Abstract
Soil heavy metal pollution has become an important environmental problem in the world. Therefore, it is particularly important to find effective remediation methods for heavy metal contaminated soil. Biochar (BC) is a kind of soil heavy metal passivator with a wide range of applications. It also has a good effect on the control of soil heavy metal pollution. However, BC does not have sufficient fixation capacity for para-anionic contaminants. Nano-zero-valent iron (nZVI) has a strong reducing ability, which can make up for this defect of BC. Therefore, to improve the passivation effect of heavy metals, nanomaterial modification is proposed to optimize biochar performance. Nanoparticles are used as carriers to impregnate biochar (BC). Biochar-supported nano-ferric zero-valent materials are prepared to repair soil contaminated by heavy metals. Results show that the physicochemical properties of modified biochar are significantly optimized. At 5%, the modified biochar (1:3) treatment group had the best remediation effect on Cd-contaminated soil, which significantly promoted soil catalase activity. The modified biochar (3:1) treatment group had the best remediation effect on As-contaminated soil, and significantly increased soil pH, Cation Exchange Capacity (CEC), and available Fe content. Modified biochar (1:3) with 3% added content was used to repair actual composite heavy metal contaminated soil, and the relative percentage content of Cu, Zn, As, Cd, and Pb residue state increased by 10.28%, 7.81%, 7.44%, 9.26%, and 12.75%, respectively. The effects of nZVI@BC on the remediation effect and soil enzymes of Cd- and As-contaminated soil under different factors such as mass ratio of carbon and iron and dosage were studied. The remediation mechanism of Cd- and As-contaminated soil was clarified, and a good solidification and stabilization effect was obtained. This provides a theoretical basis for nZVI@BC remediation of soil contaminated by Cd and As. It has good application value in the treatment and remediation of complex heavy metal contaminated soil.
1. Introduction
Heavy metal pollution in soil is getting worse with the acceleration of urban industrialization. The total excess rate of soil pollutants is 16.1%, of which 19.4% is the arable soil point level. The main pollutants include Cd, Pb, Cu, As, and some organic pollutants [1]. According to the statistics of relevant environmental protection departments, the country’s annual heavy metal pollution of grain is up to 12 million tons, resulting in direct economic losses of more than CNY 20 billion [2]. The amount of pollution is not only increasing, but the scope of pollution is also spreading. The required maintenance funds exceed several trillion yuan. Coupled with the lack of soil pollution and soil remediation laws and standards, the remediation of heavy metal pollution in the country has a long way to go [3]. Human activities are the major cause of soil pollution by heavy metals, including industrial activities, agricultural activities, transport and domestic waste, and urban construction. Heavy industrial activities, such as ore mining, are the most important contributors to the release of heavy metal pollutants [4]. Soils within the area of industrial activities are often heavily contaminated by heavy metals and environmental damage [5].
Heavy metal pollution from agricultural activities is manifested in the accumulation of heavy metals on farmland caused by sewage irrigation or inappropriate practices. Under critical soil conditions, agricultural and forestry production and normal plant growth need to be ensured. The content limits of various heavy metals are as follows. In soil, pH > 6.5, Hg ≤ 1.5 mg/kg, As (paddy field) ≤ 30 mg/kg, As (dry land) ≤ 40 mg/kg, Cu (farmland) ≤ 400 mg/kg, Cu (orchard) ≤ 400 mg/kg, Pb ≤ 500 mg/kg, Cr (paddy field) ≤ 400 mg/kg, Cr (dry land) ≤ 300 mg/kg, Zn ≤ 500 mg/kg, and Ni ≤ 200 mg/kg [6]. Cd is seen as the signature metal of agricultural activities. It is accumulated by chemical fertilizers and pesticides [7,8]. Studies have shown that emissions from transport vehicles contribute to the accretion of Pb and Zn in soils along roads. In addition, gasoline, lubricating oil, and gold-plated parts of cars also release large amounts of Zn, Cu, Pb, and Cd through combustion and wear [9,10]. In cities, heavy metal pollution is much more severe than in the natural environment, because cities are the main areas of resource overuse and industrial pollution emissions [10,11]. Therefore, it is essential to research remediation methods for heavy metal contaminated soils.
The concept of heavy metal forms in soil was introduced as early as 1958. The main link in morphological analysis is the continuous extraction method. The European Community Bureau of Reference (BCR) organized 35 European laboratories to integrate different classification and operation methods and harmonize standards. Since 1992, it has been committed to studying methods for the morphological analysis of heavy genera in soil and sediments. In recent years, the three-state continuous extraction method of the BCR has been widely used in the research of heavy metal elements in soil, sludge, and other media. Chen D et al. used the improved continuous extraction method of BCR to effectively analyze the effects of three aging methods, four types of biochar, and aging time on the chemical forms of Pb in soil [12]. Hu QQ and other researchers used Scanning Electron Microscopy-Energy Dispersive Spectrometer (SEM-EDS) and BCR to continuously extract heavy metals. The occurrence of Cd in different soil thicknesses was analyzed. The study provides a scientific basis for further understanding the activity and occurrence forms of Cd in soil profiles [13]. Ahn Y et al. used the Tessier and Wenzel sequential extraction method to separate Zn from As in soils and predicted the mobility of both. They showed a dominant contribution of As to heavy metal risk [14].
Heavy metal pollution is persistent, highly toxic, and bio-accumulative. It not only affects material exchange and energy flow in the soil system, but also affects various plants, animals, and microorganisms in the soil. It seriously threatens human health and hinders the sustainable development of the ecological environment [15]. The essential trace elements for the human body include Cu, Zn, and other metals. However, when these trace elements exceed a certain range, they can cause major functional diseases in the human body [16]. Prolonged exposure to excessive Pb can cause neurological disorders in both animals and humans [17]. Prolonged exposure to Cd can harm the kidneys, causing osteoporosis and softening [18]. Prolonged exposure to As can hinder normal physiological metabolism [19]. Therefore, it is crucial to repair contaminated soil. The process principle can be divided into physical, chemical, and bioremediation technologies [20].
He L et al. addressed recent research progress in the interaction between metals and biochar in soil, the potential risks of biochar improvement, and the remediation effect of biochar on soil pollution. Research shows that biochar application can scientifically and effectively reduce the heavy metals in soil plants [21]. Mokarram-Kashtiban S et al. analyzed the remediation techniques of heavy metal contaminated soil, namely nano-zero-valent iron (nZVI). Results show that low-dose nZVI evidently increases the bio-concentration factor of Cd. In contrast, high-dose nZVI negatively affects the bio-enrichment coefficients of Pb and Cu [22]. Wang J N and other research experts synthesized magnetic materials using a mild liquid-phase reductive oxidation method to improve the stabilization effect of biochar on heavy metals in soil. Results show that magnetic biochar could reduce the bio-effective states of Cd, Cu, Ni, Zn, and Pb in soil [23]. Fajardo C et al. proposed using physicochemical, toxicological, and molecular analyses in the context of mixed heavy metal Pb, Cd, and Zn contamination to assess the nZVI (5% w/w) of environmental restoration. The experimental results show that nZVI has a 20% fixation capacity for Pb, but it is almost ineffective for heavy metals Zn and Cd [24]. A comparison and application status of various heavy metal soil remediation methods with nZVI@BC are shown in Table 1.

