Biosorption of Engine Oil Using Rice Husk in a Filtration System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Filtration Setup
2.3. Sorption Capacity and Efficiency Evaluations
2.4. Screening of the Different RH
2.5. Identification of RH
2.5.1. Fourier Transform Reflectance Infrared Spectroscopy (FTIR)
2.5.2. Scanning Electron Microscopy (SEM)
2.6. Optimisation of Engine Oil Biosorption
2.6.1. One-Factor-at-a-Time (OFAT) Approach
2.6.2. Response Surface Methodology (RSM)
2.7. Statistical Analysis
3. Results and Discussion
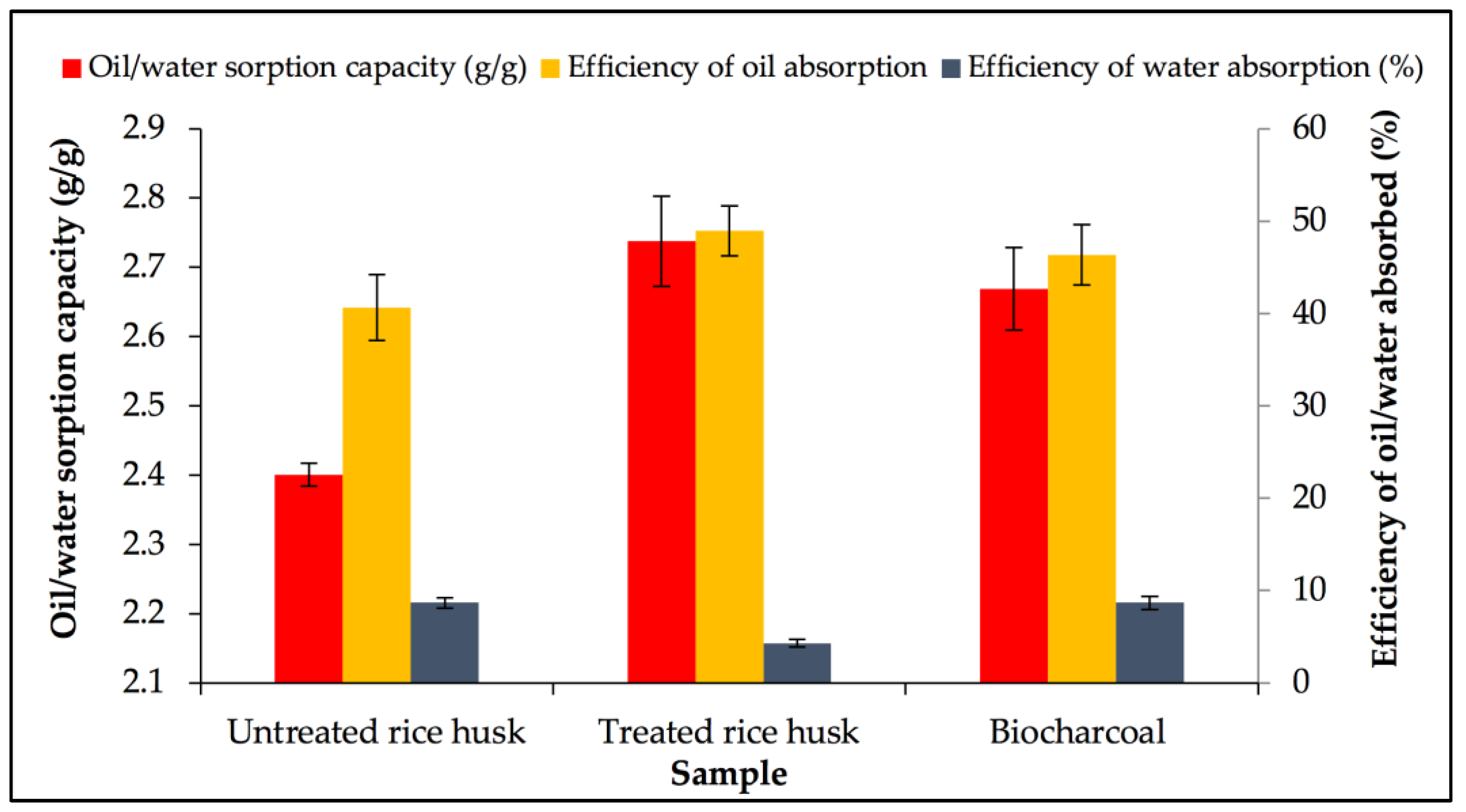
3.1. Screening of Rice Husk Samples
3.2. Characterisation of RH
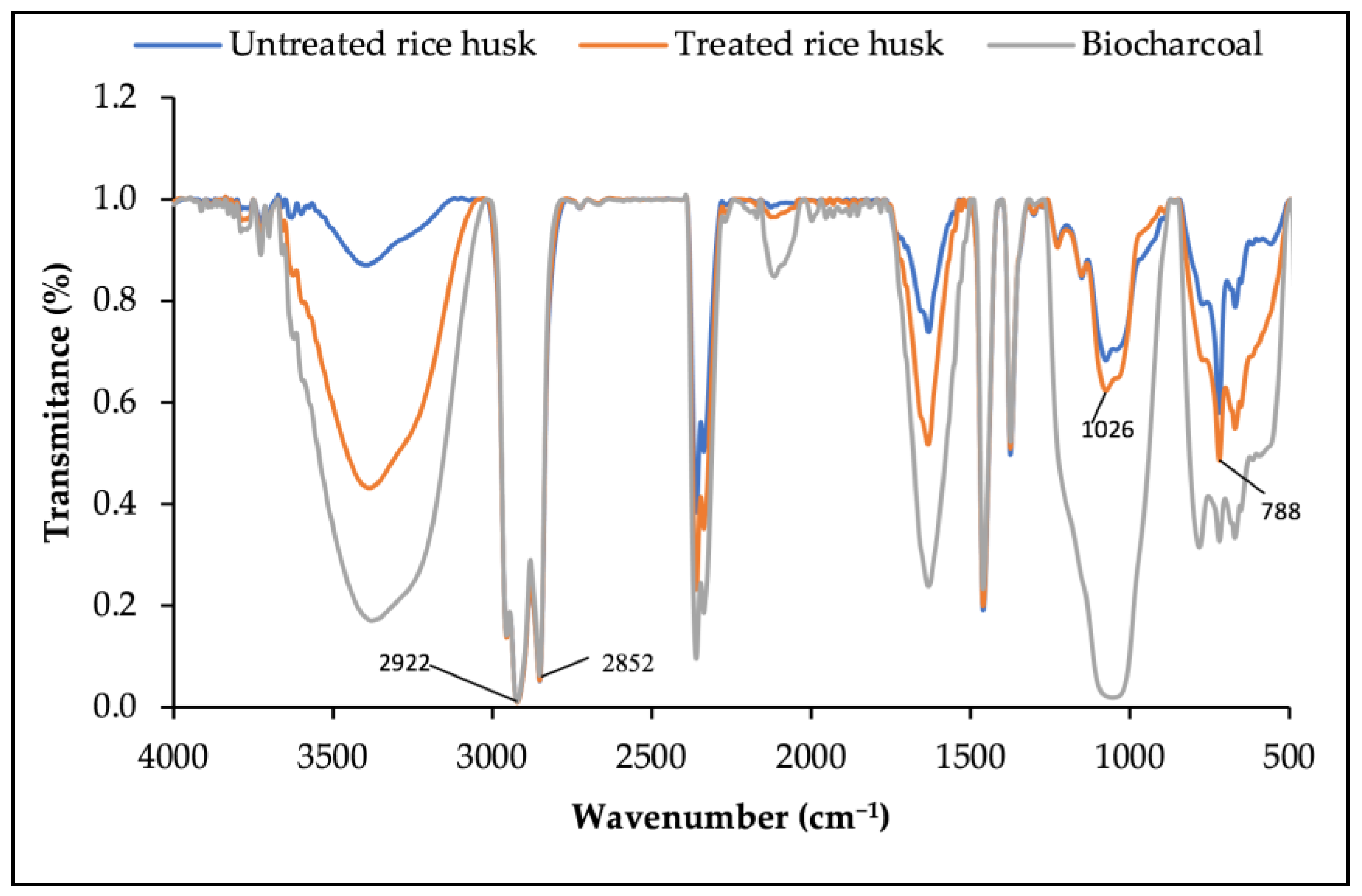
3.2.1. Spectroscopic Analysis Using Fourier Transform Infrared Spectroscopy (FTIR)
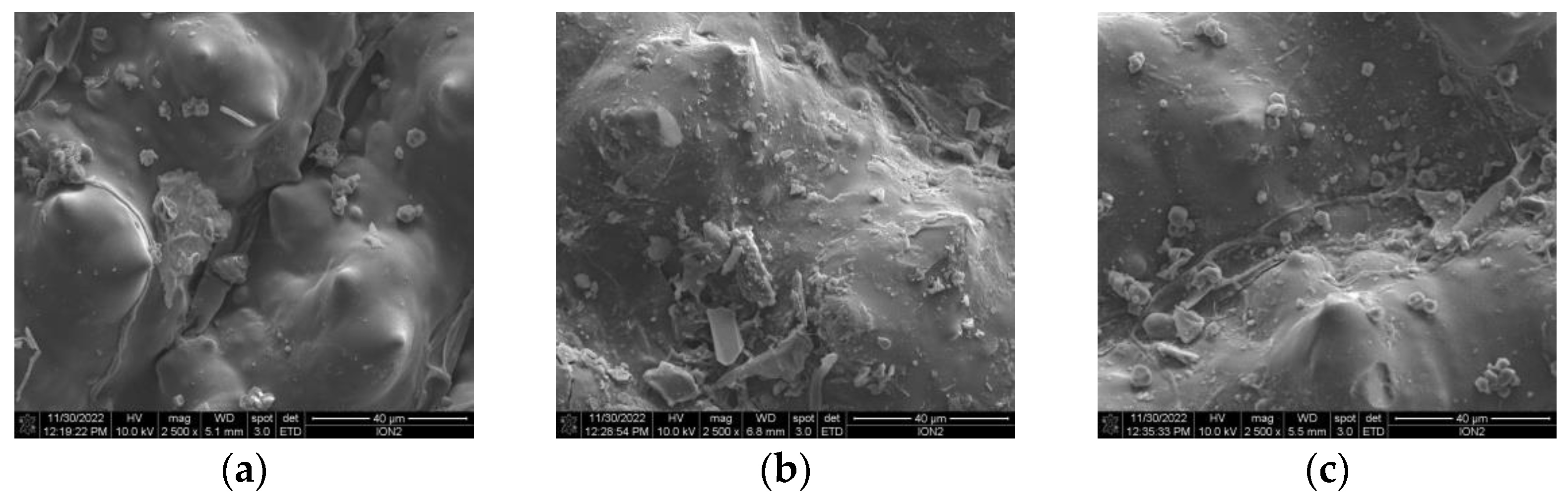
3.2.2. Surface Morphology Characterisation by Scanning Electron Microscopy (SEM)
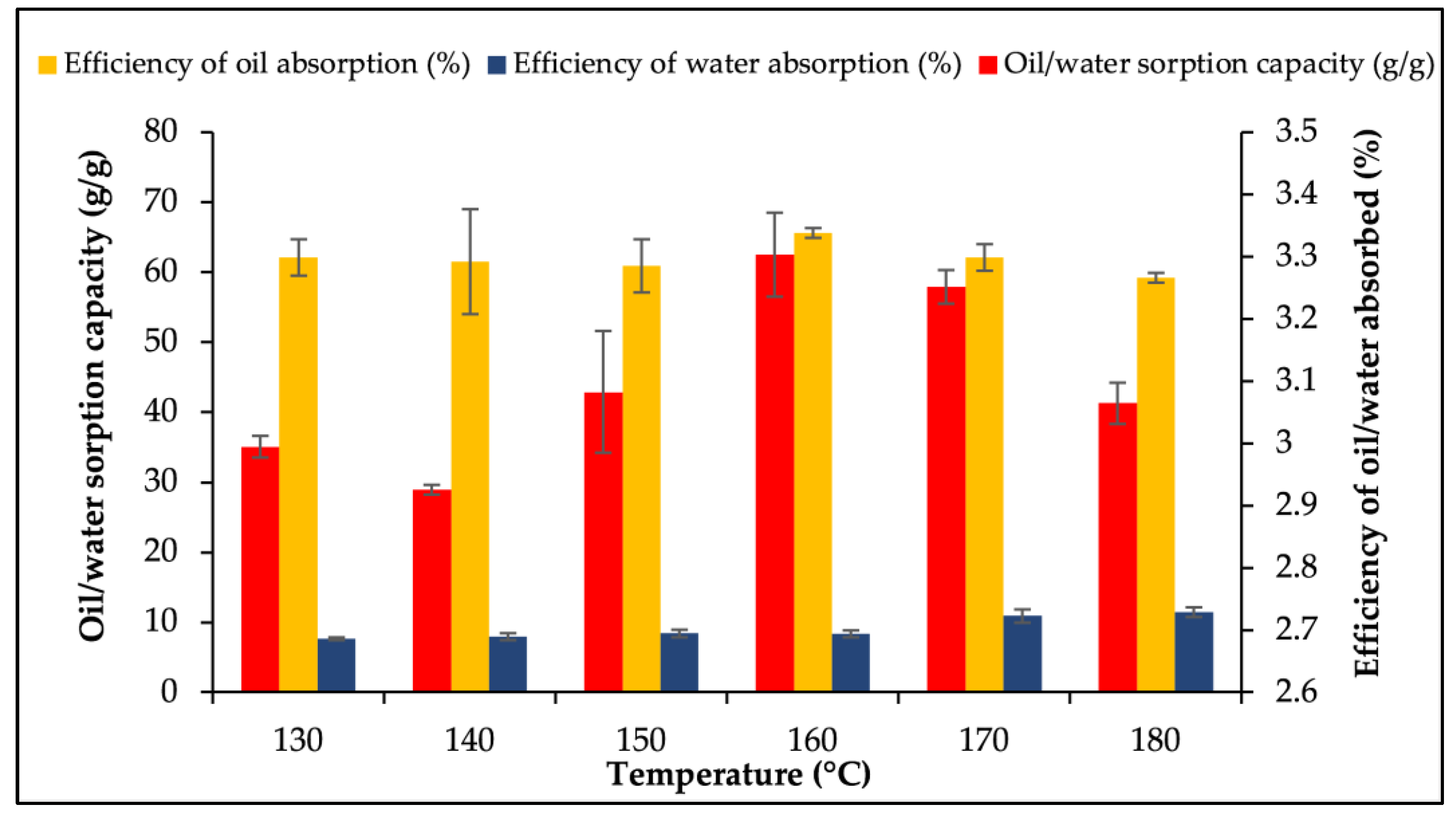
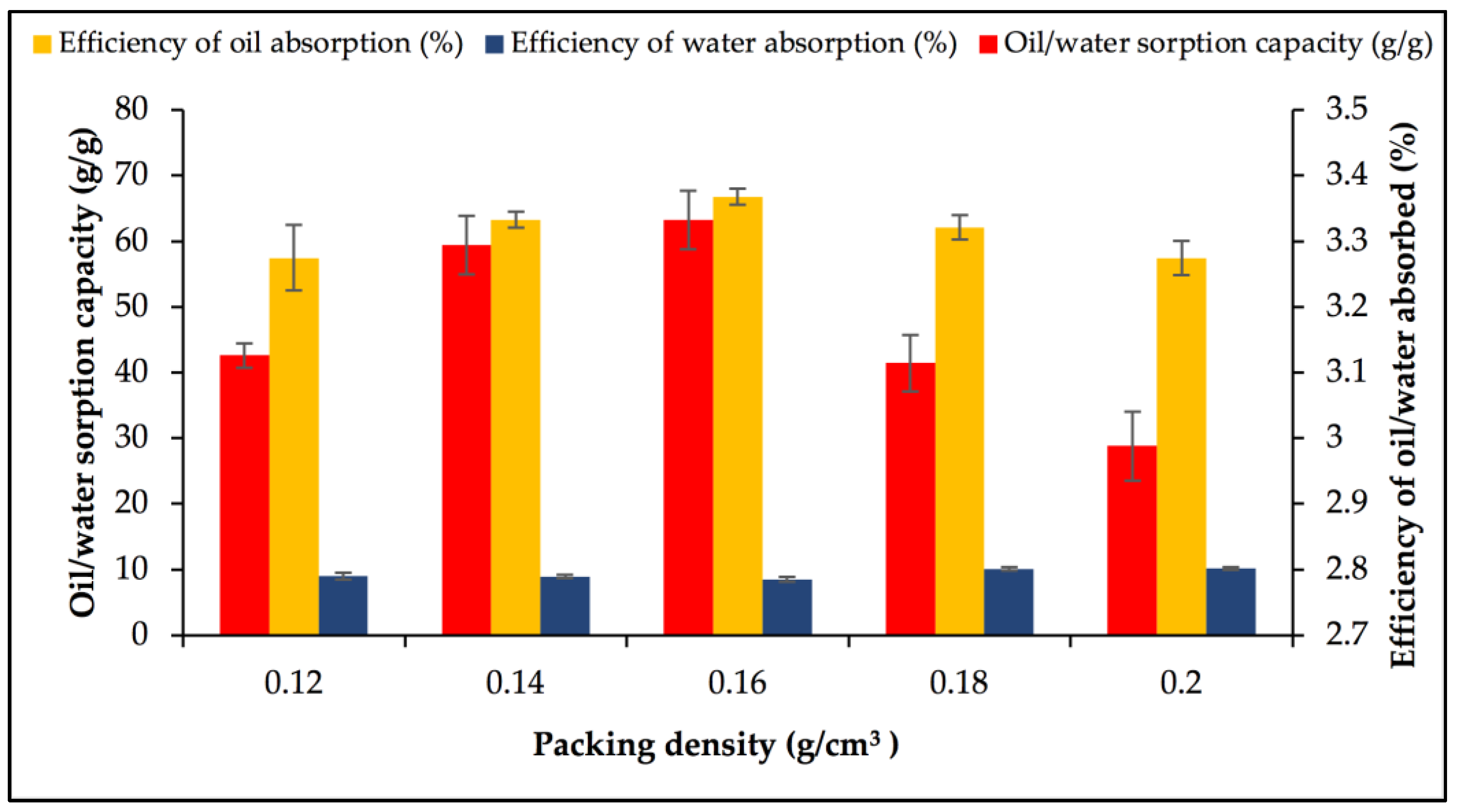
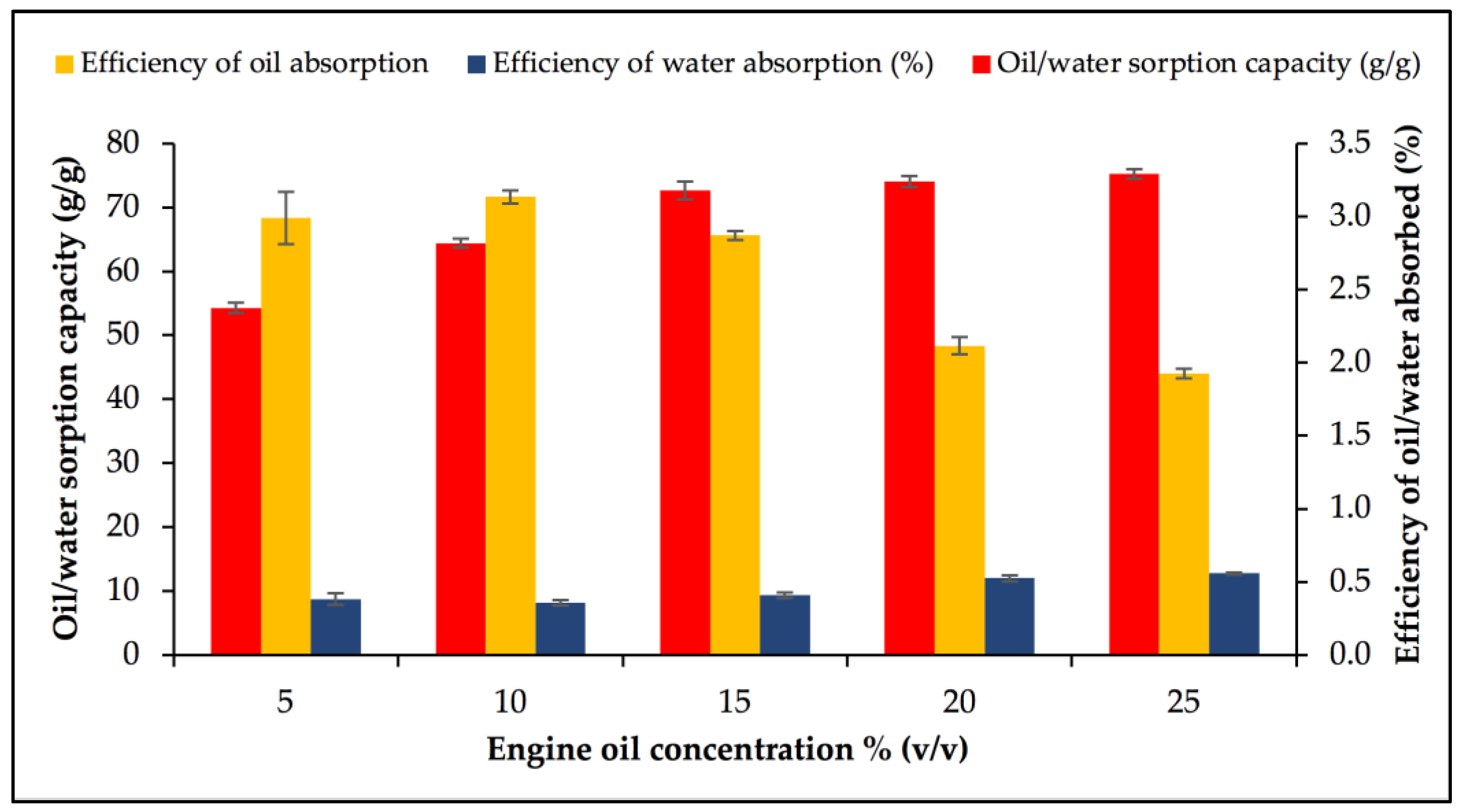
3.3. Optimisation of Factors for Oil/Water Sorption Capacity Using OFAT
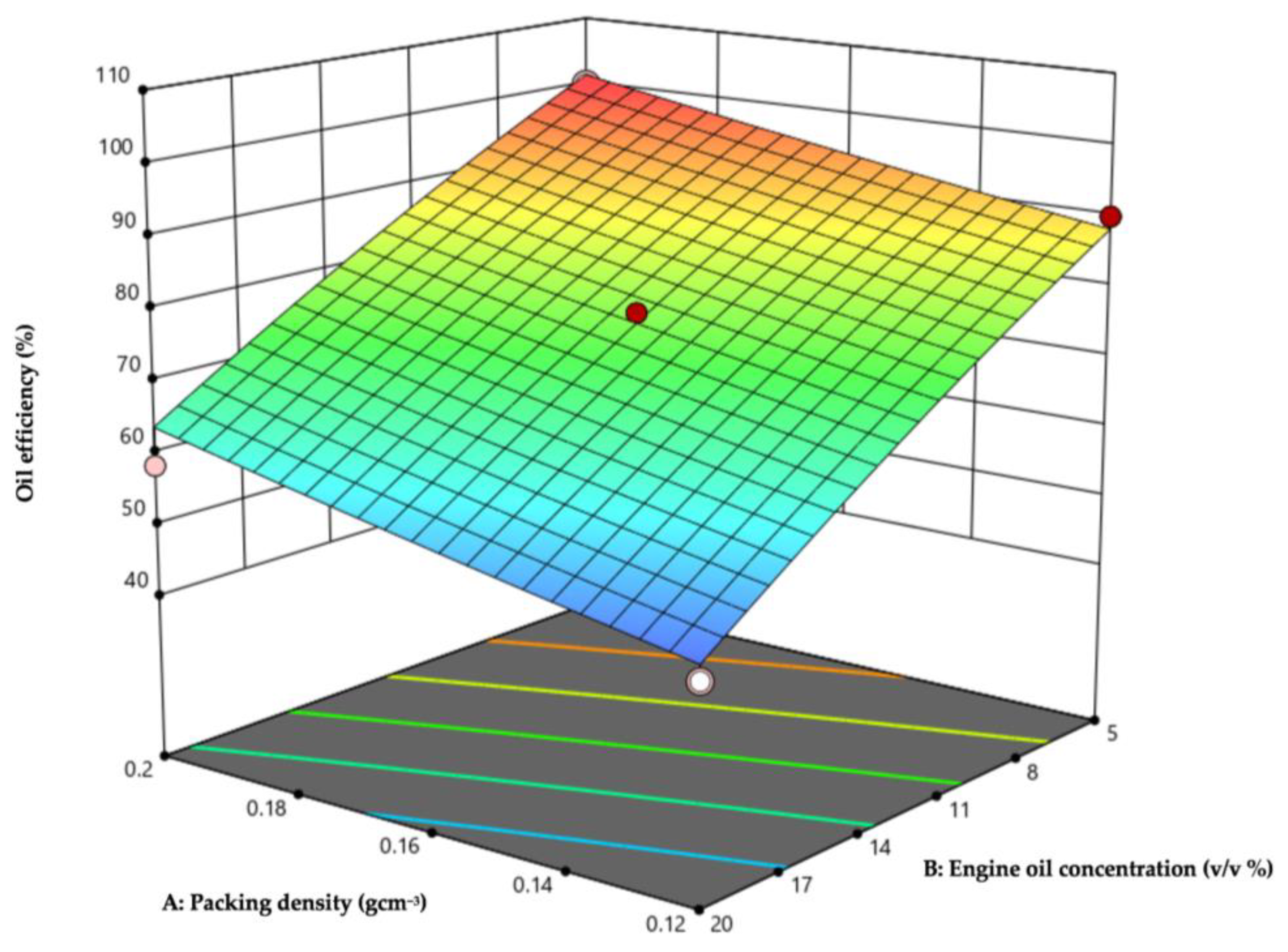
3.4. Response Surface Methodology (RSM) Optimisation
3.4.1. Optimisation of Factors by PBD
3.4.2. Interactions of Significant Factors Analysed Using CCD
3.4.3. RSM Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagy, A.L.; Knaup, J.C.; Zsoldos, I. Investigation of used engine oil lubricating performance through oil analysis and friction and wear measurements. Acta Tech. Jaurinensis. 2019, 12, 237–251. [Google Scholar] [CrossRef]
- Hasan-Zadeh, A.; Poshtiban, M. Car engine oil: Investigation of function and related challenges, and provision of environmental solutions. Asian J. Appl. Sci. 2021, 9, 35–44. [Google Scholar] [CrossRef]
- Rostek, E.; Babiak, M. The experimental analysis of engine oil degradation utilizing selected thermoanalytical methods selected thermoanalytical methods. Transp. Res. Procedia 2019, 40, 82–89. [Google Scholar] [CrossRef]
- Qurashi, I.A.; Swamy, A.K. Viscoelastic properties of recycled asphalt binder containing waste engine oil. J. Clean. Prod. 2018, 182, 992–1000. [Google Scholar] [CrossRef]
