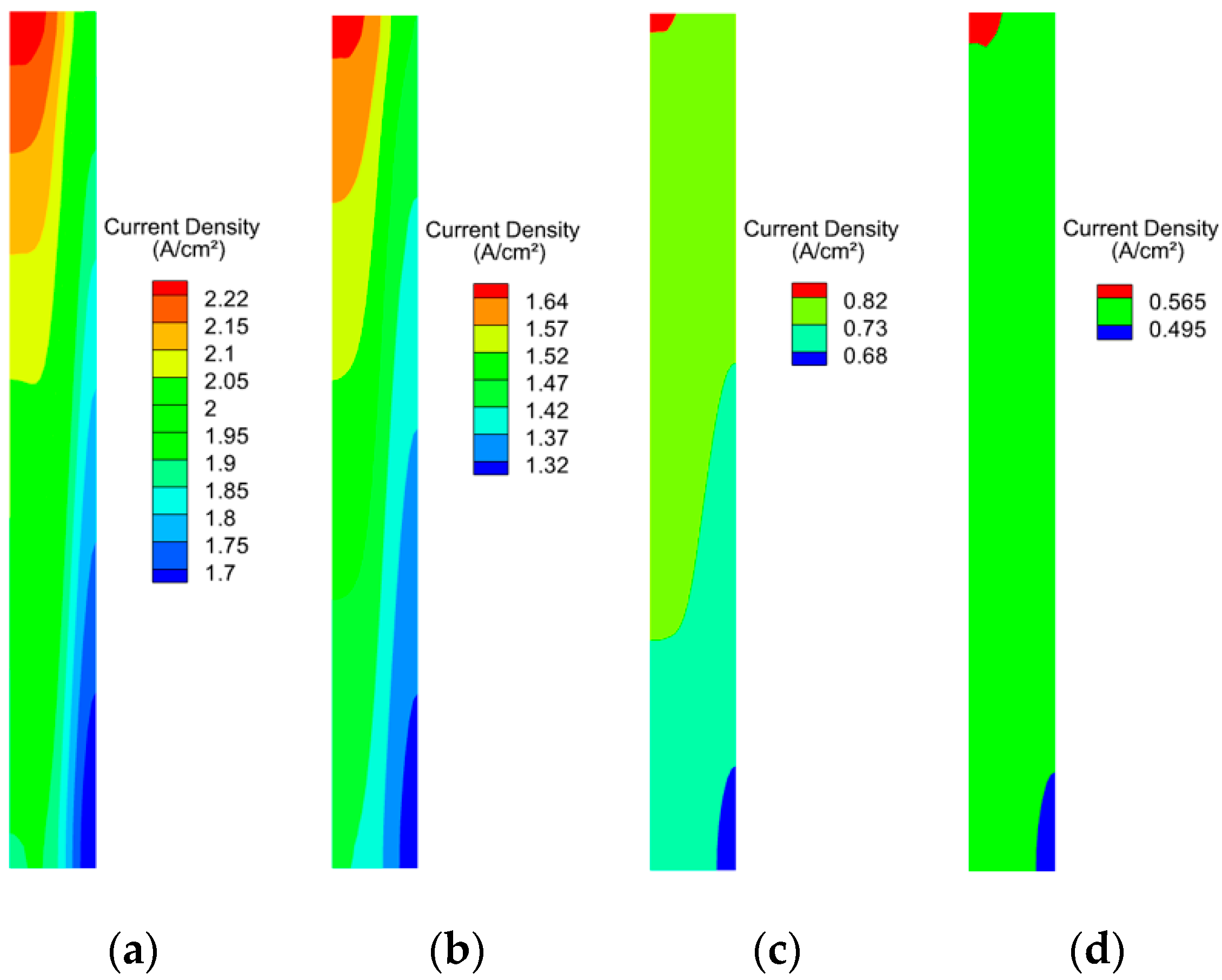
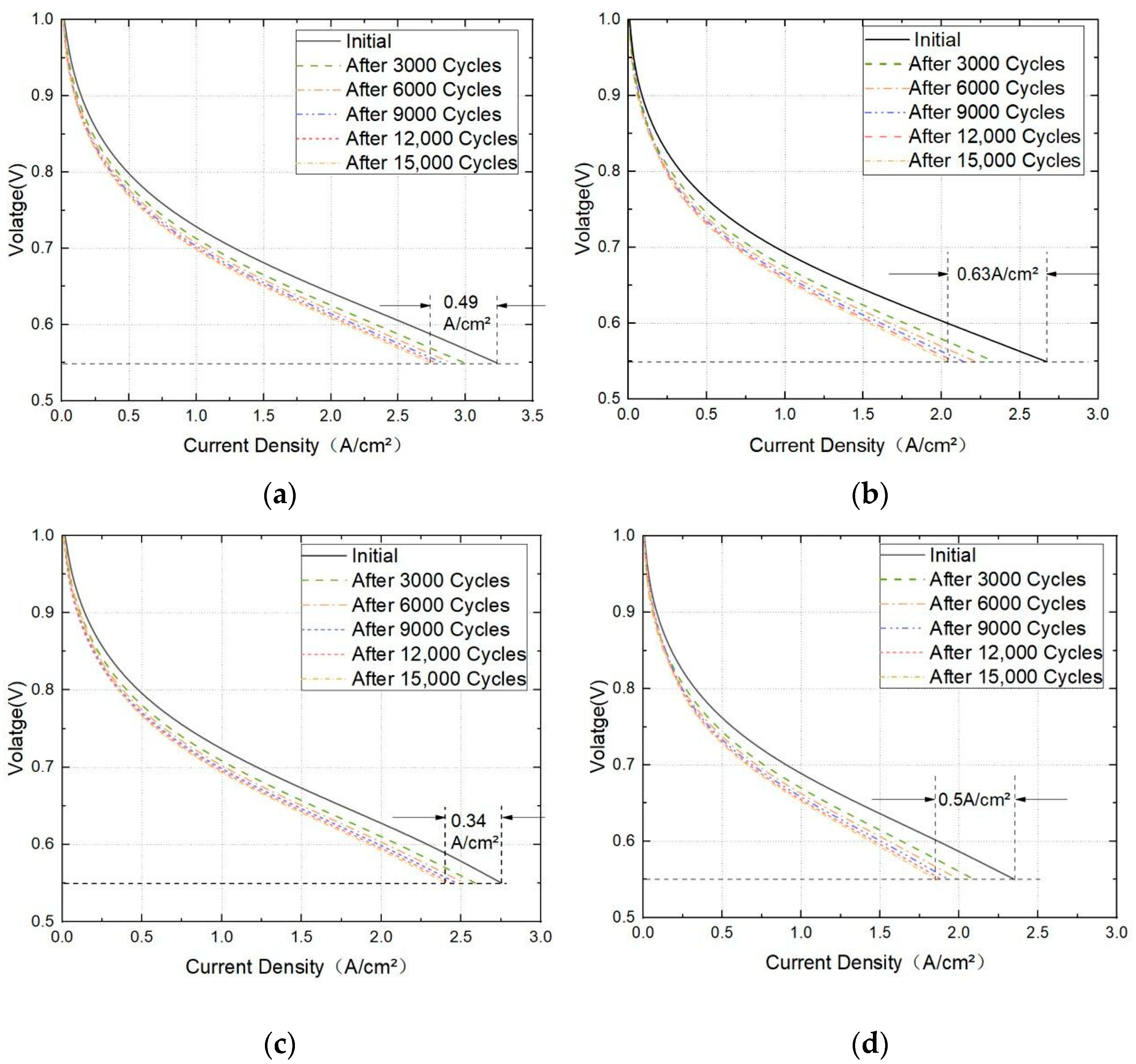
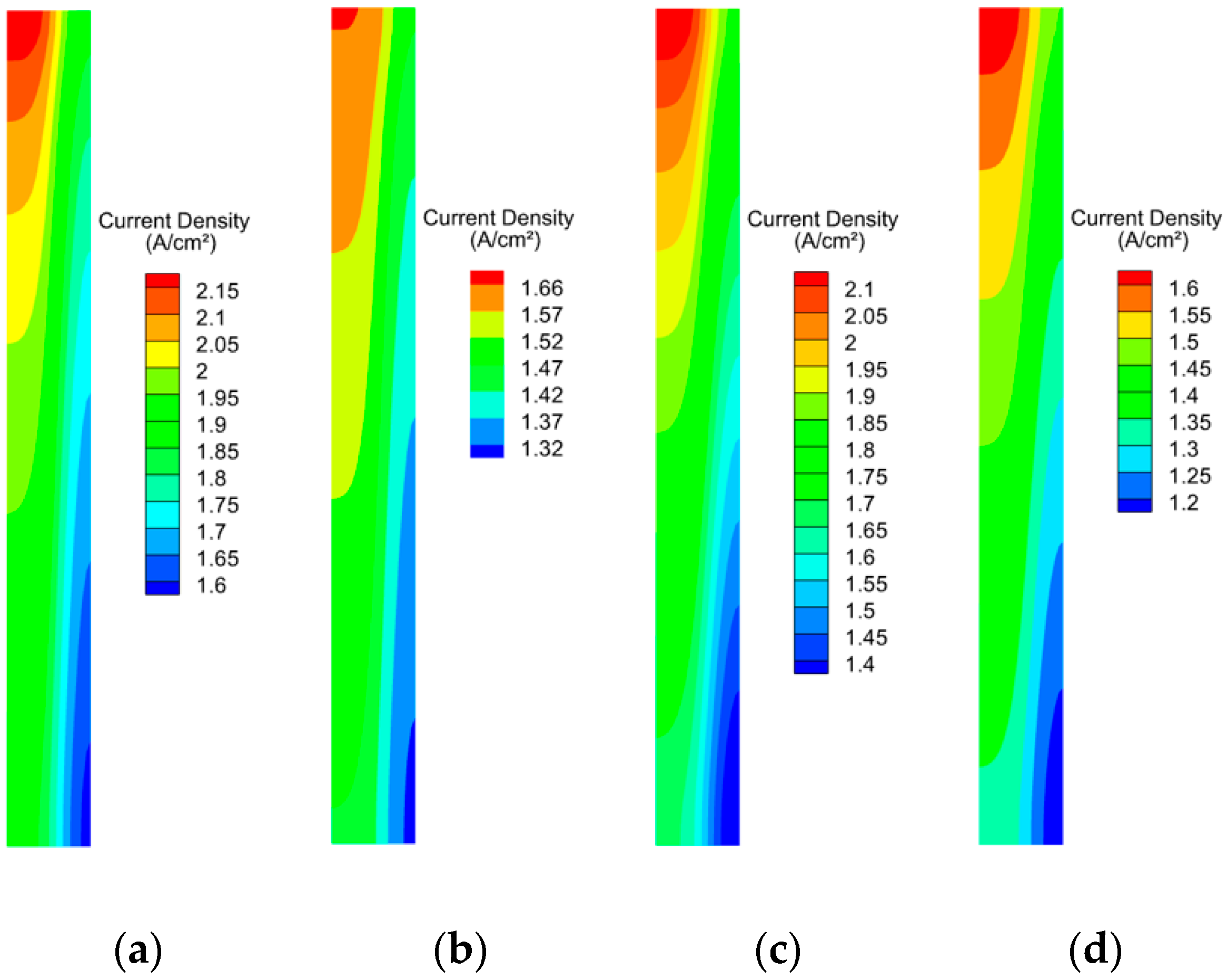
3.2. Effect of Working Pressure on PEMFC Aging
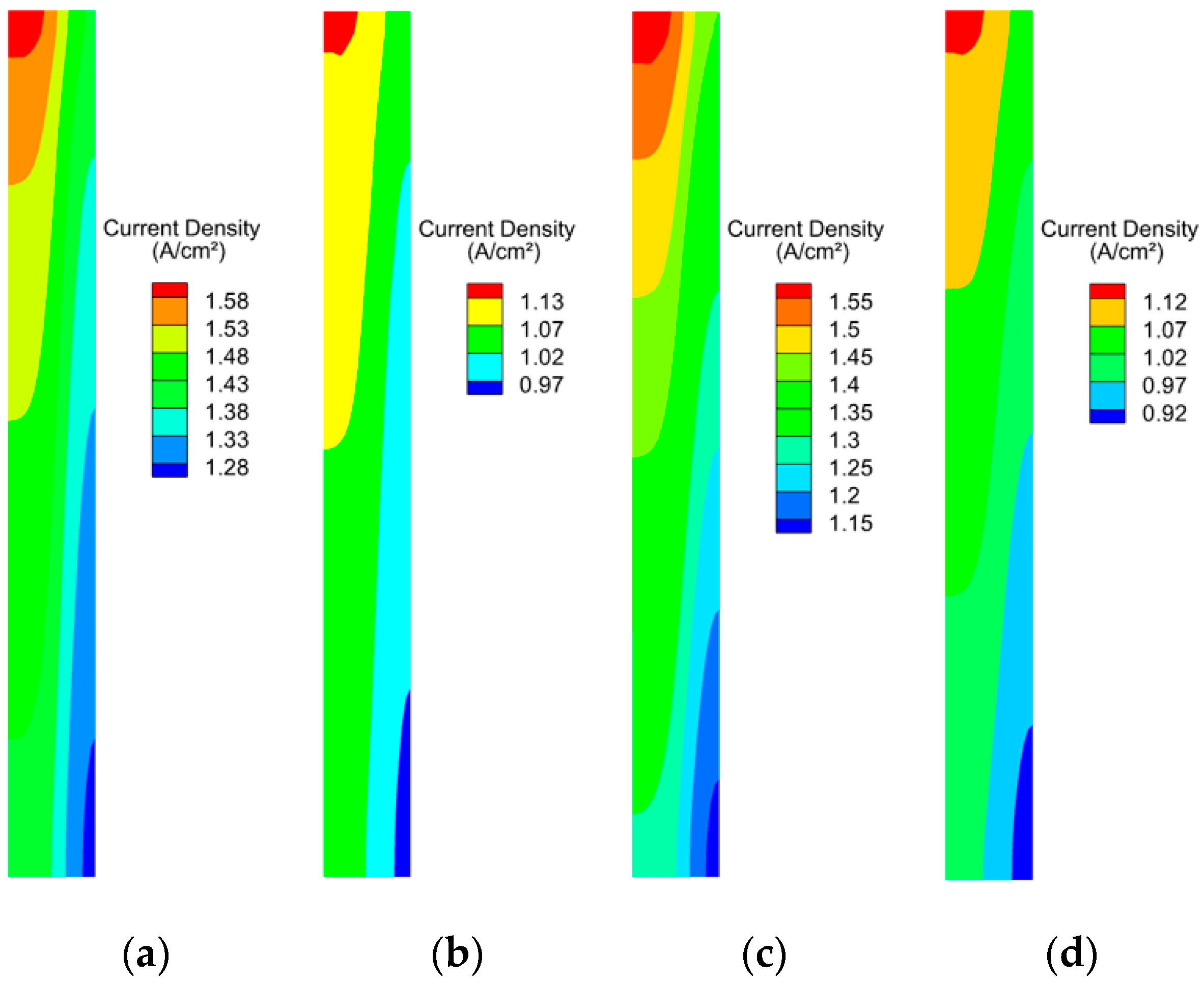
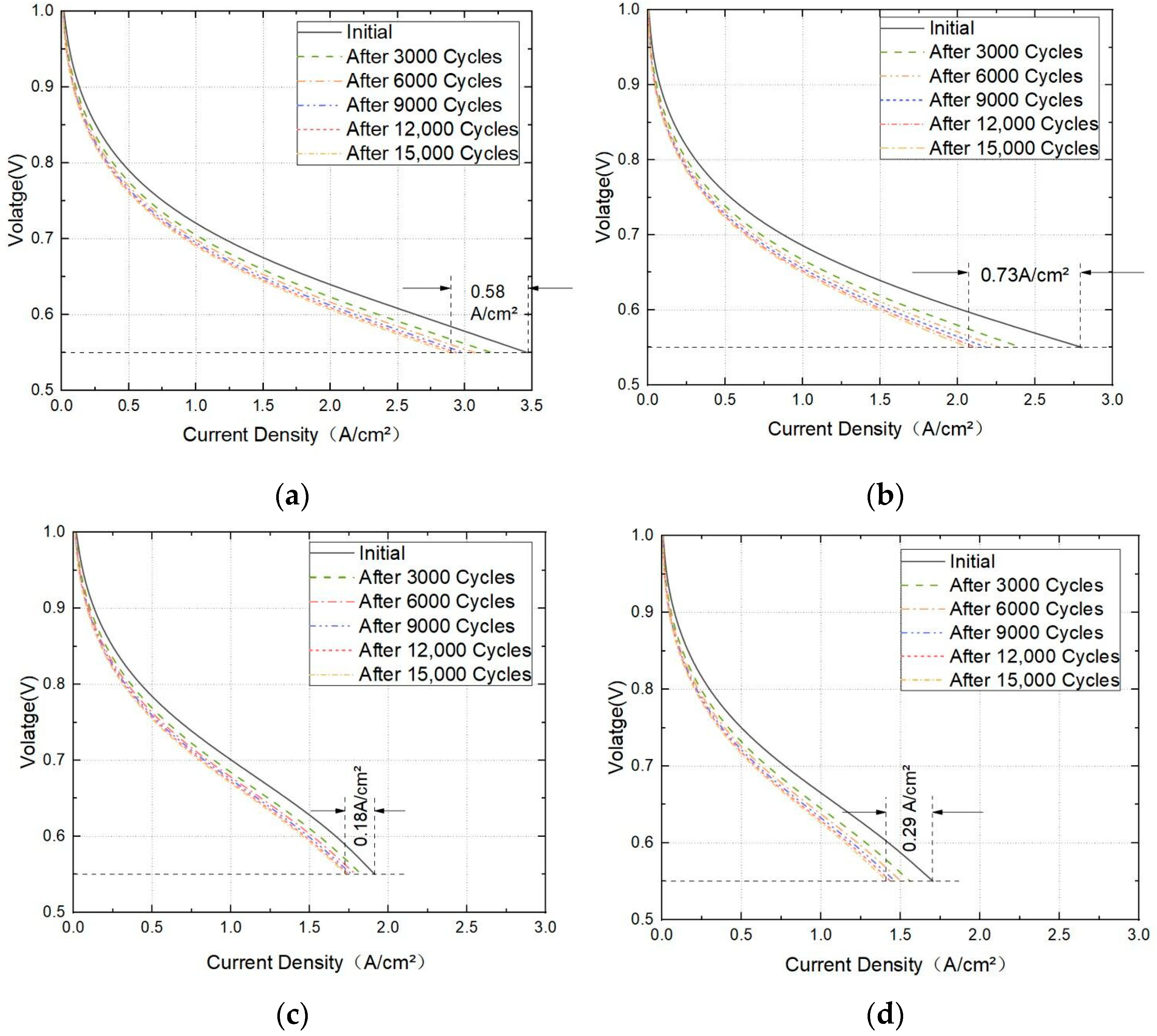
3.3. Effect of Cathodic Oxygen Stoichiometry on PEMFC Performance
3.4. Effect of Cathode Channel and Plate Width on the Performance of PEMFC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, S.T.; James, B.D.; Huya-Kouadio, J.M.; Houchins, C.; DeSantis, D.A.; Ahluwalia, R.; Wilson, A.R.; Kleen, G.; Papageorgopoulos, D. Direct hydrogen fuel cell electric vehicle cost analysis: System