New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater and Algal–Bacterial Granular Sludge
2.2. Batch Experiments
2.3. Analysis Methods
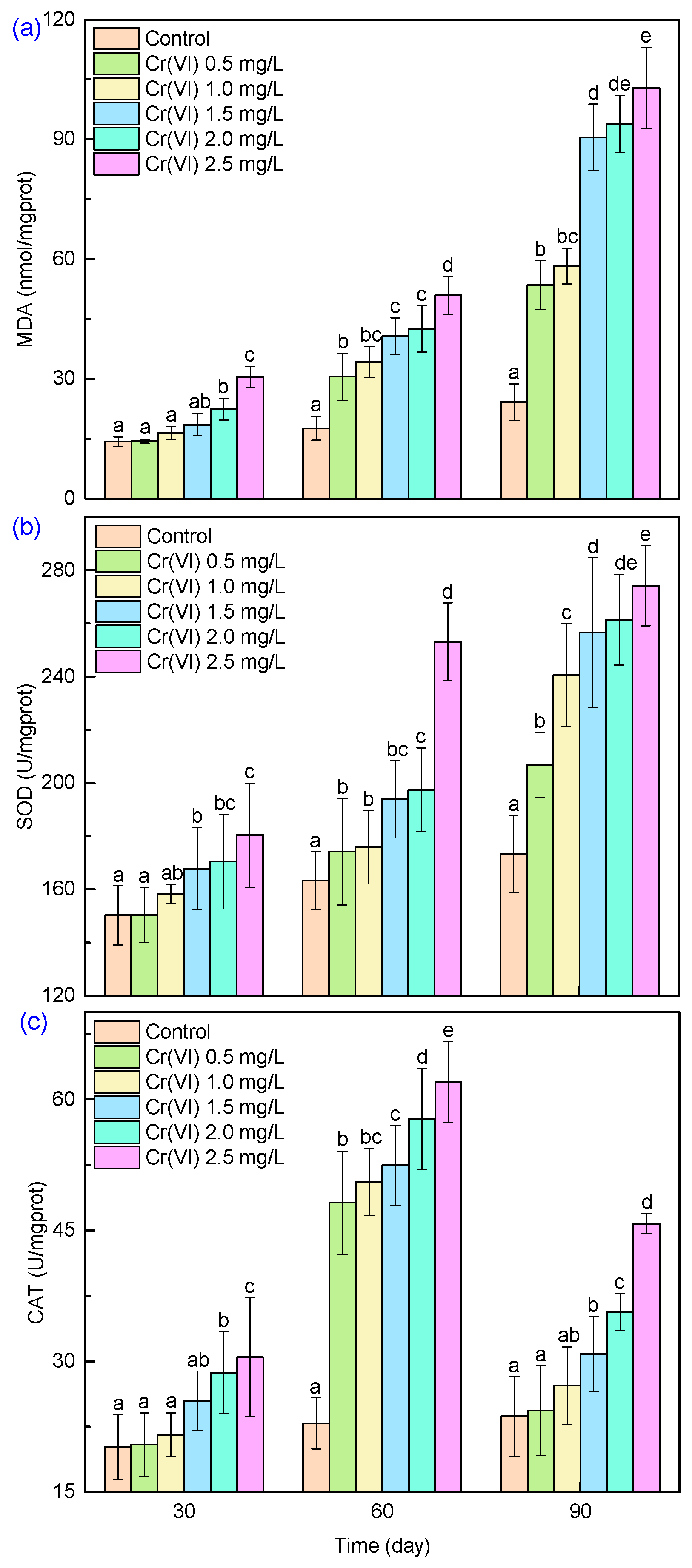
3. Results and Discussion
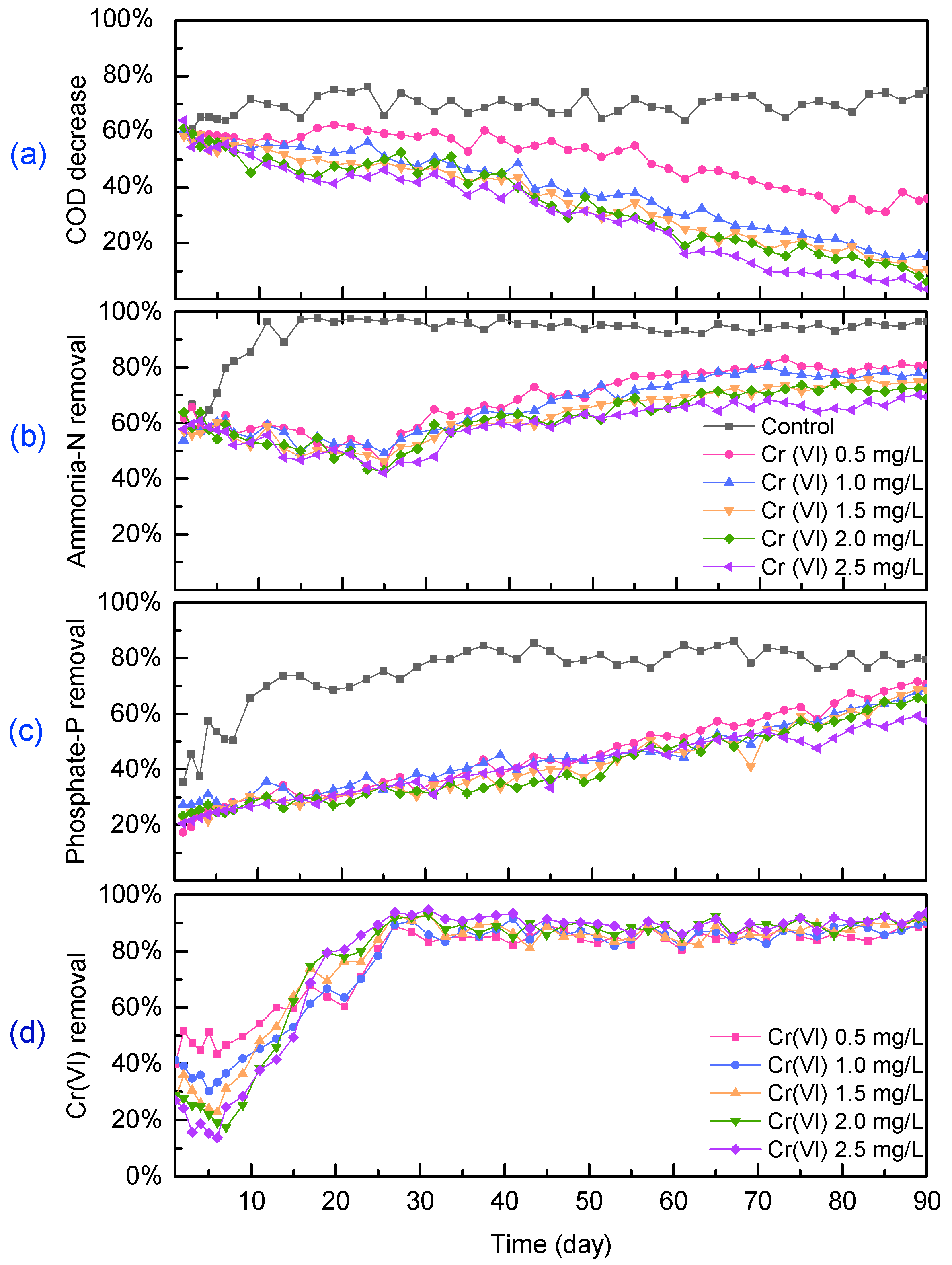
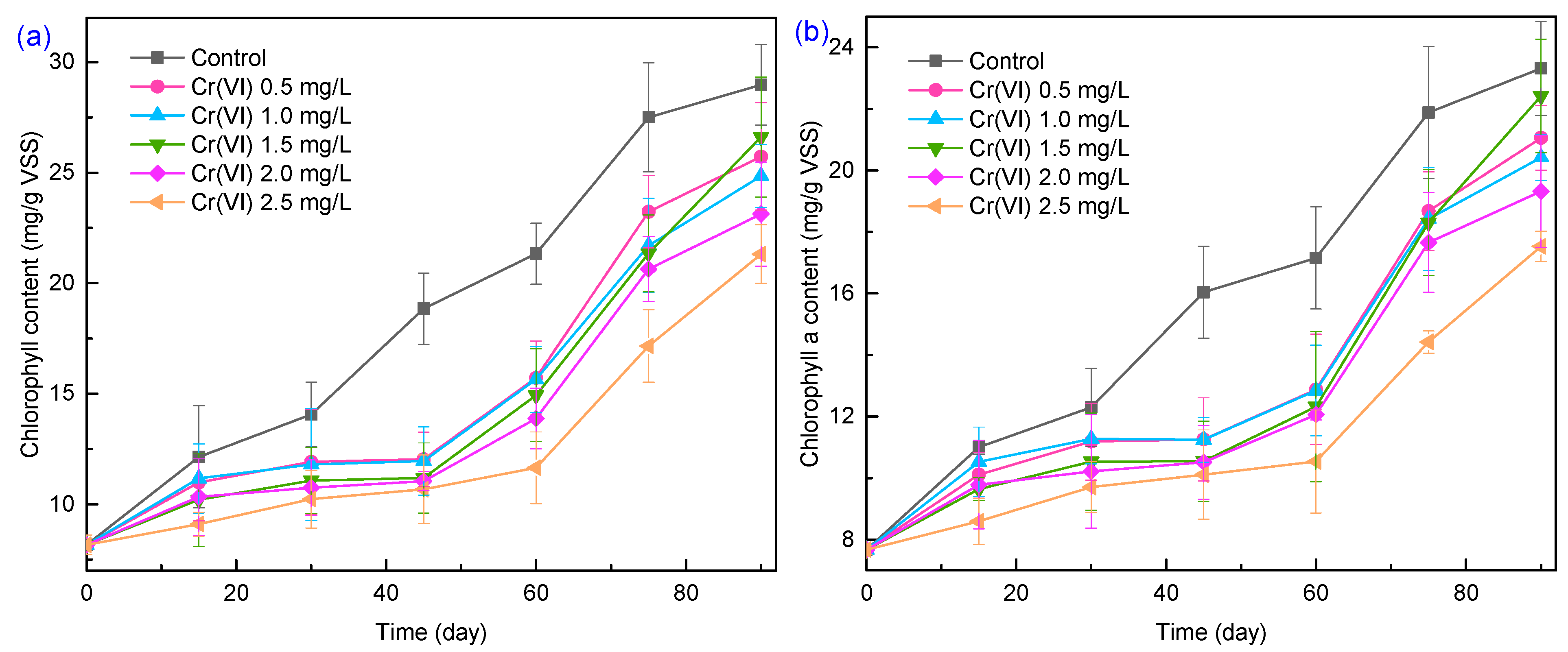
3.1. Performance of the Algal–Bacterial Granular Sludge Process on Nutrient Removal under Cr(VI) Stress
3.2. Fate of Cr(VI) in Algal–Bacterial Granular Sludge
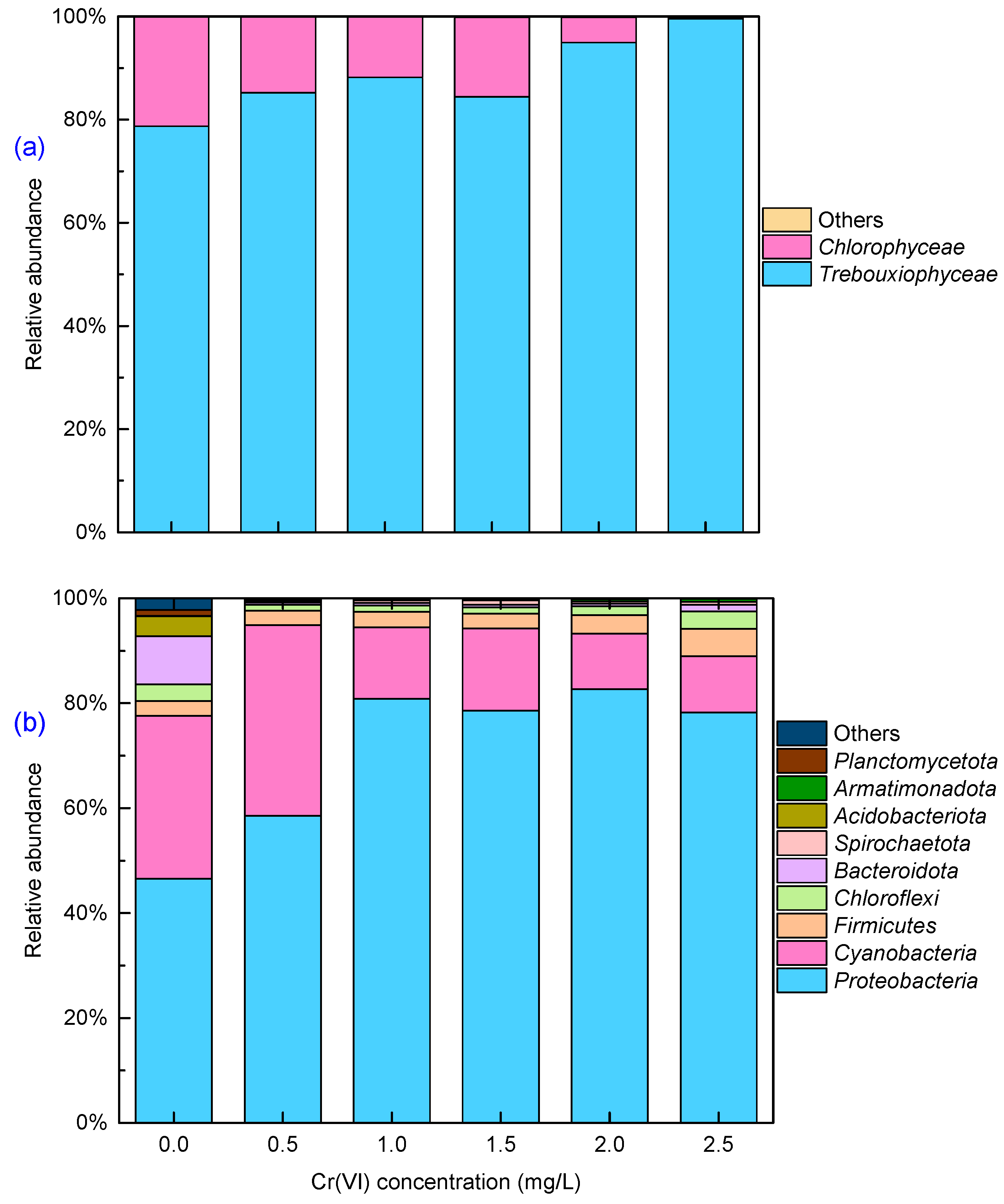
3.3. Changes in Microbial Communities under Cr(VI) Stress
3.4. Defensive Responses of Algal–Bacterial Granular Sludge under Cr(VI) Stress
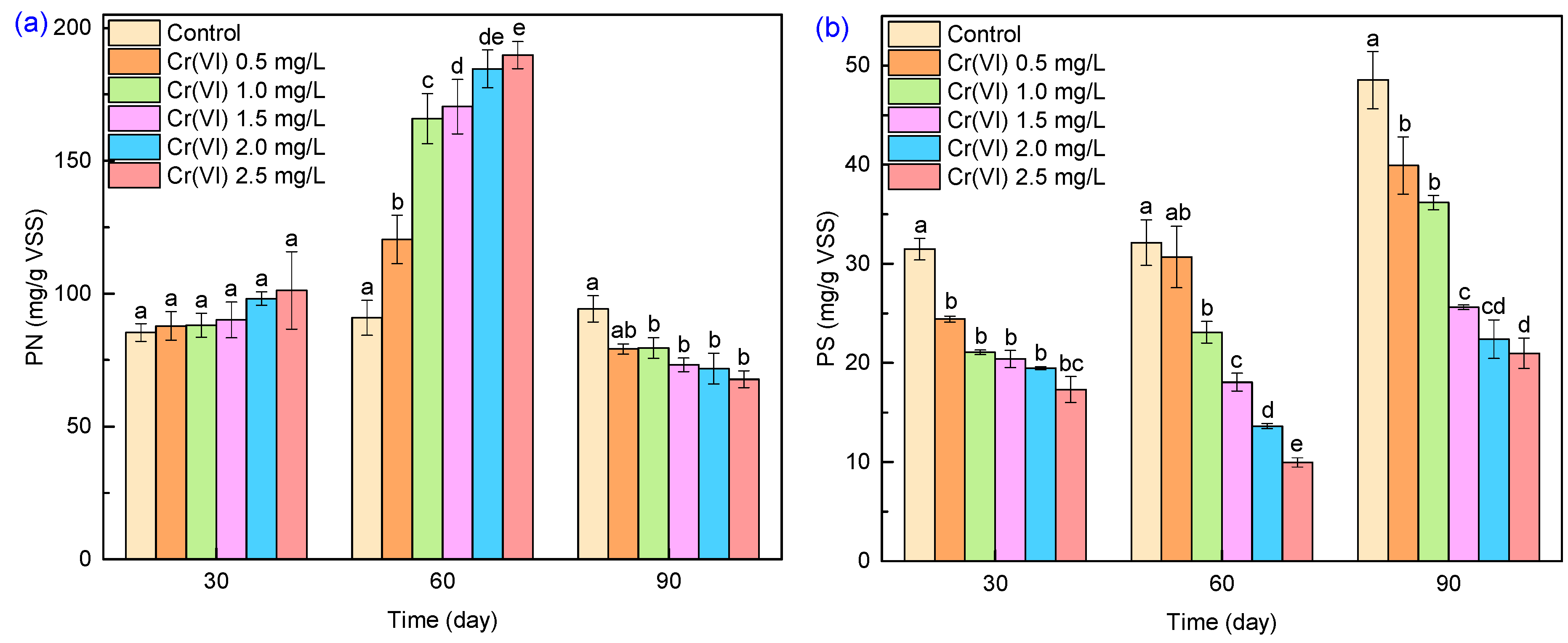
3.4.1. EPS Variations
3.4.2. Antioxidant Enzyme Activity
