Te-Doped Bi2Se3@NC Nanocomposites for High-Performance Li-Ion Battery Anodes
Abstract
:1. Introduction
2. Experimental
2.1. Material
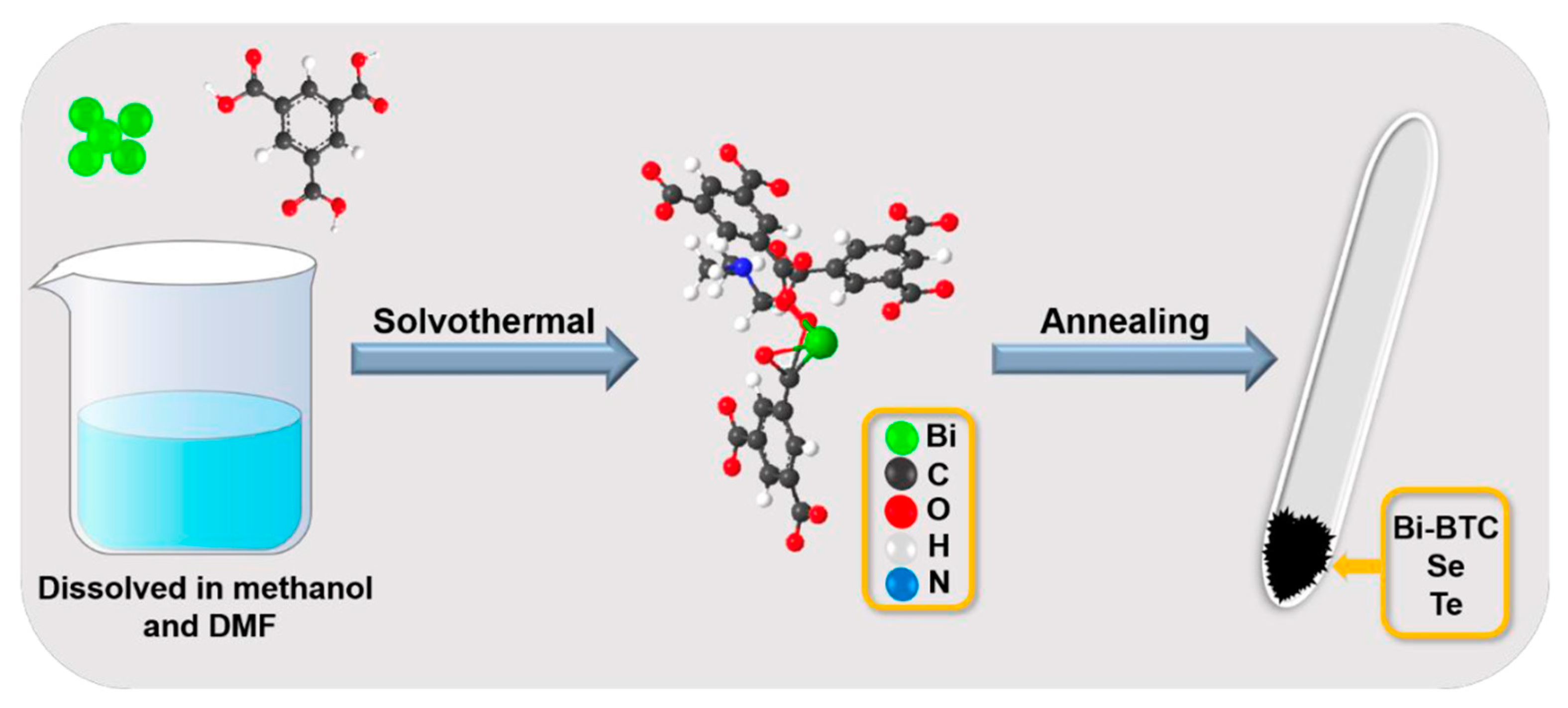
2.2. Material Synthesis
2.2.1. Synthesis of Bi- BTC Precursor
2.2.2. Synthesis of Bi2Se3 @NC
2.2.3. Synthesis of Bi2Se3−xTex@NC
2.3. Material Characterization
2.4. Electrochemical Characterization
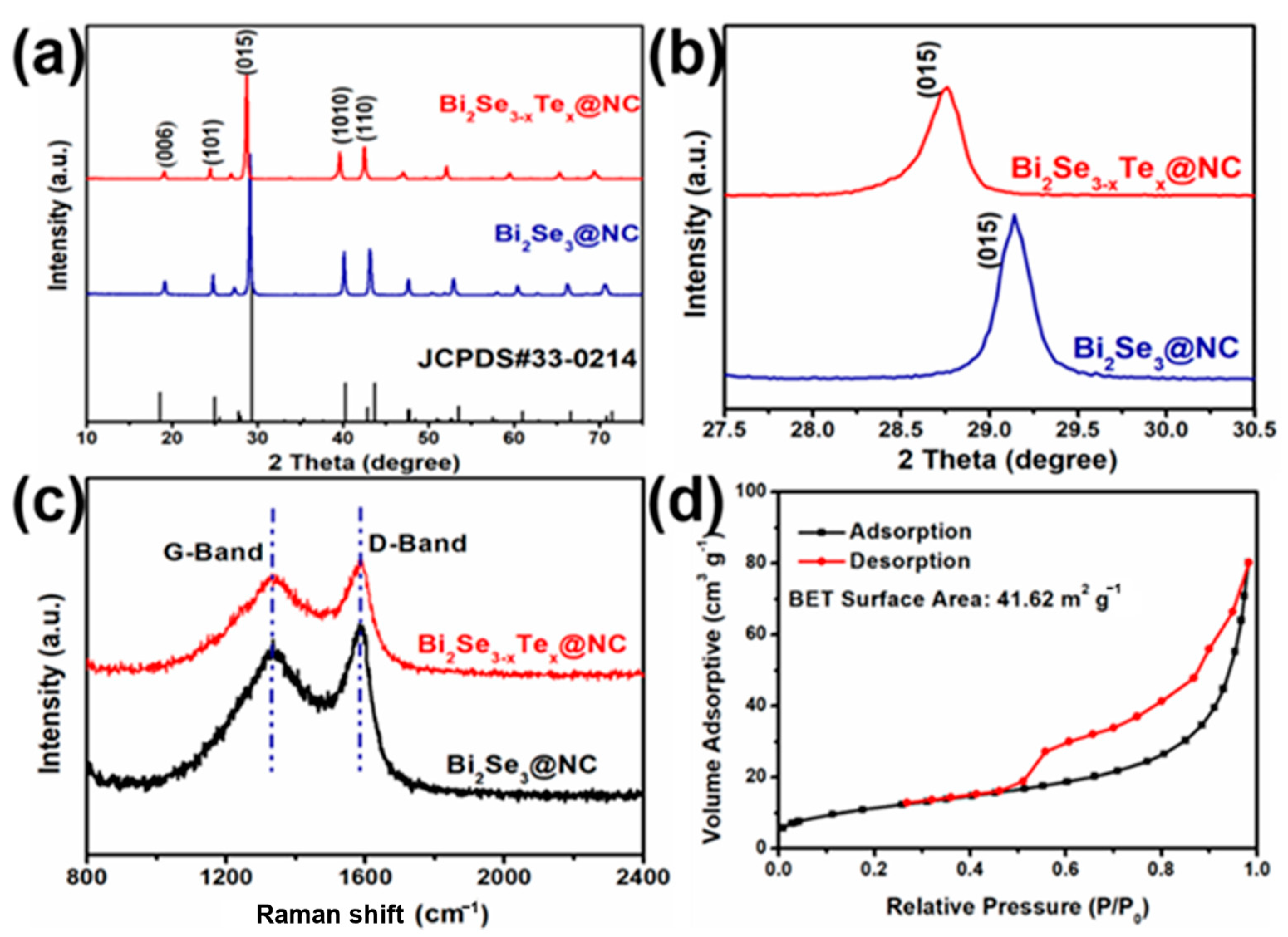
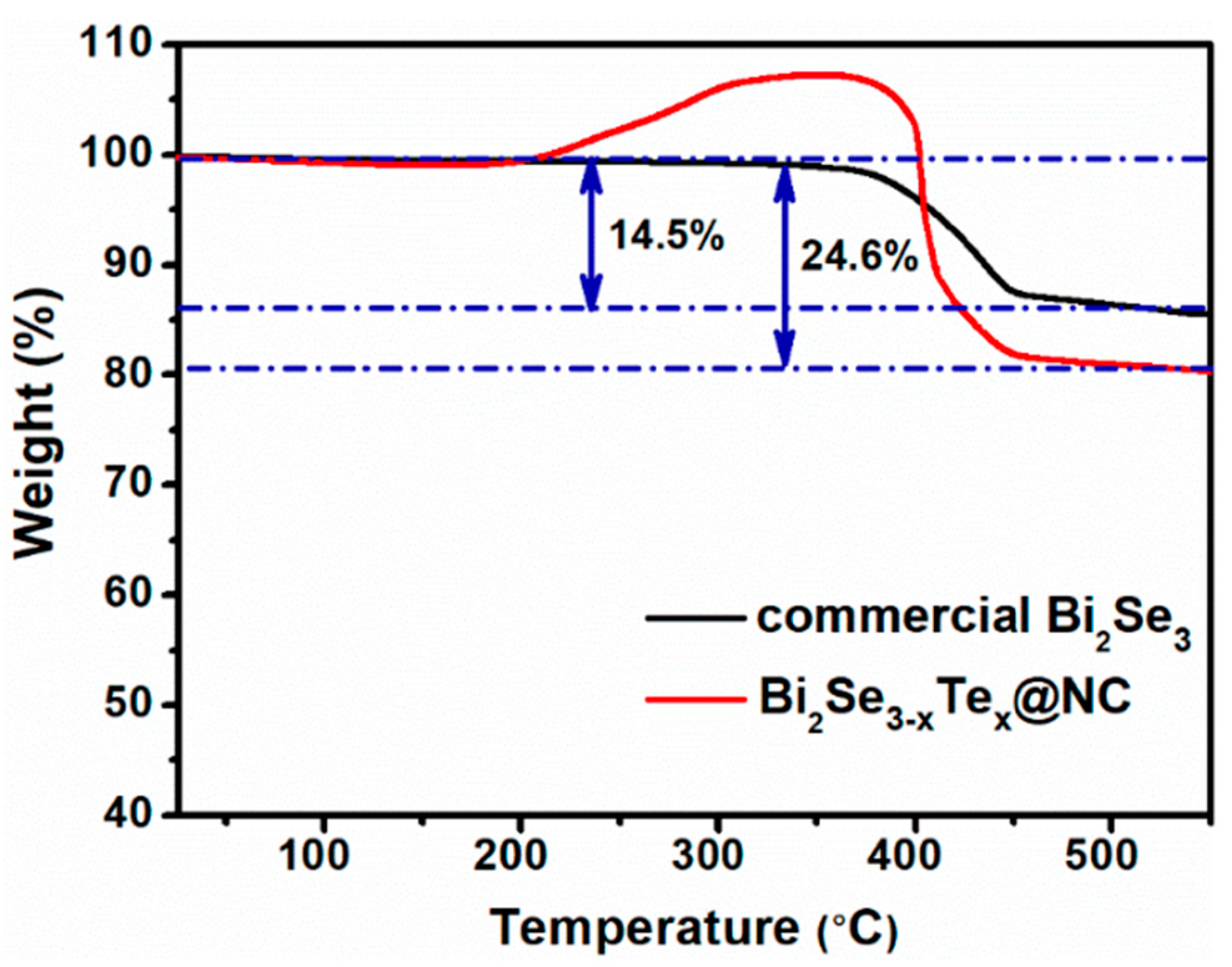
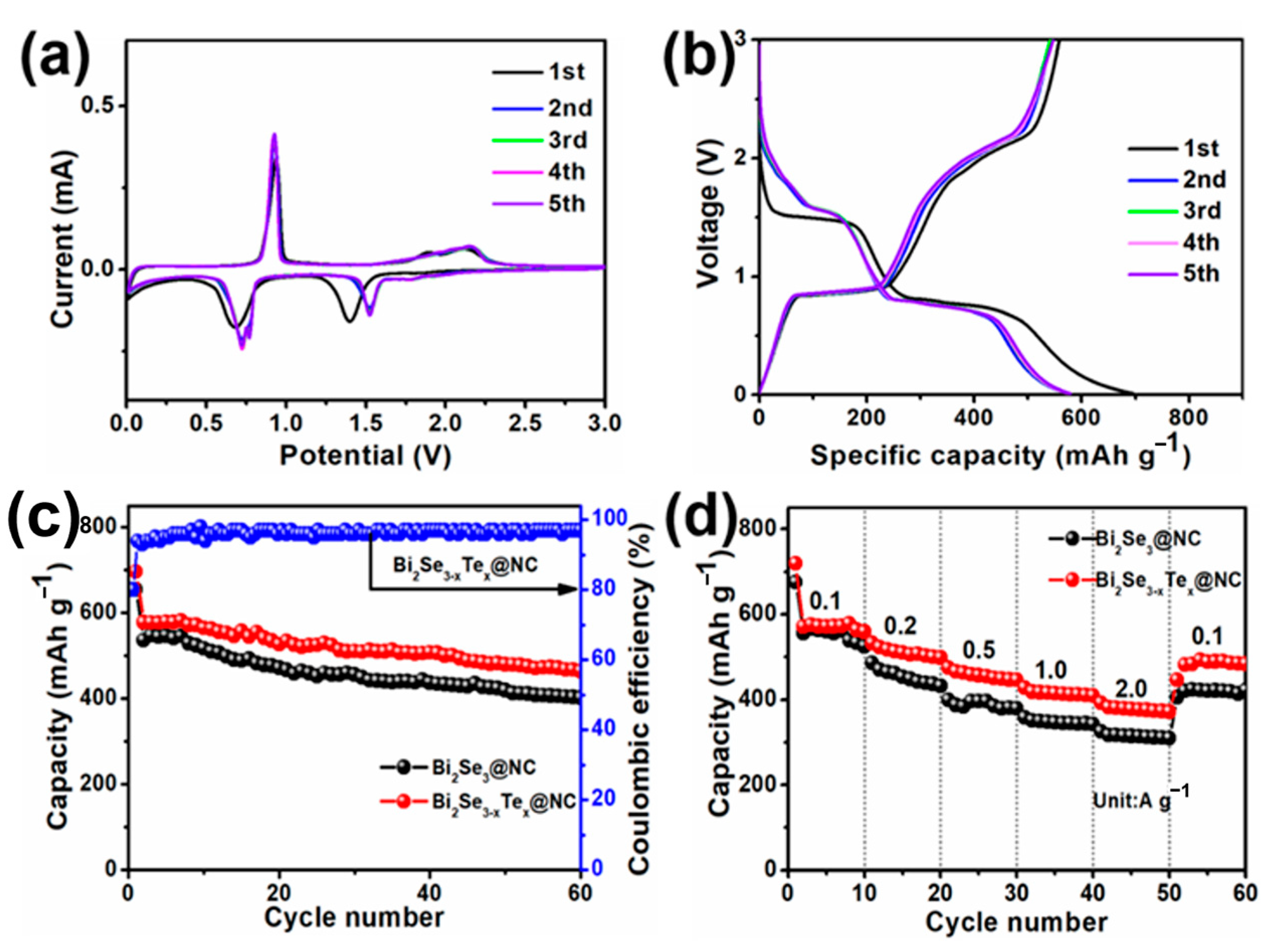
3. Results and Discussion
Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zubi, G.; Dufo-Lopez, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Song, J.; Li, G.; Dong, G.B.; Goodenough, J.B.; Yu, G.H. Sustainable Electrical Energy Storage through the Ferrocene/Ferrocenium Redox Reaction in Aprotic Electrolyte. Angew. Chem.-Int. Edit. 2014, 53, 11036–11040. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.; Plett, G.L.; Trimboli, M.S.; Oca, L.; Iraola, U.; Bekaert, E. Review of computational parameter estimation methods for electrochemical models. J. Energy Storage 2021, 44, 103388. [Google Scholar] [CrossRef]
- Rauf, H.; Khalid, M.; Arshad, N. A novel smart feature selection strategy of lithium-ion battery degradation modelling for electric vehicles based on modern machine learning algorithms. J. Energy Storage 2023, 68, 15. [Google Scholar] [CrossRef]
