Utilization of Digestate from Agricultural and Food Waste for the Production of Biochar Used to Remove Methylene Blue
Abstract
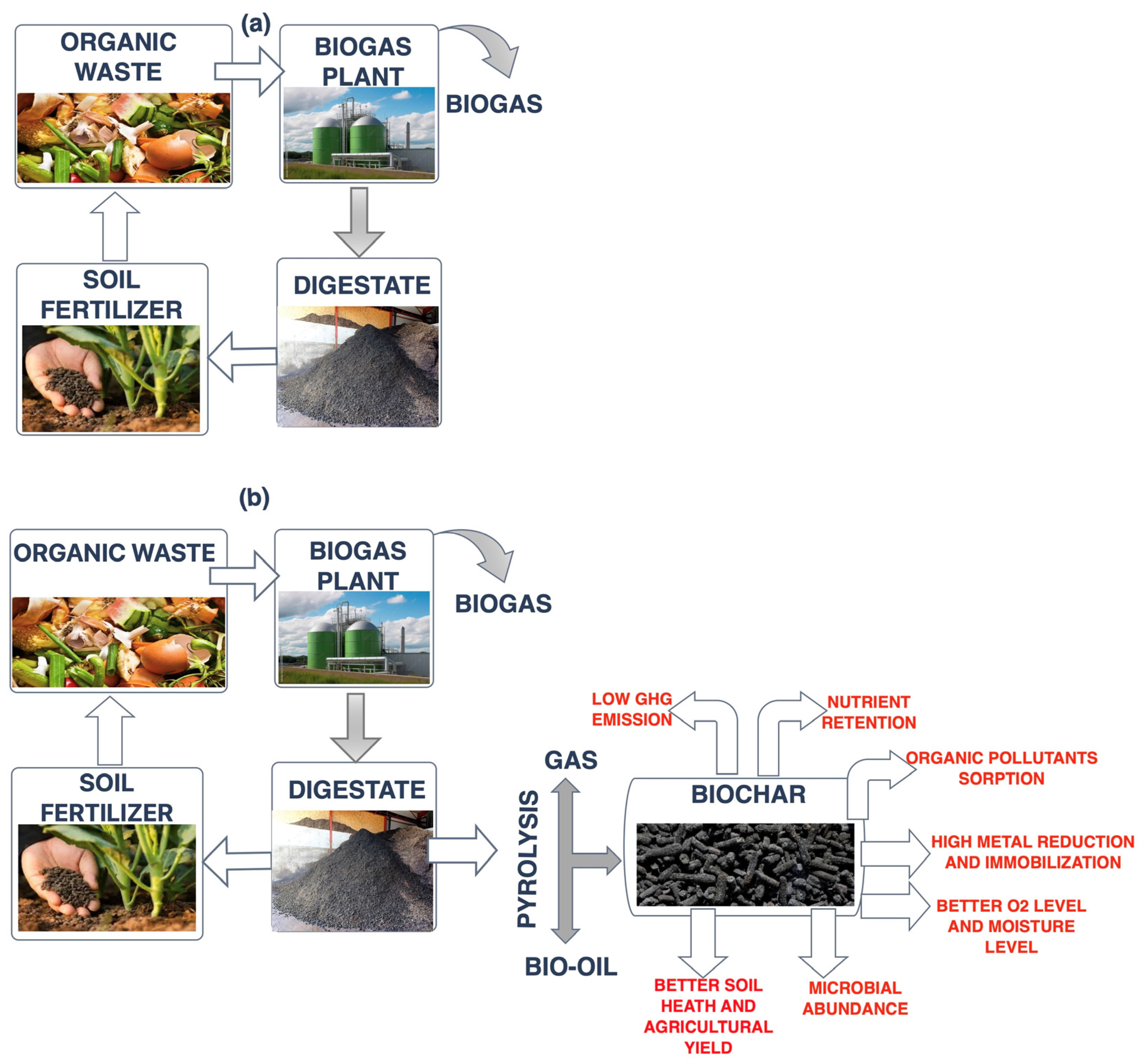
:1. Introduction
2. Materials and Methods
2.1. Substrates for Biochar Production
2.2. Characteristics of the Pyrolysis Process
2.3. Physicochemical and Physical Analyses
2.4. Methylene Blue Sorption
- C1—initial concentration methylene blue mgL−1
- C2—concentration methylene blue during balance mgL−1.
