Contamination and Risk of Heavy Metals in Sediments from Zhuzhou, Xiangtan and Changsha Sections of the Xiangjiang River, Hunan Province of China
Abstract
:1. Introduction
2. Materials and Methods
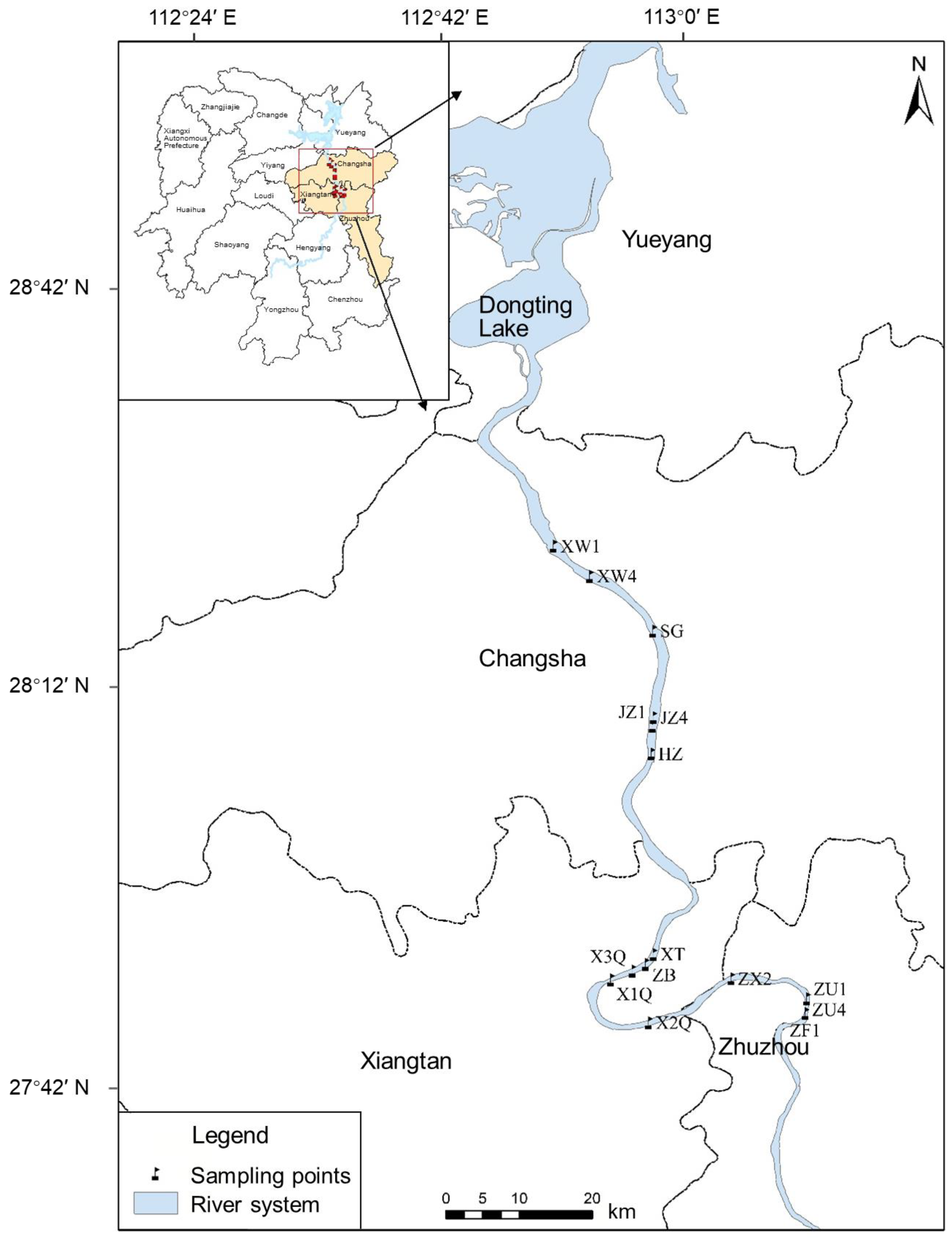
2.1. Study Area
2.2. Sampling, Analysis, and Quality Control
- (1)
- ZZ River section: including three sampling points, namely Glass Factory (ZF), Shifeng Bridge (ZU), and Xiawan (ZX).
- (2)
- XT River section: including five sampling points, namely Xiangtan Second Bridge (X2Q), Xiangtan First Bridge (X1Q), Xiangtan Three Bridges (X3Q), Zhubu Port (ZB), and Fengtan Bridge (XT).
- (3)
- CS River Section: including four sampling points, namely Monkey Stone (HZ1), Orange Island (JZ), Sanchaji (SG), and Xia Ning (XW).
- (1)
- Forty milligrams of powdered sample were added into a Teflon container with the addition of a 1:1 HNO3 (0.8 mL) and HF (0.8 mL) mixture and three times HClO4 (about 2.4 mL), followed by a seal and shake ultrasonic for 60 s, and heated at a constant 100 °C temperature for 48 h and then evaporated.
- (2)
- Furthermore, 0.8 mL of HNO3 was added, followed by heating at 100 °C for 24 h and evaporating. Furthermore, after the addition of HF (0.8 mL) and HClO4 (0.8 mL), the treated samples were sealed in an autoclave and put into an oven set to 170 °C for 48 h, where they evaporated to dry.
- (3)
- Finally, the treated samples were put into the oven at 170 °C for 4 h after adding 4 mL of 4N HNO3. After that, the mixture was diluted with HNO3 (3%), transferred into a 50 mL volumetric flask, and then used as an internal standard solution for Rh-Re, diluted with 1% HNO3 to 40 g, and reserved for the following ICP-MS analysis.
