Abstract
The transformation of forests into degraded pastures in the Amazon region has caused alterations in the soil components. Likewise, the use of organic fertilizers as an alternative to enhance soil quality and plant biomass accumulation have been poorly studied. The objective of this study was to evaluate the effects of organic fertilization on biomass production using three specific forage grasses (Urochloa decumbens, Urochloa humidicola, and Urochloa brizantha) aged 3 years in a hilly landscape. For each crop, an area of 5005 m² was delimited with a randomized complete block design consisting of four treatments and three replications. Biomass production of Urochloa spp. and the physical and biological soil properties were assessed under the influence of different fertilization treatments. The results revealed significant differences (p < 0.0001) in the biomass production of Urochloa spp., with 1920.94 ± 155.44 kg of dry matter per hectare (kg DM ha−1) of forage at the end of the study, compared to 992.19 ± 97.66 kg DM ha−1 of forage at the beginning of the organic fertilizations. Overall, the application of organic fertilizers had a significant and positive effect on Urochloa spp. forage biomass and on the physical and biological properties of soils that had historically been affected by extensive livestock farming in a deforested hill landscape in the Colombian Amazon region.
1. Introduction
Livestock farming in the department of Caqueta, Colombia, is currently consolidated with 2,175,065 heads of cattle distributed across 20,512 farms [1]. This livestock system is composed of naturalized pastures and introduced Urochloa genus grasses, resulting in a total pasture area of 3,761,400 ha [2]. The deforestation and conversion of Amazonian forests, along with high cattle density, have had adverse effects on soil quality and transformed grazing areas into degraded pastures [3].
Livestock farming in the humid tropical region relies on grazing in soils characterized by acidic pH levels ranging from 4.4 to 4.8 [4]. Since pastures are the primary source of cattle feed, they require a replenishment of nutrients in the soil. However, high grazing intensities can hinder this process [5].
Pasture fertilization plays a fundamental role in cattle farming since grass is considered the most cost-effective feed source for meat and milk production [6,7]. It has been suggested as a viable option to enhance pasture productivity and quality, recognizing that productivity hinges on water availability and the presence of essential nutrients in the soil [8].
In this context, concerning sustainability and the environmental impact of livestock production [9], organic fertilization using solid and liquid organic residues should enhance not only Urochloa spp. biomass production but also the physical and biological properties of the soil. These changes are induced by two primary mechanisms: (i) the increased nutrient input to the soil assimilated by the pastures at the end of the forage production cycle and (ii) the increased soil macrofauna density and richness, coupled with reduced soil compaction. These organic fertilizers supply a significant portion of essential plant nutrients and have a positive impact on crop productivity [10] and also could influence the physical, chemical, and biological properties of the soil [11].
Therefore, this study aimed to assess the changes in forage biomass, soil macroinvertebrate diversity, and soil physical properties induced by the application of organic fertilizers on Urochloa spp.
2. Materials and Methods
2.1. Study Area and Selection of Experimental Area
This study was carried out at La Esperanza farm, in the Las Acacias rural settlement, located 10 km from the road connecting the Municipality of Morelia (1°29′09″ north and 75°43′28″ west, and an altitude of 258 m above sea level) and the Municipality of Valparaíso (1°11′36″ north and 75°42′23″ west, and an altitude of 211 m above sea level) in the Department of Caquetá (northwestern Colombian Amazon). This site is in the Tropical Humid Forest (THF) ecosystem [12]. This zone has an average annual rainfall of 3759 mm yr−1, an average annual temperature of 25.8°C, and a relative humidity of 81%. These climatic conditions categorize it as climate type A: Tropical (Tropical Rainforest—Equatorial—Af) according to Köppen climate classification for Colombia [13].
Three paddocks and three cover crops (Urochloa decumbens, Urochloa humidicola, and Urochloa brizantha) 5 years of age were individually selected within hill landscape under traditional pasture management. For each of the cover crops, a designated area of 5005 m2 was delimited for the implementation of a randomized complete block design with four fertilization treatments and three replications. Each sampling unit covered an area of 300 m2, with a one-meter gap between plots to minimize edge effects.
2.2. Organic Fertilizers
Liquid and solid organic fertilizers derived from cattle urine and manure were used in this study. The solid fertilizer employed was Bokashi Biofertilizer (BB), which is produced by drying cattle manure, forest soil, rice husks, rice bran, and charcoal and moistening it with mixture of yeast and molasses dissolved in water. It is then fermented aerobically for a period of 14 days [14].
Three liquid fertilizers were used. (i) Simple Biofertilizer (SB) is a liquid fertilizer crafted from fresh bovine manure dissolved in water, combined with milk, molasses, and wood ash. It had an anaerobic fermentation in a plastic container for 20 days [15]. (ii) Super Lean Biofertilizer (SLB) is a liquid fertilizer prepared from fresh bovine manure dissolved in water, blended with milk and molasses, and enriched with mineral salts such as Sulfates (Zn, Mg, Mn, Cu, and Fe), phosphate rock, sodium molybdate, borax, cobalt chloride, and wood ash. It was left to ferment for 50 days in plastic tanks under an anaerobic system [15]. (iii) Cow Urine Biofertilizer (CUB) is a liquid fertilizer derived from cow urine and fermented anaerobically in 2 L plastic bottles for five days. The bottles were uncorked every morning to release the gas produced during fermentation. The chemical characteristics of these organic fertilizers are detailed in Table 1.

