Effect of Leaf Surface Regulation of Zinc Fertilizer on Absorption of Cadmium, Plumbum and Zinc in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site
2.2. Experimental Treatments
- CK: tiller stage–heading stage–filling stage
- T1: heading stage was sprayed with leaf zinc fertilizer once
- T2: filling stage was sprayed with leaf zinc fertilizer once
- T3: heading stage–filling stage was sprayed with leaf zinc fertilizer twice
- T4: Leaf zinc fertilizer was sprayed 3 times from tiller stage to heading stage to grouting stage
2.3. Sample Collection
2.4. Sample Determination
2.5. Analysis Method
3. Results
3.1. Effects of Foliar Zn Application on Rice Yield
3.2. Effects of Foliar Zn Application on Cd and Pb Concentration of Rice
3.2.1. Effects of Foliar Zn Application on Cd and Pb Concentration in Brown Rice
3.2.2. Effects of Foliar Zn Application on Cd Concentration of Rice Plants
3.2.3. Effects of Foliar Zn Application on Pb Concentration of Rice Plants
3.3. Effects of Foliar Zn Application on Cd and Pb Enrichment and Transport in Rice
3.3.1. Effects of Foliar Zn Application on Cd Enrichment and Transport in Rice
3.3.2. Effects of Foliar Zn Application on Pb Enrichment and Transport in Rice
3.4. Effects of Foliar Zn Application on Zinc, Nitrogen, Phosphorus and Potassium Concentrations in Brown Rice
3.5. Influence of Foliar Zn Application on Economic Benefits
4. Discussion
4.1. Effects of Leaf Zinc Application at Different Periods on Reducing Cd and Pb Accumulation in Rice