2.3. Enrichment of Heavy Metals
2.4. Ecological Assessment of Heavy Metals
2.4.1. Geoaccumulation Index
2.4.2. Potential Ecological Risk Index
2.4.3. Ecological Risk Index
3. Results and Discussion
3.1. Physical and Chemical Properties of Sediments
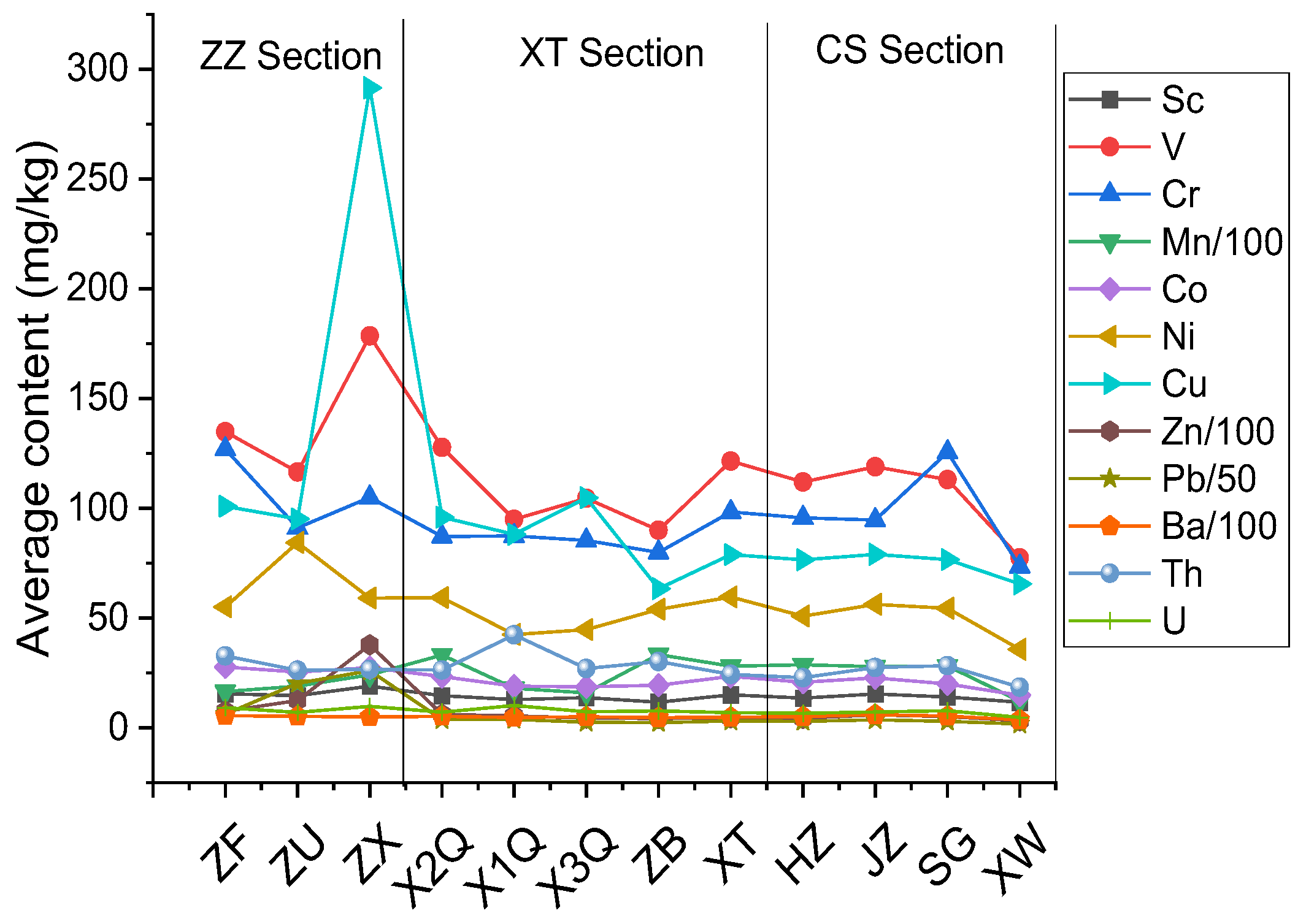
3.2. Distribution of Heavy Metals
3.3. Assessment of Heavy Metal Pollution and Potential Ecological Risk
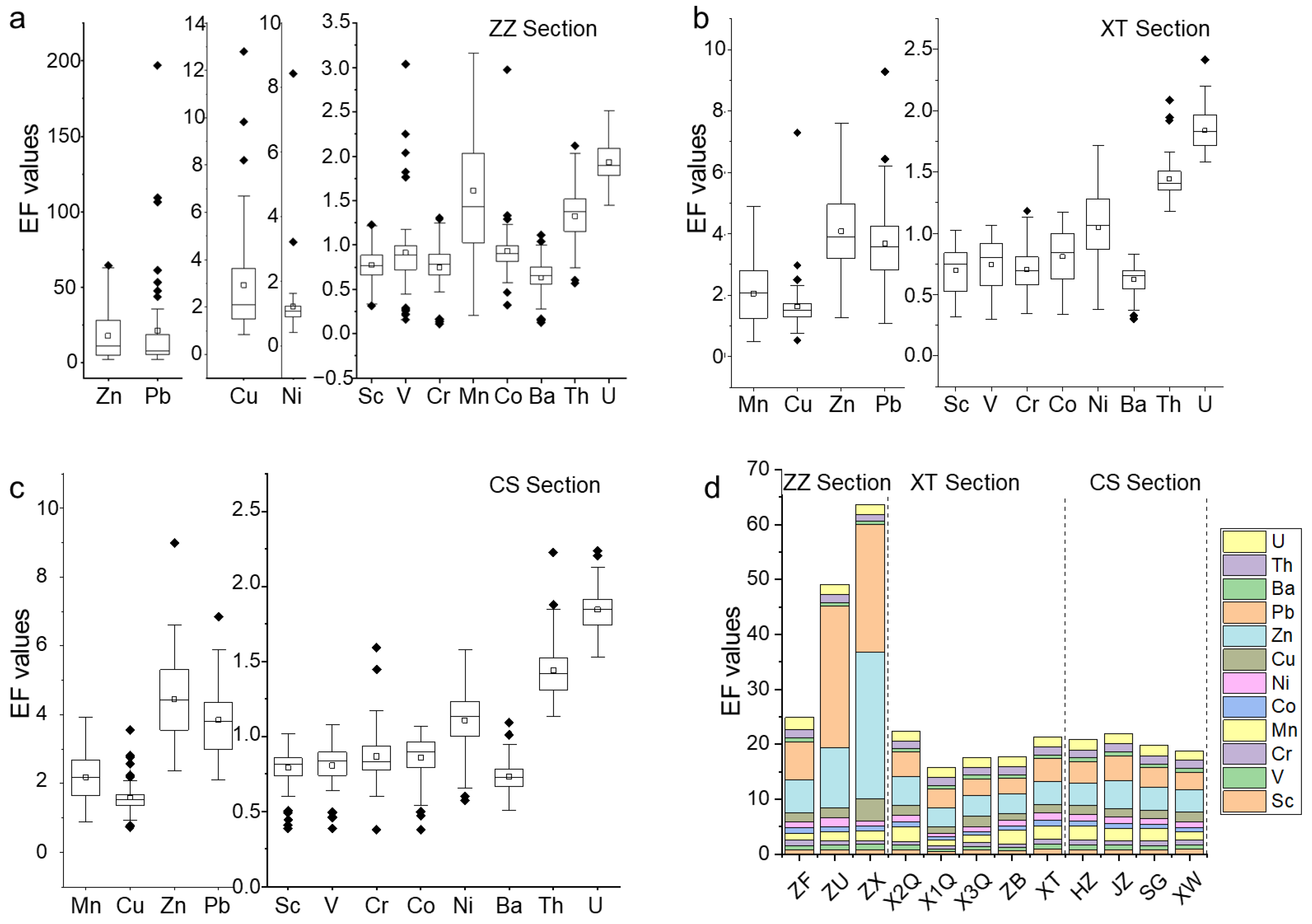
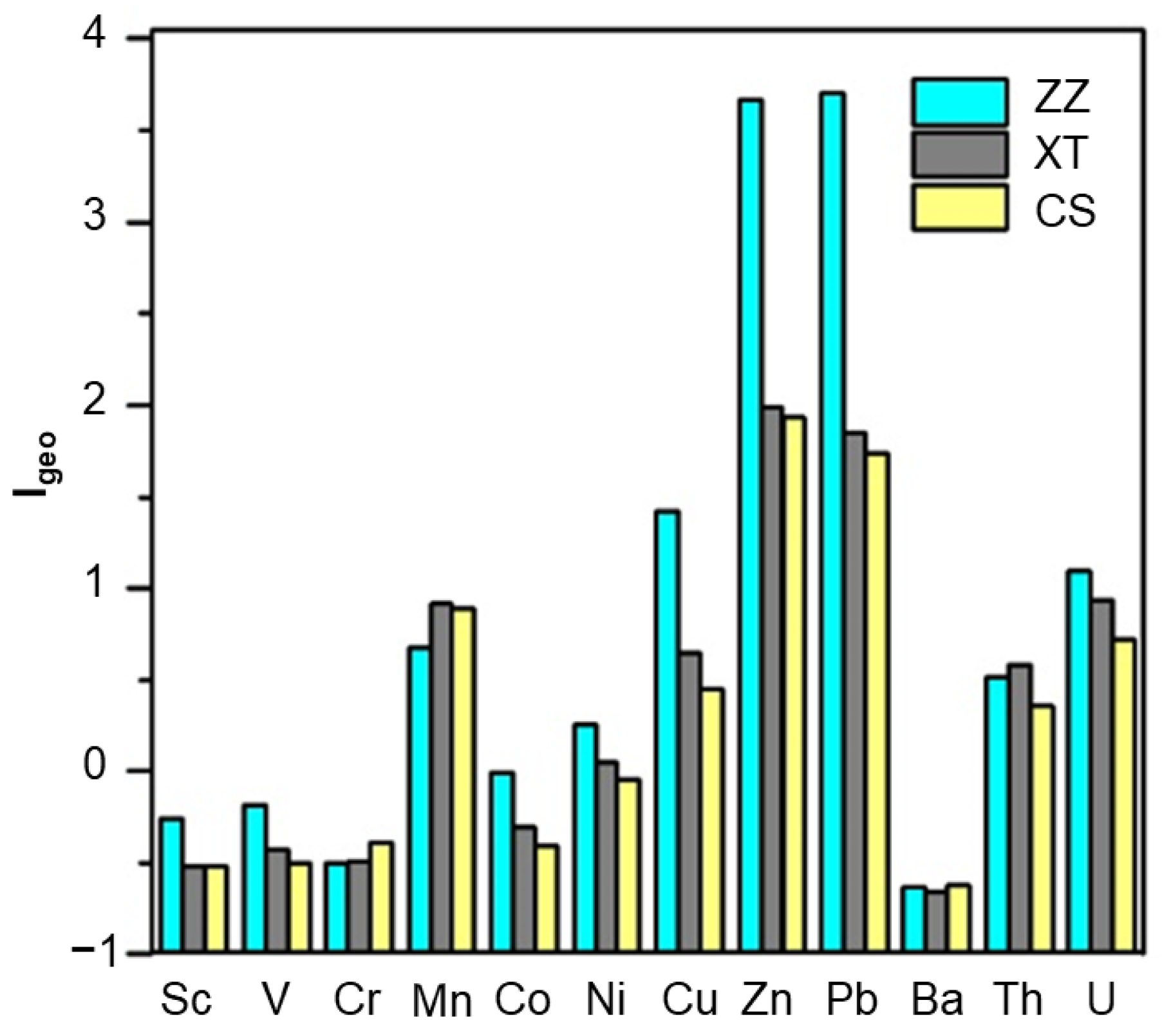
3.3.1. Assessment of Heavy Metal Pollution
3.3.2. Assessment of Potential Ecological Risk
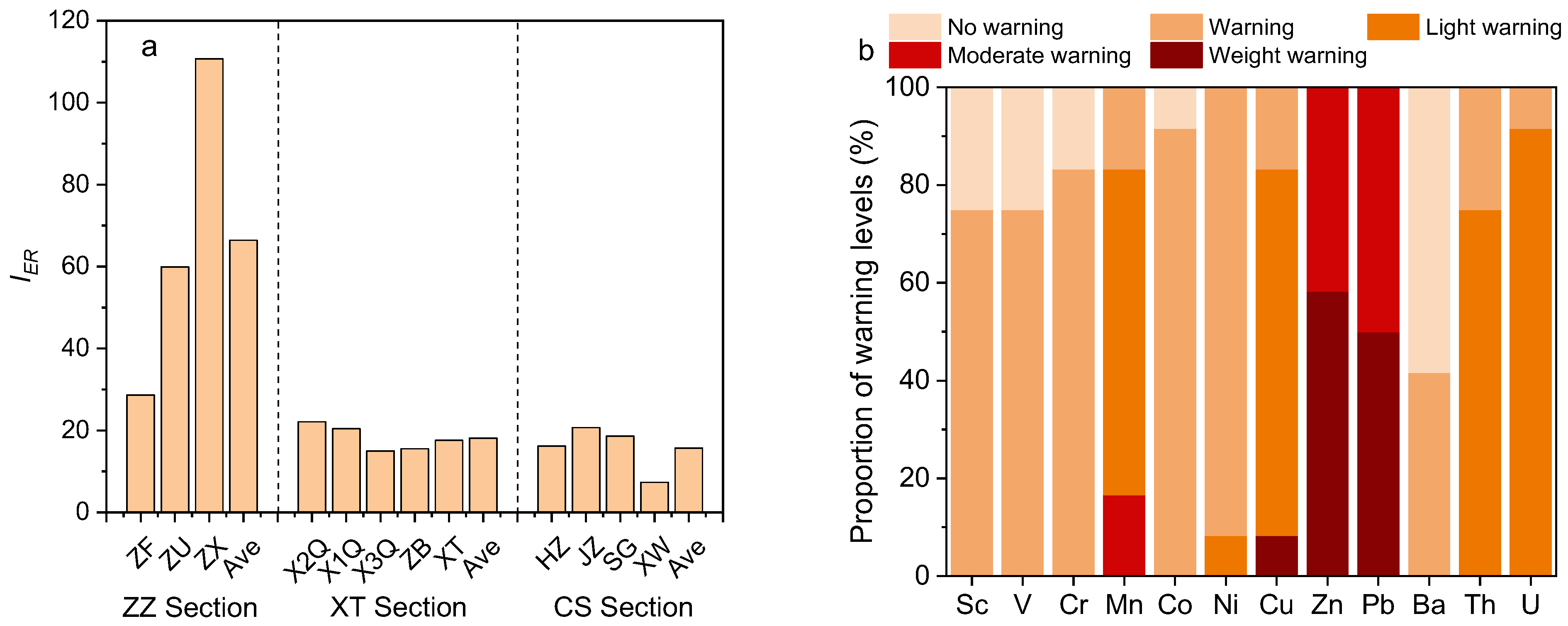
3.3.3. Ecological Risk Early Warning
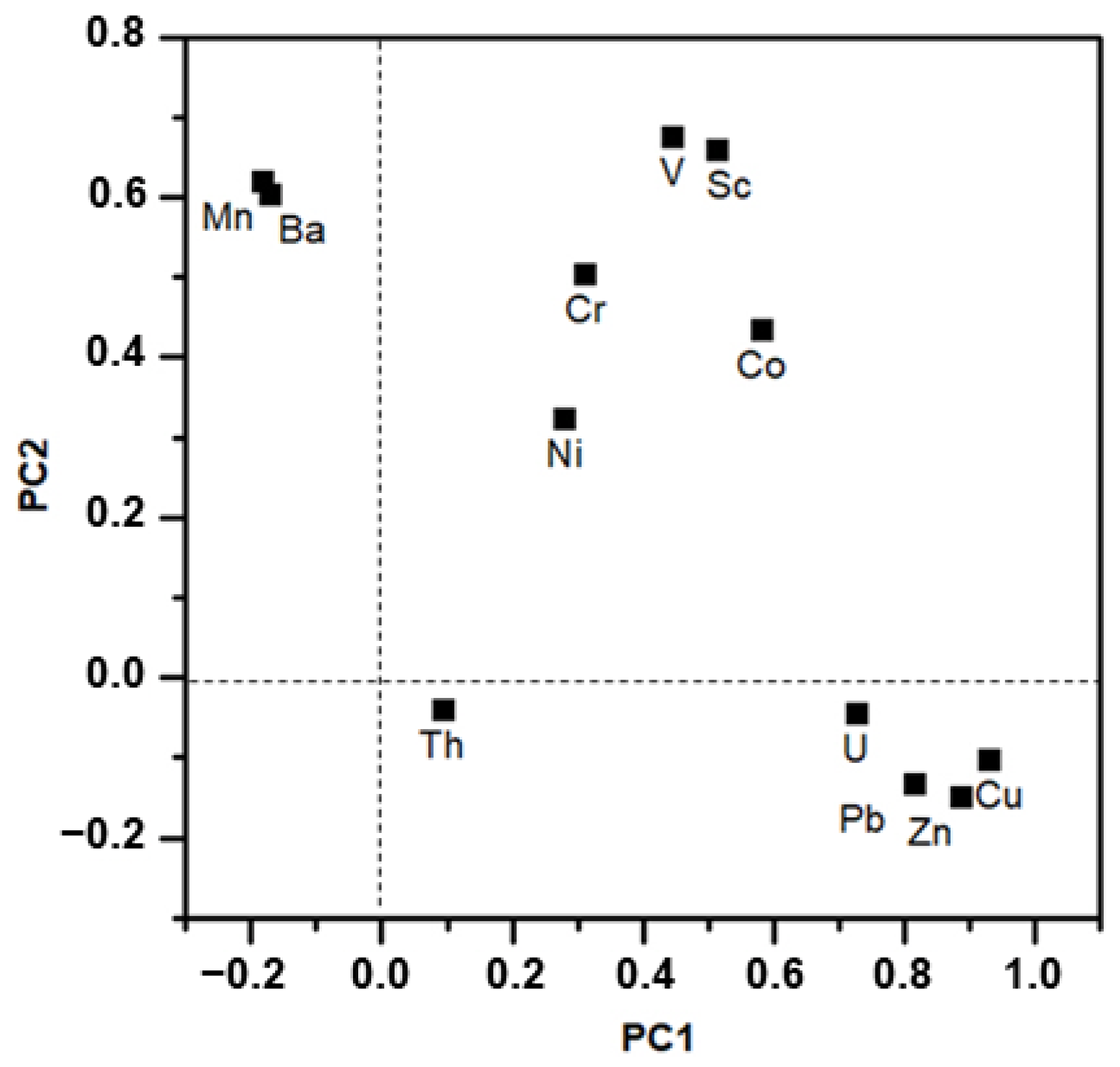
3.4. Analysis of Heavy Metal Sources
3.5. Prevention and Control Measures for Heavy Metal Pollution
- (1)
- Establish a dynamic monitoring and warning system for long-term sediment risk sources.
- (2)
- Improve the source control of industrial enterprises involved in heavy metal emissions.
- (3)
- Pollution source treatment of industrial enterprises involved in heavy metal emissions.
- (4)
- Dredging of heavily polluted river sections.
4. Conclusions
- (1)
- The heavy metal element contents of each sedimentary column in the ZZ section exhibited significant variation with a high degree of enrichment, some of which were much higher than those of other columns in the CS and XT sections. The influence of anthropogenic activities (metal mineral processing, metal product smelting, wastewater discharge in industrial parks, etc.) on the heavy metal element content was obvious.
- (2)
- Results of the Geoaccumulation index indicated that the ZZ section had the largest number of heavy metal pollution elements and the strongest degree of pollution, followed by the XT section and the CS section. Potential ecological risk index results suggested strong ecological risks of heavy metals in sediments at ZU and ZX points in the ZZ section, and the risk degree of heavy metals was Pb > Cu > Zn > Ni > Co > Mn > V > Cr.
- (3)
- The ecological risk early warning levels of heavy metal in sediments followed: ZZ section (IER = 66.4) > XT section (IER = 18.1) > CS section (IER = 15.7). Zn and Pb exhibited the highest early warning degree (severe warning proportion ≥ 50%), and thus special attention should be paid to the prevention and control of heavy metal pollution in the ZZ section, especially the pollution of Zn and Pb.
- (4)
- The distribution of studied elements in sediment included: Th, U, Pb, Zn, Cu, and Mn were obviously enriched, which were mainly controlled by natural and anthropogenic sources, while Ni, Cr, Co, V, Sc, and B had no obvious enrichment characteristics, mainly from natural sources.
- (5)
- Strategies and measures for the prevention and control of sediment heavy metal pollution, including the establishment of a dynamic monitoring and warning system, source control and pollution source treatment improvements, and suitable dredging, were proposed based on the characteristics of the heavy metal pollution.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Chen, X.; Cheng, C.; Wang, D.; Wang, W. Variability of streambed vertical hydraulic conductivity with depth along the Elkhorn River, Nebraska, USA. Chin. Sci. Bull. 2009, 54, 3892–3899. (In Chinese) [Google Scholar] [CrossRef]
- Lei, M.; Qin, P.; Tie, B. Current situation and analysis of heavy metal pollution in the Xiangjiang River basin of Hunan province. J. Agric. Resour. Environ. 2010, 27, 62–65. (In Chinese) [Google Scholar]
- Wayne Nesbitt, H.; Markovics, G. Weathering of granodioritic crust, long-term storage of elements in weathering profiles, and petrogenesis of siliciclastic sediments. Geochim. Cosmochim. Acta. 1997, 61, 1653–1670. [Google Scholar] [CrossRef]