Table 1.
Chemical characteristics of organic fertilizers.
2.3. Treatments
Four treatments were established as follows: (T1) a control treatment (TGO) represented the farmer’s traditional management with no fertilization applied. The other treatments involved combinations of organic fertilizers (both solid and liquid): (T2) Bokashi Biofertilizer + Simple Biofertilizer (BB + SB), (T3) Bokashi Biofertilizer + Cow Urine Biofertilizer (BB + CUB), and (T4) Bokashi Biofertilizer + Super Lean Biofertilizer (BB + SLB).
A uniform cut was performed in the experimental units to simulate cattle grazing before initiating the treatments. BB was applied to the soil in all three organic treatments two months later, and liquid fertilizers were sprayed on the grass at the beginning and repeated every 30 days for four months.
2.4. Evaluation of Biomass Production and the Soil’s Biological and Physical Properties
To measure biomass production in each cover crop, at each treatment, random points were selected where a 50 × 50 cm plastic frame was placed. We used the botanal technique proposed by Tothill et al. [14]. At each point, forage was harvested from a 0.25 m2 area, cut at a height of 10 cm, and weighed in the field. The harvested forage was packed in labeled paper bags and transported to the “Macagual” Research Center’s laboratory for forage evaluation. In the laboratory, 200 g of subsamples were weighed and dried at 70 °C until a constant weight was achieved.
For the biological soil characterization, soil macrofauna was evaluated in two stages: (i) Initial Moment (IM) and (ii) Final Moment (FM) for each cover crop among the sampling units per treatment. For ISO, a 23611-5 standard [16] was followed for macrofauna collection. A soil monolith measuring 25 × 25 cm at a depth of 30 cm was extracted using a metal angle frame of the same dimensions as the monolith. Samples collected were preserved in plastic bottles with 97% alcohol for morphological description and taxonomic classification.
For the physical properties of the soil, bulk density was determined using the known volume method [17]. A 50 × 50 × 50 cm test pit was dug in each cover crop at depths of 0–10, 10–20, and 20–30 cm. The soil penetration resistance was evaluated using a manual pressure penetrometer model 0601 (Eijkelkamp Agrisearch Equipment, Giesbeek, The Netherlands). Two sets of measurements were taken, the first at the beginning of the experiment (day 0, initial time) and the second at the end of the study (day 120, final time).
2.5. Statistical Analysis
Three datasets were organized: (i) soil macrofauna, (ii) forage biomass, and (iii) physical properties. To assess the effects of treatment and time, as well as their interaction on forage biomass, a Linear Mixed-Effects (LME) model was fitted. The forage species, nested blocks within the forage species, and the plots associated with treatment within the blocks were included as random effects. LME models were adjusted to analyze the fixed effects: treatment, time, soil depth, and their interactions on physical properties, where the forage species, blocks, plots, and sub-plots (i.e., soil depth within the treatment within each block) were included as random effects.
The normality and variance homogeneity assumptions were evaluated by exploratory analyses of model residuals. Data transformation was not necessary. Macrofauna density was analyzed using Generalized Linear Mixed-Effects (GLME) models with a negative binomial distribution and Poisson for taxonomic categories (richness). In both cases, the log link function was utilized along with deviance as a goodness-of-fit measure [18]. Mean separation was performed with Fisher’s LSD test based on the inverse link function (PredLin) at a 5% significance level. The LME and GLME models were fitted with the nlme [19] and lme4 packages [20] in R v.4.0.3 language [21] using the InfoStat v.2020 interface [22], respectively.
For analyzing relationships between variables, we conducted a Pearson correlation analysis. Visualization of the Pearson correlation matrix was performed with the package circlize [23] in R. Additionally, Principal Component Analysis (PCA) was performed, along with a Monte-Carlo test (999 permutations), to assess the significance (α = 0.05) of the relationships between all variables and fixed effects. The PCA and Monte-Carlo test were carried out using the ade4 [24] and factoextra [25] packages in R.
3. Results
3.1. Biomass Production
In the LME models, when comparing the moments of fertilization, significant differences in biomass production were observed (p < 0.0001). It was found that the combined averages of the three Urochloa species yielded 1920.94 kg DM ha−1 of forage at the end of the experiment, compared to 992.19 kg DM ha−1 of forage at the beginning of the organic fertilizations (Figure 1).
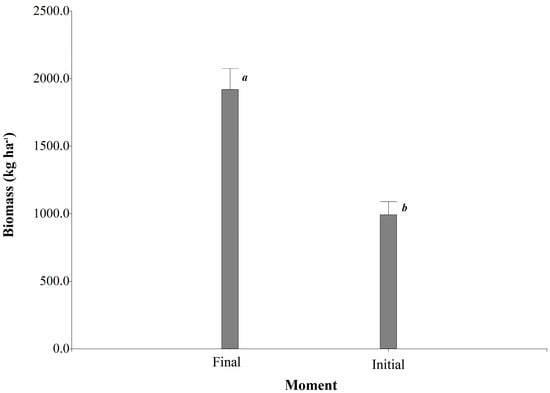
Figure 1.
Impact of organic fertilization on the biomass production of Urochloa spp. at two different evaluation times. Values followed by the same letter do not differ statistically (Fisher’s LSD test, p < 0.05).