4.2. Effects of Leaf Zinc Application at Different Periods on Rice Yield and Zn-Rich Effect of Brown Rice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Othman, Z.A.; Ali, R.; Al-Othman, A.M.; Ali, J.; Habila, M.A. Assessment of toxic metals in wheat crops grown on selected soils, rigated by diferent water sources. Arab. J. Chem. 2012, 9, 1555–1562. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Ezaki, T.; Moriguchi, J.; Furuki, K.; Shimbo, S.; Matsuda-Inoguchi, N.; Ikeda, M. Rice as the most influentialsource of cadmium intake among general Japanese population. Sci. Total Environ. 2003, 305, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Feng, A.X.; Liu, N.; Jiang, Z.; Wei, S. Silicon application improved the yield and nutritional quality while reduced cadmium concentration in rice. Environ. Sci. Pollut. Res. 2020, 27, 20370–20379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, C.; Luo, Z.C.; Zhu, H.H.; Wang, S.; Zhu, Q.H.; Huang, D.Y.; Zhang, Y.Z.; Xiong, J.; He, Y.B. Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environ. Sci. Pollut. Res. 2018, 25, 29287–29294. [Google Scholar] [CrossRef]
- Xue, W.J.; Zhang, C.B.; Wang, P.P.; Wang, C.-R.; Huang, Y.-C.; Zhang, X.; Liu, Z.-Q. Rice vegetative organs alleviate cadmium toxicity by altering the chemical forms of cadmium and increasing the ratio of calcium to manganese. Ecotoxicol. Environ. Saf. 2019, 184, 109640. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice. J. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Tian, S.Q.; Liang, S.; Qiao, K.; Wang, F.H.; Zhang, Y.X.; Chai, T.Y. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- Lv, G.H.; Xu, C.; Wang, H.; Shuai, H.; Wang, S.; Li, B.Z.; Zhu, Q.H.; Zhu, H.H.; Huang, D.Y. Effect of foliar spraying zinc on the accumulation of zinc and cadmium in rice. J. Agro-Environ. Sci. 2018, 37, 1521–1528. [Google Scholar]