2.5. Statistical Analysis
3. Results and Discussion
3.1. Pyrolysis Process Yield
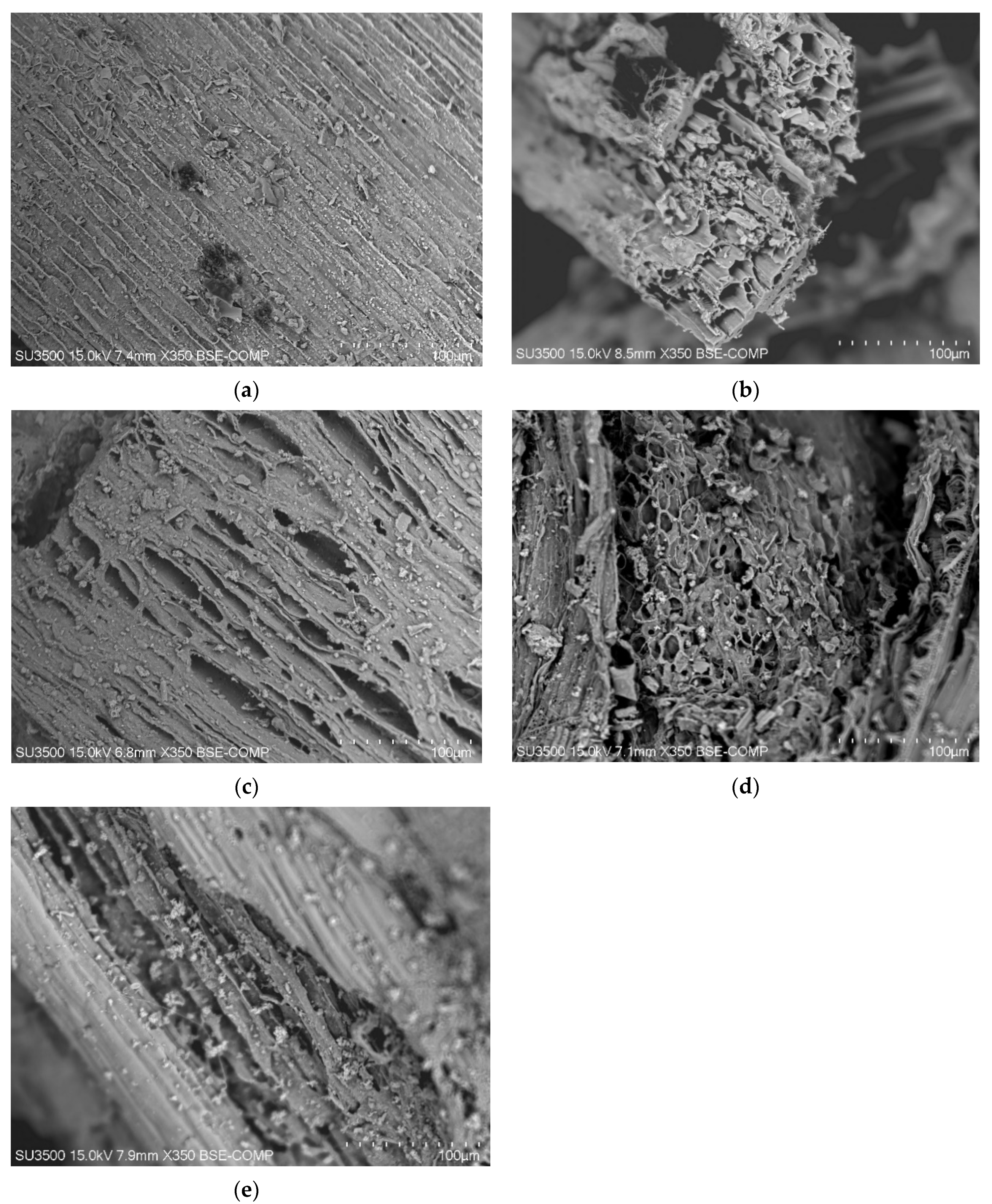
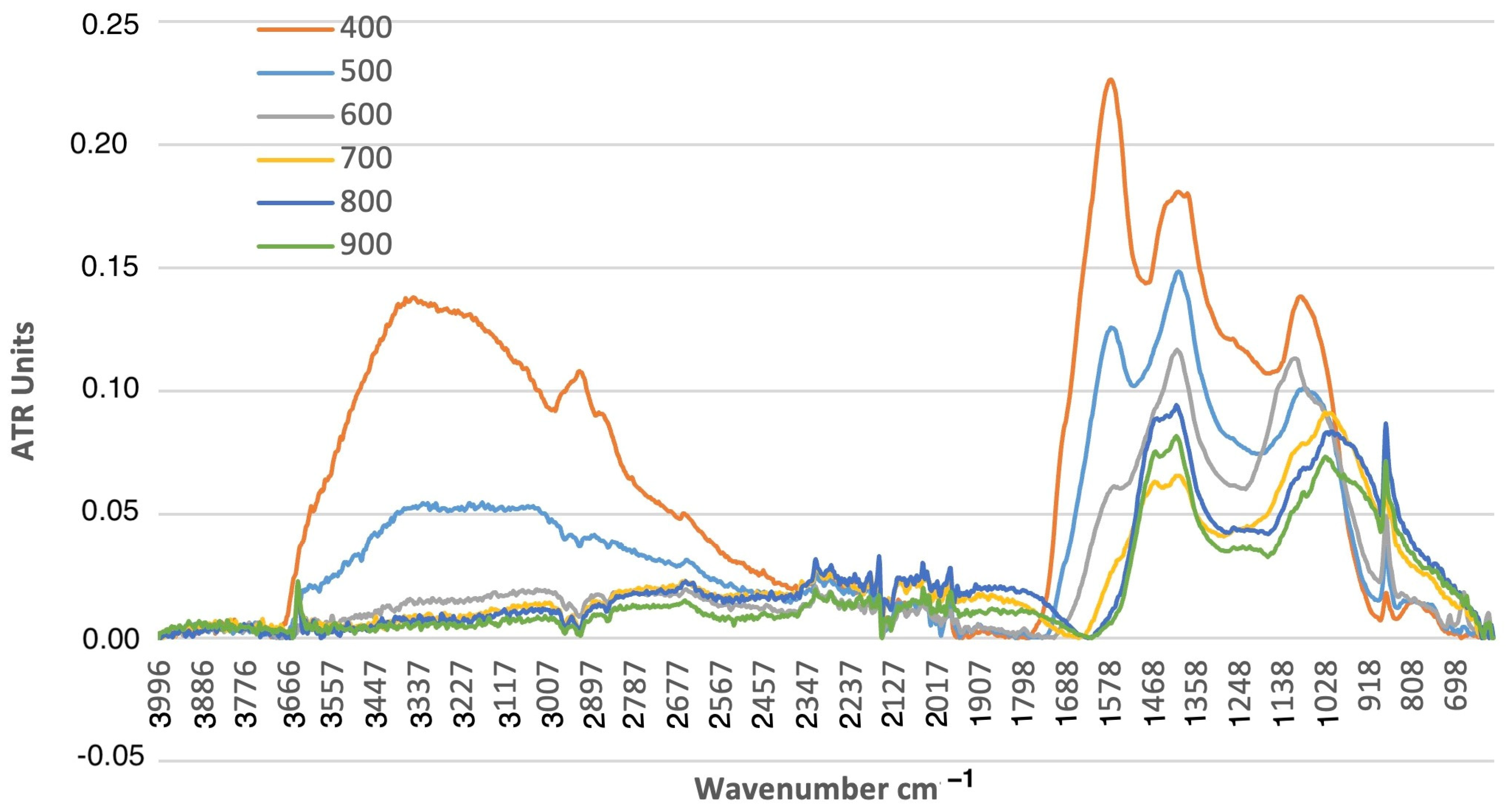
3.2. Physicochemical Characteristics of the Produced Biochar
3.3. Methylene Blue Sorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, M.T.H.; Nizamuddin, S.; Mubarak, N.M.; Shirin, K.; Aijaz, M.; Hussain, M.; Baloch, H.A. Characterization and Process Optimization of Biochar Produced Using Novel Biomass, Waste Pomegranate Peel: A Response Surface Methodology Approach. Waste Biomass Valor. 2019, 10, 521–532. [Google Scholar] [CrossRef]
- Grosser, A. Determination of methane potential of mixtures composed of sewage sludge, organic fraction of municipal waste and grease trap sludge using biochemical methane potential assays. A comparison of BMP tests and semi-continuous trial results. Energy 2018, 143, 488–499. [Google Scholar] [CrossRef]
- Neogi, S.; Sharma, V.; Khan, N.; Chaurasia, D.; Ahmad, S.C.; Singh, A.; You, S.; Pandey, A.; Bhargava, P.C. Sustainable biochar: A facile strategy for soil and environmental restoration, energy generation, mitigation of global climate change and circular bioeconomy. Chemosphere 2022, 293, 133474. [Google Scholar] [PubMed]
- Grosser, A.; Neczaj, E. Sewage sludge and fat rich materials co-digestion-Performance and energy potential. J. Clean. Prod. 2018, 198, 1076–1089. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Jasinska, A.; Celary, P. The influence of grease trap sludge sterilization on the performance of anaerobic co-digestion of sewage sludge. Renew. Energ. 2020, 161, 988–997. [Google Scholar] [CrossRef]
- Regulation of the Minister of Climate of 2 January 2020 on the Waste Catalog (Dz.U. 2020 poz.10). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200000010/O/D20200010.pdf (accessed on 30 October 2020). (In Polish)
- Miciuła, I.; Wojtaszek, H.; Bazan, M.; Janiczek, T.; Włodarczyk, B.; Kabus, J.; Kana, R. Management of the Energy Mix and Emissivity of Individual Economies in the European Union as a Challenge of the Modern World Climate. Energies 2020, 13, 5191. [Google Scholar] [CrossRef]
- Kabus, J.; Dziadkiewicz, M. Modern Management Methods in the Area of Public Housing Resources in the Community. Sustainability 2023, 15, 7776. [Google Scholar] [CrossRef]
- Kabus, J.; Dziadkiewicz, M. Residents’ Attitudes and Social Innovation Management in the Example of a Municipal Property Manager. Energies 2022, 15, 5812. [Google Scholar] [CrossRef]
- Agricultural Property Agency. Register of Agricultural Biogas Producers. Available online: https://www.kowr.gov.pl/uploads/pliki/oze/biogaz/Rejestr%20wytw%C3%B3rc%C3%B3w%20biogazu%20rolniczego%20z%20dnia%2029.12.2020%20r.pdf (accessed on 30 October 2020). (In Polish)