and high-volume manufacturing description, validation, and outlook. J. Power Sources 2018, 399, 304–313. [Google Scholar] [CrossRef]
- Han, R.; Li, J.; Guo, Z. Optimal quota in China’s energy capping policy in 2030 with renewable targets and sectoral heterogeneity. Energy 2022, 239, 121971. [Google Scholar] [CrossRef]
- Usman, M.; Balsalobre-Lorente, D.; Jahanger, A.; Ahmad, P. Pollution concern during globalization mode in financially resource-rich countries: Do financial development, natural resources, and renewable energy consumption matter? Renew. Energy 2022, 183, 90–102. [Google Scholar] [CrossRef]
- Tian, S.; Di, Y.; Dai, M.; Chen, W.; Zhang, Q. Comprehensive assessment of energy conservation and CO2 emission reduction in future aluminum supply chain. Appl. Energy 2022, 305, 117796. [Google Scholar] [CrossRef]
- Salvi, B.L.; Subramanian, K.A. Sustainable development of road transportation sector using hydrogen energy system. Renew. Sustain. Energy Rev. 2015, 51, 1132–1155. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Raza, S.S.; Janajreh, I.; Ghenai, C. Sustainability index approach as a selection criteria for energy storage system of an intermittent renewable energy source. Appl. Energy 2014, 136, 909–920. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Li, H.; Zhang, S.S.; Martin, J.; Wang, H.J. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Lin, T.; Lan, T.; Liao, X.; Maréchal, F.; Van Herle, J.; Yang, Y.; Dong, C.; Wang, L. Fuel cell-battery hybrid systems for mobility and off-grid applications: A review. Renew. Sustain. Energy Rev. 2021, 135, 110119. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Kojima, K.; Fukazawa, K. Current Status and Future Outlook of Fuel Cell Vehicle Development in TOYOTA. ECS Trans. 2015, 69, 213–219. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Zhao, J. Catalyst Layers in Polymer Electrolyte Membrane Fuel Cells: Formation, Characterization and Performance. UWSpace. 2019. Available online: http://hdl.handle.net/10012/14425 (accessed on 1 February 2023).
- Chen, H.; Pei, P.; Song, M. Lifetime prediction and the economic lifetime of Proton Exchange Membrane fuel cells. Appl. Energy 2015, 142, 154–163. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hamrock, S.J.; Garland, N.L. Fuel Cell Technologies Program Multi-Year Research, Development and Demonstration Plan. 2015. Available online: https://www.semanticscholar.org/paper/Fuel-Cell-Technologies-Program-Multi-Year-Research%2C-Kim-Hamrock/cda07117b3f06f41b536068a867185742cad616e (accessed on 1 February 2023).