- Sun, G.X.; Wang, X.J.; Hu, Q.H. Using stable lead isotopes to trace heavy metal contamination sources in sediments of Xiangjiang and Lishui Rivers in China. Environ. Pollut. 2011, 159, 3406–3410. [Google Scholar] [CrossRef]
- García-Pereira, F.J.; Giménez, R.G.; Villa, R.V.D.L.; Procopio, J.R. Heavy metal fractionation in sediments from the Jarama River (central Spain). Environ. Earth Sci. 2015, 73, 2385–2396. [Google Scholar] [CrossRef]
- Mao, M.; Liu, Z.; Wei, J. Study on the chemical morphology of heavy metals in the surface sediment of the Xiangjiang River. Environ. Sci. 1987, 5, 35–41. (In Chinese) [Google Scholar]
- Guo, Z.-H.; Song, J.; Xiao, X.-Y.; Ming, H.; Miao, X.-F.; Wang, F.-Y. Spatial distribution and environmental characterization of sediment-associated metals from middle-downstream of Xiangjiang River, southern China. J. Cent. South Univ. Technol. 2010, 17, 68–78. [Google Scholar] [CrossRef]
- Bao, Z.; Bo, P.; Xu, J.; Tan, C.; Quan, M. Geochemical study on the relation of chemical compositions to heavy metal contamination of sedimens from the lowermost Xiangjiang River, Hunan Province, China. Ceochimica 2012, 41, 545–558. (In Chinese) [Google Scholar]
- Guo, L. The Research on the Teatment of Water Pollutions and the Water Resoures Proteetion in the Urbanization Process of Changsha—Zhuzhou—Xiangtan Urban Group. Master’s Thesis, Hunan University, Changsha, China, 2004. (In Chinese). [Google Scholar]
- Huang, Z.; Zheng, S.; Liu, Y.; Zhao, X.; Qiao, X.; Liu, C.; Zheng, B.; Yin, D. Distribution, toxicity load, and risk assessment of dissolved metal in surface and overlying water at the Xiangjiang River in southern China. Sci. Rep. 2021, 11, 109. [Google Scholar] [CrossRef]
- Lu, J.; Cai, H.; Fu, Y.; Zhang, X.; Zhang, W. A study on the impacts of landscape structures on water quality under different spatial scales in the Xiangjiang River Basin. Water Air Soil Pol. 2022, 233, 164. [Google Scholar] [CrossRef]
- Wen, T.; Zhong, H.; Fu, G.; Zhong, Y. Effects of environmental factors on release amount of heavy metal and structure of microbial community in sediments. Int. J. Environ. Sci. Technol. 2022, 19, 4007–4018. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, W.; Luo, H.; He, L.; Liu, H.; Xu, R.; Zhang, L.; Wang, Y.; Wu, G.; Wei, F. Assessing the risk of total and available potentially toxic elements in agricultural soil in typical mining areas in Xiangjiang River basin, Hunan Province. Minerals 2021, 11, 953. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Liu, L.; Li, L.; Lin, H.; Wu, X.; Bi, W.; Wang, L.; Mao, D.; Luo, Y. The prevalence of ampicillin-resistant opportunistic pathogenic bacteria undergoing selective stress of heavy metal pollutants in the Xiangjiang River, China. Environ. Pollut. 2021, 268, 115362. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Peng, B.; Wang, X.; Song, Z.; Zhou, D.; Wang, Q.; Qin, Z.; Tan, C. Distribution, contamination and source identification of heavy metals in bed sediments from the lower reaches of the Xiangjiang River in Hunan province, China. Sci. Total Environ. 2019, 689, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y. Background values of some metals in rocks, soil and water in Xiangjiang basin. Acta Sci. Circumstantiae 1984, 4, 17–32. (In Chinese) [Google Scholar]