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate management in polish farms as an element of the nutrient cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Tsai, W.-T.; Chen, J.-W.; Lin, Y.-Q.; Chang, Y.-M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Teglia, C.; Tremier, A.; Martel, J.L. Characterization of Solid Digestates: Part 2, Assessment of the Quality and Suitability for Composting of Six Digested Products. Waste Biomass Valor. 2011, 2, 113–126. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R. Anaerobic co-digestion of recalcitrant agricultural wastes: Characterizing of biochemical parameters of digestate and its impacts on soil ecosystem. Sci. Total Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Characterization of biochars produced from residues from biogas production. J. Anal. Appl. Pyrol. 2015, 115, 157–165. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Guillén Fiallos, C.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar]
- Hsu, J.; Lo, S.L. Effect id composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ. Pollut. 2001, 114, 119–127. [Google Scholar] [CrossRef]
- Taurino, R.; Lancellotti, I.; Tatàno, F.; Carchesio, M.; Pozzi, P. Mechanical and chemical resistance of composite materials with addition of anaerobic digestate. Compos. Part B Eng. 2016, 92, 259–264. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Liang, J.; Nabi, M.; Cai, Y.; Wang, Q.; Zhang, P.; Zhang, G. Biochar application in anaerobic digestion: Performances, mechanisms, environmental assessment and circular economy. Resour. Conserv. Recycl. 2023, 188, 106720. [Google Scholar]
- Neumann, J.; Binder, S.; Apfelbacher, A.; Gasson, J.R.; García, P.R.; Hornung, A. Production and characterization of a new quality pyrolysis oil, char and syngas from digestate—Introducing the thermo-catalytic reforming proces. JAAP 2015, 113, 137–142. [Google Scholar]
- Tang, Y.; Alam, M.A.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.J.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. The pyrolysis and gasification of digestate from agricultural biogas plant. Arch. Environ. Protect. 2015, 41, 70–75. [Google Scholar] [CrossRef]
- Ruiz-Gomez, N.; Quispe, V.; Abrego, J.; Atienza-Martinez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kobyłecki, R. Środowiskowe Aspekty Termolizy Biomasy, Monografia nr 290, Wydawnictwo Politechniki Częstochowskiej, Częstochowa; University of Technology Publishing House: Częstochowa, Poland, 2014; ISBN 978-83-7193-612-8. (In Polish) [Google Scholar]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soli quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent development in biochor utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar]