- Hameed, D.K. Deterioration in physical engine oil properties after different trip length. Kurd J. Appl. Res. 2021, 6, 13–20. [Google Scholar] [CrossRef]
- Özçelik, A.E.; öğüt, H. Determination of the effects of safflower biodiesel and its blends with diesel fuel on lubricating oil in a single cylinder diesel engine. J. Environ. Sci. Engin. 2012, 1, 1338–1345. [Google Scholar]
- Szyszlak-Bargłowicz, J.; Zając, G.; Wolak, A. Heavy metal content in used engine oils depending on engine type and oil change interval. Arch. Environ. Prot. 2021, 47, 81–94. [Google Scholar] [CrossRef]
- Jahromi, F.A.; Kannan, N.; Zakaria, M.P.; Aris, A.Z. Persistent Contaminants in Waste Oils: A Short Review on PCBs and PAHs as Main Contaminants. In From Sources to Solution; Aris, A.Z., Tengku Ismail, T.H., Harun, R., Abdullah, A.M., Ishak, M.Y., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Aguirre, M.A.; Canals, A.; López-García, I.; Hernández-Córdoba, M. Determination of cadmium in used engine oil, gasoline and diesel by electrothermal atomic absorption spectrometry using magnetic ionic liquid-based dispersive liquid-liquid microextraction. Talanta 2020, 220, 121395. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Chidike Ezeorba, T.P.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging bio-dispersant and bioremediation technologies as environmentally friendly management responses toward marine oil spill: A comprehensive review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Combi, T.; Alexandre, M.R.; Sasaki, S.T.; Zanardi-Lamardo, E.; Yogui, G.T. Mysterious oil spill along Brazil’s northeast and southeast seaboard (2019–2020): Trying to find answers and filling data gaps. Mar. Pollut. Bull. 2020, 156, 111219. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, I.M.K. Impact of Oil Spills on Marine Life. In Emerging Pollutants in the Environment; Larramendy, M.L., Soloneski, S., Eds.; Book Metrics Overview; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Akpan, E. Environmental consequences of oil spills on marine habitats and the mitigating measures—The Niger Delta perspective. J. Geosci. Environ. Prot. 2022, 10, 191–203. [Google Scholar] [CrossRef]