3.5. Engineering Implications and Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.; Wei, Y.; Wang, Q.; Tian, C.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J. Behavior of algal-bacterial granular sludge in a novel closed photo-sequencing batch reactor under no external O2 supply. Bioresour. Technol. 2020, 318, 124190. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Liu, C. CO2 improves the microalgal-bacterial granular sludge towards carbonnegative wastewater treatment. Water Res. 2022, 208, 117865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ji, B.; Liu, Y. Microalgal-bacterial granular sludge process: A game changer of future municipal wastewater treatment? Sci. Total Environ. 2021, 752, 141957. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, H.; Liu, Y.; Liu, C.; Huang, X. Development of algae-bacteria granular consortia in photo-sequencing batch reactor. Bioresour. Technol. 2017, 232, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, B.; Cui, B.; Ma, Y.; Guo, D.; Liu, Y. Cadmium-effect on performance and symbiotic relationship of microalgal-bacterial granules. J. Clean. Prod. 2021, 282, 125383. [Google Scholar] [CrossRef]
- Yang, X.; Nguyen, V.B.; Zhao, Z.; Wu, Y.; Lei, Z.; Zhang, Z.; Le, X.S.; Lu, H. Changes of distribution and chemical speciation of metals in hexavalent chromium loaded algal-bacterial aerobic granular sludge before and after hydrothermal treatment. Bioresour. Technol. 2022, 355, 127229. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Nie, Q.; Ding, Y.; Lei, Z.; Zhang, Z.; Shimizu, K.; Yuan, T. Pb(II) bioremediation using fresh algal-bacterial aerobic granular sludge and its underlying mechanisms highlighting the role of extracellular polymeric substances. J. Hazard. Mater. 2023, 444, 130452. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Zhang, G.; Hirayama, S.; Nguyen, B.V.; Lei, Z.; Shimizu, K.; Zhang, Z. Insight into Cr(VI) biosorption onto algal-bacterial granular sludge: Cr(VI) bioreduction and its intracellular accumulation in addition to the effects of environmental factors. J. Hazard. Mater. 2021, 414, 125479. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Yu, Y.; Shimizu, K.; Zhang, Z.; Lei, Z.; Lee, D.-J. Enhanced biosorption of Cr(VI) from synthetic wastewater using algal-bacterial aerobic granular sludge: Batch experiments, kinetics and mechanisms. Sep. Purif. Technol. 2020, 251, 117323. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Nguyen, B.V.; Hirayama, S.; Tian, C.; Lei, Z.; Shimizu, K.; Zhang, Z. Cr(VI) bioremediation by active algal-bacterial aerobic granular sludge: Importance of microbial viability, contribution of microalgae and fractionation of loaded Cr. J. Hazard. Mater. 2021, 418, 126342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.M.; Wang, C.; Ruan, C.; Liu, X.L.; Song, S. Optimization of immobilized microorganisms technology to Cr(VI) in wastewater. Adv. Mater. Res. 2015, 1092–1093, 886–891. [Google Scholar] [CrossRef]
- WHO. World Health Organization Guidelines for Drinking Water Quality, 1, Recommendations, 2nd ed.; WHO: Geneva, Switzerland, 1993.