- Huang, Z.; Liu, C.; Zhao, X.; Dong, J.; Zheng, B. Risk assessment of heavy metals in the surface sediment at the drinking water source of the Xiangjiang River in South China. Environ. Sci. Eur. 2020, 32, 23. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Xu, G.; Chen, B. Distribution and transport of heavy metals in surface sediments of the Zhejiang nearshore area, East China Sea: Sedimentary environmental effects. Mar. Pollut. Bull. 2019, 146, 542–551. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Xiao, X.; Chen, T.; Liao, X.; Song, J.; Wu, B. Heavy metal pollution of soils and vegetables in the midstream and downstream of the Xiangjiang River, Hunan Province. J. Geogr. Sci. 2008, 18, 353–362. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Wu, H.; Wang, S.; Li, F. Probabilistic ecological risk assessment of heavy metals in sediments from China’s major aquatic bodies. Stoch. Environ. Res. Risk Assess. 2016, 30, 271–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, F.; Du, J.; Shi, P.; Yu, D.; Meng, Y.; Sun, Y. Surface water quality and its control in a river with intensive human impacts–A case study of the Xiangjiang River, China. J. Environ. Manag. 2010, 91, 2483–2490. [Google Scholar] [CrossRef]
- Sun, W.; Sang, L.; Jiang, B. Trace metals in sediments and aquatic plants from the Xiangjiang River, China. J. Soils Sed. 2012, 12, 1649–1657. [Google Scholar] [CrossRef]
- Li, J.; Zeng, B.; Yao, Y.; Zhang, L.; Qiu, C. Study on the environmental background value of the Dongting Lake water system. J. Environ. Sci. 1986, 7, 62–68. (In Chinese) [Google Scholar]
- Wu, S.; Peng, B.; Fang, X.; Xie, S.; Li, X.; Jiang, C.; Dai, Y. Distribution and assessment of cadmium contamination in sediments from the Four River inlets to Dongting Lake, China. Environ. Sci. Pollut. Res. 2021, 286, 6072–6085. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Tittonell, P.A. An Evaluation of the Loss-on-Ignition Method for Determining the Soil Organic Matter Content of Calcareous Soils. Commun. Soil Sci. Plant Anal. 2018, 49, 1541–1552. [Google Scholar] [CrossRef]
- Ying, L.; Haichen, L.; Xianhua, L. Simultaneous and precise determination of 40 trace elements in rock samples using ICP-MS. Geochimica 1996, 25, 552–558. (In Chinese) [Google Scholar]
- Duzgoren-Aydin, N.S. Sources and characteristics of lead pollution in the urban environment of Guangzhou. Sci. Total Environ. 2007, 385, 182–195. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhu, X.; Xi, D.; Li, J. Distribution of anthropogenic lead estimated by Pb isotopic composition in the upper layers of soil from a mixed forest at Dinghushan, southern China. J. Soils Sed. 2013, 13, 394–402. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Rapant, S.; Kordík, J. An environmental risk assessment map of the Slovak Republic: Application of data from geochemical atlases. Environ. Geol. 2003, 44, 400–407. [Google Scholar] [CrossRef]
- Gao, S.; Luo, T.; Zhang, B.; Zhang, H.; Han, Y.; Zhao, Z. The Structure and Composition of the Crust in Eastern China. Sci. China Ser. D 1999, 29, 204–213. (In Chinese) [Google Scholar]
- Yao, Z.; Bao, Z.; Gao, P. Environmental geochemistry of heavy metals in the Dongting Lake sediments. Geochimica 2006, 35, 629–638. (In Chinese) [Google Scholar]
- Shi, C.; Yan, M.; Chi, Q. Abundances of chemical elements of granitoids in different geotectonic units of China and their characteristics. Acta Geol. Sin. 2007, 81, 47–59. (In Chinese) [Google Scholar] [CrossRef]
- Yan, M.; Gu, T.; Chi, Q.; Wang, C. Abundances of elements of China soils and surface geochemical properties of elements. Inst. Geophys. Geochem. Explor. 1997, 21, 161–167. (In Chinese) [Google Scholar]
- Bayon, G.; Toucanne, S.; Skonieczny, C.; André, L.; Bermell, S.; Cheron, S.; Dennielou, B.; Etoubleau, J.; Freslon, N.; Gauchery, T.; et al. Rare earth elements and neodymium isotopes in world river sediments revisited. Geochim. Cosmochim. Acta 2015, 170, 17–38. [Google Scholar] [CrossRef]