Table 1.
Comparison of remediation methods for soil heavy metal pollution.
Through research on the source harm of heavy metal pollution in soil and existing remediation technology, five heavy metals, Cu, Zn, As, Cd, and Pb, are explored. With the deepening of research, some disadvantages of biochar have gradually appeared. The adsorption efficiency of biochar for heavy metals is lower than that of activated carbon, and there are fewer adsorption sites. In addition, fixation on some anionic pollutants, such as As, cannot achieve the expected effect. Commonly used biochar modification methods mainly include functional group modification, metal oxide modification, and nanomaterial modification. nZVI is a zero-valent iron particle with a particle size between 1 and 100 nm, which has a large specific surface area and strong reduction capacity [25]. In environmental remediation, nZVI has good application prospects. Biochar modified by nano-zero-valent iron has great potential for the fixation of heavy metals [26]. Therefore, a method for remediating heavy metal contaminated soil by biochar-loaded nZVI is proposed in this study. At the same time, the remediation effect and mechanisms of the research method are observed and evaluated in the light of current science and technology. The innovation of this research is in exploring the influence of nZVI@BC on the remediation effect and soil enzymes of Cd- and As-contaminated soil. Under different factors such as carbon iron mass ratio and dosage, the remediation mechanisms of cadmium- and arsenic-contaminated soil are clarified nZVI@BC is used for remediation of Cu, Zn, As, Cd, and Pb composite polluted soil. The purpose of this research is to clarify the remediation mechanism of biochar-supported nano-zero-valent iron materials on composite heavy metal contaminated soil and to provide more reasonable and effective technical and theoretical support for the remediation of heavy metal contaminated soil.
2. Materials and Methods
2.1. Test Materials
2.1.1. Test Reagents
The main reagents used in the experiment are shown in Table 2.

Table 2.
Test reagents.
2.1.2. Principal Instruments
The main instruments and equipment used in the experiment are shown in Table 3.

Table 3.
Instruments and equipment.
2.2. Soil for Testing
Firstly, contaminated soil samples with exogenous additions of Cd and As were prepared. Raw soil was obtained from a test farm at a northern university, sampled at a depth in the range of 0–20 cm. The maximum field capacity was 18.50% [27]. Soils contaminated with a variety of heavy metals such as Cu, Zn, As, Cd, and Pb were selected from a southern region. They were used as raw soil for preparing soil contaminated by complex heavy metals. This soil was also sampled at a depth of 0–20 cm. The maximum field capacity was 20.06%. Some 10 g of air-dried soil samples were weighed using a 1 mm sieve and placed in a 25 mL beaker. Some 10 mL of distilled water was added and thoroughly mixed, and then the sample solution was allowed to stand for 30 min. A calibrated pH meter was used to measure the pH value of the suspension. The bulb (or bottom) of the glass electrode was immersed in the suspension mud layer, and the plug on the side hole of the calomel electrode was pulled out. The calomel electrode was immersed in the upper suspension liquid to read the pH value. At present, commonly used methods for detecting heavy metals in soil include boiling soil samples with strong acids, flame atomic absorption spectroscopy, graphite furnace atomic absorption spectroscopy, atomic fluorescence spectroscopy, and plasma emission spectroscopy. Physical and chemical property test results of the two soils are shown in Table 4 [28].

Table 4.
Physical and chemical properties of two test soils.
In Table 4, the two types of soil tested are brown loam and yellowish-brown loam. After removing plant residues and gravel from the soil, the remaining parts were mixed together. Then it was placed indoors for natural air drying and then ground. Finally, it was screened with 2 mm nylon for a backup.
To prepare simulated Cd-contaminated soil, 10 kg of brown soil was weighed in a pot. Some 1 L of 100 mg/L CdCl2 solution was added and mixed well to reach an exogenous Cd content of 10 mg/kg. To prepare simulated As-contaminated soil, 10 kg of brown soil was also weighed in a pot and 1 L of 400 mg/L Na2HAsO4 solution was added and mixed well to reach an exogenous As content of 10 mg/kg. The maximum water holding capacity in the field was adjusted to 65%. The simulated Cd-and As-contaminated soils were incubated for a fortnight in a 25 °C incubator. The two contaminated soils were dried indoors, ground, and sieved.
2.3. Test Program
2.3.1. Material Synthesis
The optimal temperature for biochar preparation was 500 °C to 600 °C. The organic material was placed in a state of hypoxia for controlled high-temperature decomposition. In nitrogen atmosphere, rice straw biochar was prepared by slow pyrolysis at 500 °C. The resulting biochar was sieved through a 100 mesh screen and then ground. It was soaked in a 1 mol/L hydrochloric acid solution for 1 d. Impurities such as ash were removed, and it was washed with deionized water and dried at 105 °C. Biochar-supported nZVI composites were prepared by liquid-phase reduction, and the biochar was proportionally put into 100 mL (0.05 mol·L−1) FeCl3 solution. The mass varied with the proportion of composite, as shown in Table 5 [29,30,31].

Table 5.
Change in biochar mass with composite ratio in 0.05 mol/L FeCl3.
After the proportional solutions were configured according to the proportions in Table 5, they were dispersed well by ultrasonication and then packed into a three-necked flask. Under the protection of nitrogen, 125 mL solution (0.2 mol·L−1) was added dropwise using a funnel while stirring. After the reaction was complete, the solid material was centrifuged and washed several times with anhydrous ethanol, followed by a small amount of cleaning. The nZVI@Biochar (BC) composites were dried at 60 °C under a vacuum. The obtained nZVI@BC composites were noted as nZVI@BC, 3:1, 1:1, and 1:3, respectively.
2.3.2. Soil Culture Trials
Some 100 g of exogenously added Cd-contaminated soil was weighed, placed in a 250 mL glass beaker, and then added at mass fractions of 1%, 3%, and 5%, respectively. Four remediation materials, BC, nZVI@BC 3:1, 1:1, and 1:3, were mixed with this soil and incubated for 20 days and 40 days, respectively. Pure Cd soil without any additional material was used as a control, with three parallel groups in each group. Some 65% deionized water with a maximum on-site field capacity was added to the beaker and incubated in a thermostat at 25 °C. After a few days, the water was replenished by weighing. Soil samples were taken at appropriate times after 20 days and 40 days of incubation for testing. Similarly, 100 g of external As-contaminated soil was weighed and placed in a 250 mL glass beaker. Pure As soil without any material added was used as a control. The remaining steps were the same as those for the external Cd-contaminated soil [32].