3.2. Soil Biological Properties Changes
When comparing the two evaluation times, significant differences were observed for Coleoptera (p < 0.0187), which had the highest number of individuals at the initial moment (IM) of the experiment compared to the final moment (FM), with average counts of 5.33 ± 2.42 and 2.37 ± 2.42, respectively. Similarly, Isoptera showed statistical differences (p < 0.0158), with a higher number of individuals at the FM of the experiment than at the IM, averaging 2.67 ± 1.27 and 0.15 ± 1.27, respectively (Table 2).

Table 2.
Density of soil macrofauna (individuals m−2) at two distinct evaluation times (moments) and across three soil depths under the influence of organic fertilization in Urochloa spp. cover crops.
The model showed significant differences (p < 0.05) for four invertebrate taxa (Coleoptera, Haplotaxida, Hymenoptera, and Isoptera).
Hymenoptera (ants) had the highest number of individuals in the 0–10 cm depth, followed by 10–20 and 20–30 cm depths, with average counts of 312.67, 194.89, and 88.22 individuals. Haplotaxida (earthworm) showed a high number of individuals in the 0–10 cm depth, followed by 10–20 and 20–30 depths, with average counts of 93.33, 9.33, and 0.44 individuals, respectively. (Table 2).
The total density per m2 was 51,808 individuals, and a total of 12 invertebrate groups were identified, defining the richness in this study (Table 3). The GLME models showed significant difference (p < 0.05) in the interaction between treatments and time for total density (p < 0.0300) and richness (p < 0.0300). It was observed that the combined averages for total density at T4 for FM was 539.26 individuals, whereas at IM, it was 169.48 individuals. Richness showed that the averages were higher at T3 and T4 for FM, with 1.30 and 1.26 ± 0.16 invertebrate taxa, respectively, compared to IM, where it was 0.93 ± 0.16 and 0.81 ± 0.16 invertebrate taxa, respectively (Table 3).

Table 3.
Total density (individuals m−2) and richness of soil macrofauna in the interaction between treatment and evaluation time, as well as soil depth, under Urochloa spp. cover crops.
Statistical analysis also revealed significant differences (p < 0.05) for depth in total density (p < 0.0001) and richness (p < 0.0001). The total density was higher at the 0–10 cm with average counts of 4.62 ± 0.48. The richness of invertebrate taxa was also higher at 0–10 cm, with average counts of 1.45 ± 0.04 (Table 3).
3.3. Changes in Soil’s Physical Properties
The LME models showed significant differences for depth, evaluation time (p < 0.0001), and the interaction between treatment and depth (p < 0.0187). Across the three depths assessed, the model indicated that bulk density was lower in the 0–10 cm depth compared to the 10–20 cm and 20–30 cm depths, with average values of 1.51 ± 0.14 g cm−3, 1.59 ± 0.14 g cm−3, and 1.62 ± 0.14 g cm−3, respectively.
Additionally, it was observed that the average bulk density was lower at the FM compared to the IM, with average values of 1.39 ± 0.14 g cm−3 and 1.76 ± 0.14 g cm−3, respectively (Figure 2). Table 4 shows the average bulk density (g cm−3) in the interaction between treatment and depth, highlighting that treatments T4 and T1 at a depth of 0–10 cm had the lowest results, with values of 1.51 ± 0.14 g cm−3 and 1.45 ± 0.14 g cm−3, respectively.
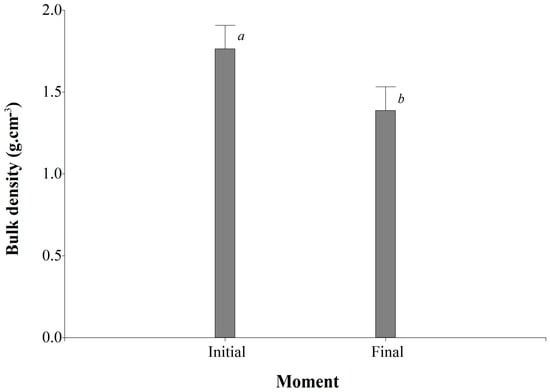
Figure 2.
Influence of organic fertilization on soil bulk density in Urochloa spp. Cover crops at two different evaluation times. Values followed by the same letter do not differ statistically (Fisher’s LSD test, p < 0.05).

Table 4.
Soil bulk density in the interaction between treatment and soil depth under Urochloa spp. Cover crops.
For the soil penetration resistance, the LME model revealed significant differences in depth (p < 0.0001) and also in the interaction between depth and evaluation time (p < 0.0466). The results indicated that at the 0–10 cm depth, soil penetration resistance was lower compared to the 10–20 cm and 20–30 cm depths, with average values of 111.17 ± 2.95 kPa cm−2, 123.63 ± 2.95 kPa cm−2, and 127.01 ± 2.95 kPa cm−2, respectively. Furthermore, the combined averages for penetration resistance at the 0–10 cm depth during the FM were lower, with an average of 107.50 ± 3.85 kPa cm−2, while at the IM, it exhibited an average of 114.84 ± 3.85 kPa cm−2 (Figure 3).
