Study and Application Status of Ultrasound in Organic Wastewater Treatment
Abstract
:1. Introduction
2. Cavitation Mechanism of Ultrasound in the Treatment of Organic Wastewater
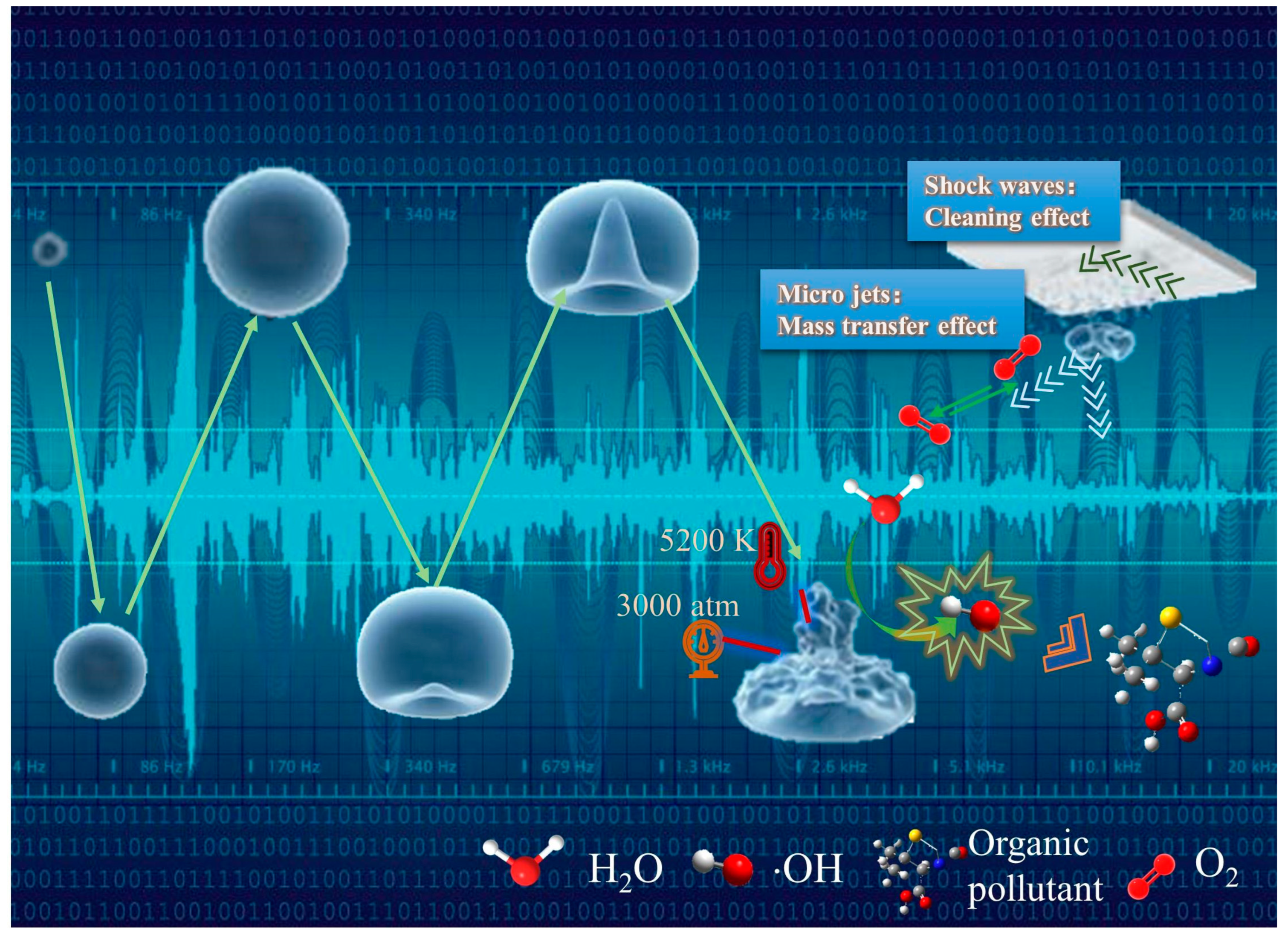
2.1. Mechanical Effect
2.2. Chemical Effect
2.3. Thermal Effect
3. Working Mode of Ultrasound in the Treatment of Organic Wastewater
3.1. Work Alone
3.2. Combination with Other Physical Fields
3.2.1. Combination of Ultrasound Field with EC Process
3.2.2. Combination of Ultrasound Field with Photocatalytic Oxidation Process
| No. | Study System | Pollutants | Ultrasound | Experimental Conditions | Removal Efficiency | Ref. | |
|---|---|---|---|---|---|---|---|
| Power (W) | Frequency (kHz) | ||||||
| 1 | Ultrasound–visible light–N–TiO2 | Amoxicillin | - | 20 | pH = 5.8, N-TiO2 = 0.5 g L−1, amoxicillin = 10 mg L−1 | 37.0% | [52] |
| 2 | Ultrasound–visible light–F–TiO2 | Crystal violet | 285 | 44–55 | pH = 7.0, reaction time = 120 min, F-TiO2 = 0.1 g/L | >80.0% | [53] |
| 3 | Ultrasound–visible light–Ca–ZnO | Tetracycline | 100 | 40 | LED = 1.6 W, Ca-ZnO = 0.5 g/L | 99.0% | [48] |