- Huang, G.X.; Ding, C.F.; Zhou, Z.G.; Zhang, T.L.; Wang, X.X. A tillering application of zinc fertilizer based on basal stabilization reduces Cd accumulation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.H.; Wang, H.; Xu, C.; Shuai, H.; Luo, Z.C.; Zhang, Q.; Zhu, H.H.; Wang, S.; Zhu, Q.H.; Zhang, Y.Z.; et al. Effectiveness of simultaneous foliar application of Zn and Mn or P to reduce Cd concentration in rice grains: A field study. Environ. Sci. Pollut. Res. 2019, 26, 9305–9313. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.M.; Wang, S.; Huang, D.Y.; Zhu, Q.H.; Liu, S.L.; Zhang, Q.; Zhu, H.H.; Xu, C. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef]
- Han, X.X.; Ren, X.H.; Wang, P.P.; Huang, Y.C.; Zhang, C.B.; Liu, Z.Q. Effects of foliar application with zinc on the characteristics of cadmium accumulation in organs of rice plants. J. Agro-Environ. Sci. 2019, 38, 1809–1817. [Google Scholar]
- Suo, Y.Y.; Wu, S.; Zhu, J.J.; Pan, F.S.; Feng, Y. Effects of foliar Zn application on rice yield and element contents under different Cd levels. J. Zhejiang Univ. Agric. Life Sci. 2012, 38, 449–458. [Google Scholar]
- Gu, J.F.; Yang, W.T.; Zhou, H.; Zhang, P.; Peng, P.Q.; Liao, B.H. Provoking effects of exogenous Zn on Cd accumulation in rice. Chin. J. Environ. Sci. 2016, 37, 3554–3561. [Google Scholar]
- Fontanili, L.; Lancilli, C.; Suzui, N.; Dendena, B.; Yin, Y.G.; Ferri, A.; Ishii, S.; Kawachi, N.; Lucchini, G.; Fujimaki, S.; et al. Kinetic analysis of zinc/cadmium reciprocal competitions suggests a possible Zn-insensitive pathway for root-to shoot cadmium translocation in rice. Rice 2016, 9, 16–28. [Google Scholar] [CrossRef]