- Hou, J.; Qu, S.; Yang, M.; Zhang, J. Materials and electrode engineering of high capacity anodes in lithium ion batteries. J. Power Sources 2020, 450, 227697. [Google Scholar] [CrossRef]
- Xiong, R.; Cao, J.; Yu, Q.; He, H.; Sun, F. Critical Review on the Battery State of Charge Estimation Methods for Electric Vehicles. IEEE Access 2018, 6, 1832–1843. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Yang, F.H.; Yu, F.; Zhang, Z.A.; Zhang, K.; Lai, Y.Q.; Li, J. Bismuth Nanoparticles Embedded in Carbon Spheres as Anode Materials for Sodium/Lithium-Ion Batteries. Chem.-A Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.W.; Wang, A.N.; Li, L.; Qiu, T.Y.; Li, J.Y.; Jiang, Y.L.; Zou, G.Q.; Peng, H.J.; Hou, H.S.; Ji, X.B. Bi Dots Confined by Functional Carbon as High-Performance Anode for Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 10. [Google Scholar] [CrossRef]
- Zhong, Y.T.; Li, B.; Li, S.M.; Xu, S.Y.; Pan, Z.H.; Huang, Q.M.; Xing, L.D.; Wang, C.S.; Li, W.S. Bi Nanoparticles Anchored in N-Doped Porous Carbon as Anode of High Energy Density Lithium Ion Battery. Nano-Micro Lett. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; van Duin, A.C.T.; Koposov, A.Y. Toward Atomistic Understanding of Materials with the Conversion-Alloying Mechanism in Li-Ion Batteries. Chem. Mater. 2023, 35, 2835–2845. [Google Scholar] [CrossRef]