| 4 | Ultrasound–UV–TiO2 | Phenol | 50 | 24 | UV = 11 W, TiO2 = 0.1 g L−1 | - | [54] |
| 5 | Ultrasound–UV–MCs | Sulfadiazine | 200 | - | pH = 11.0, reaction time = 150 min, UV = 150 W, MCs = 0.9 g L−1 | 100% TOC = 89.0% COD = 96.0% | [55] |
| 7 | Ultrasound–UV–O3 | Atrazine | 142.5 | - | UV = 75 W, O3 = 10.75 g h−1 | 97.7% | [50] |
| 8 | Ultrasound–UV–PS | Trichloroethylene | 95 | 24 | pH = 5.5, reaction time = 20 min, PS = 61 µmol L−1, trichloroethylene = 61 µmol L−1 | 96.3% | [56] |
| 9 | Ultrasound–UV–PMS | Direct orange 26 | 125 | 20 | pH = 7.0, PMS = 1.5 mmol L−1 | 100% | [57] |
| 10 | Ultrasound–UV–PMS–MNPs@C | BPA | 200 | 20 | pH = 6.0, UV-C lamp = 6 W, mechanical stirrer = 200 r min−1, T = 25 ± 1 °C | 100% | [58] |
| 11 | Ultrasound–UV–FE | E. coli | 20 | 275 | pH = 4.5–5.0, ferrozine solution = 4.9 mmol L−1, hydroxylamine hydrochloride solution = 10.0% (w/w) | 100% | [51] |
| 12 | Toluidine blue | 70 | - | pH = 4.0, PVP/Fe3O4@SiO2 = 0.07 g, H2O2 = 1.0 mol L−1, toluidine blue = 4 × 10−4 mol/L | 95.3% | [59] | |
| 13 | 17 emerging pollutants | - | 375 | pH = 7.48, Fe2+ = 5 mg L−1, oxalic acid = 2 mg L−1, power density = 88 W L−1 | - | [60] | |