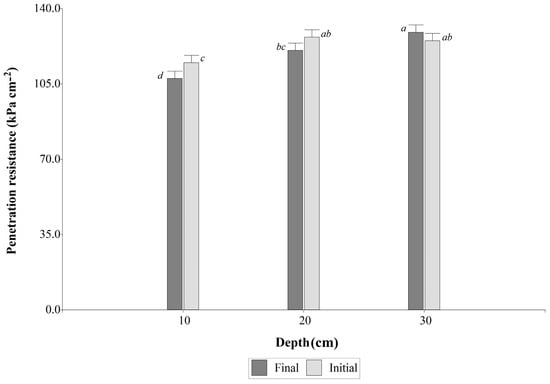
Figure 3.
Soil penetration resistance in the interaction between evaluation time and soil depth under Urochloa spp. cover crops. Values followed by the same letter do not differ statistically (Fisher’s LSD test, p < 0.05).
3.4. Multivariate Analysis of Relationships between Studied Variables
A total of 24.2% of the correlations were statistically significant (p < 0.05), with a r > 0.2. Of these, 87.5% were positive correlations, while the remaining 12.5% were negative correlations (Figure 4). The highest positive correlations (r > 0.5) were observed between total density and Hymenoptera, Orthoptera, and Spirobolida, and richness with Coleoptera and total density. As for the negative correlations, the most relevant (r < −0.2) were observed between biomass and penetration resistance, as well as bulk density and other taxonomic groups.
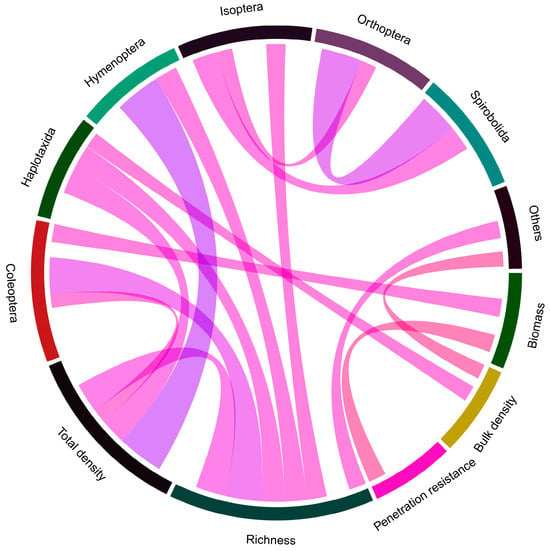
Figure 4.
Chord diagram of Pearson’s correlation coefficients for significant relationships of studied variables (p < 0.05). Colors and width of ribbons represent direction and strength of correlation. Blue ribbons indicate positive coefficients, and red ribbons indicate negative coefficients.
Principal component analysis (PCA) was performed using parameter treatment and evaluation time, along with invertebrate taxa, and correlated with bulk density, forage biomass, and penetration resistance. The first two components explained 42.4% of the total variability (Figure 5). The first principal component, accounting for 23.3% of the variability, separated taxonomic groups (Isoptera and Hymenoptera) with the highest richness and biomass production associated with T2 and T4 during the FM.
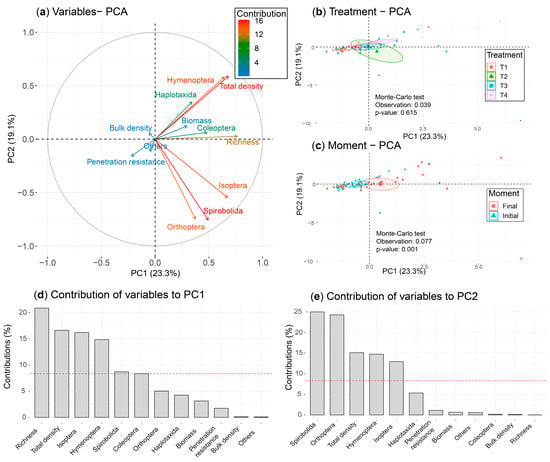
Figure 5.
Projection of the variables (soil macrofauna, biomass, and physical properties) and observations of the PC1/PC2 ordination plane of the PCA, grouped according to treatment and time of evaluation. (a) Correlation circle of the variables; (b,c) observations grouped according to treatment and time of evaluation, respectively; (d,e) contribution of the variables to the formation of PC1/PC2 of the PCA, respectively.
The second component explained 19.1% of the variability, and separated the Orthoptera and Spirobolida groups linked to T2 and T4. Taxonomic groups Orthoptera, Spirobolida, and Isoptera were negatively correlated with soil bulk density. The Monte Carlo test indicated a significant difference in the three groups of variables studied (soil macrofauna, forage biomass, and soil physical properties) between the two evaluation moments (p < 0.01; 7.7%), but not between the four treatments (p > 0.05; 3.9%).
The PCA of physical properties explained 47.3% of the total variability, with the first two axes distinguishing between different soil depths (p < 0.01; 8.8%) (Figure 6). PC1 (27.4%) separated the 20–30 cm depth, characterized by higher bulk density and penetration resistance, from the shallower soil depths (0–10 and 10–20 cm), which had greater richness and density of soil macrofauna. Taxonomic groups such as Coleoptera, Isoptera, and Haplotaxida were predominantly found in the shallower soil layers. PC2 (19.9%) separated groups such as Spirobolida and Hymenoptera, mainly concentrated in the 0–10 cm depth.