- Verhage, A.J.L.; Coolegem, J.F.; Mulder, M.J.J.; Yildirim, M.H.; de Bruijn, F.A. 30,000 h operation of a 70 kW stationary PEM fuel cell system using hydrogen from a chlorine factory. Int. J. Hydrogen Energy 2013, 38, 4714–4724. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.; Le, D.D. Review of the durability of polymer electrolyte membrane fuel cell in long-term operation: Main influencing parameters and testing protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Hou, J.B.; Yang, M.; Ke, C.C.; Wei, G.H.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J.L. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Wang, Y.J.; Long, W.Y.; Wang, L.L.; Yuan, R.S.; Ignaszak, A.; Fang, B.Z.; Wilkinson, D.P. Unlocking the door to highly active ORR catalysts for PEMFC applications: Polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Wang, G.J.; Yu, Y.; Liu, H.; Gong, C.L.; Wen, S.; Wang, X.H.; Tu, Z.K. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Guan, D.; Xu, M.; Ran, R.; Ni, M.; Zhou, W.; Hayre, R.O.; Shao, Z. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 2021, 591, 246–251. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel cell application in the automotive industry and future perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Mirzaei, F.; Parnian, M.J.; Rowshanzamir, S. Durability investigation and performance study of hydrothermal synthesized platinum-multi walled carbon nanotube nanocomposite catalyst for proton exchange membrane fuel cell. Energy 2017, 138, 696–705. [Google Scholar] [CrossRef]
- Zhang, F.; Advani, S.G.; Prasad, A.K. Performance of a metallic gas diffusion layer for PEM fuel cells. J. Power Sources 2008, 176, 293–298. [Google Scholar] [CrossRef]
- Kong, I.M.; Jung, A.; Kim, Y.S.; Kim, M.S. Numerical investigation on double gas diffusion backing layer functionalized on water removal in a proton exchange membrane fuel cell. Energy 2017, 120, 478–487. [Google Scholar] [CrossRef]
- Ekiz, A.; Camci, T.; Türkmen, I.; Sankir, M.; Uslu, S.; Baker, D.K.; Aǧar, E. Modeling of bipolar plates for proton exchange membrane fuel cells. J. Fac. Eng. Archit. Gazi Univ. 2011, 26, 591–605. [Google Scholar]
- Ozden, A.; Shahgaldi, S.; Zhao, J.; Li, X.; Hamdullahpur, F. Assessment of graphene as an alternative microporous layer material for proton exchange membrane fuel cells. Fuel 2018, 215, 726–734. [Google Scholar] [CrossRef]
- Wang, J. System integration, durability and reliability of fuel cells: Challenges and solutions. Appl. Energy 2017, 189, 460–479. [Google Scholar] [CrossRef]
- Yue, M.L.; Jemei, S.; Gouriveau, R.; Zerhouni, N. Review on health conscious energy management strategies for fuel cell hybrid electric vehicles: Degradation models and strategies. Int. J. Hydrogen Energ. 2019, 44, 6844–6861. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.G. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Panels, J.E.; Yan, S.G. Dependence of PEM fuel cell performance on catalyst loading. J. Power Sources 2004, 127, 162–171. [Google Scholar] [CrossRef]