- Skurtveit, A.; Brennhagen, A.; Park, H.; Cavallo, C.; Koposov, A.Y. Benefits and Development Challenges for Conversion-Alloying Anode Materials in Na-Ion Batteries. Front. Energy Res. 2022, 10, 7. [Google Scholar] [CrossRef]
- Chen, K.T.; Chong, S.K.; Yuan, L.L.; Yang, Y.C.; Tuan, H.Y. Conversion-alloying dual mechanism anode: Nitrogen-doped carbon-coated Bi2Se3 wrapped with graphene for superior potassium-ion storage. Energy Storage Mater. 2021, 39, 239–249. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, M.; Khan, R.; Huang, L.; Wu, Y.; Zhou, Y. Strain engineering of Bi2Se3 anode for ultrafast sodium storage. Mater. Today Chem. 2023, 29, 101401. [Google Scholar] [CrossRef]
- Dang, Z.Z.; Meng, W.J.; Zuo, D.P.; Li, D.S.; Jiang, L.; Fang, D.N. Synthesis of pomegranate-like Bi2Se3@C composite for high volume specific capacity lithium storage. Electrochim. Acta 2022, 425, 9. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.W.; Yang, D.X.; Mao, Q.N.; Zhong, J.S.; Yuan, Y.J.; Li, X.Y.; Zheng, X.; Ji, Z.G.; Liu, H.; et al. Bi2Se3@C Rod-like Architecture with Outstanding Electrochemical Properties in Lithium/Potassium-Ion Batteries. ACS Appl. Energ. Mater. 2020, 3, 11073–11081. [Google Scholar] [CrossRef]
- Chong, S.K.; Yuan, L.L.; Qiao, S.Y.; Ma, M.; Li, T.; Huang, X.L.; Zhou, Q.W.; Wang, Y.K.; Huang, W. Chemical bonding in multiple encapsulation geometry of Bi2Se3-based conversion-alloying anode materials for superior sodium-ion storage. Sci. China-Mater. 2023, 66, 2641–2651. [Google Scholar] [CrossRef]