- Cao, F.B.; Wang, R.F.; Cheng, W.D.; Zeng, F.R.; Ahmed, I.M.; Hu, X.N.; Zhang, G.P.; Wu, F.B. Genotypic and environmental variation in cadmium, chromium, lead and copper in rice and approaches for reducing the accumulation. Sci. Total Environ. 2014, 496, 275–281. [Google Scholar]
- Liu, Y.; Zhang, C.B.; Zhao, Y.L.; Sun, S.J.; Liu, Z.Q. Effects of growing seasons and genotypes on the accumulation of cadmium and mineral nutrients in rice grown in cadmium contaminated soil. Sci. Total Environ. 2017, 579, 1282–1288. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol. Plant. 2002, 116, 73–78. [Google Scholar] [CrossRef]
- Saifullah; Sarwar, N.; Bibi, S.; Ahmad, M.; Ok, Y.S. Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ. Earth. Sci. 2014, 71, 1663–1672. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Khan, F.; Wu, C.; Saud, S.; Hassan, S.; Ahmad, N.; Gang, D.; Ullah, A.; Huang, J.L. Effects of tire rubber ash and zinc sulfate on crop productivity and cadmium accumulation in five rice cultivars under field conditions. Environ. Sci. Pollut. Res. 2015, 22, 12424–12434. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Ishaq, W.; Farid, G.; Shaheen, M.R.; Imran, M.; Geng, M.J.; Hussain, S. Zinc-cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotoxicol. Environ. Saf. 2015, 122, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zeng, J.; Ming, X.Y.; He, Q.L.; Tao, Q.; Jiang, M.Y.; Gao, S.P.; Li, X.; Lei, T.; Pan, Y.Z.; et al. The presence of zinc reduced cadmium uptake and translocation in Cosmos bipinnatus seedlings under cadmium/zinc combined stress. Plant Physiol. Bioch. 2020, 151, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Zinc deficiency and human health: Etiology, health consequences, and future solutions. Plant Soil 2012, 361, 291–299. [Google Scholar] [CrossRef]