- Salva, J.A.; Iranzo, A.; Rosa, F.; Tapia, E.; Lopez, E.; Isorna, F. Optimization of a PEM fuel cell operating conditions: Obtaining the maximum performance polarization curve. Int. J. Hydrogen Energy 2016, 41, 19713–19723. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Thompson, J.; Olabi, A.G. Modelling and simulation of Proton Exchange Membrane fuel cell with serpentine bipolar plate using MATLAB. Int. J. Hydrogen Energy 2017, 42, 25639–25662. [Google Scholar] [CrossRef]
- Kanani, H.; Shams, M.; Hasheminasab, M.; Bozorgnezhad, A. Model development and optimization of operating conditions to maximize PEMFC performance by response surface methodology. Energy Convers. Manag. 2015, 93, 9–22. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M. PEM fuel cell model and simulation in Matlab–Simulink based on physical parameters. Energy 2016, 116, 1131–1144. [Google Scholar] [CrossRef]
- Kongkanand, A.; Gu, W.; Mathias, M.F. Proton-Exchange Membrane Fuel Cells with Low-Pt Content. Fuel Cells Hydrog. Prod. 2017. [Google Scholar] [CrossRef]
- Zhao, J.J.; Tu, Z.K.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, L. Parametric study of the porous cathode in the PEM fuel cell. Int. J. Energy Res. 2009, 33, 52–61. [Google Scholar] [CrossRef]
- Wang, X.; Duan, Y.; Yan, W.; Peng, X. Effects of flow channel geometry on cell performance for PEM fuel cells with parallel and interdigitated flow fields. Electrochim. Acta 2008, 53, 5334–5343. [Google Scholar] [CrossRef]
- Macauley, N.; Papadias, D.D.; Fairweather, J.; Spernjak, D.; Langlois, D.; Ahluwalia, R.; More, K.L.; Mukundan, R.; Borup, R.L. Carbon Corrosion in PEM Fuel Cells and the Development of Accelerated Stress Tests. J. Electrochem. Soc. 2018, 165, F3148–F3160. [Google Scholar] [CrossRef]
- Le, A.D.; Zhou, B. A general model of proton exchange membrane fuel cell. J. Power Sources 2008, 182, 197–222. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Multi-phase models for water and thermal management of proton exchange membrane fuel cell: A review. J. Power Sources 2018, 391, 120–133. [Google Scholar] [CrossRef]
- Meng, H. Numerical studies of liquid water behaviors in PEM fuel cell cathode considering transport across different porous layers. Int. J. Hydrogen Energy 2010, 35, 5569–5579. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Pan, M.; Li, Z.; Tang, B. Model prediction of effects of operating parameters on proton exchange membrane fuel cell performance. Renew. Energy 2010, 35, 656–666. [Google Scholar] [CrossRef]
- Mann, R.F.; Amphlett, J.C.; Peppley, B.A.; Thurgood, C.P. Application of Butler–Volmer equations in the modelling of activation polarization for PEM fuel cells. J. Power Sources 2006, 161, 775–781. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.G.; Wang, C.Y. Ultrahigh fuel utilization in polymer electrolyte fuel cells—Part II: A modeling study. Int. J. Green Energy 2022, 19, 166–174. [Google Scholar] [CrossRef]
- Turhan, A.; Heller, K.; Brenizer, J.S.; Mench, M.M. Passive control of liquid water storage and distribution in a PEFC through flow-field design. J. Power Sources 2008, 180, 773–783. [Google Scholar] [CrossRef]
- Holby, E.F.; Morgan, D. Application of Pt Nanoparticle Dissolution and Oxidation Modeling to Understanding Degradation in PEM Fuel Cells. J. Electrochem. Soc. 2012, 159, B578–B591. [Google Scholar] [CrossRef]