- Xu, C.; Jiao, C.; Yao, R.; Lin, A.; Jiao, W. Adsorption and regeneration of expanded graphite modified by CTAB-KBr/H3PO4 for marine oil pollution. Environ. Poll. 2018, 233, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lutfee, T.; Al-Najar, J.A.; Abdulla, F.M. Removal of oil from produced water using biosorbent. IOP Conf. Ser. Mater. Sci. Eng. 2020, 737, 012198. [Google Scholar] [CrossRef]
- Dagde, K.K. Biosorption of crude oil spill using groundnut husks and plantain peels as adsorbents. Adv. Chem. Engin. Sci. 2018, 8, 161–175. [Google Scholar] [CrossRef]
- Annam, A.R.; Jagadeesan, A.K.; Deivasigamani, P.; Sundararaman, S.; Balakrishna Pillai Sankari, N.P. The bio-adsorption competence of tailor made lemon grass adsorbents on oils: An in-vitro approach. Environ. Res. 2023, 222, 115332. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a process for produced water treatment: A review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Erkey, C.; Türk, M. Thermodynamics and kinetics of adsorption of metal complexes on surfaces from supercritical solutions. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 8, pp. 73–127. [Google Scholar] [CrossRef]
- Andrade, C.A.; Zambrano-Intriago, L.A.; Oliveira, N.S.; Vieira, J.S.; Quiroz-Fernández, L.S.; Rodríguez-Díaz, J.M. Adsorption behavior and mechanism of oxytetracycline on rice husk ash: Kinetics, equilibrium, and thermodynamics of the process. Water Air Soil Pollut. 2020, 231, 103. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil and Gas Produced Water Treatment Technologies; EPA: Washington, DC, USA, 2005.
- El-Nafaty, U.A.; Muhammad, I.M.; Abdulsalam, S. Biosorption and kinetic studies on oil removal from produced water using banana peel. Civil Environ. Res. 2013, 3, 125–136. [Google Scholar]
- Pandia, S.; Hutagalung, A.T.; Siahaan, A.D. Utilization of Cocoa Peel as Biosorbent for oil and color removal in palm oil mill effluent (POME). IOP Conf. Ser. Mater. Sci. Eng. 2018, 300, 012066. [Google Scholar] [CrossRef]