Some 100 g of composite heavy metal contaminated soil was weighed, placed in a 250 mL glass beaker, and then added at a mass fraction of 3% [33]. Remediation materials BC and nZVI@BC (1:3) were mixed with this soil separately and incubated for 40 days. Heavy metal contaminated soil without any added materials was used as a control, and three parallels were set for each group. The remaining steps were the same as those for the exogenous Cd and As-contaminated soil culture. Soil samples were taken after 40 days of incubation for testing.
When the reaction was complete, the soil samples were freeze-dried using a vacuum and grounded through a 100 mesh sieve [34]. Then magnetic separation and recovery of nZVI@BC (1:3) were carried out by repeating the above steps. The nZVI@BC (1:3) was characterized by EDS and XRD [35]. The soil particles were characterized by XPS to explore the remediation mechanism of the composite material on heavy metal contaminated soil [36]. The nZVI@BC (1:3) of Cd and As content for different heavy metals was measured using an EDS.
2.3.3. Characterization and Determination
The experiments were characterized by EDS, FTIR, and XRD to determine the changes in biochar before and after demodeling. The characterization tools and methods used are shown in Table 6 [37,38,39].

Table 6.
Characterization analysis and determination methods.
Various measuring tools used in the experiment are shown in Table 6. Their function was to characterize the characteristics of the material. The fugitive forms of heavy metals were measured and analyzed. The Cd form was extracted from the soil based on the BCR method by grinding the soil sample. Then, 1.0 g of the soil sample was weighed into a 100 mL centrifuge tube. The corresponding extraction procedure is shown in Table 7.

Table 7.
BCR sequential extraction procedure.
The Cd form was extracted from the soil by adding reagents according to the steps in Table 7. Then it was determined by ICP-OES [40,41,42]. The Wenzel method was used for extracting the As form from the soil. Then, 1.0 g of the ground soil sample was weighed through a 100 mesh sieve. This was placed in a 50 mL centrifuge tube. The corresponding extraction steps are shown in Table 8.

Table 8.
Wenzel sequential extraction procedure.
The Wenzel method was used to extract from the soil by adding reagents according to the procedure in Table 8. Then it was determined by ICP-OES. The contaminated soil contains five heavy metals, Cu, Zn, As, Cd, and Pb. BCR was used to simultaneous extract forms containing multiple heavy metals. The total amount of the three heavy metals was determined by the HJ 803–2016 [43] aqua regia extraction method—inductively coupled plasma mass spectrometry [44,45]. The recovery rates of the BCR extraction method and Wenzel extraction method were 89~106% and 88~114%, respectively, which met the standard (85~115%) [46]. Characterization data of the materials were processed by Omnic, MDI Jade, and Avantage software. SPSS 24.0 software was used for statistical analysis. The statistical method used was LSD ANOVA [47]. The significance level of the difference was 0.05.
3. Experimental Results on the Remediation Effect and Mechanism of Biochar-Loaded nZVI on Heavy Metal Contaminated Soil
3.1. Characterization of Biochar-Loaded nZVI
To understand the physical and chemical property changes of biochar before and after modification, the following characterization analysis was carried out. SEM maps are shown in Figure 1.

Figure 1.
SEM images of (a) BC, (b) nZVI@BC (3:1), (c) nZVI@BC (1:1), and (d) nZVI@BC (1:3).
From Figure 1a, BC presented a honeycomb structure with abundant pores, which was conducive to the adsorption of pollutants. From Figure 1b–d, there were many white particles attached to the surface of the BC. These were nano-zero-valent iron. Nano-zero-valent iron existed in a spherical or chain structure, distributed in the surface or pores of the biochar, indicating that biochar was a good carrier to reduce the aggregation of white particles. The results showed that Fe appeared on the surface of the composite after modification. From Figure 1b, when the mass ratio of nZV@BC was 3:1, nZV on the BC surface presented an agglomeration phenomenon. From Figure 1c, when the mass ratio of nZV@BC was 1:1, nZVI phase pairs were evenly distributed on the surface of the BC. From Figure 1d, when the mass ratio of nZV@BC was 1:3, the amount of nZVI decreased, and excessive BC meant an abundant pore structure, resulting in most nZVI being distributed in the inner pores of the biochar. EDS analysis of the biochar before and after modification is shown in Figure 2.
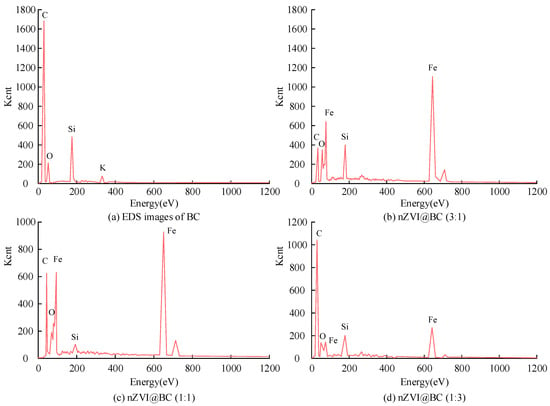
Figure 2.
EDS images of BC, nZVI@BC (3:1), 1:1, and 1:3.
From Figure 2a, the EDS spectrum showed that the BC was mainly composed of four elements: C, O, Si, and K. From Figure 2b–d, EDS spectra showed that the main components of nZVI@BC were C, Fe, O, and Si. Fe appeared on the surface of the modified composite material. When the mass ratio of nZVI to BC was 3:1, the Fe content in the composite was the highest. This content was nearly 1180. When the mass ratio of nZVI to BC was 1:1, the content of Fe in the composite was 970. When the mass ratio of nZVI to BC was 1:3, the quantity of nZVI decreased. At 400~4000 cm−1, the FTIR of BC and nZVI@BC was analyzed, as shown in Figure 3.
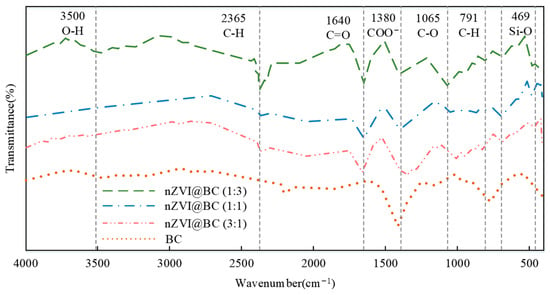
Figure 3.