- Hinrichs, J.; Dellwig, O.; Brumsack, H.J. Lead in sediments and suspended particulate matter of the German Bight: Natural versus anthropogenic origin. Appl. Geochem. 2002, 17, 621–632. [Google Scholar] [CrossRef]
- Peng, B.; Tang, X.; Yu, C.; Tan, C.; Tu, X.; Liu, Q.; Yang, K.; Xiao, M.; Xu, J. Heavy metal contamination of inlet sediments of the Xiangjiang River and Pb isotopic geochemical implication. Acta Geol. Sin. 2011, 85, 282–299. (In Chinese) [Google Scholar]
- Song, J.; Yang, X.; Zhang, J.; Long, Y.; Zhang, Y.; Zhang, T. Assessing the variability of heavy metal concentrations in liquid-solid two-phase and related environmental risks in the Weihe River of Shaanxi Province, China. Int. J. Environ. Res. Public Health 2015, 12, 8243–8262. [Google Scholar] [CrossRef]
- Ahamad, M.I.; Song, J.; Sun, H.; Wang, X.; Mehmood, M.S.; Sajid, M.; Su, P.; Khan, A.J. Contamination level, ecological risk, and source identification of heavy metals in the hyporheic zone of the Weihe River, China. Int. J. Environ. Res. Public Health. 2020, 17, 1070. [Google Scholar] [CrossRef]
- Wang, X.; Su, P.; Lin, Q.; Song, J.; Sun, H.; Cheng, D.; Wang, S.; Peng, J.; Fu, J. Distribution, assessment and coupling relationship of heavy metals and macroinvertebrates in sediments of the Weihe River Basin. Sustain. Cities Soc. 2019, 50, 101665. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, L.; Ma, J.; Cai, Z. Ree Geochemistry of Sediments from West Philippine Sea. Acta Geol. Sin. 1996, 16, 260–267. (In Chinese) [Google Scholar]
- Singh, P. Major, trace and REE geochemistry of the Ganga River sediments: Influence of provenance and sedimentary processes. Chem. Geol. 2009, 266, 242–255. [Google Scholar] [CrossRef]
- Rousseau, T.C.C.; Roddaz, M.; Moquet, J.-S.; Handt Delgado, H.; Calves, G.; Bayon, G. Controls on the geochemistry of suspended sediments from large tropical South American rivers (Amazon, Orinoco and Maroni). Chem. Geol. 2019, 522, 38–54. [Google Scholar] [CrossRef]
- Lin, S.; Hsieh, I.J.; Huang, K.M.; Wang, C.H. Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem. Geol. 2002, 182, 377–394. [Google Scholar] [CrossRef]

| Major Elements (%) | Zhuzhou (ZZ, n = 72) | Xiangtan (XT, n = 132) | Changsha (CS, n = 94) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Ave | SD | CV | Min | Max | Ave | SD | CV | Min | Max | Ave | SD | CV | |
| SiO2 | 26.98 | 77.99 | 56.562 | 14.35 | 0.254 | 53.61 | 74.82 | 64.067 | 4.824 | 0.075 | 55.78 | 78.07 | 64.928 | 5.593 | 0.086 |
| TiO2 | 0.74 | 5.87 | 1.511 | 1.237 | 0.819 | 0.78 | 1.13 | 0.906 | 0.077 | 0.085 | 0.62 | 1 | 0.863 | 0.073 | 0.085 |
| Al2O3 | 8.34 | 18.61 | 13.468 | 2.711 | 0.201 | 9.59 | 18.71 | 14.394 | 2.48 | 0.172 | 8.65 | 18.4 | 14.715 | 2.458 | 0.167 |
| Fe2O3 | 4.39 | 44.05 | 11.735 | 11.731 | 1 | 5.21 | 10.93 | 7.18 | 1.151 | 0.16 | 4.07 | 9.25 | 6.645 | 1.12 | 0.169 |
| MnO | 0.07 | 0.32 | 0.204 | 0.055 | 0.268 | 0.06 | 0.64 | 0.309 | 0.129 | 0.416 | 0.11 | 0.61 | 0.304 | 0.114 | 0.377 |
| MgO | 0.76 | 1.29 | 0.987 | 0.127 | 0.129 | 0.76 | 1.43 | 1.083 | 0.157 | 0.145 | 0.72 | 1.49 | 1.066 | 0.164 | 0.154 |
| CaO | 0.56 | 12.53 | 2.62 | 2.892 | 1.104 | 0.29 | 1.76 | 0.884 | 0.279 | 0.316 | 0.49 | 1.98 | 0.772 | 0.22 | 0.286 |
| K2O | 0.68 | 2.6 | 1.809 | 0.521 | 0.288 | 1.81 | 2.67 | 2.297 | 0.188 | 0.082 | 1.95 | 2.64 | 2.356 | 0.146 | 0.062 |
| Na2O | 0.28 | 1.83 | 0.764 | 0.409 | 0.535 | 0.24 | 2.12 | 0.581 | 0.382 | 0.658 | 0.28 | 1.23 | 0.511 | 0.207 | 0.406 |
| P2O5 | 0.1 | 0.7 | 0.215 | 0.107 | 0.497 | 0.12 | 0.46 | 0.224 | 0.066 | 0.293 | 0.11 | 0.28 | 0.21 | 0.043 | 0.205 |