- Li, Y.B.; Moriyama, K.; Gu, W.B.; Arisetty, S.; Wang, C.Y. A One-Dimensional Pt Degradation Model for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2015, 162, F834–F842. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W.B. Impact of Platinum Loading and Catalyst Layer Structure on PEMFC Performance. J. Electrochem. Soc. 2013, 160, F824–F833. [Google Scholar] [CrossRef]
- Ota, K.; Koizumi, Y. Platinum dissolution models and voltage cycling effects: Platinum dissolution in polymer electrolyte fuel cell (PEFC) and low-temperature fuel cells. Handb. Fuel Cells 2010. [Google Scholar] [CrossRef]
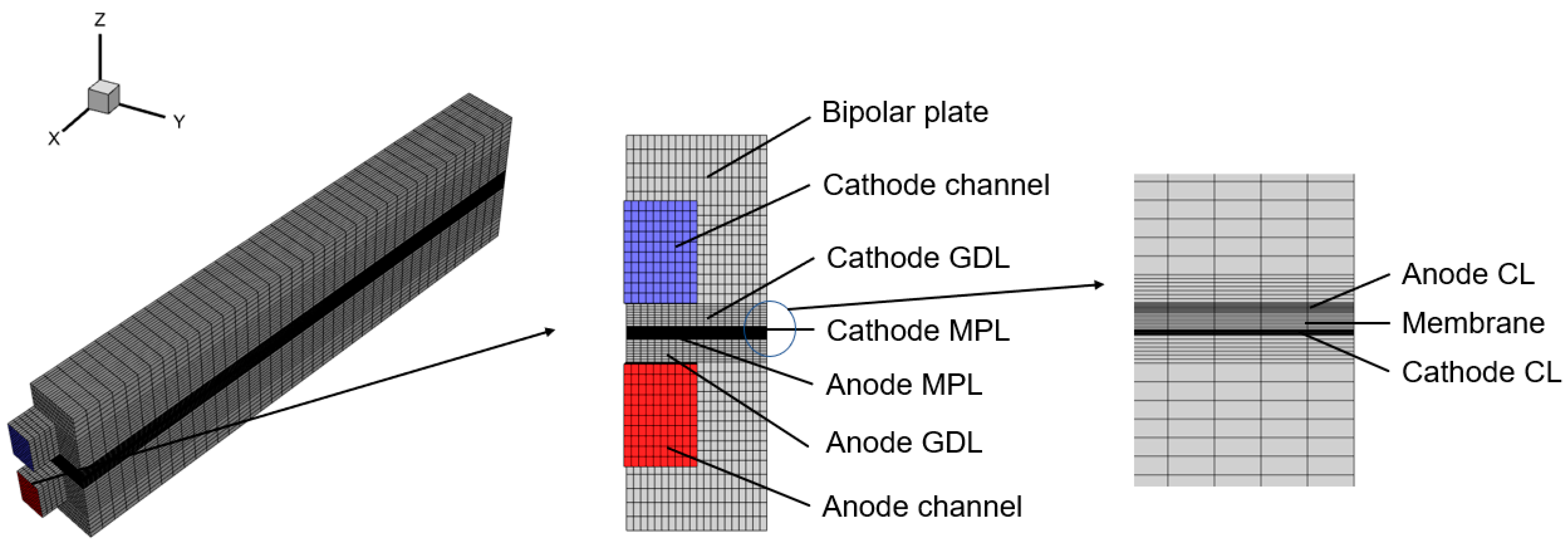







| Parameter | Value |
|---|---|
| Channel length | 110 mm |
| Channel width | 1.0 mm |
| Channel height | 0.7 mm |
| Land width | 1.0 mm |
| GDL thickness | 160 μm |
| MPL thickness | 30 μm |
| ACL thickness | 6 μm |
| Membrane thickness | 18 μm |
| CCL thickness | 11 μm |
| PEMFC length | 100 mm |
| Parameter | Value |
|---|---|
| Reference exchange current density at anode, | 1.2 × 103 A/m² |
| Reference exchange current density at cathode, | 13.2 A/m² |
| Specific active surface area at anode/cathode, | 1.6 × 105 1/m/9.1 × 104 1/m |
| Activation energy, | 8314 J/mol |
| Reference temperature, | 353.15 K |
| Reference pressure, | 2.02 × 105 Pa |
| Anode/cathode transfer coefficients of the anode/cathode electrodes, | 1.0/1.0 |
| Concentration dependence, | 1.0/1.0 |
| Permeability of GDL/MPL/CL, | 3 × 10−12 m2/1 × 10−12 m2/2 × 10−13 m2 |
| Thermal conductivity of BP/GDL/MPL/CL membrane, | 100.0 W/m K/10.0 W/m K/10.0 W/m K/10.0 W/m K/2.0 W/m K |
| Cathode ionomer resistance, | 7.954 s/m |
| Equivalent weight, EW | 1100 kg/kmol |
| Porosity of GDL/MPL/CL, | 0.7/0.3/0.15 |
| Contact angle of GDL/MPL/CL | 110°/130°/95° |
| Electrical conductivity of BP/GDL/MPL/CL, | 1.0 × 106 S/m/5.0 × 103 S/m/5.0 × 103 S/m/5.0 × 103 S/m |
| Anode (50% RH)/cathode (10% RH) inlet velocity for gas channel | 0.109 m/s/0.314 m/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Su, L.; Yang, Y.; Gao, L.; Liu, X.; Huang, H.; Li, Y.; Song, Y. Three-Dimensional Simulation on the Effects of Different Parameters and Pt Loading on the Long-Term Performance of Proton Exchange Membrane Fuel Cells. Sustainability 2023, 15, 2902. https://doi.org/10.3390/su15042902
Huang Z, Su L, Yang Y, Gao L, Liu X, Huang H, Li Y, Song Y. Three-Dimensional Simulation on the Effects of Different Parameters and Pt Loading on the Long-Term Performance of Proton Exchange Membrane Fuel Cells. Sustainability. 2023; 15(4):2902. https://doi.org/10.3390/su15042902
Chicago/Turabian StyleHuang, Zheng, Laisuo Su, Yunjie Yang, Linsong Gao, Xinyu Liu, Heng Huang, Yubai Li, and Yongchen Song. 2023. "Three-Dimensional Simulation on the Effects of Different Parameters and Pt Loading on the Long-Term Performance of Proton Exchange Membrane Fuel Cells" Sustainability 15, no. 4: 2902. https://doi.org/10.3390/su15042902
APA StyleHuang, Z., Su, L., Yang, Y., Gao, L., Liu, X., Huang, H., Li, Y., & Song, Y. (2023). Three-Dimensional Simulation on the Effects of Different Parameters and Pt Loading on the Long-Term Performance of Proton Exchange Membrane Fuel Cells. Sustainability, 15(4), 2902. https://doi.org/10.3390/su15042902