FTIR spectra of BC and n ZVI@BC.
Figure 3 shows that the BC had an O-H stretching vibrational peak near 3500 cm. The vibrational peaks at 2364 cm and 790 cm were both caused by C-H, both corresponding to C-H in long-chain aliphatic and aromatic groups, respectively. The 1642 cm and 1381 cm peaks were characterized by telescopic vibrations of C=O and COO−. A vibrational peak of C-O was present at 1065 cm. A peak of S-O was observed at 468 cm. In summary, the biochar surface was rich in O-H, C=O, S-O, etc., which was conducive to the complexation and fixation of heavy gold. The Fe-O stretching vibration peak near 678 cm in nZVI@BC indicated that there might be FeO or Fe3O in the composite4, tentatively demonstrating the existence of an nZVI core–shell structure. After loading the nZVI, the composites not only retained the characteristic functional groups of biochar, but also showed new Fe-O functional groups. They provided new adsorption sites for heavy metals. Because the surface of BC contained more base ions (K+, Nat, Ca2+, Mg2+, etc.), it exchanged with H+ and AI3+ after entering the soil, which reduced the content of exchangeable acids and aluminum ions in the soil. Soil pH value in all nZVI@BC treatment groups was significantly higher than that in the BC group. The process can be expressed by reaction Equation (1).
As the soil pH increases, the amount of negative charge on the soil surface increases. Therefore, it shows strong electrostatic attraction to metal cations. Electrostatic attraction is an effective way to stabilize Cd2+. At the same time, the increase in pH is conducive to the formation of Cd(OH)2 and other precipitates, thereby reducing the migration of Cd. The XRD pattern of the material nZVI@BC is shown in Figure 4.
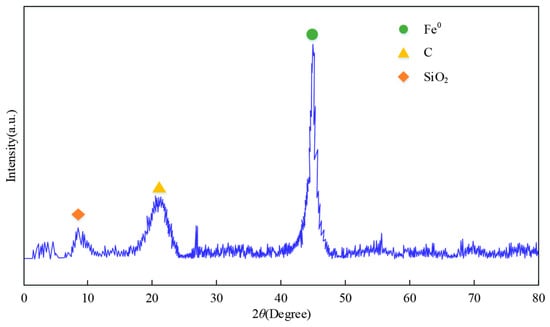
Figure 4.
XRD pattern of nZVI@BC (1:3).
In Figure 4, the diffraction peak at 7.95° is attributed to SiO2. The characteristic peak at 20.84° is attributed to the amorphous structure of the biochar. There was a significant Fe0 characteristic peak in nZVI@BC at 44.7°. In the XRD spectrum, the characteristic peaks of C and Fe0 appeared simultaneously. This meant that nZVI was successfully attached to the biochar.
3.2. The Remediation Effect and Mechanism Analysis of Biochar-Loaded nZVI on Heavy Metal Contaminated Soil
The effects of different treatments on the heavy metal forms in soil after 20 days and 40 days of cultivation were studied. The remediation effect of nZVI loaded with biochar on heavy metal contaminated soil was analyzed. Table 9 shows the change results of Cd occurrence forms.

Table 9.
Effects on Cd speciation under different treatments after 20 days and 40 days incubation.
From Table 9, after 20 days and 40 days of soil cultivation, the amount of material added and the remediation time were positively correlated with the passivation effect of Cd when the addition amount increased from 1% to 5%. The nZVI@BC group showed a better passivation effect on Cd in soil than the BC group. After 40 days of soil cultivation, the nZVI@BC (1:3) group at 5% addition had the best immobilization of Cd, and the synthetic composite had more biochar content. The changes in As fugitive morphology were statistically analyzed using the same means. The results are shown in Table 10.

Table 10.
Effects on As speciation under different treatments after 20 days and 40 days incubation.
From Table 10, when the addition level increased from 1% to 5%, there was a positive correlation between the amount of material added, remediation time, and the passivation effect of As. This was similar to the change in Cd fugacity. The nZVI@BC group showed a better passivation effect of As in the soil than the BC group. The nZVI@BC (3:1) group showed the best immobilization of As at 40 days and 5% addition. nZVI@BC immobilization of As in soil was mainly related to Fe conversion. The composite material with the highest nZVI content synthesized by this carbon to Fe ratio was very helpful for the immobilization of As. In the experiment, 3% addition of nZVI@BC (1:3) was used to analyze the remediation effect of composite heavy metal contaminated soil after 40 days of soil cultivation. Cation Exchange Capacity (CEC) refers to the amount of exchangeable cations adsorbed by soil per unit mass. Soil CEC can reflect the ability to retain heavy metals. The effects of different treatments on soil CEC after 20 days and 40 days of soil culture are shown in Figure 5.
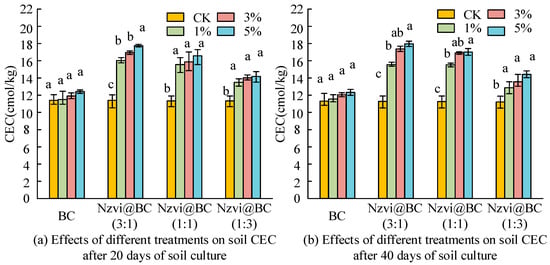
Figure 5.
Effects of different treatments on soil CEC after 20 days and 40 days of soil culture. Note: Those with the same letters in each group indicated that the difference was not significant (p > 0.05), while those with different letters indicated that the difference was significant (p < 0.05).
From Figure 5, soil CEC in the BC group increased by 0.152 to 1.011 cmol/kg after soil culture for 20 days, and the addition rate increased from 1% to 5%. The effect was not significant. Soil CEC in the nZVI@BC treatment group significantly increased by 2.086~6.369 cmol/kg. The CEC value of soil in the nZVI@BC (3:1) treatment group significantly increased by 4.590~6.369 cmol/kg. The nZVI@BC (1:1) treatment group increased by 4.235~5.146 cmol/kg. The nZVI@BC (1:3) group increasedby 2.085~2.791 cmol/kg. Figure 5b shows that, after 40 days of soil culture, soil CEC in the nZVI@BC treatment group significantly increased by 1.577~6.691 cmol/kg with an increase in soil addition. The soil CEC value of the nZVI@BC (3:1) treatment group significantly increased by 4.269~6.691 cmol/kg. The nZVI@BC (1:1) treatment group significantly increased by 4.191~5.654 cmol/kg. The nZVI@BC (1:3) treatment group significantly increased by 1.577~3.156 cmol/kg. Soil CEC in all treatment groups showed an increasing trend with an increase in addition amount. Under the same addition amount, CECnZVI@BC (3:1) > CECnZVI@BC(1:1) > CECnZVI@BC (3:1) > CECBC, which was consistent with the nano-zero-valent iron content in the relevant material. The original biochar itself has a large cation exchange capacity. At the same time, the surface functional groups of biochar are oxidized to form carbonyl, phenolic, and quinone groups under the action of soil organisms or abiotics, which is also conducive to enhancing the soil’s poor CEC. The nZVI@BC treatment group had a more significant effect on the improvement in soil CEC. This was due to the effect of the biochar base. In addition, it was due to the release of Fe3+ or Fe2+ by nZVI oxidation, which resulted in a substantial increase in soil CEC. Cd2+ can be ion exchanged with cations (K+, Na+, Ca2+, Mg2+, etc.) on the surface of the composite material, so that Cd2+ can be adsorbed and fixed. An increase in soil CEC is conducive to reducing Cd migration in the soil through ion exchange and promoting Cd immobilization. The available iron content in soil can be used as an index to evaluate soil fertility. However, excessive availability of iron has toxicity to organisms. Table 11 shows the effective iron content measured after 40 days of soil cultivation using different methods.

