Synthesis and Characterization of MnWO4-CNT for Supercapacitor Applications
Abstract
:1. Introduction
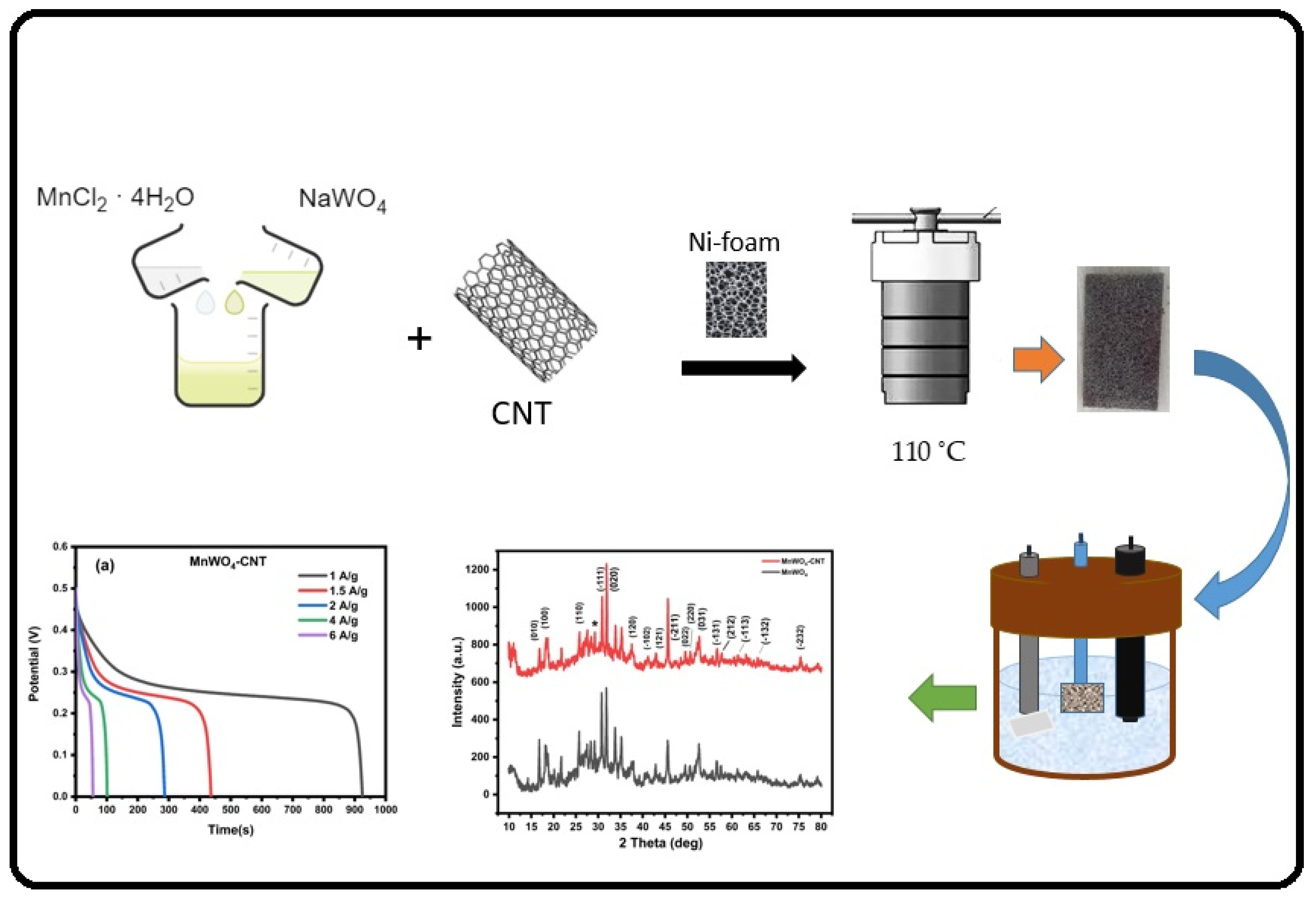
2. Materials and Methods
3. Results
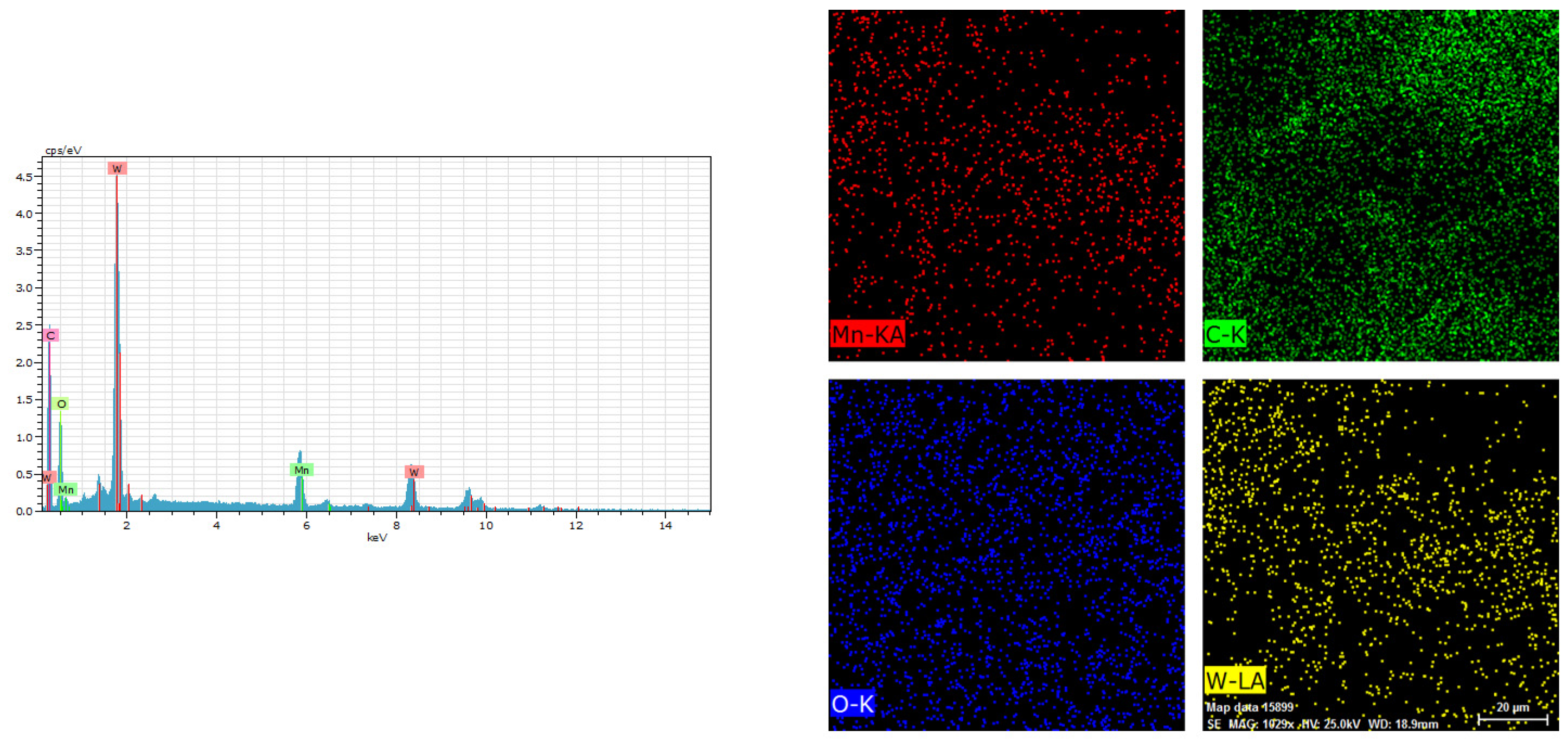
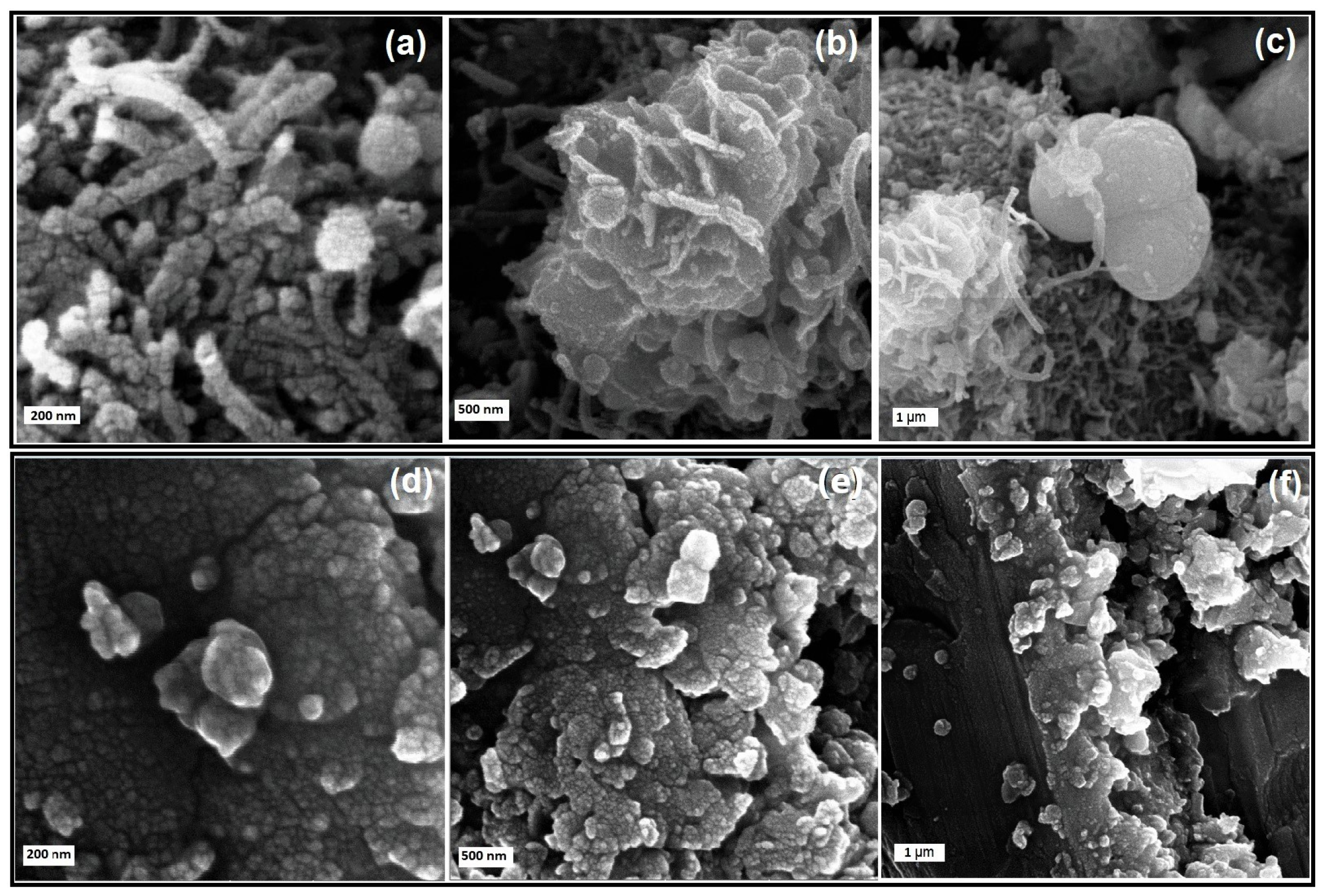
3.1. Physical Characterization
3.2. Electrochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Aftab, W.; Usman, A.; Shi, J.; Yuan, K.; Qin, M.; Zou, R. Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 2021, 14, 4268–4291. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; de Larramendi, I.R.; Goikolea, E. Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes. Batteries 2022, 8, 263. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and electrochemical performances of TiO2-coated Mg–Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef]
- Moghadam, M.T.T.; Seifi, M. Fabrication and investigation of ZnO-CNT@ Fe3O4/NF as supercapacitor electrode by using a novel preparation method of CNT. Diam. Relat. Mater. 2022, 125, 108962. [Google Scholar] [CrossRef]
- Shulga, R.N.; Putilova, I.V. Multi-agent direct current systems using renewable energy sources and hydrogen fuel cells. Int. J. Hydrog. Energy 2020, 45, 6982–6993. [Google Scholar] [CrossRef]
- Lim, J.M.; Jang, Y.S.; Nguyen, H.V.T.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, H.D.; Lee, K.-K.; Han, Z.; Ostrikov, K.; et al. Advances in high-voltage supercapacitors for energy storage systems: Materials and electrolyte tailoring to implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, Z.; Cao, X.; Wang, N. The Integration of Triboelectric Nanogenerators and Supercapacitors: The Key Role of Cellular Materials. Materials 2023, 16, 3751. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Kunmming, L.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Dinesh, M.; Haldorai, Y.; Kumar, R.T.R. Mn–Ni binary metal oxide for high-performance supercapacitor and electro-catalyst for oxygen evolution reaction. Ceram. Int. 2020, 46, 28006–28012. [Google Scholar] [CrossRef]