| LOI | 3.65 | 22.78 | 9.861 | 3.4 | 0.345 | 4.08 | 11.24 | 8.083 | 1.818 | 0.225 | 3.05 | 12.56 | 7.73 | 1.954 | 0.253 |
| K2O/Na2O | 0.421 | 7 | 3.206 | 1.877 | 0.585 | 0.991 | 9.889 | 5.039 | 2.177 | 0.432 | 1.806 | 8.484 | 5.261 | 1.774 | 0.337 |
| Na2O/K2O | 0.143 | 2.373 | 0.542 | 0.509 | 0.94 | 0.101 | 1.01 | 0.257 | 0.18 | 0.702 | 0.118 | 0.554 | 0.22 | 0.098 | 0.444 |
| Al2O3/TiO2 | 2.276 | 19.356 | 12.368 | 5.453 | 0.441 | 8.513 | 20.575 | 15.994 | 2.949 | 0.184 | 9.362 | 21.714 | 17.055 | 2.47 | 0.145 |
| SiO2/Al2O3 | 1.984 | 9.318 | 4.316 | 1.397 | 0.324 | 2.975 | 7.778 | 4.653 | 1.226 | 0.263 | 3.038 | 9.025 | 4.641 | 1.423 | 0.307 |
| K2O/Al2O3 | 0.068 | 0.21 | 0.135 | 0.033 | 0.244 | 0.115 | 0.22 | 0.163 | 0.021 | 0.129 | 0.136 | 0.256 | 0.164 | 0.026 | 0.159 |
| Fe2O3/K2O | 2.494 | 64.779 | 10.666 | 17.06 | 1.599 | 2.203 | 5.28 | 3.139 | 0.54 | 0.172 | 1.893 | 3.587 | 2.807 | 0.36 | 0.128 |
| Samples | Sc | V | Cr | Mn | Co | Ni | Cu | Zn | Pb | Ba | Th | U | El | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhuzhou (ZZ) section | ||||||||||||||
| ZF | Ave | 15.52 | 134.80 | 126.90 | 1646.56 | 27.69 | 55.05 | 100.87 | 743.83 | 310.32 | 548.68 | 32.69 | 9.18 | 5.76 |
| CV | 0.06 | 0.09 | 0.29 | 0.27 | 0.18 | 0.16 | 0.16 | 0.22 | 0.29 | 0.12 | 0.19 | 0.13 | 0.12 | |
| ZU | Ave | 14.66 | 116.49 | 91.10 | 1900.61 | 25.39 | 84.29 | 95.18 | 1244.52 | 1014.57 | 519.07 | 26.17 | 6.98 | 8.81 |
| CV | 0.18 | 0.20 | 0.16 | 0.28 | 0.70 | 1.33 | 0.62 | 1.36 | 1.85 | 0.19 | 0.17 | 0.13 | 1.07 | |
| ZX | Ave | 18.93 | 178.56 | 104.98 | 2407.50 | 27.39 | 59.12 | 291.50 | 3781.01 | 1299.05 | 493.71 | 26.56 | 9.73 | 14.15 |
| CV | 0.33 | 0.72 | 0.72 | 0.48 | 0.26 | 0.16 | 0.79 | 0.55 | 1.07 | 0.29 | 0.30 | 0.40 | 0.55 | |
| Xiangtan (XT) section | ||||||||||||||
| X2Q | Ave | 14.61 | 127.68 | 87.10 | 3316.08 | 23.38 | 59.24 | 95.89 | 614.06 | 184.63 | 519.33 | 26.27 | 7.12 | 5.14 |
| CV | 0.14 | 0.15 | 0.10 | 0.25 | 0.15 | 0.15 | 0.30 | 0.23 | 0.27 | 0.14 | 0.11 | 0.11 | 0.15 | |
| X1Q | Ave | 12.82 | 94.98 | 87.40 | 1799.57 | 18.93 | 42.44 | 88.09 | 539.67 | 190.90 | 473.03 | 42.36 | 10.11 | 4.57 |
| CV | 0.22 | 0.28 | 0.14 | 0.10 | 0.18 | 0.20 | 0.30 | 0.24 | 0.56 | 0.24 | 0.23 | 0.17 | 0.22 | |
| X3Q | Ave | 13.75 | 104.52 | 85.29 | 1593.48 | 18.65 | 44.70 | 104.70 | 443.93 | 127.83 | 499.57 | 27.03 | 7.28 | 4.23 |
| CV | 0.14 | 0.13 | 0.17 | 0.56 | 0.20 | 0.15 | 0.84 | 0.53 | 0.29 | 0.07 | 0.23 | 0.19 | 0.17 | |
| ZB | Ave | 11.67 | 90.02 | 79.89 | 3331.38 | 19.36 | 53.95 | 63.34 | 418.59 | 117.85 | 459.75 | 30.13 | 7.72 | 4.12 |
| CV | 0.12 | 0.16 | 0.13 | 0.43 | 0.19 | 0.23 | 0.16 | 0.27 | 0.21 | 0.15 | 0.30 | 0.27 | 0.13 | |
| XT | Ave | 15.06 | 121.47 | 98.33 | 2813.33 | 23.40 | 59.57 | 78.86 | 452.23 | 153.88 | 481.90 | 24.34 | 6.90 | 4.72 |
| CV | 0.15 | 0.15 | 0.16 | 0.29 | 0.15 | 0.19 | 0.25 | 0.16 | 0.23 | 0.11 | 0.13 | 0.12 | 0.14 | |
| Changsha (CS) section | ||||||||||||||
| HZ | Ave | 13.49 | 111.99 | 95.66 | 2871.50 | 20.79 | 50.85 | 76.48 | 435.94 | 144.03 | 510.54 | 22.85 | 6.57 | 4.41 |
| CV | 0.12 | 0.18 | 0.11 | 0.30 | 0.17 | 0.25 | 0.55 | 0.32 | 0.40 | 0.18 | 0.16 | 0.22 | 0.21 | |
| JZ | Ave | 15.38 | 118.92 | 94.59 | 2790.35 | 22.72 | 56.22 | 79.00 | 587.13 | 180.91 | 601.89 | 27.49 | 7.14 | 5.01 |
| CV | 0.10 | 0.11 | 0.10 | 0.14 | 0.10 | 0.11 | 0.17 | 0.21 | 0.18 | 0.12 | 0.18 | 0.14 | 0.09 | |
| SG | Ave | 14.07 | 113.09 | 125.53 | 2822.36 | 19.95 | 54.42 | 76.62 | 510.48 | 147.08 | 534.71 | 28.27 | 7.80 | 4.68 |
| CV | 0.20 | 0.32 | 0.32 | 0.40 | 0.27 | 0.34 | 0.34 | 0.43 | 0.24 | 0.13 | 0.26 | 0.15 | 0.23 | |
| XW | Ave | 11.65 | 77.44 | 73.48 | 1217.85 | 14.78 | 35.75 | 65.57 | 325.06 | 86.75 | 361.39 | 18.52 | 4.74 | 3.22 |