Table 11.
Effects of different treatments on available iron content in soil after 40 days of soil culture (mg/kg).
From Table 11, compared with CK, BC treatment had no significant effect on soil available iron content with an increase in addition amount. However, nZVI@BC treatment significantly increased the available iron content in soil by 102~285 mg/kg. Among treatments, the nZVI@BC (3:1) treatment increased by 163~285 mg/kg, the nZVI@BC (1:1) treatment increased by 157~279 mg/kg, and the nZVI@BC (1:3) treatment increased by 102~246 mg/kg. With an increase in material addition, the available iron content in soil of the nZVI@BC treatment group increased. Under the same supplemental level, the available Fe content can be sorted as nZVI@BC (3:1) > nZVI@BC (1:1) > nZVI@BC (1:3) > BC. This was related to the content of nano-zero-valent iron in passivated materials. After 40 days of soil cultivation and 5% addition, the nZVI@BC (3:1) treatment group had the highest content of soil available iron. The effect of BC and nZVI@BC (1:3) on the morphology of Cu, Zn, As, Cd, and Pb in the soil at 3% addition is shown in Figure 6.
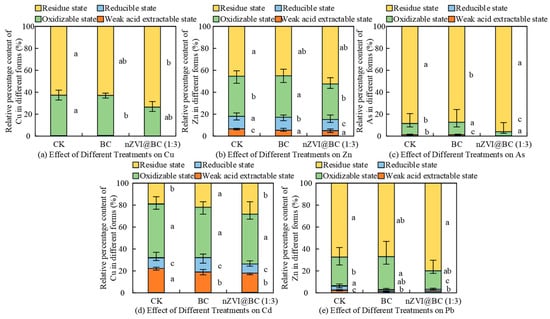
Figure 6.
Effects on Cu, Zn, As, Cd, and Pb speciation under different treatments after 40 days incubation. Note: Those with the same letters in each group indicated that the difference was not significant (p > 0.05), while those with different letters indicated that the difference was significant (p < 0.05).
Zn was mainly present in the residual state (45.71%) and Cd was mainly present in the oxidizable state (48.00%). After 40 days of soil cultivation, the relative content of oxidizable Cu and Cd in the BC-treated soil decreased by 0.06% and 2.57%, respectively. The relative content of residual Cu and Cd increased by 0.36% and 2.87%, respectively. The relative content of residual Zn, As, and Pb decreased by 0.34%, 1.37%, and 0.28%, respectively.
After 40 days of soil cultivation, the nZVI@BC (1:3) group was more effective than the BC group in passivating multiple heavy metals. The relative percentages of Cu, Zn, As, Cd, and Pb in the oxidizable state decreased by 9.87 mg/kg, 38.82 mg/kg, 2.81 mg/kg, 2.37 mg/kg, and 22.43 mg/kg, respectively. The relative percentages of Cu, Zn, As, Cd, and Pb in the residue state increased by 10.28%, 7.81%, 7.44%, 9.26%, and 12.75%, respectively. In conclusion, the nZVI@BC (1:3) passivation treatment effectively increased the residual Cu, Zn, As, Cd, and Pb in soil. The reaction results are shown in Figure 7.
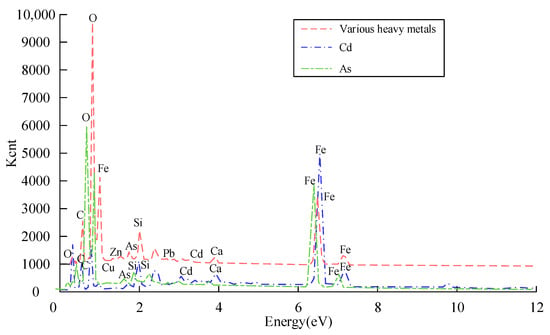
Figure 7.
After-reaction nZVI@BC (1:3) of Cd, As, and various heavy metals.
From Figure 7, the nZVI@BC (1:3) material had Cu, Zn, As, Cd, and Pb immobilized by adsorption on the surface. The nZVI@BC (1:3) material is shown in Figure 8. It shows the XRD patterns of Cd, As, and various heavy metals in the nZVI@BC (1:3) material.
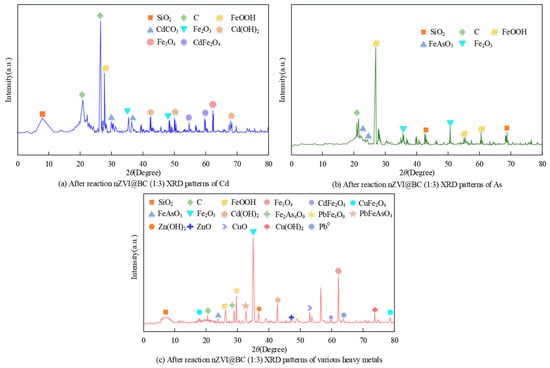
Figure 8.
After-reaction nZVI@BC (1:3) XRD patterns of Cd, As, and various heavy metals in the material.
As shown in Figure 8a, the characteristic Fe0 peaks of the composites disappeared after the reaction. The formation of new minerals FeOOH (2θ = 27.66°), Fe2O3 (2θ = 35.3°, 48.57°), and Fe3O4 (2θ = 62.48°) meant that Fe0 was involved in the reaction and was oxidized. The characteristic peak near 57.1° is attributed to CdO. The peaks near 30.4° and 36.3° were caused by CdCO3 Cd(OH)2 at 42.48°, 50.06°, and 68.23°, and there is a new characteristic peak for CdFe2O4 (2θ = 54.84°, 60.02°). This represents a complexation reaction between the nZVI@BC (1:3) surface iron minerals and Cd. In summary, the nZVI@BC (1:3) material immobilized Cd in the soil by complexation, ion exchange, and co-precipitation.