- Moghadam, M.T.T.; Seifi, M.; Askari, M.B.; Azizi, S. ZnO-MWCNT@ Fe3O4 as a novel catalyst for methanol and ethanol oxidation. J. Phys. Chem. Solids 2022, 165, 110688. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Shahid, M.; Agboola, P.O.; Al-Khalli, N.F.; Warsi, M.F.; Shakir, I. Fabrication of CNTs supported binary nanocomposite with multiple strategies to boost electrochemical activities. Electrochim. Acta 2021, 383, 138332. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Ya, K.Z.; Tan, W.K.; Kawamura, G.; Matsuda, A. Microwave-assisted synthesis of Mn3O4-Fe2O3/Fe3O4@ rGO ternary hybrids and electrochemical performance for supercapacitor electrode. Diam. Relat. Mater. 2020, 101, 107622. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chakrabarty, N.; Senapati, A.; Chakraborty, A.K. CuO@ NiO/Polyaniline/MWCNT nanocomposite as high-performance electrode for supercapacitor. J. Phys. Chem. C 2018, 122, 27180–27190. [Google Scholar] [CrossRef]
- Singu, B.S.; Goda, E.S.; Yoon, K.R. Carbon Nanotube–Manganese oxide nanorods hybrid composites for high-performance supercapacitor materials. J. Ind. Eng. Chem. 2021, 97, 239–249. [Google Scholar] [CrossRef]
- Tourchi Moghadam, M.T.; Babamoradi, M.; Azimirad, R. Effect of hydrothermal reaction temperature on the photocatalytic properties of CdWO4-RGO nanocomposites. J. Nanostructures 2019, 9, 600–609. [Google Scholar]
- Ma, C.; Wang, R.; Tetik, H.; Gao, S.; Wu, M.; Tang, Z.; Lin, D.; Ding, D.; Wu, W. Hybrid nanomanufacturing of mixed-dimensional manganese oxide/graphene aerogel macroporous hierarchy for ultralight efficient supercapacitor electrodes in self-powered ubiquitous nanosystems. Nano Energy 2019, 66, 104124. [Google Scholar] [CrossRef]
- Tseng, L.-H.; Hsiao, C.-H.; Nguyen, D.D.; Hsieh, P.-Y.; Lee, C.-Y.; Tai, N.-H. Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes. Electrochim. Acta 2018, 266, 284–292. [Google Scholar] [CrossRef]