3.2.3. Combination of Ultrasound Field with MW Field
3.3. Ultrasound-Enhanced Organic Wastewater Treatment Methods
3.3.1. Ultrasound–Heterogeneous Fenton-like System
| No. | Study System | Pollutants | Ultrasound | Experimental Conditions | Removal Efficiency | Ref. | |
|---|---|---|---|---|---|---|---|
| Power (W) | Frequency (kHz) | ||||||
| 1 | Ultrasound–FE–Fe2+ | Emerging contaminants | - | 400 | Fe2+ = 0.5 mmol L−1, SDZ = 0.1 mmol L−1 | - | [68] |
| 2 | Acid scarlet | - | - | pH = 4.5, Fe2+ = 0.045 mmol L−1, H2O2 = 66 mmol L−1, GR = 300 mg L−1, GR: H2O2: Fe2+ = 100:15:41 | 96.6% | [66] | |
| 3 | C.I. reactive yellow 145 | 80 | 35 | pH = 3.0, reaction time = 60 min, Fe2+ = 20 mg L−1, H2O2 = 20 mg L−1 | 95.0% COD = 51.0% | [71] | |
| 4 | Reactive blue 19 | 100 | 53 | pH = 3.67, Fe2+ = 48.98 mg L−1, H2O2 = 300 mg L−1 | 85.0% | [72] | |
| 5 | Carbofuran | - | 40 | pH = 3.0, reaction time = 120 min, Fe2+ = 20 mg L−1, H2O2 = 100 mg L−1, initial carbofuran = 20 mg L−1 | 99.0% mineralisation = 46.0% | [73] | |