As shown in Figure 8b, the characteristic peaks of Fe0 disappeared after the reaction. The formation of new minerals Fe2O3 (2θ = 35.32°, 50.21°) and FeOOH (2θ = 26.58°, 54.84°, 59.89°) indicated that Fe0 was involved in the reaction and was oxidized by corrosion. The obtained FeOOH provided more new adsorption sites for As. Due to the strong affinity of As for FeO (hydroxide) compounds, As could be co-precipitated with this compound to form FeAsO4 (2θ = 22.3°, 24.2°). Then, it was immobilized. Experiments showed that As was adsorbed and co-precipitated by amorphous iron oxides to form Fe(As)OOH due to a decrease in the amount of non-specific adsorbed As, specific adsorbed As, and an increase in the amount of amorphous iron aluminum oxide bound As.
From Figure 8c, after 40 days of soil cultivation, when the characteristic peaks of Fe0 disappeared, new peaks of FeOOH (2θ = 26.59°), Fe2O3 (2θ = 35.68°), and Fe3 O4 (2θ = 62.58°) were detected simultaneously. This indicated that there was oxidative corrosion on the nZVI surface. Zn(OH)2 (2θ = 37.08°), Cd(OH)2 (2θ = 43.02), and Cu(OH)2 (2θ = 74.03°) exhibited characteristic peaks, indicating that Zn, Cd, and Cu were immobilized by precipitation. Characteristic peaks of Pb0 were detected at 64.22°, and the formation of PbFeAsO4OH was detected at 33.28°. New characteristic peaks for ZnO (2θ = 47.49°), CuO (2θ = 53.45°), CdO (2θ = 57.10°), CuFeO24 (2θ = 18.41°, 79.18°), FeAsO4 (2θ = 24.28°), PbFe4O7 (2θ = 30.13°), and CdFe2O4 (2θ = 60.02°) appeared after the reactions. This indicated that complexation and co-precipitation between the heavy metals and the nZVI@BC (1:3) composites appeared. The formation of insoluble minerals resulted in unstable to stable performance of heavy metals.
4. Discussion
SEM images showed that there were many white particles in the shape of spheres or chains attached to the modified BC. EDS showed that Fe appeared on the surface of the material. XRD detected a strong diffraction peak of FeO, which jointly proved that the white particles were composed of nZVI. This indicated that nZVI was successfully loaded on the surface of the biochar. FTIR showed that the surface of the BC contained abundant oxygen-containing functional groups, such as O-H, C=O, COO−,and C-O, etc. Heavy metals can be fixed by complexing with oxygen-containing functional groups. After loading nZVI, nZVI@BC not only retains the original characteristic functional groups of biochar, but also produces new Fe-O functional groups. XPS detected the presence of FeO, Fe2O3, and Fe3O4. This not only reconfirmed the successful loading of nZVI, but also proved the existence of an nZVI putamen structure on the surface of the composite material. The iron oxide layer outside the putamen structure not only provided a new adsorption site, but also stabilized the heavy metal through electrostatic interaction and complexation. After nZVI modification, the physical and chemical properties of the biochar were significantly optimized, which was conducive to the adsorption and fixation of heavy metals in the soil.
To assess the ecological risk of heavy metals more accurately, the form can be analyzed. The mobility of Cd in soil is closely related to the morphology. The environmental risk of Cd in a weak acid extractable state is much higher than that of other non-extractable Cd forms. The results of the soil cultivation experiments showed that all nZVI@BC groups are more effective in passivating Cd in the soil than the BC treatment group. After 40 days of soil cultivation, the nZVI@BC (1:3) treatment group at 5% addition had the best immobilization effect on Cd. At this time, the relative percentage of weak acid extractable Cd decreased by 37%, reducible Cd decreased by 12%, oxidizable Cd increased by 4%, and residual Cd increased by 46%, effectively promoting the conversion of weak acid extractable and reducible Cd to oxidizable and residual Cd. To investigate the remediation mechanism of nZVI@BC (l:3) composites on Cd-contaminated soil, the reacted composites were characterized. EDS showed that Cd was successfully adsorbed on the surface of the material. XRD characterization showed that CdO and CdCO3 were produced with the oxidation of nZVI, demonstrating the occurrence of complexation, ion exchange, and co-precipitation. The effects of nZVI@BC (1:3) on the remediation mechanism of Cd-contaminated soil mainly included surface adsorption, ion exchange, complexation, and co-precipitation.
The effect and mechanisms of biological carbon loaded with nZVI on the remediation of As-contaminated soil by exogenous addition were investigated by soil cultivation experiments. The experiments showed that all nZVI@BC treatment groups are more effective than the BC treatment group in passivating As in the soil, because the fixation of As in soil is related to the transformation of iron. After 40 days of soil cultivation, the nZVI@BC (3:1) group at 5% addition had the highest nZVI content, so it has the best passivation effect on As of the seven soils. At this time, the As removal rate of the non-specific adsorption state was close to 100%. The relative percentage of As in the specific adsorbed state was reduced by 30%. The composite material effectively promotes the conversion of As from the non-specific and specific adsorbed states to the amorphous iron-aluminum oxide bound and residual states. To explore the remediation mechanism of As-contaminated soil by nZVI@BC (3:1) composites, the reacted composites and soil particles were characterized. EDS demonstrated the successful adsorption of As on the composite surface. XRD characterization detected As2 O5 and FeAsO4, demonstrating the occurrence of complexation and co-precipitation reactions between As. Moreover, nZVI corrosion produces a large amount of FeOOH.
The results of the soil cultivation experiments with actual complex heavy metal contamination showed that the nZVI@BC (1:3) treatment group is more effective than the BC treatment group in passivating soil heavy metals Cu, Zn, As, Cd, and Pb. After 40 days of soil cultivation, nZVI@BC (1:3) at 3% addition is effective in promoting the conversion of heavy metals from other easily extractable forms to the remaining forms. Compared with the CK control, the relative percentage of Cu, Zn, As, Cd, and Pb in the residue state increased by 10.28%, 7.81%, 7.44%, 9.26%, and 12.75%, respectively. Experiments showed that nZVI@BC (1:3) achieves immobilization of Cu, Zn, As, Cd, and Pb.