- Mane, V.J.; Malavekar, D.B.; Ubale, S.B.; Bulakhe, R.N.; In, I.; Lokhande, C.D. Binder free lanthanum doped manganese oxide @ graphene oxide composite as high energy density electrode material for flexible symmetric solid state supercapacitor. Electrochim. Acta 2020, 335, 135613. [Google Scholar] [CrossRef]
- Tang, J.; Shen, J.; Li, N.; Ye, M. Facile synthesis of layered MnWO4/reduced graphene oxide for supercapacitor application. J. Alloys Compd. 2016, 666, 15–22. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Huo, J.; Wang, W. A simple synthesis of MnWO4 nanoparticles as a novel energy storage material. Mater. Chem. Phys. 2015, 167, 22–27. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Wang, Y.; Wang, X.; Han, G. Shape-controlled synthesis of MnWO4 nanocrystals by a surfactant-free hydrothermal method. Ceram. Int. 2014, 40, 5085–5090. [Google Scholar] [CrossRef]
- Mal, D.D.; Khilari, S.; Pradhan, D. Efficient and selective oxidation of toluene to benzaldehyde on manganese tungstate nanobars: A noble metal-free approach. Green Chem. 2018, 20, 2279–2289. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Yoo, K.; Ramaraghavulu, R.; Shim, J. Hydrothermal Synthesis of MnWO4@ GO Composite as Non-Precious Electrocatalyst for Urea Oxidation. Nanomaterials 2021, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fu, P.; Ruan, B.; Wu, M.; Wu, M.; Wu, R. Performances of MnWO4@AC mixed oxide composite materials as Pt-free counter electrodes for high efficiently dye sensitized solar cells. New J. Chem. 2021, 45, 1686–1694. [Google Scholar] [CrossRef]
- Yang, S.; Song, X.; Zhang, P.; Gao, L. Facile synthesis of nitrogen-doped graphene–ultrathin MnO2 sheet composites and their electrochemical performances. ACS Appl. Mater. Interfaces 2013, 5, 3317–3322. [Google Scholar] [CrossRef]
- Donolikar, P.D.; Patil, S.; Sadale, S.B.; Ryu, J.; Patil, D.R. Redox-active electrolyte-based MnWO4//AC asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 8054–8063. [Google Scholar] [CrossRef]