- Pagnucco, R.; Phillips, M.L. Comparative effectiveness of natural by-products and synthetic sorbents in oil spill booms. J. Environ. Manag. 2018, 225, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Fingas, M. Physical Spill Countermeasures. In Oil Spill Science and Technology; Fingas, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Michel, J.; Fingas, M. Oil spills: Causes, consequences, prevention, and countermeasures. Fossil Fuels 2015, 1, 159–201. [Google Scholar] [CrossRef]
- Taufik, S.H.; Ahmad, S.A.; Zakaria, N.N.; Shaharuddin, N.A.; Azmi, A.A.; Khalid, F.E.; Merican, F.; Convey, P.; Zulkharnain, A.; Khalil, A.K. Rice straw as a natural sorbent in a filter system as an approach to bioremediate diesel pollution. Water 2021, 13, 3317. [Google Scholar] [CrossRef]
- Oliveira, L.M.T.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.S.; McKay, G.; Meili, L. Sorption as a rapidly response for oil spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2021, 407, 124842. [Google Scholar] [CrossRef]
- Dong, T.; Wang, F.; Xu, G. Theoretical and experimental study on the oil sorption behavior of kapok assemblies. Ind. Crops Prod. 2014, 325–330. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhaskarwar, A.N. A review on sorbent devices for oil-spill control. Environ. Poll. 2018, 243, 1758–1771. [Google Scholar] [CrossRef]
- Khalid, F.E.; Ahmad, S.A.; Zakaria, N.N.; Shaharuddin, N.A.; Sabri, S.; Azmi, A.A.; Khalil, K.A.; Verasoundarapandian, G.; Gomez-Fuentes, C.; Zulkharnain, A. Application of cogon grass (Imperata cylindrica) as biosorbent in diesel-filter system for oil spill removal. Agronomy 2021, 11, 2273. [Google Scholar] [CrossRef]
- Puasa, N.A.; Ahmad, S.A.; Zakaria, N.N.; Shaharuddin, N.A.; Khalil, K.A.; Azmi, A.A.; Gomez-Fuentes, C.; Merican, F.; Zulkharnain, A.; Kok, Y.-Y.; et al. Utilisation of oil palm’s empty fruit bunch spikelets for oil-spill removal. Agronomy 2022, 12, 535. [Google Scholar] [CrossRef]