5. Conclusions
Biochar-supported nano-zero-valent iron materials with different ratios of carbon to iron were prepared by the liquid-phase reduction method. Their structural characteristics and physicochemical properties were studied by modern chemical methods and instrument analysis. The results showed that biochar was a good carrier to prevent nZVI agglomeration. nZVI was distributed uniformly in the biochar after modification. The addition of nZVI@BC significantly increased soil pH and significantly increased soil CEC and available iron content. After 20 days of soil culture, soil CEC increased by 0.024~0.650 cmol/kg after BC treatment. nZVI@BC soil CEC significantly increased by 2.348~5.851 cmol/kg after treatment. The CEC of soil treated with nZVI@BC (3:1) increased by 4.193~5.851 cmol/kg. After soil culture for 40 days, nZVI@BC changed the CEC value of soil by 3.386~6.421 cmol/kg.After soil culture for 40 days, the group with 5% nZVI@BC (1:3) added had the best remediation effect on the soil contaminated by exogenous Cd, and the residual Cd increased by 46%. The group with 5% nZVI@BC (3:1) added had the best remediation effect on the soil contaminated by exogenous arsenic. The non-specific adsorption of As was almost completely removed. The residual arsenic increased by 8%. nZVI@BC (3:1) had the most effective remediation effect on Cd- and As-contaminated soil. The repair mechanisms of Cd and As mainly include surface adsorption, complexation, and co-precipitation. After 40 days of cultivation in the same soil, nZVI@BC (1:3) effectively promoted the transformation of heavy metals from an easily extracted state to a residual state. At this time, the relative percentages of Cu, Zn, As, Cd, and Pb in the residual state increased by 10.28%, 7.81%, 7.44%, 9.26%, and 12.75%, respectively. This research investigated the effects of nZVI@BC on the remediation effect and soil enzymes of Cd- and As-polluted soil under different factors such as mass ratio of carbon and iron and dosage. The remediation mechanism of soil contaminated by Cd and As was expounded. nZVI@BC was applied to the remediation of soil contaminated by Cu, Zn, As, Cd, and Pb.Good solidification and stabilization effects were obtained. The research method has good application value for the treatment and restoration of soil contaminated by complex heavy metals. It could be used for risk assessment of the ecological environment to ensure the ecological safety of the soil environment. Although this research explored the short-term effective restoration of nZVI@BC to heavy metal polluted soil, the long-term restoration effect and stability of nZVI@BC still need to ensure the ecological security of the soil environment.
Author Contributions
Software, L.T.; Formal analysis, H.R.; Investigation, C.D. and J.R.; Data curation, M.W.; Writing—original draft, L.T.; Writing—review & editing, B.W.; Supervision, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
Authors Jun Ren, Ling Tao and Hanru Ren were employed by the company Gansu Hanxing Environmental Protection Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Raab, T.; Sut-Lohmann, M.; Ramezany, S.; Kästner, F. Feasibility of pXRF to evaluate chosen heavy metals in soil highly influenced by municipal waste disposal-A monitoring study of a former sewage farm. Land Degrad. Dev. 2022, 33, 439–451. [Google Scholar] [CrossRef]
- Chorol, L.; Gupta, S.K. Evaluation of groundwater heavy metal pollution index through analytical hierarchy process and its health risk assessment via Monte Carlo simulation. Process Saf. Environ. Prot. 2023, 170, 855–864. [Google Scholar] [CrossRef]
- Yang, H.; Dong, C.; Zhang, H.; Luo, H.; Li, J.; Yin, J.; Dong, X.; Wei, Z.; Zhang, N.; Bao, L. Characteristics and Source Analysis of Soil Heavy Metal Pollution in a Mining Area. J. Earth Sci. Environ. Prot. 2022, 10, 159–176. [Google Scholar] [CrossRef]
- Meng, M.; Yang, L.; Yu, J.; Cao, Z. Identification of spatial patterns and sources of heavy metals in greenhouse soils using geostatistical and positive matrix factorization (PMF) methods. Land Degrad. Dev. 2021, 32, 5412–5426. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, X.R. Impact of industrial activities on heavy metal contamination in soils in three major urban agglomerations of China. J. Clean. Prod. 2019, 230, 5–10. [Google Scholar] [CrossRef]
- Quds, T.; Ahmed, M.; Shakeel, S.; Jalbani, N.; Azhar, I. Determination of the heavy metal contents of frequently used herbal products in Pakistan. Trop. J. Pharm. Res. 2021, 20, 377–382. [Google Scholar] [CrossRef]
- Rplabc, D.; Ynxac, D.; Jhzac, D.; Rmef, G. Effects of heavy metal pollution on farmland soils and crops: A case study of the Xiaoqinling Gold Belt, China-ScienceDirect. China Geol. 2020, 3, 402–410. [Google Scholar] [CrossRef]
- Sheeja, K.M.; Harilal, C.C. Spatial distribution and seasonal variation of heavy metal contaminants and pollution indices in a coastal landmass of Kerala, peninsular India. Chem. Ecol. 2022, 38, 211–232. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, Y.; Li, M.; Lin, L.; Hu, Y. Effects of the lubricating oil and diesel mixture combustion on the oxidation and microphysical properties of particulate matter. Energy Rep. 2020, 6, 308–314. [Google Scholar] [CrossRef]
- Shukla, L.; Jain, N. Contamination of Heavy Metal in Soil Due to Industrial Activity. J. Environ. Pollut. Hum. Health 2022, 10, 1–5. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.; Han, Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. Engl. 2021, 3, 217–226. [Google Scholar] [CrossRef]
- Huang, J.; Peng, J. Rare earth element (REE) geochemistry of different colored fluorites from the Baoshan Cu-Pb-Zn deposit, Southern Hunan, South China. Chin. J. Geochem. Engl. 2022, 41, 419–433. [Google Scholar] [CrossRef]
- Chen, D.; Liu, W.; Wang, Y.; Lu, P. Effect of biochar aging on the adsorption and stabilization of Pb in soil. J. Soils Sediments 2021, 22, 56–66. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Shen, Q.; Chen, F. Reconstructed Soil Vertical Profile Heavy Metal Cd Occurrence and Its Influencing Factors. Huan Jing Ke Xue 2020, 41, 2878–2888. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Yun, H.S.; Pandi, K.L. Heavy metal speciation with prediction model for heavy metal mobility and risk assessment in mine-affected soils. Environ. Sci. Pollut. Res. 2020, 27, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, J.; Wang, S. Sensor-based characteristics of kaolin and the adsorption of heavy metal ions. Arab. J. Geosci. 2021, 14, 2–16. [Google Scholar] [CrossRef]