- Ferreira, S.D.; Manera, C.; Silvestre, W.P.; Pauletti, G.F.; Altafini, C.R.; Godinho, M. Use of biochar produced from elephant grass by pyrolysis in a screw reactor as a soil amendment. Waste Biomass Valori. 2018, 10, 3089–3100. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Zheng, Y.; Hu, X.; Creamer, A.E.; Annable, M.D.; Li, Y. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017, 245, 606–614. [Google Scholar] [CrossRef]
- Czekała, W.; Jeżowska, A.; Chełkowski, D. The use of biochar for the production of organic fertilizers. J. Ecol. Eng. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Zama, E.F.; Zhu, Y.-G.; Reid, B.J.; Sun, G.-X. The role of biochar properties in influencing the sorption and desorption of Pb (II), Cd (II) and as (III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, R.; Ma, F.; Li, Y.; Wang, L. Effects of biochar derived from chiken manure and rape straw on speciation and phytoavailability of Cd to maize in artificially contaminated loess soli. J. Environ. Manag. 2016, 184, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludg composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E. Effects of pyrolysis temperature on soybean stover—peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activetd biochar from solid waste of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pitman, C.U., Jr. Modeling and evaluation of chromium remediation from using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increase nitrogen retention and lowers greenhouse gas emission when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing nitrogen loss and phytotoxicity during beer vinasse composting with biochar addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Wang, M.; Chen, H.; Zhang, Z. Combing biochar, zeolite and wood vinegar for composting of pig manure: The effect on greenhouse gas emission and nitrogen conservation. Waste Manag. 2018, 74, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Dinelli, F.D.; Kenar, J.A.; Jackson, M.A.; Thomas, A.J.; Peterson, S.C. Physical and chemical properties of pyrolyzed biosolids for utilization in sand-based turfgrass rootzones. Waste Manag. 2018, 76, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrol. 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Włodarczyk, R.; Chajczyk, O. Effects of pyrolysis parameters on the yield and properties of biochar from pelletized sunflower husk. EKO-DOK 2018. E3S Web Conf. 2018, 44, 00197. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Barczak, M. Poultry manure derived biochars—The impact of pyrolysis temperature on selected properties and potentials for further modifications. JSDEWES 2021, 9, 1080337. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochor governing end use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Giudicianni, P.; Pindozzi, S.; Grottola, C.M.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Pyrolysis for exploitation of biomasses selected for soil phytoremediation: Characterization of gaseous and solid products. Waste Manag. 2017, 61, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manage. 2012, 32, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Roberst, D.A.; Cole, A.J.; Whelan, A.; de Nys, R.; Paul, N.A. Slow pyrolysis enhances the recovery and reuse of phosphorus and reduces metal leaching from biosolids. Waste Manag. 2017, 64, 133–139. [Google Scholar]