- Han, Y.X.; Jiu, H.; Zhang, L.X.; Wang, C.D.; Yue, L.C.; Wang, C.L.; Guo, Z.X.; Che, S.C.; Ma, J.F.; Li, H. Facile synthesis of Bi2Se3/nitrogen-doped carbon dot nanoplates for aqueous zinc ion battery cathodes. Phys. Chem. Chem. Phys. 2023, 25, 21350–21357. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Chen, G.; Jin, R.C.; Pei, J.; Wang, Y.; Chen, D.H. Hierarchical Bi2Se3 microrods: Microwave-assisted synthesis, growth mechanism and their related properties. Crystengcomm 2013, 15, 1618–1625. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.J.; Shen, J.W.; Li, X.; Chen, Z.X.; Cao, S.A.; Li, T.; Xu, F. Facile synthesis and electrochemical Mg-storage performance of Sb2Se3 nanowires and Bi2Se3 nanosheets. Dalton Trans. 2019, 48, 17516–17523. [Google Scholar] [CrossRef]
- Han, G.; Chen, Z.G.; Ye, D.L.; Yang, L.; Wang, L.Z.; Drennan, J.; Zou, J. In-doped Bi2Se3 hierarchical nanostructures as anode materials for Li-ion batteries. J. Mater. Chem. A 2014, 2, 7109–7116. [Google Scholar] [CrossRef]
- Mao, F.X.; Guo, J.; Zhang, S.H.; Yang, F.; Sun, Q.; Ma, J.M.; Li, Z. Solvothermal synthesis and electrochemical properties of S-doped Bi2Se3 hierarchical microstructure assembled by stacked nanosheets. RSC Adv. 2016, 6, 38228–38232. [Google Scholar] [CrossRef]