| CV | 0.19 | 0.23 | 0.27 | 0.27 | 0.23 | 0.22 | 0.27 | 0.24 | 0.20 | 0.18 | 0.29 | 0.23 | 0.21 | |
| UCC | 15 | 98 | 80 | 774.5 | 17 | 38 | 32 | 70 | 18 | 678 | 8.95 | 1.55 | 2.81 | |
| GR | 4 | 23 | 6.6 | 320 | 3 | 5.2 | 5.5 | 40 | 26 | 680 | 17 | 2.9 | 1.01 | |
| ACS | 11 | 82 | 65 | 600 | 13 | 26 | 24 | 68 | 23 | 500 | 12.5 | 2.7 | 2.22 | |
| WRS | 11 | 82 | 65 | 600 | 13 | 26 | 24 | 68 | 23 | 500 | 12.5 | 2.7 | 2.22 | |
| YZ | 13 | 97 | 82 | 810 | 17 | 33 | 35 | 78 | 27 | 512 | 12.4 | 2.6 | 2.66 | |
| SSW | 18.2 | 129 | 130 | 1679 | 22.5 | 74.5 | 75.9 | 208 | 61.1 | 522 | 12.1 | 3.3 | 4.18 | |
| BV | 0.8 | 42 | 44 | 450 | 10.3 | 21.2 | 20 | 76 | 554.1 | 22 | 14.8 | 3.6 | 1.00 | |
| Sc | V | Cr | Mn | Co | Ni | Cu | Zn | Pb | Ba | Th | U | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZZ | ZF (n = 9) | Min | −0.455 | −0.324 | −0.523 | 0.021 | −0.248 | −0.189 | 0.626 | 1.962 | 2.540 | −0.717 | 0.451 | 1.004 |
| Max | −0.195 | 0.096 | 0.528 | 0.987 | 0.463 | 0.399 | 1.224 | 2.983 | 3.661 | −0.187 | 1.212 | 1.467 | ||
| Ave | −0.331 | −0.116 | −0.012 | 0.394 | 0.098 | 0.136 | 0.927 | 2.634 | 2.892 | −0.494 | 0.790 | 1.225 | ||
| ZU (n = 18) | Min | −1.194 | −1.311 | −0.823 | −0.019 | −1.163 | −0.788 | 0.092 | 1.064 | 1.700 | −1.216 | 0.028 | 0.505 | |
| Max | −0.069 | −0.010 | 0.036 | 1.253 | 1.893 | 3.396 | 2.294 | 5.760 | 7.471 | −0.152 | 1.113 | 1.232 | ||
| Ave | −0.436 | −0.354 | −0.449 | 0.593 | −0.170 | 0.327 | 0.684 | 2.576 | 3.317 | −0.591 | 0.475 | 0.829 | ||
| ZX (n = 27) | Min | −0.914 | −2.341 | −3.257 | −0.858 | −0.491 | −0.185 | 0.746 | 2.454 | 2.223 | −1.796 | −0.172 | 0.605 | |
| Max | 0.801 | 2.023 | 1.287 | 1.908 | 0.853 | 0.703 | 3.840 | 6.126 | 7.043 | 0.110 | 1.381 | 2.497 | ||
| Ave | −0.117 | −0.087 | −0.696 | 0.820 | 0.062 | 0.238 | 2.085 | 4.735 | 4.230 | −0.703 | 0.457 | 1.234 | ||
| XT | X2Q (n = 12) | Min | −0.796 | −0.569 | −0.717 | 0.659 | −0.601 | −0.088 | 0.466 | 1.855 | 1.634 | −0.871 | 0.236 | 0.610 |
| Max | −0.220 | 0.159 | −0.291 | 1.972 | 0.096 | 0.627 | 1.686 | 2.862 | 2.747 | −0.252 | 0.690 | 1.144 | ||
| Ave | −0.431 | −0.204 | −0.504 | 1.406 | −0.142 | 0.244 | 0.821 | 2.356 | 2.144 | −0.577 | 0.490 | 0.861 | ||
| X3Q (n = 13) | Min | −0.907 | −0.921 | −1.055 | −1.172 | −0.786 | −0.442 | 0.086 | 0.815 | 1.094 | −0.832 | 0.257 | 0.607 | |
| Max | −0.368 | −0.244 | −0.190 | 1.383 | 0.067 | 0.236 | 0.890 | 2.915 | 2.287 | −0.484 | 1.099 | 1.411 | ||
| Ave | −0.591 | −0.544 | −0.591 | 0.516 | −0.446 | −0.154 | 0.520 | 2.044 | 1.741 | −0.643 | 0.612 | 0.946 | ||
| X1Q (n = 7) | Min | −0.919 | −1.030 | −0.726 | 0.286 | −0.753 | −0.553 | 0.372 | 1.663 | 1.457 | −0.963 | 0.694 | 1.138 | |
| Max | −0.075 | 0.016 | −0.173 | 0.752 | −0.015 | 0.237 | 1.461 | 2.763 | 3.359 | −0.118 | 1.555 | 1.665 | ||
| Ave | −0.632 | −0.656 | −0.505 | 0.560 | −0.449 | −0.245 | 0.702 | 2.174 | 2.090 | −0.728 | 1.151 | 1.358 | ||
| ZB (n = 8) | Min | −0.951 | −1.027 | −0.829 | 0.856 | −0.860 | −0.684 | −0.217 | 1.052 | 0.824 | −0.995 | 0.392 | 0.712 | |
| Max | −0.472 | −0.370 | −0.256 | 2.471 | −0.068 | 0.544 | 0.496 | 2.416 | 1.865 | −0.341 | 1.431 | 1.680 | ||
| Ave | −0.751 | −0.708 | −0.632 | 1.369 | −0.422 | 0.086 | 0.252 | 1.793 | 1.509 | −0.754 | 0.651 | 0.949 | ||
| XT (n = 12) | Min | −0.791 | −0.770 | −0.963 | 0.591 | −0.630 | −0.403 | 0.198 | 1.535 | 1.512 | −0.972 | 0.080 | 0.486 | |
| Max | −0.112 | −0.013 | −0.083 | 1.729 | 0.138 | 0.523 | 1.296 | 2.404 | 2.530 | −0.456 | 0.817 | 1.101 | ||
| Ave | −0.388 | −0.278 | −0.342 | 1.154 | −0.141 | 0.240 | 0.550 | 1.934 | 1.893 | −0.681 | 0.378 | 0.813 | ||
| CS | HZ (n = 14) | Min | −0.906 | −0.711 | −0.634 | 0.703 | −0.716 | −0.435 | −0.166 | 1.086 | 1.120 | −1.066 | −0.001 | 0.425 |