- Ali, N.; El-Harbawi, M.; Abo Jabal, A.; Yin, C.-Y. Environmental technology characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: Oil removal suitability matrix. Environ. Technol. 2012, 33, 481–486. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Pete, A.J.; Bharti, B.; Benton, M.G. Nano-enhanced bioremediation for oil spills: A review. ACS EST Engg. 2021, 1, 928–946. [Google Scholar] [CrossRef]
- Azat, S.; Korobeinyk, A.V.; Moustakas, K.; Inglezakis, V.J. Sustainable production of pure silica from rice husk waste in Kazakhstan. J. Clean. Prod. 2019, 217, 352–359. [Google Scholar] [CrossRef]
- Al-Doury, M.K.W.; Hettiarachchy, N.S.; Horax, R. Rice-endosperm and rice-bran proteins: A review. J. Am. Oil Chem. Soc. 2018, 95, 943–956. [Google Scholar] [CrossRef]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of biogenic silica generated by thermo chemical treatment of rice husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Liu, J.; Xie, C.; Fu, C.; Wei, X.; Wu, D. Hydrochloric acid pretreatment of different types of rice husk ash influence on the properties of cement paste. Materials 2020, 13, 1524. [Google Scholar] [CrossRef]
- Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of sorbents for oil spill cleanup focusing on natural-based modified materials: A review. Molecules 2020, 25, 4522. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.-H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef] [PubMed]
- El Gheriany, I.A.; Ahmad El Saqa, F.; Abd El Razek Amer, A.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Zakaria, N.N.; Shaharuddin, N.A.; Khalil, K.A.; Puasa, N.A.; Azmi, A.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Wong, C.Y.; Rahman, M.F.; et al. Coco peat as agricultural waste sorbent for sustainable diesel-filter system. Plants 2021, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Hamid, R.; Osman, S.A. Physical and chemical modifications of plant fibres for reinforcement in cementitious composites. Adv. Civ. Eng. 2019, 2019, 5185806. [Google Scholar] [CrossRef]