- Goncalves, S.; Almeida, S.F.P.; Figueira, E.; Kahlert, M. Valve teratologies and Chl c in the freshwater diatom Tabellaria flocculosa as biomarkers for metal contamination. Ecol. Indic. 2019, 101, 476–485. [Google Scholar] [CrossRef]
- Al-Saadi, M.R.; Al-Fartusie, F.S.; Thani, M.Z. Evaluation of lead, cadmium, copper and zinc levels and studying their toxic effect in sera of private electrical generator workers. J. Phys. Conf. Ser. 2021, 1853, 012044. [Google Scholar] [CrossRef]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377. [Google Scholar] [CrossRef]
- Tang, B.; Tong, P.; Xue, K.S. High-throughput assessment of toxic effects of metal mixtures of cadmium(Cd), lead(Pb), and manganese(Mn) in nematode Caenorhabditis elegans. Chemosphere 2019, 234, 232–241. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Mokarram-Kashtiban, S.; Hosseini, S.M.; Tabari Kouchaksaraei, M.; Younesi, H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ. Sci. Pollut. Res. 2019, 26, 10776–10789. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Cheng, S.S.; Zhan, W.H.; Ren, Q.; Wang, Y.Y. Synthesis of Magnetic Biochar and Its Application in the Remediation of Heavy-Metal-Contaminated Soils. Huan Jing Ke Xue 2020, 41, 2381–2389. [Google Scholar] [CrossRef]
- Fajardo, C.; Sanchez-Fortun, S.; Costa, G.; Nande Met Botias, P.; Garcia-Cantalejo, J. Evaluation of nanoremediation strategy in a Pb, Zn and Cd contaminated soil. Sci. Total Environ. 2020, 706, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Popoola, B.M.; Olanbiwoninu, A.A.; Fashogbon, R.O. Bioremediation of Vegetable Oil Contaminated Soil with Two Microbial Isolates. Microbiology 2022, 12, 218–241. [Google Scholar] [CrossRef]
- Li, X.; Tan, B.; Ma, Q.; Zeng, Y.; Wang, Y.; Tao, S.; Zhang, H.; Zhang, X.; Luo, X.; Deng, Q. Effects of applying Bidens species straw to Cd-contaminated soil on growth and cadmium accumulation of Ziziphus Acidojujuba seedlings. Environ. Prog. Sustain. Energy 2022, 41, e13730. [Google Scholar] [CrossRef]
- Osu, S.R.; Udofia, G.E.; Ndaeyo, N.U. Improving Crude Oil Contaminated Soil with Organic Amendments: Effect of Oil Palm Bunch Ash and Dried Poultry Litters on Soil Properties and Cassava Growth and Yields. J. Appl. Sci. Environ. Manag. 2022, 26, 1647–1656. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Shi, Z. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Environ. Pollut. Bioavailab. 2021, 33, 55–65. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, Y.H.; Eom, H. Effect of preparation method of noble metal supported catalyts on formaldehyde oxidation at room temperature: Gas or liquid phase reduction. J. Environ. Sci. 2022, 122, 201–216. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, J.; Ren, X.; Schaeffer, S.M. Effects of phosphorus modified nZVI-biochar composite on emission of greenhouse gases and changes of microbial community in soil. Environ. Pollut. 2021, 274, 116483. [Google Scholar] [CrossRef]
- Zou, L.; Liu, Q.; Zhang, Q. Synthesis of Bimetallic Pd-Based/Activated Carbon Catalyst by Biomass-Reduction Method for Highly Efficient Hydrogen Storage System Based on CO2/Formate. Ind. Eng. Chem. Res. 2022, 14, 2455–2468. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Z.; Yu, A. Phytoremediation of cadmium-contaminated soils by Solanum nigrum L. enhanced with biodegradable chelating agents. Environ. Sci. Pollut. Res. 2022, 29, 56750–56759. [Google Scholar] [CrossRef] [PubMed]
- Helaoui, S.; Boughattas, I.; Mkhinini, M. Biochar improves the adaptability of Vicia faba L in cadmium contaminated soil. Soil Sediment Contam. Int. J. 2023, 32, 496–517. [Google Scholar] [CrossRef]
- Zoufan, P.; Bavani, M.R.Z.; Tousi, S.; Rahnama, A. Effect of exogenous melatonin on improvement of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mol. Biol. Plants 2023, 29, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Verma, A.K.; Sangal, V.K. Synergistic degradation employing photocatalysis and photo-Fenton process of real industrial pharmaceutical effluent utilizing the Iron-Titanium dioxide composite. Process Saf. Environ. Prot. 2020, 146, 564–576. [Google Scholar] [CrossRef]
- Khan, M.N.; Ullah, H.; Naeem, S.; Uddin, J.; Hamid, Y.; Ahmad, W. Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights. Sustainability 2021, 13, 8817. [Google Scholar] [CrossRef]
- Paul, D.; Maiti, S.; Sethi, D.P. Bi-functional NiO-ZnO nanocomposite: Synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 2021, 32, 131–143. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Aguirre, M.; Nóbrega, J.A.; Canals, A. Dispersive liquid-liquid microextraction of Cd, Hg and Pb from medicines prior to ICP OES determination according to the United States Pharmacopeia. Anal. Methods 2021, 13, 5670–5678. [Google Scholar] [CrossRef]
- Taheri, A.; Cheniany, M.; Ganjeali, A.; Arefi-Oskouie, A. ICP-OES assessment of trace and toxic elements in Ziziphora clinopodioides Lam. from Iran by chemometric approaches. Biometals 2022, 35, 1169–1186. [Google Scholar] [CrossRef]
- Xiong, Q.; Lin, Y.; Wu, W. Chemometric intraregional discrimination of Chinese liquors based on multi-element determination by ICP-MS and ICP-OES. Appl. Spectrosc. Rev. 2021, 56, 115–127. [Google Scholar] [CrossRef]
- HJ 803-2016; Soil and Sediment-Determination of Aqua Regia Extracts of 12 Metal Elements-Inductively Coupled Plasma Mass Spectrometry. Ministry of Health Environmental Protection: Beijing, China, 2016.
- Liu, Y.; Fu, Y.; Zheng, L. Leaching characteristics and solidification strategy of heavy metals in solid waste from natural graphite purification. Environ. Sci. Pollut. Res. 2023, 30, 30892–30904. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Tang, Q.; Khan, A. Identification and speciation of nanoscale silver in complex solid matrices by sequential extraction coupled with inductively coupled plasma optical emission spectrometry. Anal. Chem. 2021, 93, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Z.; Xie, Y.; Tan, Z. Method for determination of Pu isotopes in soil and sediment samples by inductively coupled plasma mass spectrometry after simple chemical separation using TK200 resin. Anal. Chim. Acta 2019, 1090, 151–158. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Y.; Lin, J.; Li, Z.; Tan, Z. Rapid method for sequential determination of Pu and Am in soil and sediment samples by sector-field inductively coupled plasma mass spectrometry. J. Radioanal. Nucl. Chem. 2021, 328, 137–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).