| 6 | Textile wastewater | 120 | 40 | pH = 3.0, reaction time = 60 min, Fe2+ = 0.05 g L−1, H2O2 = 1 g L−1 | 97.5% | [74] | |
| 7 | Olive mill wastewater | 100 | 20 | pH = 3.5, H2O2/Fe = 1.5, H2O2/COD = 0.73 | TPH = 80.0% .toxicity = 13.0-17.0% COD = 26.0% | [69] | |
| 8 | Ciprofloxacin | - | 580 | pH = 3.0, H2O2 = 14.2 mmol L−1, H2O2/Fe2+ = 6, T = 30 ± 1 °C | mineralisation rate = 60.0%. | [75] | |
| 9 | Industrial textile wastewater | - | 35 | pH = 3.0, reaction time = 60 min, Fe2+ = 0.05 g L−1, H2O2 = 1.65 g L−1 | 99.0% | [76] | |
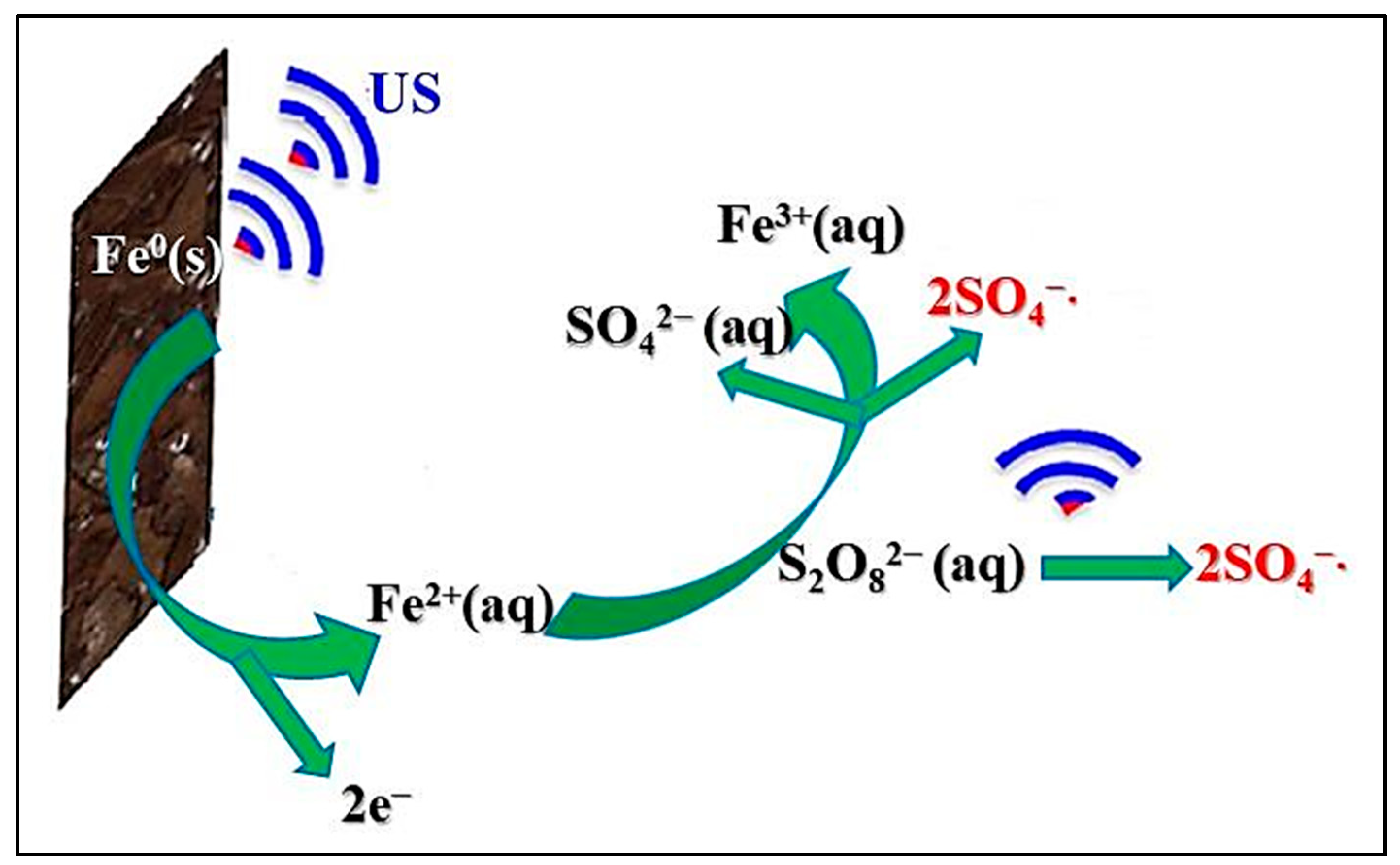
| 10 | Ultrasound–FE–nZVI | Decabromodiphenyl ether | - | 20 | pH = 2.0, Fe2+ = 0.5 g L−1, H2O2 = 150 mg L−1 | TOC = 60.0% | [70] |
| 11 | Azo dyes | - | - | pH = 2.0, reaction time = 60 min, H2O2 = 20 mg L−1, Fe2+ = 1 g L−1, dye: H2O2:Fe2+ = 1:3.6:2.4 | 99.9% COD = 63.4% | [77] | |
| 12 | Ultrasound–FE–magnetite nanoparticles | Acid blue15 | 1200 | 50 | pH = 3.0, magnetite nanoparticles = 1 g L−1, H2O2 = 10 mmol L−1 | 99.3% TOC = 40.4% | [29] |
| 13 | Ultrasound–FE–sponge iron | Chloramphenicol | 200 | 20 | pH = 3.0, Fe2+ = 2.26 g L−1, H2O2 = 3.19 mmol L−1 | 99.97% | [78] |