- Kleemann, R.; Chenoweth, J.; Clift, R.; Morse, S.; Pearce, P. Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag. 2017, 60, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, B.; Sun, M.; Chen, X.; Zhang, M.; Li, H.; Shaucong, X. Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis. Waste Manag. 2017, 62, 91–100. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolisis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solution: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Das, R.K.; Satinde, K.B.; Ramirez, A.A.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag. 2016, 49, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.; Gharaibeh, M.A.; Mohawesh, O.; Alajlouni, M.; Quzaih, M.; Masad, M.; Hanandeh, A.E. Characterization and Artificial Neural Networks Modelling of methylene blue adsorption of biochar derived from agricultural residues: Effect of biomass type, pyrolysis temperature, particle size. J. Saudi Chem. Soc. 2020, 24, 811–823. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Smernik, R.; Das, O.; Farid, M.; Gao, W. A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: Production, characterization and potential application. Sci. Total Environ. 2015, 512–513, 495–505. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Zhang, S.; Song, Y.; Xue, S.; Liu, L.; Lvy, X.; Wang, X.; Yang, G. Coupling biochar with anaerobic digestion in a circular economy perspective: A promising way to promote sustainable energy, environment and agriculture development in China. Renew. Sustain. Energy Rev. 2021, 144, 110973. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.T.; Yin, J.; Draper, K.; Trabold, T.A. Closing nutrient cycles with biochar- from filtration to fertilizer. J. Clean. Prod. 2018, 197, 1597–1606. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.L.; Mai, T.L.A.; Brandão, M.; de la Rosa, R.A.; Kristiansen, P.; Joseph, S. Climate-change and health effects of using rice husk for biochar-compost: Comparing three pyrolysis systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.-E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanism of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Chuanming, X.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering application of biochar for removal organic pollutants. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Oreoluwa, A.T.; Yetunde, A.T.; Joseph, U.E.; Chengsen, Z.; Hongyan, W. Effect of Biochar and Poultry litter Application on Chemical Properties and Nutrient Availability of an Acidic Soil. Commun. Soil Sci. Plant Anal. 2020, 51. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Lopez-Capel, E.; Pena, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Ezz, H.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Enhanced Removal of Methylene Blue Dye by Sustainable Biochar Derived from Rice Straw Digestate. KEM 2022, 932, 119–129. [Google Scholar] [CrossRef]