- Levenson, C.W.; Morris, D. Zinc and neurogenesis: Making new neurons from development to adulthood. Adv. Nutr. 2011, 2, 96–100. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Abdoli, M.; Esfandiari, E.; Mousavi, S.B.; Bahman, S.; Behzad, S. Effects of foliar application of zinc sulfate at different phenological stages on yield formation and grain zinc content of bread wheat (cv. Kohdasht). Azarian J. Agric. 2014, 1, 11–16. [Google Scholar]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.-C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Khoshgoftarmanesh, A.H.; Afyuni, M.; Norouzi, M.; Ghiasi, S.; Schulin, R. Fractionation and bioavailability of zinc (Zn) in the rhizosphere of two wheat cultivars with different Zn deficiency tolerance. Geoderma 2018, 309, 1–6. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.J.; Sayers, Z.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant. 2006, 128, 144–152. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci. 2011, 53, 118–125. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.Y.; Tang, Q.; Li, H.X.; Zhang, C.B.; Liu, Z.Q. Effects of foliar modulators on cadmium accumulation and transport of nutrient elements in rice. J. Agro-Environ. Sci. 2018, 37, 2507–2513. [Google Scholar]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.J.; Li, M.L.; Wu, G.Q. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.Y.; Cui, H.B.; Fan, X.J.; Zhou, D.M.; Zhou, J. Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars. Environ. Pollut. 2020, 265, 115045. [Google Scholar] [CrossRef]