- Li, W.C.; Law, F.Y.; Chan, Y.H.M. Biosorption studies on copper (II) and cadmium (II) using pretreated rice straw and rice husk. Environ. Sci. Pollut. Res. 2015, 24, 8903–8915. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Yu, C.-Y.; Chen, Y.-C. Preparation and characteristics of polyurethane made with polyhydric alco-hol-liquefied rice husk. J. Appl. Polym. Sci. 2017, 135, 45910. [Google Scholar] [CrossRef]
- Anuzyte, E.; Vaisis, V. Natural oil sorbents modification methods for hydrophobicity improvement. Energy Procedia 2018, 147, 295–300. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Yarmo, M.A.; Sopian, K. Effect of chemical treatments on rice husk (RH) water absorption property. Int. J. Chem. Eng. Appl. 2015, 6, 273. [Google Scholar] [CrossRef]
- Nassar, H.N.; El-azab, W.I.M.; El-Gendy, N.S. Sustainable ecofriendly recruitment of bioethanol fermentation lignocellulosic spent waste biomass for the safe reuse and discharge of petroleum production produced water via biosorp-tion and solid biofuel production. J. Hazard. Mater. 2022, 422, 126845. [Google Scholar] [CrossRef]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Alam, M.K.; Bell, R.W.; Hasanuzzaman, M.; Salahin, N.; Rashid, M.H.; Akter, N.; Akhter, S.; Islam, M.S.; Islam, S.; Naznin, S.; et al. Rice (Oryza sativa L.) establishment techniques and their implications for soil properties, global warming potential mitigation and crop yields. Agronomy 2020, 10, 888. [Google Scholar] [CrossRef]
- Wong, C.; McGowan, T.; Bajwa, S.G.; Bajwa, D.S. Impact of fiber treatment on the oil absorption characteristics of plant fibers. Bioresources 2016, 11, 6452–6463. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Shen, H.; Xu, G. Evaluation of oil sorption kinetics behavior and wetting characteristic of cattail fiber. Cellulose 2020, 27, 1531–1541. [Google Scholar] [CrossRef]



| Run | A | B | C | D | Response 1 Oil Efficiency (%) | Response 2 Water Efficiency (%) |
|---|---|---|---|---|---|---|
| 1 | 180 | 30 | 0.12 | 5.0 | 81.67 | 9.17 |
| 2 | 165 | 45 | 0.16 | 12.5 | 76.67 | 9.33 |
| 3 | 165 | 45 | 0.16 | 12.5 | 76.00 | 8.83 |
| 4 | 180 | 60 | 0.20 | 5.0 | 97.33 | 10.08 |
| 5 | 150 | 60 | 0.20 | 20.0 | 62.50 | 9.25 |
| 6 | 150 | 30 | 0.20 | 5.0 | 98.50 | 9.75 |