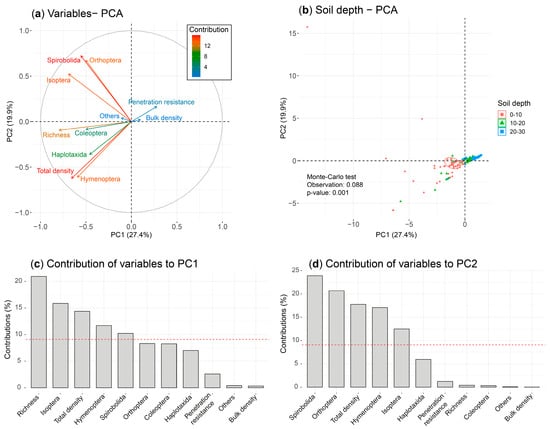
Figure 6.
Projection of the variables (soil macrofauna and physical properties) and observations of the PC1/PC2 ordination plane of the PCA, grouped according to soil depth. (a) Correlation circle of the variables; (b) observations grouped according to soil depth; and (c,d) contribution of the variables to the formation of PC1/PC2 of the PCA, respectively.
4. Discussion
The biomass production of Urochloa showed a positive response to the application of organic fertilizers, and this finding could be linked to other studies on various grasses subjected to organic fertilizers [26,27]. This effect is attributed to factors such as the quality and rate of organic fertilizer application, rainfall patterns, and organic matter decomposition patterns [28]. Moreover, organic fertilizers have a favorable impact on forage quality and the nutritional composition of grasses. They increase protein percentages and other essential nutrients for cattle (e.g., Crude Protein PC and Dry Matter DM), thereby directly influencing meat and milk production in livestock systems [29].
The application of organic fertilizers also contributes to the improvement of soil properties [30,31] by increasing the nutrient composition of the soil through processes such as nitrogen fixation and mineral ion solubilization. This stimulates plant growth through the synthesis of substandard growth promoters [32]. The genus Urochloa reveals positive characteristics related to soil quality and health in agroecosystems. These include higher nutrient use efficiency, reduced erosion risk, enhanced soil structure, increased organic matter levels, and greater biological activity [33]. These characteristics are clearly demonstrated through the application of organic fertilizers in our study.
The high biomass production and elevated nutrient concentrations in Urochloa plant shoots [34] enhance livestock performance and health. Efficient nutrient utilization in agriculture is crucial for system sustainability and is closely linked to nutrient cycling, which can be optimized through the adoption of management practices aimed at maintaining soil fertility, biodiversity, increasing soil carbon sequestration, and minimizing adverse climatic effects [35]. The effects of organic fertilizers on plant production align with the principles of sustainable agriculture, which seek to meet economic, social, and environmental standards [36].
The viability of an agroecosystem hinges on the synergy among its components, primarily the soil biodiversity, which governs ecological processes related to biotic regulation, nutrient recycling, and productivity [37]. Within this framework, the utilization of organic fertilizers in pastures serves to fortify and enhance both the macrofauna and microbial populations responsible for decomposing organic matter. This decomposition process releases nutrients into the soil, subsequently benefiting plant growth [38].
By identifying taxonomic groups with a total density of 5808 individuals, we can establish a connection with Noguera et al. [39], who reported 5296 individuals. It is important to mention that, after fertilizer application, the population increased to 9856 individuals in our experiment.
According to Lopez [40], the identified taxa are differentiated based on their ecosystem functions. For instance, Haplotaxide organisms play a role in soil transformation, regulating soil dynamics and structure [37]. Others, such as Hymenoptera and Isoptera, contribute to pore formation, while Coleoptera, Blattodea, Spirobolida, and Orthoptera participate in plant residue shredding and the transformation of organic matter. They use their specialized mouthparts to shred leaves [41,42]. These organisms also act as epigean species, aiding in the decomposition process by consuming leaf litter, which increases the surface area for microbial attack [43].
This observed increase in soil macrofauna can be attributed to the contributions of organic fertilizers. These fertilizers help retain water and nutrients, thus promoting plant health and resilience during dry periods. Want et al. [44] suggested that changes in the quality and quantity of soil organic matter and immediate chemical properties following fertilizer application can lead to an increase in soil microarthropod abundance. However, they may have limited influence on microarthropod diversity in the alkaline coastal soils of eastern China.
Thus, soil macrofauna is considered an indicator of soil condition, serving both to assess the impact of human activities on the soil and to describe ecosystem functioning [45]. Consequently, organic fertilization emerges as an effective and sustainable alternative for maintaining and enhancing the presence of this vital biological component in pasture-covered agricultural systems, as opposed to conventional chemical fertilizers [43].
The soil physical variables assessed, including bulk density and penetration resistance, had lower values at a depth of 0 to 10 cm when compared to the other depths. This effect is attributed to the application of organic fertilizers and the subsequent accumulation of organic matter on the soil surface. This accumulation significantly increases the population of soil macrofauna and taxonomic richness in Urochloa spp. pastures.
The functional group with the highest population representation was soil engineers, followed by herbivores and detritivores. According to Lavelle et al. [43], soil engineers comprise groups that consume organic matter, such as earthworms and termites, as well as omnivorous organisms, such as ants, which have a specific impact on the soil’s physical properties.
The application of organic fertilizers induces transformations in the physical properties of the soil, leading to improvements in soil aggregation, reduced compaction, and prevention of crusting [46]. It also enhances water retention and transmission characteristics on the proportion of macroaggregates [47].