- Wang, S.; Ji, B.; Zhang, M.; Ma, Y.; Gu, J.; Liu, Y. Defensive responses of microalgal-bacterial granules to tetracycline in municipal wastewater treatment. Bioresour. Technol. 2020, 312, 123605. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Ritchie, R.J. Measurement of chlorophylls a and b and bacteriochlorophyll a in organisms from hypereutrophic auxinic waters. J. Appl. Phycol. 2018, 30, 3075–3087. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Wang, S. Over-produced extracellular polymeric substances and activated antioxidant enzymes attribute to resistance of Pb(II) for algal–bacterial granular sludge in municipal wastewater treatment. Water 2023, 15, 3833. [Google Scholar] [CrossRef]
- Herbert, D.; Phipps, P.; Strange, R. Chapter III Chemical Analysis of Microbial Cells. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 1971; pp. 209–344. [Google Scholar]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plants. China Environment Press: Beijing, China, 2002.
- Council of European Communities. Directive Concerning Urban Wastewater Treatment (91/271/EEC); Council of European Communities: Columbus, OH, USA, 1991. [Google Scholar]
- Wang, L.; Liu, X.; Lee, D.J.; Tay, J.H.; Zhang, Y.; Wan, C.L.; Chen, X.F. Recent advances on biosorption by aerobic granular sludge. J. Hazard. Mater. 2018, 357, 253–270. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012006. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Mishra, B.B.; Devi, N. Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J. Clean. Prod. 2019, 209, 617–629. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Alhewairini, S.S.; Peng, C.; Naz, I. Application of microbial immobilization technology for remediation of Cr(VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Kafil, M.; Berninger, F.; Koutra, E.; Kornaros, M. Utilization of the microalga Scenedesmus quadricauda for hexavalent chromium bioremediation and biodiesel production. Bioresour. Technol. 2022, 346, 126665. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, L.; Cerdeira, S.B.; Vullo, D.L. Chromium (VI) biotransformation by β- and γ-Proteobacteria from natural polluted environments: A combined biological and chemical treatment for industrial wastes. J. Hazard. Mater. 2010, 175, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Meng, S.; Wang, Y.; Yan, L.; Zhang, X.; Wei, D. Microbial fuel cell enhanced pollutants removal in a solid-phase biological denitrification reactor: System performance, bioelectricity generation and microbial community analysis. Bioresour. Technol. 2021, 341, 125909. [Google Scholar] [CrossRef] [PubMed]
- Abouhend, A.S.; Milferstedt, K.; Hamelin, J.; Ansari, A.A.; Butler, C.; Carbajal-González, B.I.; Park, C. Growth progression of oxygenic photogranules and its impact on bioactivity for aeration-free wastewater treatment. Environ. Sci. Technol. 2020, 54, 486–496. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Colica, G.; Mecarozzi, P.C.; De Philippis, R. Treatment of Cr(VI)-containing wastewaters with exopolysaccharide-producing cyanobacteria in pilot flow through and batch systems. Appl. Microbiol. Biotechnol. 2010, 87, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Hedayatkhah, A.; Cretoiu, M.S.; Emtiazi, G.; Stal, L.J.; Bolhuis, H. Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. J. Environ. Manag. 2018, 227, 313–320. [Google Scholar] [CrossRef]