- Sardar, K.; Thakur, S.; Maiti, S.; Besra, N.; Bairi, P.; Chanda, K.; Majumdar, G.; Chattopadhyay, K.K. Amalgamation of MnWO4 nanorods with amorphous carbon nanotubes for highly stabilized energy efficient supercapacitor electrodes. Dalton Trans. 2021, 50, 5327–5341. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.F.; Gao, J.F.; Kong, L.B. A crystalline nickel vanadium oxide@ amorphous cobalt boride nanocomposites with enhanced specific capacity for hybrid supercapacitors. Electrochim. Acta 2021, 377, 138086. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Acharya, J.; Seo, M.-K.; Khil, M.-S.; Kim, H.-Y.; Kim, B.-S. One-pot sonochemical synthesis of hierarchical MnWO4 microflowers as effective electrodes in neutral electrolyte for high performance asymmetric supercapacitors. Int. J. Hydrog. Energy 2019, 44, 10838–11085. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Huang, J.; Liu, M. Low Temperature Tolerant Organohydrogel Electrolytes for Flexible Solid-State Supercapacitors. Adv. Energy Mater. 2018, 8, 1801967. [Google Scholar] [CrossRef]
- Askari, M.B.; Rozati, S.M.; Salarizadeh, P.; Azizi, S. Reduced graphene oxide supported Co3O4–Ni3S4 ternary nanohybrid for electrochemical energy storage. Ceram. Int. 2022, 48, 16123–16130. [Google Scholar] [CrossRef]
- Moghadam, M.T.T.; Seifi, M.; Jamali, F.; Azizi, S.; Askari, M.B. ZnWO4-CNT as a superior electrode material for ultra-high capacitance supercapacitor. Surf. Interfaces 2022, 32, 102134. [Google Scholar] [CrossRef]
- Li, S.; Fan, J.; Liao, H.; Xiao, G.; Gao, S.; Cui, K.; Nu, C.; Jin, H.G.; Luo, W.; Chao, Z. MnCoP/(Co, Mn)(Co, Mn) 2O4 nanocomposites for all-solid-state supercapacitors with excellent electrochemical energy storage. J. Electroanal. Chem. 2022, 924, 116866. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, C.; Xiao, Z.; Xu, F.; Sun, L.; Zhang, J.; Zou, Y. Phosphidated Ni-Mn layered double hydroxide–based electrode material with superior electrochemical performance for supercapacitors. J. Energy Storage 2021, 44, 103311. [Google Scholar] [CrossRef]