- Dong, R.Y.; Xu, Y.M.; Wang, L.; Zhao, M.Y.; Liang, X.F.; Sun, Y.B. Effects of soil application and foliar spray of zinc fertilizer on cadmium uptake in a pakchoi cultivar with lower cadmium accumulation. Acta Sci. Circumstantiae 2015, 35, 2589–2596. [Google Scholar]
- Yang, J.X.; Wang, L.Q.; Wei, D.P.; Chen, S.B.; Ma, Y.B. Foliar spraying and seed soaking of zinc fertilizers decreased cadmium accumulation in cucumbers grown in Cd-contaminated soils. Soil Sediment Contam. 2011, 20, 400–410. [Google Scholar] [CrossRef]
- Tan, L.T.; Zhu, Y.X.; Fan, T.; Peng, C.; Wang, J.R.; Sun, L.; Chen, C.Y. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Bioph. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol. 2020, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Barabasz, A.; Klimecka, M.; Kendziorek, M.; Weremczuk, A.; Ruszczyńska, A.; Bulska, E.; Antosiewicz, D.M. The ratio of Zn to Cd supply as a determination of metal-homeostasis gene expression in tobacco and its modulation by overexpressing the metal exporter AtHMA4. J. Exp. Bot. 2016, 67, 6201–6214. [Google Scholar] [CrossRef]
- Akay, A.; Koleli, N. Interaction between cadmium and zinc in barley (Hordeum vulgare L.) grown under field conditions. Bangl. J. Bot. 2007, 36, 13–19. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.B.; Zhao, Y.L.; Huang, Y.C.; Liu, Z.Q. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilization with the plant and root uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.W.; Tian, X.H.; Wang, S.X.; Chen, Y.L. Effect of nitrogen fertilizer and foliar zinc application at different growth stages on zinc translocation and utilization efficiency in winter wheat. Cereal Res. Commun. 2014, 42, 81–90. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]

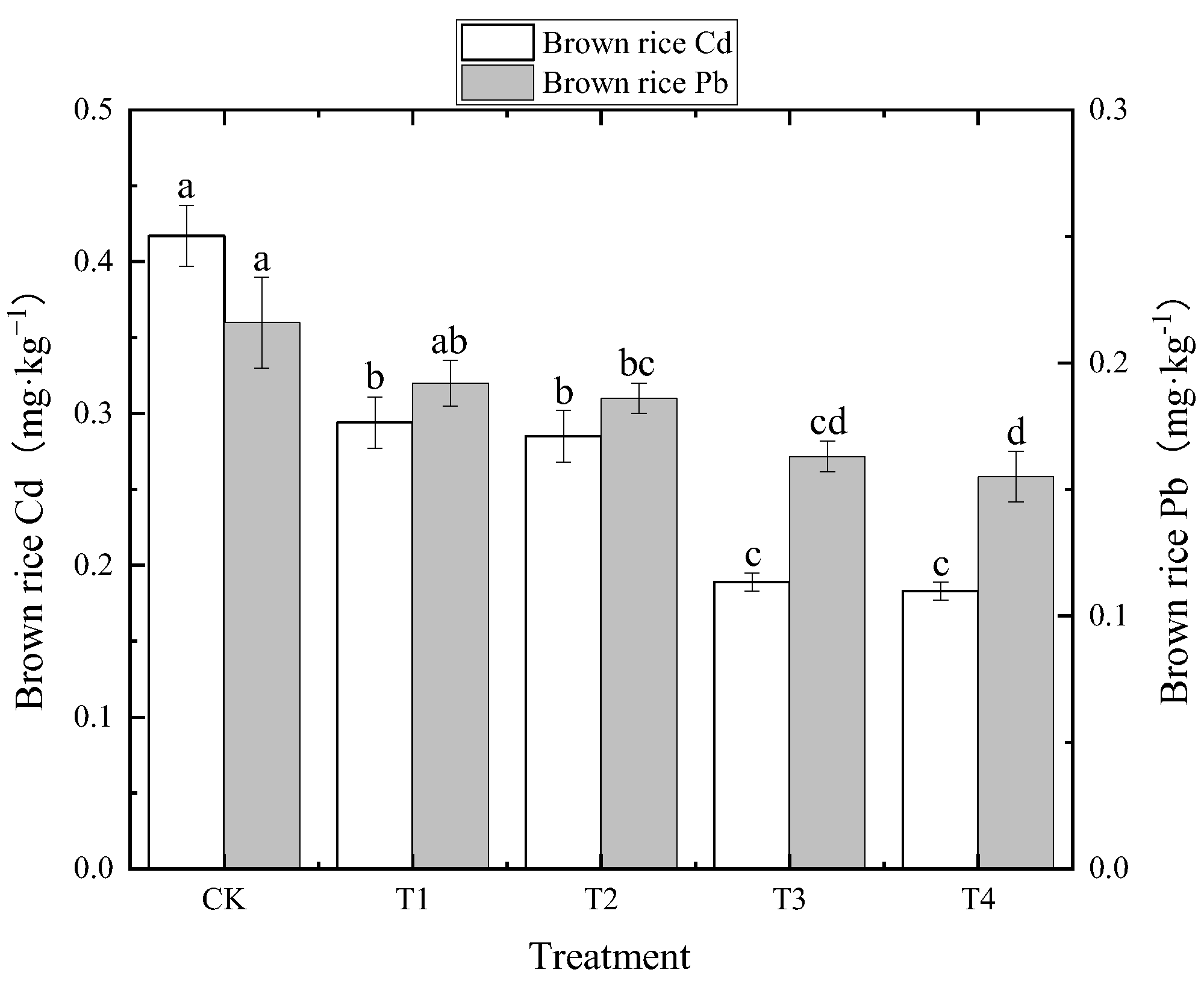
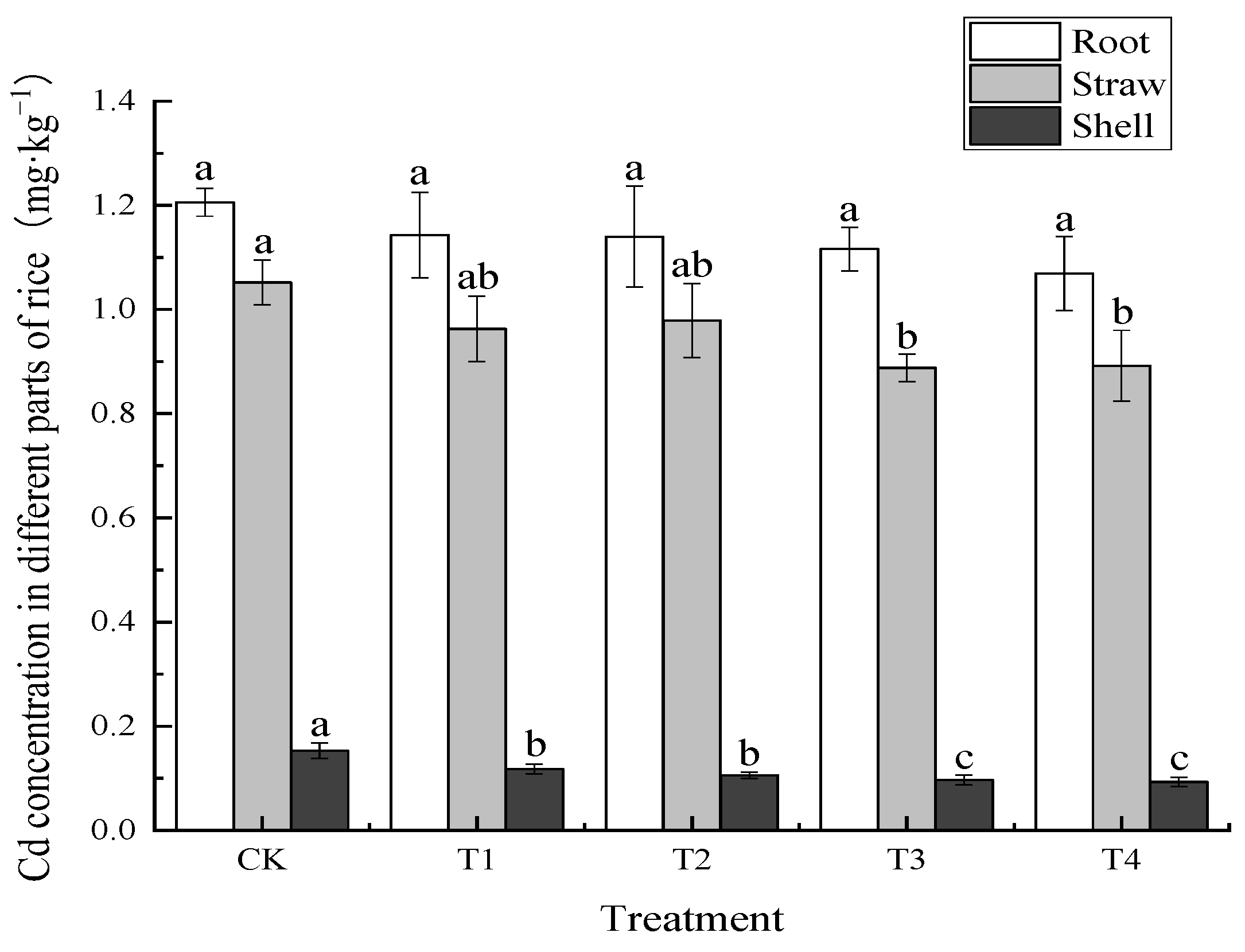

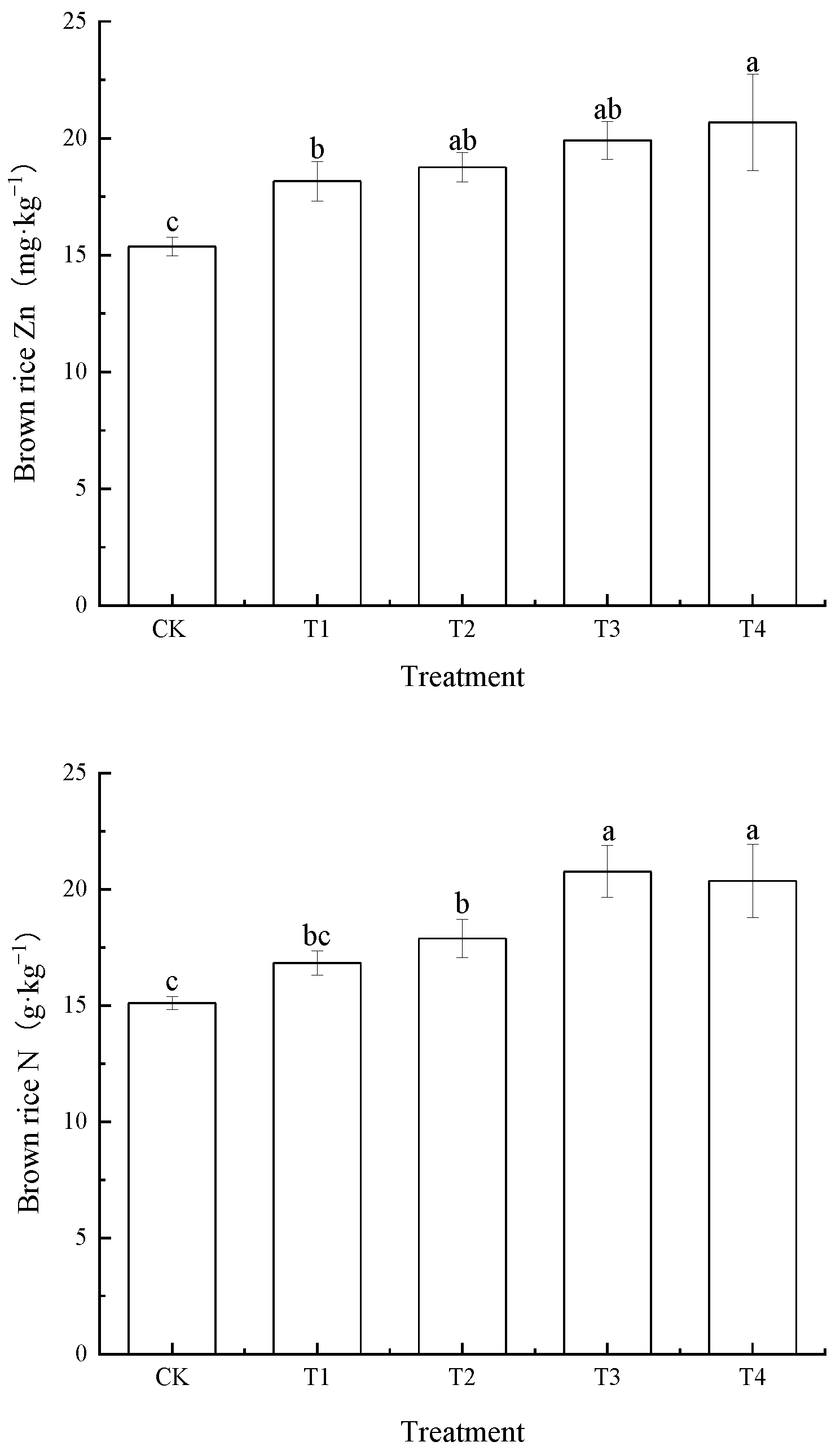
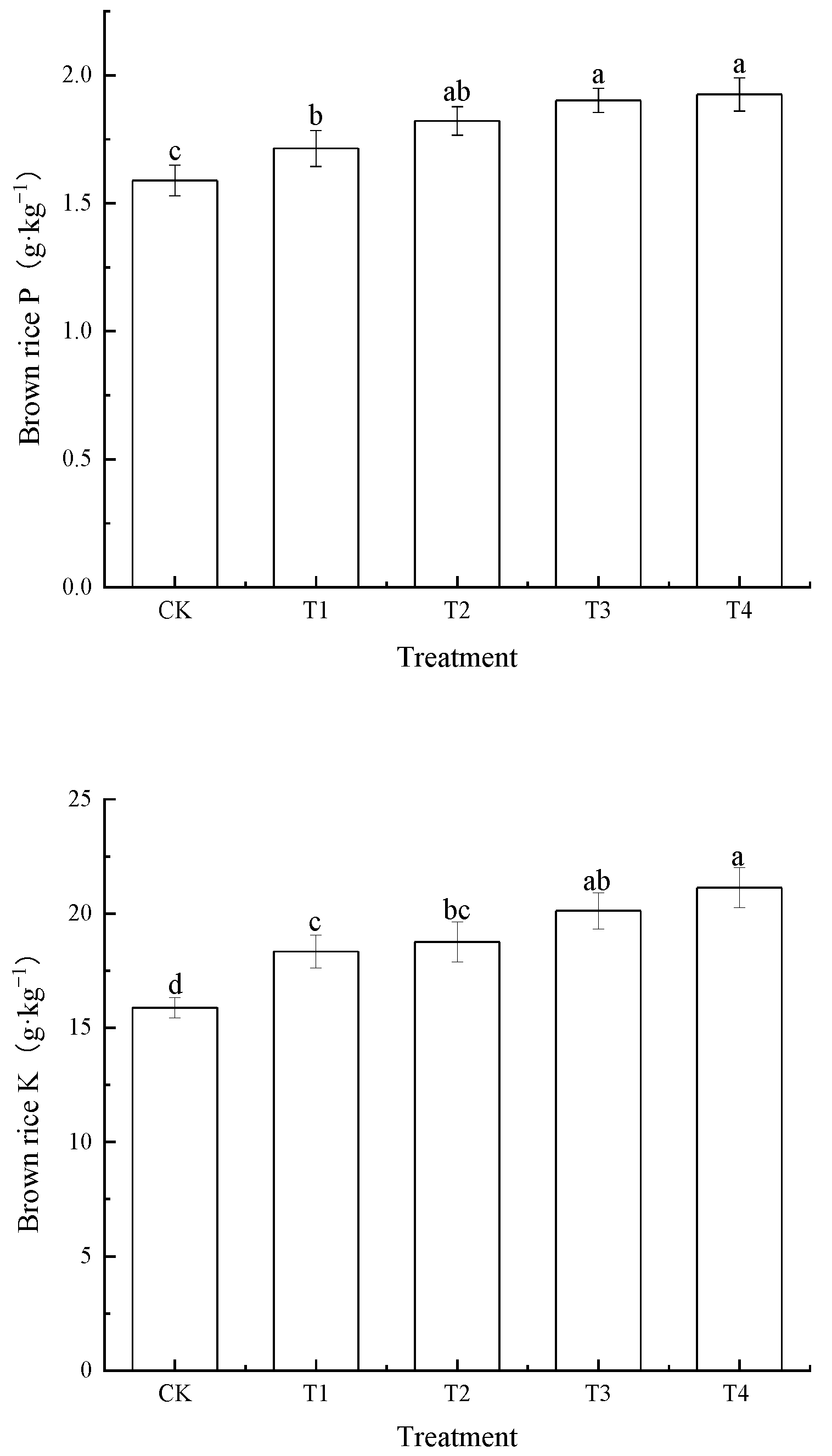
| Treatment | Soil Cd | BCFBrown Rice | TFStraw-Root | TFBrown Rice-Straw | TFBrown Rice-Root |
|---|---|---|---|---|---|
| CK | 1.545 ± 0.020 a | 0.270 ± 0.015 a | 0.872 ± 0.018 a | 0.396 ± 0.025 a | 0.346 ± 0.020 a |
| T1 | 1.516 ± 0.030 a | 0.194 ± 0.012 b | 0.844 ± 0.052 a | 0.306 ± 0.033 b | 0.257 ± 0.021 b |
| T2 | 1.509 ± 0.024 a | 0.189 ± 0.012 b | 0.864 ± 0.114 a | 0.293 ± 0.037 b | 0.251 ± 0.022 b |
| T3 | 1.539 ± 0.016 a | 0.122 ± 0.005 c | 0.796 ± 0.032 a | 0.212 ± 0.007 c | 0.169 ± 0.011 c |
| T4 | 1.518 ± 0.061 a | 0.121 ± 0.010 c | 0.839 ± 0.111 a | 0.206 ± 0.008 c | 0.172 ± 0.016 c |
| Treatment | Soil Pb | BCFBrown Rice | TFStraw-Root | TFBrown Rice-Straw | TFBrown Rice-Root |
|---|---|---|---|---|---|
| CK | 93.54 ± 2.602 a | 0.002 ± 0.001 a | 1.867 ± 0.193 a | 0.077 ± 0.009 c | 0.142 ± 0.005 b |
| T1 | 92.42 ± 1.830 a | 0.002 ± 0.000 a | 1.842 ± 0.159 ab | 0.091 ± 0.009 bc | 0.168 ± 0.030 ab |
| T2 | 91.09 ± 1.361 a | 0.002 ± 0.000 a | 1.969 ± 0.085 a | 0.092 ± 0.008 b | 0.180 ± 0.008 a |
| T3 | 90.74 ± 1.892 a | 0.002 ± 0.000 a | 1.584 ± 0.158 bc | 0.110 ± 0.001 a | 0.174 ± 0.016 ab |
| T4 | 90.70 ± 2.891 a | 0.002 ± 0.000 a | 1.532 ± 0.020 c | 0.109 ± 0.007 a | 0.166 ± 0.009 ab |