- Chen, J.; Vacchio, M.J.; Wang, S.; Chernova, N.; Zavalij, P.Y.; Whittingham, M.S. The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ion. 2008, 178, 1676–1693. [Google Scholar] [CrossRef]
- Hayner, C.M.; Zhao, X.; Kung, H.H. Materials for Rechargeable Lithium-Ion Batteries. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Ed.; Annual Reviews: Palo Alto, CA, USA, 2012; Volume 3, pp. 445–471. [Google Scholar]
- Jheng, S.L.; Chen, J.Y.; Tuan, H.Y. Solution-liquid-solid growth of CuInTe2 nanowires as lithium-ion battery anodes. Mater. Des. 2018, 149, 113–121. [Google Scholar] [CrossRef]
- Guo, X.; Jia, X.P.; Jie, K.K.; Sun, H.R.; Zhang, Y.W.; Sun, B.; Ma, H.G. Thermoelectric transport properties and crystal growth of BiSbTe3 bulk materials produced by a unique high-pressure synthesis. Crystengcomm 2013, 15, 7236–7242. [Google Scholar] [CrossRef]
- Teng, Y.Q.; Zhao, H.L.; Zhang, Z.J.; Zhao, L.N.; Zhang, Y.; Li, Z.L.; Xia, Q.; Du, Z.H.; Swierczek, K. MoS2 nanosheets vertically grown on reduced graphene oxide via oxygen bonds with carbon coating as ultrafast sodium ion batteries anodes. Carbon 2017, 119, 91–100. [Google Scholar] [CrossRef]
- Wen, S.Y.; Zhao, J.C.; Zhu, Y.Q.; Mao, J.F.; Wang, H.X.; Xu, J.L. Carbon-encapsulated Bi2Te3 derived from metal-organic framework as anode for highly durable lithium and sodium storage. J. Alloys Compd. 2020, 837, 155536. [Google Scholar] [CrossRef]