- Harichandran, G.; Divya, P.; Radha, S.; Yesuraj, J. Facile and controllable CTAB-assisted sonochemical synthesis of one-dimensional MnWO4 nanorods for supercapacitor application. J. Mol. Struct. 2020, 1199, 126931. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]








| Nanocomposite | Specific Capacitance (F g−1) | Potential Range (V) | Current Density (Ag−1) | Scan Rate (mVs−1) | Reference |
|---|---|---|---|---|---|
| MnWO4/CNT | 1849 | 0–0.5 | 1 | _ | This work |
| MnWO4/RGO | 288 | −0.35–0.55 | _ | 5 | [23] |
| ZnWO4/CNT | 4552 | −0.1–0.8 | 1 | _ | [36] |
| MnCoP/(Co,Mn)(Co Mn)2O4 | 123.43 | _ | 0.5 | _ | [37] |
| ACNFs@Ni-Mn | 1077 | _ | 1 | _ | [38] |
| MnWO4 nanorods | 247 | 0–1.1 | _ | 5 | [39] |
| CNPFs/MoS2/rGO | 144.3 | 0–1.0 | 2 | [40] | |
| Ni-Co-S@N-pCNFs | 520.2 | _ | 0.2 | _ | [40] |
| N-doping grape-based honeycomb-like porous carbon (NGHPC) | 275 | −1.0–0 | 0.5 | _ | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, M.B.; Jamali, F.; Tourchi Moghadam, M.T.; Azizi, S.; Seifi, M. Synthesis and Characterization of MnWO4-CNT for Supercapacitor Applications. Sustainability 2023, 15, 14910. https://doi.org/10.3390/su152014910
Askari MB, Jamali F, Tourchi Moghadam MT, Azizi S, Seifi M. Synthesis and Characterization of MnWO4-CNT for Supercapacitor Applications. Sustainability. 2023; 15(20):14910. https://doi.org/10.3390/su152014910
Chicago/Turabian StyleAskari, Mohammad Bagher, Fatemeh Jamali, Mohammad Taghi Tourchi Moghadam, Sadegh Azizi, and Majid Seifi. 2023. "Synthesis and Characterization of MnWO4-CNT for Supercapacitor Applications" Sustainability 15, no. 20: 14910. https://doi.org/10.3390/su152014910
APA StyleAskari, M. B., Jamali, F., Tourchi Moghadam, M. T., Azizi, S., & Seifi, M. (2023). Synthesis and Characterization of MnWO4-CNT for Supercapacitor Applications. Sustainability, 15(20), 14910. https://doi.org/10.3390/su152014910