This positive effect is consistent with findings that show an increase in the proportion of macroaggregates and a rise in the percentage of aggregates within different size ranges [48]. These changes benefit physical properties and subsequently improve crop yields. Thus, the use of organic fertilizers offers a sustainable and effective approach to enhancing soil health and crop productivity [49].
The physical improvement process of the soil resulting from the applied treatments results in porous soils with excellent aeration, good drainage, and enhanced root penetration. This outcome aligns with the findings of Ramírez et al. [50], who observed lower bulk density than 2.30 g cm−3, indicating improved soil quality attributed to the effectiveness of organic fertilizers. Bulk density is commonly used as a measure of soil structure, indicative of increased porosity, aggregate stability, reduced compaction, and higher moisture content [51].
In this regard, García-Ruiz and Lana-Renault [52] found that organic fertilizers lead to an increase in organic carbon, enhance soil physical quality, and result in lower bulk density, distinguishing them from traditional fertilizers.
In this context, organic fertilizers bolster soil structure by promoting biological activity within the soil, consequently facilitating the formation of aggregates. Simultaneously, they enrich the soil with humus, thereby enhancing its water retention capacity, as observed by González-Salas et al. [53]. Furthermore, this aeration effect improves air circulation, activating the microorganisms residing in the soil, which are essential for plant growth and overall health. These combined benefits contribute positively to plant growth by aiding in nutrient retention and reducing the leaching process [54]
5. Conclusions
The organic fertilizers improved forage production at the plant level and influenced the physical and biological properties of the soil. This was reflected in the increased biomass of Urochloa spp. forage, an increase in the richness and density of soil macrofauna, and a reduction in soil compaction.
Our study offers valuable insights into the utilization of on-farm organic resources within the Amazon region. It sheds light on their potential for pasture restoration in livestock systems within the productive landscapes with high levels of deforestation, such as the Caquetá department, promoting the sustainability of agroecosystems and the conservation of natural resources.
It is necessary to begin using organic fertilizers, as they are cost-effective alternatives that make use of the residues generated in cattle production. Additionally, they are effective for plant growth and nutrition and for improving soil quality.
Author Contributions
Conceptualization, F.A. and P.R.; methodology, F.A. and P.R.; software, F.A. and A.S.; validation, F.A. and A.S.; formal analysis, A.S.; investigation, F.A. and P.R.; resources, F.A.; data curation, F.A., P.R., and A.S.; writing—original draft preparation, F.A., P.R., and A.S.; writing—review and editing, F.A., P.R., and A.S.; visualization, F.A. and A.S.; supervision, F.A. and A.S.; project administration, F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Vicerrectoría de Investigaciones y Posgrados—Universidad de la Amazonia, Florencia, Caquetá (Colombia).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
The authors thank the Universidad de la Amazonia, which made this research possible.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Torrijos, R. Cifras de Contexto Ganadero Caquetá 2022 by Rafael Torrijos—Issuu. Available online: https://issuu.com/rafaeltorrijos/docs/contexto_2022_imp (accessed on 31 May 2023).
- Armenteras, D.; Murcia, U.; González, T.M.; Barón, O.J.; Arias, J.E. Scenarios of Land Use and Land Cover Change for NW Amazonia: Impact on Forest Intactness. Glob. Ecol. Conserv. 2019, 17, e00567. [Google Scholar] [CrossRef]
- Hernández, Á.S. Agricultural Law and Development of Rural Areas: Food Challenges, Natural Resources and Climate Change; Wydawnictwo NAUKOWE UAM: Poznan, Poland, 2017. [Google Scholar]
- Jimenez, O.; Granados, L.; Oliva, J.; Quiroz, J.; Barrón, M. Calidad Nutritiva de Brachiaria Humidicola Con Fertilización Orgánica e Inorgánica En Suelos Ácidos. Available online: https://scielo.isciii.es/scielo.php?pid=S0004-05922010000400009&script=sci_arttext&tlng=en (accessed on 28 May 2023).