- Chen, X.J.; Hong, Y.; Ge, X.L.; Li, C.X.; Miao, X.G.; Wang, P.; Zhang, Z.W.; Yin, L.W. Se-doped Bi2S3 nanoneedles grown on the three-dimensional carbon foam as a self-supported anode for high-performance sodium ion batteries. J. Alloys Compd. 2020, 825, 153901. [Google Scholar] [CrossRef]
- Yi, Z.; Qian, Y.; Jiang, S.; Li, Y.; Lin, N.; Qian, Y.T. Self-wrinkled graphene as a mechanical buffer: A rational design to boost the K-ion storage performance of Sb2Se3 nanoparticles. Chem. Eng. J. 2020, 379, 122352. [Google Scholar] [CrossRef]
- Xu, Z.P.; Huang, Y.; Chen, C.; Ding, L.; Zhu, Y.D.; Zhang, Z.; Guang, Z.X. MOF-derived hollow Co(Ni)Se-2/N-doped carbon composite material for preparation of sodium ion battery anode. Ceram. Int. 2020, 46, 4532–4542. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.S.; Chen, X.H.; Song, H.H. Graphene-Loaded Bi2Se3: A Conversion-Alloying-type Anode Material for Ultrafast Gravimetric and Volumetric Na Storage. Acs Appl. Mater. Interfaces 2018, 10, 30379–30387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Zhao, J.C.; Li, L.J.; Xu, J.L.; Zhao, X.X.; Mi, Y.M.; Jin, J. Multi-core yolk-shell-structured Bi2Se3@C nanocomposite as an anode for high-performance lithium-ion batteries. Dalton Trans. 