| 7 | 165 | 45 | 0.16 | 12.5 | 74.67 | 8.42 |
| 8 | 180 | 60 | 0.12 | 5.0 | 96.50 | 9.33 |
| 9 | 150 | 30 | 0.12 | 20.0 | 45.00 | 8.67 |
| 10 | 165 | 45 | 0.16 | 12.5 | 76.67 | 9.41 |
| 11 | 150 | 60 | 0.12 | 20.0 | 49.58 | 9.25 |
| 12 | 180 | 30 | 0.20 | 20.0 | 62.50 | 9.17 |
| 13 | 150 | 30 | 0.12 | 5.0 | 81.67 | 7.58 |
| 14 | 165 | 45 | 0.16 | 12.5 | 77.33 | 9.47 |
| 15 | 180 | 60 | 0.12 | 20.0 | 54.58 | 10.92 |
| 16 | 165 | 45 | 0.16 | 12.5 | 80.00 | 9.58 |
| 17 | 150 | 60 | 0.20 | 5.0 | 98.67 | 9.25 |
| 18 | 180 | 30 | 0.20 | 20.0 | 66.67 | 9.50 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 4384.84 | 4 | 1096.21 | 96.73 | <0.0001 | significant |
| A: Temperature | 45.37 | 1 | 45.37 | 4.00 | 0.0685 | |
| B: Time | 44.72 | 1 | 44.72 | 3.95 | 0.0703 | |
| C: Packing density | 496.22 | 1 | 496.22 | 43.79 | <0.0001 | |
| D: Engine oil concentration | 3798.52 | 1 | 3798.52 | 335.20 | <0.0001 | |
| Curvature | 21.01 | 1 | 21.01 | 1.85 | 0.1984 | |
| Residual | 135.99 | 12 | 11.33 | |||
| Lack of Fit | 111.60 | 6 | 18.60 | 4.58 | 0.0433 | significant |
| Pure Error | 24.38 | 6 | 4.06 | |||
| Cor Total | 4541.83 | 17 | ||||
| Std. Dev. | 3.37 | R2 | 0.9699 | |||
| Mean | 75.36 | Adjusted R2 | 0.9599 | |||
| C.V.% | 4.47 | Predicted R2 | 0.9168 | |||
| Adeq precision | 20.125 | |||||
| Run | A | B | Response 1 Oil Efficiency (mL) | Response 2 Water Efficiency (mL) |
|---|---|---|---|---|
| 1 | 0.12 | 5.00 | 90.00 | 7.92 |
| 2 | 0.16 | 12.50 | 75.33 | 9.00 |
| 3 | 0.16 | 12.50 | 78.00 | 8.92 |
| 4 | 0.16 | 12.50 | 78.00 | 8.92 |
| 5 | 0.12 | 20.00 | 46.25 | 7.67 |
| 6 | 0.20 | 20.00 | 58.33 | 8.83 |
| 7 | 0.20 | 5.00 | 99.83 | 9.50 |
| 8 | 0.16 | 1.89 | 100.00 | 8.25 |
| 9 | 0.16 | 12.50 | 78.00 | 8.67 |
| 10 | 0.16 | 12.50 | 73.33 | 8.83 |
| 11 | 0.22 | 12.50 | 91.33 | 9.42 |
| 12 | 0.10 | 12.50 | 66.67 | 8.67 |
| 13 | 0.16 | 23.11 | 50.96 | 9.42 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 3408.87 | 5 | 681.77 | 49.14 | <0.0001 | significant |
| A-Packing density | 403.29 | 1 | 403.29 | 29.07 | 0.0010 | |