| Treatment | Input (¥·hm−2) | Total Input (¥·hm−2) | Total Output (¥·hm−2) | Input–Output Ratio | ||
|---|---|---|---|---|---|---|
| Blade Material | Fertilizer | Other | ||||
| CK | 0 | 1215 | 9500 | 10,715 | 11,345 ± 43 c | 1.059 ± 0.004 c |
| T1 | 60 | 1215 | 9500 | 10,775 | 11,831 ± 68 b | 1.098 ± 0.006 b |
| T2 | 60 | 1215 | 9500 | 10,775 | 11,902 ± 88 b | 1.105 ± 0.008 b |
| T3 | 120 | 1215 | 9500 | 10,835 | 20,189 ± 322 a | 1.863 ± 0.030 a |
| T4 | 180 | 1215 | 9500 | 10,895 | 20,286 ± 215 a | 1.862 ± 0.020 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Tao, R.; Cao, C.; Xie, J.; Gao, Y.; Hu, H.; Ma, Z.; Ma, Y. Effect of Leaf Surface Regulation of Zinc Fertilizer on Absorption of Cadmium, Plumbum and Zinc in Rice (Oryza sativa L.). Sustainability 2023, 15, 1877. https://doi.org/10.3390/su15031877
Hu J, Tao R, Cao C, Xie J, Gao Y, Hu H, Ma Z, Ma Y. Effect of Leaf Surface Regulation of Zinc Fertilizer on Absorption of Cadmium, Plumbum and Zinc in Rice (Oryza sativa L.). Sustainability. 2023; 15(3):1877. https://doi.org/10.3390/su15031877
Chicago/Turabian StyleHu, Jingyi, Ronghao Tao, Chi Cao, Junhao Xie, Yuxin Gao, Hongxiang Hu, Zhongwen Ma, and Youhua Ma. 2023. "Effect of Leaf Surface Regulation of Zinc Fertilizer on Absorption of Cadmium, Plumbum and Zinc in Rice (Oryza sativa L.)" Sustainability 15, no. 3: 1877. https://doi.org/10.3390/su15031877
APA StyleHu, J., Tao, R., Cao, C., Xie, J., Gao, Y., Hu, H., Ma, Z., & Ma, Y. (2023). Effect of Leaf Surface Regulation of Zinc Fertilizer on Absorption of Cadmium, Plumbum and Zinc in Rice (Oryza sativa L.). Sustainability, 15(3), 1877. https://doi.org/10.3390/su15031877