- Gohoho, H.; Noby, H.; Hayashi, J.I.; El Shazly, A.H. Adsorption of Methylene Blue Dye Using Raw and Carbonized Peanut Shell. KEM 2022, 932, 131–137. [Google Scholar] [CrossRef]
- Sawalha, H.; Bader, A.; Sarsour, J.; Al-Jabari, M.; Rene, E.R. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes 2022, 10, 2039. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, C.; Liu, H.; Liu, H.; Tang, Y.; Tang, C.; Wang, A.; Wang, C. Adsorption Characteristics and Mechanism of Methylene Blue in Water by NaOH-Modified Areca Residue Biochar. Processes 2022, 10, 2729. [Google Scholar] [CrossRef]
- PN-EN ISO 18122:2016-01; Polish Version, Solid Biofuels—Determination of Ash Content. PKN: Warszawa, Poland, 2019.
- PN-ISO 10694:2002; Soil Quality—Determination of Organic Carbon Content and Total Carbon Content after dry Combustion (Elemental Analy-sis). PKN: Warszawa, Poland, 2002.
- PN-EN 16169:2012; Polish Standard. Sewage Sludge, Treated Bio-Waste and Soil. Determination of Nitrogen by the Kjeldahl Method. PKN: Warszawa, Poland, 2012.
- Shariff, A.; Aziz, N.S.M.; Saleh, N.M.; Ruzali, N.S.I. The Effect of Feedstock Type and Slow Pyrolysis Temperature on Biochar Yield from Coconut Wastes. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2016, 10, 1335. [Google Scholar]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of Sewage Sludge Properties on the Biochar Characteristic. JAAP 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Bavariani, M.Z.; Ronaghi, A.; Ghasemi, R. Influence of pyrolysis tempeartures on FTIR analysis, nutrient bioavailability, and agricultural use of poultry manure biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterizaton of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Li, J.; Cao, L.; Yuan, Y.; Wang, R.; Wen, Y.; Man, J. Comparative study for microcystin-LR sorption onto biochars produced from various plant- and animal- wastes at different pyrolysis temperatures: Influencing mechanisms of biochar properties. Bioresour. Technol. 2018, 247, 794–803. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Effects of biochar and poultry manure on soil characteristics and the yield of radish. Sci. Hortic. 2019, 243, 457–463. [Google Scholar] [CrossRef]
- EBC (2012)’ European Biochar Certificate—Guidelines for Sustainable Production of Biochar. European Biochar Foundation (EBC): Arbaz, Switzerland Version 6.5E of 30th August 2018. Available online: http://www.european-biochar.org/en/download (accessed on 1 June 2023).