| B-Engine oil concentration | 2988.10 | 1 | 2988.10 | 215.36 | <0.0001 | |
| AB | 1.27 | 1 | 1.27 | 0.0912 | 0.7714 | |
| A2 | 0.7337 | 1 | 0.7337 | 0.0529 | 0.8247 | |
| B2 | 14.36 | 1 | 14.36 | 1.04 | 0.3429 | |
| Residual | 97.12 | 7 | 13.87 | |||
| Lack of Fit | 78.99 | 3 | 26.33 | 5.81 | 0.0611 | not significant |
| Pure Error | 18.13 | 4 | 4.53 | |||
| Cor Total | 3505.99 | 12 | ||||
| Std. Dev. | 3.72 | R2 | 0.9723 | |||
| Mean | 75.85 | Adjusted R2 | 0.9525 | |||
| C.V.% | 4.91 | Predicted R2 | 0.8317 | |||
| Adeq precision | 21.7175 | |||||
| Optimised Factors | Value | Predicted Value | Experimental Value |
|---|---|---|---|
| Packing density | 0.16 g/cm3 | 49 mL of engine oil with 30 mL of water * | 49 mL of engine oil with 30 mL of water ±1 mL * |
| Engine oil concentration | 12.5% (v/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminuddin, I.H.; Taufik, S.H.; Puasa, N.A.; Radziff, S.B.M.; Zamree, N.D.; Shaharudddin, N.A.; Che Abdullah, C.A.; Rahman, M.F.; Azmi, A.A.; Ahmad, S.A. Biosorption of Engine Oil Using Rice Husk in a Filtration System. Sustainability 2023, 15, 14599. https://doi.org/10.3390/su151914599
Aminuddin IH, Taufik SH, Puasa NA, Radziff SBM, Zamree ND, Shaharudddin NA, Che Abdullah CA, Rahman MF, Azmi AA, Ahmad SA. Biosorption of Engine Oil Using Rice Husk in a Filtration System. Sustainability. 2023; 15(19):14599. https://doi.org/10.3390/su151914599
Chicago/Turabian StyleAminuddin, Irfan Hafeez, Siti Hajar Taufik, Nurul Aini Puasa, Syahirah Batrisyia Mohamed Radziff, Nur Diyanah Zamree, Noor Azmi Shaharudddin, Che Azurahanim Che Abdullah, Muhammad Fahdli Rahman, Alyza Azzura Azmi, and Siti Aqlima Ahmad. 2023. "Biosorption of Engine Oil Using Rice Husk in a Filtration System" Sustainability 15, no. 19: 14599. https://doi.org/10.3390/su151914599
APA StyleAminuddin, I. H., Taufik, S. H., Puasa, N. A., Radziff, S. B. M., Zamree, N. D., Shaharudddin, N. A., Che Abdullah, C. A., Rahman, M. F., Azmi, A. A., & Ahmad, S. A. (2023). Biosorption of Engine Oil Using Rice Husk in a Filtration System. Sustainability, 15(19), 14599. https://doi.org/10.3390/su151914599