| 14 | Ultrasound–FE–MnFe2O4/BC | MB | 665 | 40 | pH = 5.0, reaction time = 20 min, MnFe2O4/BC = 0.7 g L−1, H2O2 = 15 mmol L−1, MB = 20 mg L−1 | 100% | [62] |
| 15 | Ultrasound–FE–Fe2O3/SBA-15 | Phenol | - | 584 | H2O2 = 0.6 g L−1 | TOC = 30.0% | [67] |
| 16 | Ultrasound-nZVI-activated carbon | [AMIM]Cl [BMBIM]Br[BMBIM]Br | - | 45 | pH = 3.0, zero-valent iron = 3 g L−1, activated carbon = 6 g L−1 | [AMIM]Cl = 92.9% [BMBIM]Br = 96.1% | [79] |
| 17 | Ultrasound–chitosan-stabilized nZVI | Aid fuchsine | - | 45 | pH = 4.96, chitosan-stabilized nZVI = 100 mg L−1, reaction time = 15 min, Acid fuchsine = 0.4 g L−1, T = 30 °C | 99.0% | [80] |
| 18 | Ultrasound–nZVI–PMS | 4-Chlorophenol | 200 | - | pH = 3.0, nZVI = 0.4 g L−1, PMS = 1.25 mmol L−1 | 95.0% | [81] |
| 19 | Ultrasound–FE–waste antivirus Cu film | BPA | 96.57 | 37 | pH = 5.0, H2O2 = 100 mmol L−1, mechanical stirrer = 250 r min−1, TA = 0.5 mmol L−1, NaOH = 2 mmol L−1, T = 60 °C | 100% | [82] |
| 20 | Ultrasound–FE–Cu–C3N4 | MB | 150 | 40 | pH = 6.7, reaction time = 30 min, Cu-C3N4 = 0.4 g L−1, H2O2 = 20 mmol L−1 | 96.0% | [83] |