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lü, F.; Zhang, H.; Shao, L.; Chen, D.; He, P. Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci. Rep. 2015, 5, 9406. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Idrees, M.; Hussain, Q.; Kong, J. Adsorption of copper (II) by using derived-farmyard and poultry manure biochars: Efficiency and mechanism. Chem. Phys. Lett. 2017, 689, 160–198. [Google Scholar] [CrossRef]
- Schulzki, G.; Nublein, B.; Sievers, H. Transition rates of selected metals determined in various types of teas (Camellia sinensis L. Kuntze) and herbal/fruit infusions. Food Chem. 2017, 215, 22–30. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, H.; Zhang, T.; Nan, X.; Ma, F. Effect of pyrolysis temperature on sulfur content, extractable fraction and release of sulfate in corn straw biochar. RSC Adv. 2018, 8, 35611–35617. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Hasnan, F.I.; Iamail, K.N.; Musa, M.; Jaapar, J.; Alwi, H.; Hamid, K.K.K. Characterization of bio char derived from tapioca skin. IOP Conf. Series Mater. Sci. Eng. 2018, 334, 012016. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference; Routledge: London, UK, 2019. [Google Scholar]


| pHH2O | MC | Ash | TC | NK |
|---|---|---|---|---|
| % | ||||
| 8.45 ± 0.06 | 9.38 ± 0.27 | 26.08 ± 2.16 | 37.98 ± 0.40 | 1.99 ± 0.03 |
| Extraction Substances | Cellulose | Lignin | Hemicellulose |
|---|---|---|---|
| % | |||
| 4.12 ± 0.12 | 30.57 ± 0.67 | 38.72 ± 0.53 | 55.50 ± 31 |
| Type of Biochar | Yield | pHH2O | MC | Ash | N | C | H | S | TOC | H/C | BET |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % dm | m2·g−1 | ||||||||
| BSD400 | 51.09 ± 0.67 | 11.40 ± 0.56 | 0.51 ± 0.27 | 39.74 ± 1.20 | 2.14 ± 0.03 | 44.53 ± 0.09 | 3.13 ± 0.02 | 0.45 ± 0.04 | 44.1 ± 8.20 | 0.84 | 4.62 ± 0.19 |
| BSD450 | 47.95 ± 1.87 | 11.30 ± 0.20 | 2.51 ± 0.10 | 44.04 ± 3.00 | 2.02 ± 0.11 | 43.56 ± 1.35 | 2.42 ± 0.13 | 0.85 ± 0.007 | 45.0 ± 11.7 | 0.67 | 9.43 ± 0.32 |
| BSD500 | 46.76 ± 1.33 | 11.58 ± 0.33 | 0.62 ± 0.20 | 44.50 ± 1.86 | 1.98 ± 0.005 | 49.95 ± 0.62 | 2.33 ± 0.05 | 0.46 ± 0.03 | 46.7± 8.90 | 0.56 | 5.07 ± 0.23 |
| BSD550 | 45.56 ± 1.29 | 12.06 ± 0.62 | 0.61 ± 0.02 | 53.57 ± 2.05 | 1.72 ± 0.005 | 45.20 ± 0.68 | 1.68 ± 0.07 | 0.49 ± 0.01 | 44.1 ± 11.5 | 0.44 | 9.66 ± 0.43 |
| BSD600 | 45.30 ± 2.66 | 12.28 ± 0.42 | 0.37 ± 0.09 | 49.74 ± 3.56 | 1.79 ± 0.04 | 46.21 ± 0.35 | 1.44 ± 0.03 | 1.60 ± 0.19 | 42.5 ± 11.0 | 0.37 | 6.39 ± 0.25 |
| BSD650 | 43.49 ± 0.29 | 12.50 ± 0.25 | 0.72 ± 0.17 | 53.12 ± 3.03 | 1.73 ± 0.03 | 52.19 ± 1.16 | 1.28 ± 0.005 | 0.60 ± 0.005 | 48.5 ± 9.40 | 0.29 | 4.98 ± 0.68 |