2021, 50, 10758–10764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Yang, Q.L.; Zhou, F.; Wu, J.; Wang, J.; Li, Y.J. SnSe coupled with nitrogen/sulfur dual-doped rGO for superior anode of lithium ion batteries. Ionics 2021, 27, 3801–3809. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Wang, S.W.; Du, J.; Jin, Q.; Zhang, T.R.; Cheng, F.Y.; Chen, J. Ultrasmall Sn Nanoparticles Embedded in Nitrogen-Doped Porous Carbon As High-Performance Anode for Lithium-Ion Batteries. Nano Lett. 2014, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wu, Y.; Zhao, J.; Zeng, X.; Mao, J.; Chen, J. Te-Doped Bi2Se3@NC Nanocomposites for High-Performance Li-Ion Battery Anodes. Sustainability 2023, 15, 16210. https://doi.org/10.3390/su152316210
Zhu Y, Wu Y, Zhao J, Zeng X, Mao J, Chen J. Te-Doped Bi2Se3@NC Nanocomposites for High-Performance Li-Ion Battery Anodes. Sustainability. 2023; 15(23):16210. https://doi.org/10.3390/su152316210
Chicago/Turabian StyleZhu, Yaqin, Yan Wu, Jiachang Zhao, Xiaohui Zeng, Jianfeng Mao, and Jiajun Chen. 2023. "Te-Doped Bi2Se3@NC Nanocomposites for High-Performance Li-Ion Battery Anodes" Sustainability 15, no. 23: 16210. https://doi.org/10.3390/su152316210
APA StyleZhu, Y., Wu, Y., Zhao, J., Zeng, X., Mao, J., & Chen, J. (2023). Te-Doped Bi2Se3@NC Nanocomposites for High-Performance Li-Ion Battery Anodes. Sustainability, 15(23), 16210. https://doi.org/10.3390/su152316210