- Pandey, L.K.; Bergey, E.A. Metal toxicity and recovery response of riverine periphytic algae. Sci. Total Environ. 2018, 642, 1020–1031. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, Y.; Chen, M.; Wan, L.; Zhao, Y.; Gao, J.; Liao, M.; Tian, X. The humic acid-like substances released from Microcystis aeruginosa contribute to defending against smaller-sized microplastics. Chemosphere 2022, 303, 135034. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, L.; Yin, X.; Pei, Y. Responses of Chlorella vulgaris exposed to boron: Mechanisms of toxicity assessed by multiple endpoints. Environ. Toxicol. Pharmacol. 2019, 70, 103208. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.K.; Mandotra, S.K.; Kumar, N.; Singh, N.K.; Singh, L.; Rai, U.N. Augmentation of arsenic enhances lipid yield and defense responses in alga Nannochloropsis sp. Bioresour. Technol. 2016, 221, 430–437. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, S.; Lin, X.; Li, J.; Yi, Z.; Al-Rasheid, K.A. Recognizing the importance of exposure-dose-response dynamics for ecotoxicity assessment: Nitrofurazone-induced antioxidase activity and mRNA expression in model protozoan Euplotes vannus. Environ. Sci. Pollut. Res. 2015, 22, 9544–9553. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pandey, L.K.; Kumar, D.; Yadav, A.; Rai, J.; Gaur, J.P. Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecol. Indic. 2014, 36, 272–279. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Liu, Y. One step further to closed water loop: Reclamation of municipal wastewater to high-grade product water. Chin. Sci. Bull. 2020, 65, 1358–1367. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. A self-sustaining synergetic microalgal-bacterial granular sludge process towards energy-efficient and environmentally sustainable municipal wastewater treatment. Water Res. 2020, 179, 115884. [Google Scholar] [CrossRef]
- Hu, G.; Fan, S.; Wang, H.; Ji, B. Adaptation responses of microalgal-bacterial granular sludge to sulfamethoxazole. Bioresour. Technol. 2022, 364, 128090. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Z.; Liu, Y. Microalgal-bacterial granular sludge for municipal wastewater treatment: From concept to practice. Bioresour. Technol. 2022, 354, 127201. [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. State Administration for Market Regulation: Beijing, China, 2022.
| Parameters (mg/L) | Cr(VI) Concentrations in this Study (mg/L) | China Class I-A [19] a | Europe [20] | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | |||
| COD | 68 | 222 | 292 | 308 | 324 | 333 | 50 | 125 |
| Ammonia-N | 1.04 | 5.74 | 7.07 | 7.63 | 8.25 | 9.16 | 5 (8) b | NA c |
| Phosphate-P/Total phosphate d | 0.98 | 1.47 | 1.56 | 1.58 | 1.73 | 2.12 | 1 | 1 (2) e |
| Cr(VI) | / | 0.05 | 0.10 | 0.12 | 0.15 | 0.16 | 0.05 | NA |
| Total Cr | / | 0.26 | 0.37 | 0.63 | 0.81 | 0.85 | 0.1 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, S.; Pi, K.; Gerson, A.R. New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress. Sustainability 2023, 15, 16754. https://doi.org/10.3390/su152416754
Zhang Y, Wang S, Pi K, Gerson AR. New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress. Sustainability. 2023; 15(24):16754. https://doi.org/10.3390/su152416754
Chicago/Turabian StyleZhang, Yu, Shulian Wang, Kewu Pi, and Andrea R. Gerson. 2023. "New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress" Sustainability 15, no. 24: 16754. https://doi.org/10.3390/su152416754
APA StyleZhang, Y., Wang, S., Pi, K., & Gerson, A. R. (2023). New Insight into the Performance and Self-Defensive Responses of the Algal–Bacterial Granular Sludge Process under Cr(VI)-Induced Stress. Sustainability, 15(24), 16754. https://doi.org/10.3390/su152416754