| BSD700 | 41.96 ± 0.65 | 12.53 ± 0.24 | 0.42 ± 0.15 | 53.32 ± 3.51 | 1.64 ± 0.01 | 48.20 ± 0.95 | 1.12 ± 0.005 | 1.00 ± 0.02 | 45.0 ± 11.7 | 0.28 | 8.58 ± 0.44 |
| BSD750 | 41.29 ± 0.35 | 12.64 ± 0.31 | 0.94 ± 0.28 | 54.40 ± 1.20 | 1.44 ± 0.06 | 46.43 ± 1.32 | 0.97 ± 0.02 | 1.02 ± 0.05 | 45.2 ± 8.70 | 0.25 | 11.68 ± 0.38 |
| BSD800 | 40.71 ± 0.10 | 12.65 ± 0.09 | 0.69 ± 0.29 | 51.50 ± 1.34 | 1.22 ± 0.11 | 45.50 ± 1.22 | 0.82 ± 0.13 | 1.46 ± 0.08 | 45.0 ± 11.0 | 0.22 | 21.09 ± 0.43 |
| BSD850 | 40.63 ± 0.24 | 12.63 ± 0.39 | 0.65 ± 0.21 | 57.43 ± 6.94 | 1.62 ± 0.01 | 48.13 ± 0.02 | 0.65 ± 0.03 | 1.63 ± 0.03 | 44.4 ± 9,50 | 0.16 | 47.90 ± 0.35 |
| BSD900 | 40.66 ± 0.23 | 12.69 ± 0.18 | 0.97 ± 0.04 | 55.76 ± 1.91 | 1.01 ± 0.01 | 42.39 ± 0.52 | 0.68 ± 0.04 | 1.65 ± 0.27 | 35.9 ± 9.30 | 0.19 | 20.39 ± 0.12 |
| Percentage of MB removal | M | Me | SD | Sk | Kurt | Min | Maks | W | p |
| 92.30 | 92.99 | 4.59 | −0.66 | −0.48 | 81.41 | 99.13 | 0.93 | <0.001 |
| Temperature—Types of MB Concentration | rho Spearmana | Percentage of MB Removal |
|---|---|---|
| Significance | ||
| Temperature—all types of MB concentration | rho Spearmana | 0.64 |
| significance | <0.001 | |
| Temperature—concentration 50 mgL−1 | rho Spearmana | 0.33 |
| significance | 0.063 | |
| Temperature—concentration 100 mgL−1 | rho Spearmana | 0.80 |
| significance | <0.001 | |
| Temperature—concentration 200 mgL−1 | rho Spearmana | 0.92 |
| significance | <0.001 |
| MB Concentration | M | SD | F | p | η2 |
|---|---|---|---|---|---|
| 50 mgL−1 | 93.40 | 4.65 | 5.07 | 0.008 | 0.10 |
| 100 mgL−1 | 90.31 | 5.44 | |||
| 200 mgL−1 | 93.20 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wystalska, K.; Kwarciak-Kozłowska, A. Utilization of Digestate from Agricultural and Food Waste for the Production of Biochar Used to Remove Methylene Blue. Sustainability 2023, 15, 14723. https://doi.org/10.3390/su152014723
Wystalska K, Kwarciak-Kozłowska A. Utilization of Digestate from Agricultural and Food Waste for the Production of Biochar Used to Remove Methylene Blue. Sustainability. 2023; 15(20):14723. https://doi.org/10.3390/su152014723
Chicago/Turabian StyleWystalska, Katarzyna, and Anna Kwarciak-Kozłowska. 2023. "Utilization of Digestate from Agricultural and Food Waste for the Production of Biochar Used to Remove Methylene Blue" Sustainability 15, no. 20: 14723. https://doi.org/10.3390/su152014723
APA StyleWystalska, K., & Kwarciak-Kozłowska, A. (2023). Utilization of Digestate from Agricultural and Food Waste for the Production of Biochar Used to Remove Methylene Blue. Sustainability, 15(20), 14723. https://doi.org/10.3390/su152014723