- Rodríguez-León, C.H.; Peña-Venegas, C.P.; Sterling, A.; Castro, D.; Mahecha-Virguez, L.K.; Virguez-Díaz, Y.R.; Silva-Olaya, A.M. Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy 2021, 11, 2484. [Google Scholar] [CrossRef]
- Finch, S.; Samuel, A.; Lane, G.P. Lockhart and Wiseman’s Crop Husbandry Including Grassland; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Borges, J.A.; Barrios, M.; Escalona, O. Efecto de La Fertilización Orgánica e Inorgánica Sobre Variables Agroproductivas y Composición Química del Pasto Estrella (Cynodon Nlemfuensis). Zootec. Trop. 2012, 30, 17–25. [Google Scholar]
- Gastal, F.; Durand, J.L. Effects of Nitrogen and Water Supply on N and C Fluxes and Partitioning in Defoliated Swards. In Grassland Ecophysiology and Grazing Ecology; CABI: Wallingford, UK, 2000; pp. 15–39. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Wiśniewska, E. Possibilities of Coffee Spent Ground Use as a Slow Action Organo-Ineral Fertilizer. Rocz. Ochr. Srodowiska 2015, 17, 422–437. [Google Scholar]
- Díaz-Gutiérrez, J.P.; Quila-Bonoso, K.M.; Zambrano-Gavilanes, F.; Bravo-Zamora, R. Efectos de la fertilización orgánica en el cultivo de algodón (Gossypium hirsutum). Biotempo 2022, 19, 291–301. [Google Scholar] [CrossRef]
- Barnwal, P.; Devika, S.; Singh, S.; Behera, T.; Chourasia, A.; Pramanick, B.; Meena, V.S.; Rakshit, A. Soil Fertility Management in Organic Farming. In Advances in Organic Farming Agronomic Soil Management Practices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–46. [Google Scholar] [CrossRef]
- Holdridge, L. Ecología Basada En Zonas de Vida; Agroamerica: Guatemala City, Guatemala, 1987. [Google Scholar]
- IDEAM—Ministerio de Ambiente. Vivienda y Desarrollo Territorial; IDEAM—Ministerio de Ambiente: Bogotá, Colombia, 2017. [Google Scholar]
- Tothill, J.; Hargreaves, J.; Jones, R.; McDonald, C. BOTANAL—A comprehensive sampling and computing procedure for estimating pasture yield and composition. 1. Field sampling. Trop. Agron. Tech. Memo. 1992, 78. [Google Scholar]
- Restrepo, J.; Hensel, J. Manual Práctico de Agricultura Orgánica y Panes de Piedra Contenido General. 2009; 436p. [Google Scholar]
- ISO 23611-5:2011(En); Soil Quality—Sampling of Soil Invertebrates—Part 5: Sampling and Extraction of Soil Macro-Invertebrates. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/obp/ui/#iso:std:iso:23611:-5:ed-1:v1:en (accessed on 1 June 2023).
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–382. [Google Scholar]
- Rodríguez, L.; Suárez Salazar, J.C.; Casanoves, F.; Ngo Bieng, M.A. Cacao Agroforestry Systems Improve Soil Fertility: Comparison of Soil Properties between Forest, Cacao Agroforestry Systems, and Pasture in the Colombian Amazon. Agric. Ecosyst. Environ. 2021, 314, 107349. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-131.1; The Comprehensive R Archive Network: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing v. 4.0.3; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat 2020; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circular Visualization [R Package Circlize Version 0.4.15]. Bioinformatics 2022, 30, 2811–2812. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B.; Thioulouse, J. Ade4: Analysis of Ecological Data: Exploratory and Euclidean Methods in Environmental Sciences; R Package Version 1.7-16; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Ryals, R.; Silver, W.L. Effects of Organic Matter Amendments on Net Primary Productivity and Greenhouse Gas Emissions in Annual Grasslands. Ecol. Appl. 2013, 23, 46–59. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Radovich, T.J.K.; Nguyen, H.V.; Uyeda, J.; Arakaki, A.; Cadby, J.; Paull, R.; Sugano, J.; Teves, G. Use of Organic Fertilizers to Enhance Soil Fertility, Plant Growth, and Yield in a Tropical Environment. In Organic Fertilizers—From Basic Concepts to Applied Outcomes; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Peinetti, H.R.; Menezes, R.S.C.; Tiessen, H.; Perez Marin, A.M. Simulating Plant Productivity under Different Organic Fertilization Practices in a Maize/Native Pasture Rotation System in Semi-Arid NE Brazil. Comput. Electron. Agric. 2008, 62, 204–222. [Google Scholar] [CrossRef]
- Štýbnarová, M.; Mičová, P.; Fiala, K.; Karabcová, H.; Látal, O.; Pozdíšek, J. Effect of Organic Fertilizers on Botanical Composition of Grassland, Herbage Yield and Quality. Agriculture 2014, 60, 87–97. [Google Scholar] [CrossRef][Green Version]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-Term Incorporation of Organic Manures and Biofertilizers Influences Biochemical and Microbial Characteristics of Soils under an Annual Crop [Turmeric (Curcuma Longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.B.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Role of Organic Fertilizers in Improving Soil Fertility. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Cham, Switzerland, 2020; pp. 61–77. [Google Scholar]
- Shaji, H.; Chandran, V.; Mathew, L. Chapter 13—Organic Fertilizers as a Route to Controlled Release of Nutrients; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–245. [Google Scholar]