| Max | −0.321 | 0.139 | −0.053 | 2.112 | 0.071 | 0.767 | 1.967 | 2.664 | 2.918 | −0.166 | 0.813 | 1.471 | ||
| Ave | −0.542 | −0.397 | −0.370 | 1.188 | −0.315 | 0.002 | 0.404 | 1.830 | 1.744 | −0.611 | 0.282 | 0.726 | ||
| JZ (n = 20) | Min | −0.736 | −0.720 | −0.664 | 0.715 | −0.656 | −0.298 | 0.213 | 1.604 | 1.684 | −0.797 | 0.055 | 0.594 | |
| Max | −0.179 | −0.081 | −0.136 | 1.658 | −0.006 | 0.460 | 1.310 | 2.677 | 2.495 | −0.029 | 1.087 | 1.366 | ||
| Ave | −0.350 | −0.300 | −0.386 | 1.186 | −0.174 | 0.174 | 0.573 | 2.295 | 2.135 | −0.362 | 0.543 | 0.860 | ||
| SG (n = 14) | Min | −1.188 | −1.293 | −0.777 | 0.014 | −1.250 | −0.903 | −0.151 | 1.199 | 1.099 | −1.008 | 0.101 | 0.720 | |
| Max | −0.134 | 0.339 | 0.790 | 2.062 | 0.053 | 0.917 | 1.472 | 3.260 | 2.363 | −0.320 | 1.418 | 1.426 | ||
| Ave | −0.501 | −0.439 | −0.031 | 1.093 | −0.415 | 0.057 | 0.476 | 2.022 | 1.816 | −0.535 | 0.568 | 0.987 | ||
| XW (n = 14) | Min | −1.115 | −1.383 | −1.467 | −0.586 | −1.218 | −0.954 | −0.247 | 0.858 | 0.682 | −1.495 | −0.692 | −0.267 | |
| Max | −0.290 | −0.441 | −0.073 | 0.479 | −0.270 | 0.007 | 0.906 | 1.975 | 1.563 | −0.788 | 0.723 | 0.894 | ||
| Ave | −0.768 | −0.943 | −0.791 | −0.045 | −0.822 | −0.501 | 0.275 | 1.434 | 1.072 | −1.110 | −0.056 | 0.249 |
| RI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V | Cr | Mn | Co | Ni | Cu | Zn | Pb | |||
| ZZ | ZF (n = 9) | 3.206 | 4.327 | 2.974 | 10.341 | 9.892 | 17.514 | 11.863 | 94.852 | 145.883 |
| ZU (n = 18) | 2.979 | 3.076 | 3.575 | 27.859 | 78.970 | 36.771 | 81.282 | 1330.741 | 1461.715 | |
| ZX (n = 27) | 12.192 | 7.322 | 5.630 | 13.544 | 12.208 | 107.400 | 104.769 | 988.889 | 1230.539 | |
| XT | X2Q (n = 12) | 3.351 | 2.451 | 5.884 | 8.015 | 11.582 | 24.129 | 10.903 | 50.333 | 112.839 |
| X1Q (n = 7) | 3.033 | 2.661 | 2.526 | 7.424 | 8.841 | 20.643 | 10.181 | 76.944 | 131.555 | |
| X3Q (n = 13) | 2.542 | 2.805 | 3.912 | 7.856 | 8.835 | 56.371 | 11.314 | 36.593 | 100.212 | |
| ZB (n = 8) | 2.322 | 2.512 | 8.316 | 7.153 | 10.938 | 10.576 | 8.005 | 27.315 | 70.863 | |
| XT (n = 12) | 2.973 | 2.832 | 4.974 | 8.253 | 10.776 | 18.414 | 7.938 | 43.315 | 97.864 | |
| CS | HZ (n = 14) | 3.303 | 2.893 | 6.483 | 7.879 | 12.767 | 29.314 | 9.506 | 56.685 | 128.384 |
| JZ (n = 20) | 2.837 | 2.729 | 4.732 | 7.471 | 10.314 | 18.600 | 9.595 | 42.278 | 90.798 | |
| SG (n = 14) | 3.796 | 5.188 | 6.263 | 7.782 | 14.159 | 20.800 | 14.372 | 38.593 | 100.258 | |
| XW (n = 14) | 2.210 | 2.851 | 2.090 | 6.221 | 7.535 | 14.057 | 5.897 | 22.167 | 62.328 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Peng, B.; Yang, X. Contamination and Risk of Heavy Metals in Sediments from Zhuzhou, Xiangtan and Changsha Sections of the Xiangjiang River, Hunan Province of China. Sustainability 2023, 15, 14239. https://doi.org/10.3390/su151914239
Zhang K, Peng B, Yang X. Contamination and Risk of Heavy Metals in Sediments from Zhuzhou, Xiangtan and Changsha Sections of the Xiangjiang River, Hunan Province of China. Sustainability. 2023; 15(19):14239. https://doi.org/10.3390/su151914239
Chicago/Turabian StyleZhang, Kun, Bo Peng, and Xia Yang. 2023. "Contamination and Risk of Heavy Metals in Sediments from Zhuzhou, Xiangtan and Changsha Sections of the Xiangjiang River, Hunan Province of China" Sustainability 15, no. 19: 14239. https://doi.org/10.3390/su151914239
APA StyleZhang, K., Peng, B., & Yang, X. (2023). Contamination and Risk of Heavy Metals in Sediments from Zhuzhou, Xiangtan and Changsha Sections of the Xiangjiang River, Hunan Province of China. Sustainability, 15(19), 14239. https://doi.org/10.3390/su151914239