- Rao, I.; Kerridge, P.C.; Macedo, M.C.; Miles, J.W.; Mass, B.l.; do Valle, C.B. Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., Valle, C.B., Eds.; CIAT: Cali, Colombia, 1996. [Google Scholar]
- Baptistella, J.L.C.; de Andrade, S.A.L.; Favarin, J.L.; Mazzafera, P. Urochloa in Tropical Agroecosystems. Front. Sustain. Food Syst. 2020, 4, 119. [Google Scholar] [CrossRef]
- Filser, J.; Faber, J.H.; Tiunov, A.V.; Brussaard, L.; Frouz, J.; De Deyn, G.; Uvarov, A.V.; Berg, M.P.; Lavelle, P.; Loreau, M.; et al. Soil Fauna: Key to New Carbon Models. Soil 2016, 2, 565–582. [Google Scholar] [CrossRef]
- Lal, R. Beyond Copenhagen: Mitigating Climate Change and Achieving Food Security through Soil Carbon Sequestration. Food Secur. 2010, 2, 169–177. [Google Scholar] [CrossRef]
- Gutierrez-Bermudez, C.; Mendieta, B.; Noguera, A. Trophic Composition of Edaphic Macrofauna in Animal Husbandry Systems in the Dry Corridor of Nicaragua. Pastos Forrajes 2020, 43, 30–37. [Google Scholar]
- Masin, C.E.; Cruz, M.S.; Rodríguez, A.R.; Demonte, M.J.; Vuizot, L.A.; Maitre, M.I.; Godoy, J.L.; Almada, M.S. Macrofauna Edáfica Asociada a Diferentes Ambientes de Un Vivero Forestal (Santa Fe, Argentina). Cienc. Suelo 2017, 35, 21–33. [Google Scholar]
- Noguera-Talavera, A.; Reyes-Sánchez, N.; Mendieta-Araica, B. Diversidad y Distribución de La Macrofauna Edáfica En Dos Sistemas de Manejo de Moringa Oleifera (Lam.): Relación Con Las Propiedades Del Suelo. Calera 2017, 17, 78–86. [Google Scholar] [CrossRef]
- López, G. Macrofauna y Microbiología Edáfica: Relación Con Servicios Ecosistémicos Y Físicoquímicos Del Suelo En Dos Con Café, San Ramón, Matagalpa, 2016. Ph.D. Thesis, Universidad Nacional Agraria, Lima, Peru, 2022. [Google Scholar]
- McGlynn, T.P.; Poirson, E.K. Ants Accelerate Litter Decomposition in a Costa Rican Lowland Tropical Rain Forest. J. Trop Ecol. 2012, 28, 437–443. [Google Scholar] [CrossRef]
- Hedde, M.; Blight, O.; Briones, M.J.I.; Bonfanti, J.; Brauman, A.; Brondani, M.; Calderón Sanou, I.; Clause, J.; Conti, E.; Cortet, J.; et al. A Common Framework for Developing Robust Soil Fauna Classifications. Geoderma 2022, 426, 116073. [Google Scholar] [CrossRef]
- Lavelle, P.; Mathieu, J.; Spain, A.; Brown, G.; Fragoso, C.; Lapied, E.; De Aquino, A.; Barois, I.; Barrios, E.; Barros, M.E.; et al. Soil Macroinvertebrate Communities: A World-Wide Assessment. Glob. Ecol. Biogeogr. 2022, 31, 1261–1276. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Fan, H.; Ruan, H.; Zheng, A. Responses of Soil Microarthropods to Inorganic and Organic Fertilizers in a Poplar Plantation in a Coastal Area of Eastern China. Appl. Soil Ecol. 2015, 89, 69–75. [Google Scholar] [CrossRef]
- Watson-Zink, V. Making the Grade: Physiological Adaptations to Terrestrial Environments in Decapod Crabs. Arthropod Struct. Dev. 2021, 64, 101089. [Google Scholar] [CrossRef] [PubMed]
- Hati, K.; Bandyoopadhay, K. Soil-Plant-Atmosphere Continuum. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Encyclopedia of Earth Sciences; Springer: Dordrecht, The Netherlands, 2011; pp. 805–810. [Google Scholar]
- Du, S.; Ma, Z.; Chen, J.; Xue, L.; Tang, C.; Shareef, T.M.E.; Siddique, K.H.M. Effects of Organic Fertilizer Proportion on the Distribution of Soil Aggregates and Their Associated Organic Carbon in a Field Mulched with Gravel. Sci. Rep. 2022, 12, 11513. [Google Scholar] [CrossRef] [PubMed]
- García, Y.; Ramírez, W.; Sánchez, S. Indicadores de La Calidad de Los Suelos: Una Nueva Manera de Evaluar Este Recurso. Pastos Forrajes 2012, 35, 125–138. [Google Scholar]
- Villanueva, C.; Ibrahim, M. Evaluacion_del_impacto. Agroforestería Américas 2019, 9, 35–36. [Google Scholar]
- Ramírez, J.; Fernandez, Y.; González, P. Influencia de La Fertilización En Las Propiedades Físico-Químicas de Un Suelo Dedicado a La Producción de Semilla de Megathyrsus Maximus. Pastos Forrajes 2015, 38, 393–402. [Google Scholar]
- Salazar-Calvo, C.; González-Venegas, J.P.; Corrales-Valverde, D.; Lacayo-Vega, J.; Carrillo-Montoya, K.; Montero-González, H. Comparación de Dos Metodos Para La Determinación de La Densidad Aparente Del Suelo. Alcances Tecnológicos 2020, 13, 5–12. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Lana, R. Hydrological and Erosive Consequences of Farmland Abandonment in Europe, with Special Reference to the Mediterranean Region—A Review. Agric. Ecosyst. Environ. 2011, 140, 317–338. [Google Scholar] [CrossRef]
- González-Salas, U.; Gallegos-Robles, M.Á.; Vázquez-Vazquez, C.; Luis García-Hernandez, J.; Fortis-Hernández, M.; Shesareli Mendoza-Retana, S. Productividad de Genotipos de Maíz Forrajero Bajo Fertilización Orgánica y Propiedades Físico-Químicas Del Suelo. Rev. Mex. Cienc. Agríc. Agrícolas 2018, 9, 4331–4341. [Google Scholar] [CrossRef][Green Version]
- del Montenegro, A.C.E.; Filella, J.B.; Valdivia, N.A.G. Estudio Comparativo Macrofauna Del Suelo En Sistema Agroforestal, Potrero Tradicional y Bosque Latifoliado En Microcuenca Del Trópico Seco, Tomabú, Nicaragua. Rev. Científica FAREM-Estelí 2017, 22, 39–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).