3.3.2. Ultrasound–PS Oxidation System
3.3.3. Ultrasound-Membrane System
3.4. Ultrasound–Physical Fields–Chemical Treatment Methods
3.4.1. Ultrasound–EC–PS Process
3.4.2. Ultrasound–EC–FE Process
3.4.3. Ultrasound–UV–FE Process
3.4.4. Ultrasound–UV–PS/PMS Process
3.5. Advantages and Disadvantages of Ultrasound in the Treatment of Organic Wastewater
4. Typical Ultrasound-Enhanced Organic Wastewater Treatment Equipment
4.1. Classification by Reactor Type
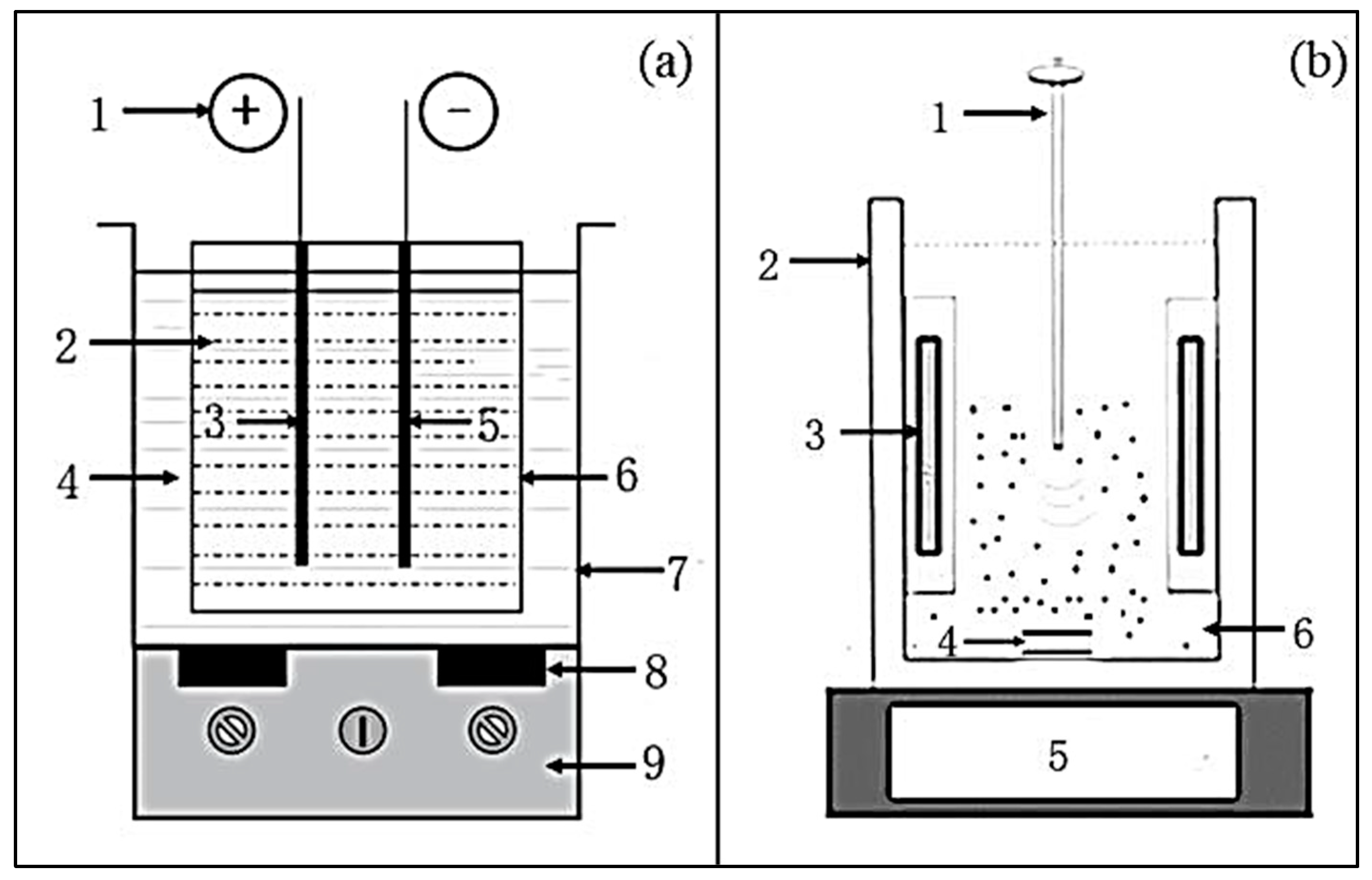
4.1.1. Ultrasound Cleaning Tank-Type Reactor
4.1.2. Ultrasound Probe Reactor
4.1.3. Comparison of Ultrasound Reactors with Different Types
4.2. Classified According to Liquid Flow Mode
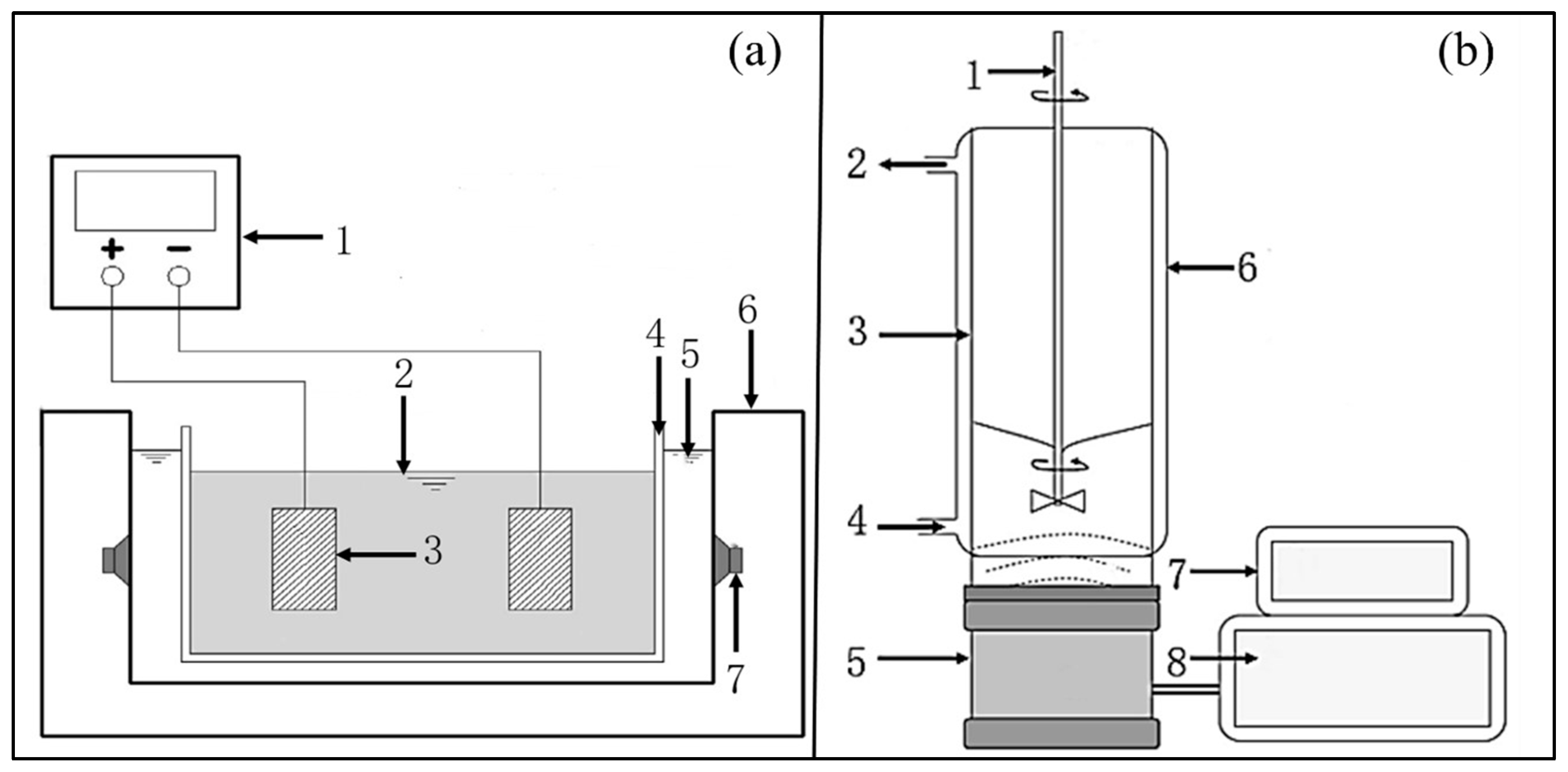
4.2.1. Fully Mixed Reactor
4.2.2. Continuous Reactor
4.2.3. Comparison of Ultrasound Reactors with Different Flow Modes
5. Development Prospect of Ultrasound in the Treatment of Organic Wastewater
- (1)
- Deeply investigate the advantages and disadvantages of the coupling of ultrasound with other treatment methods in order to provide a reference of the potential novel coupling of ultrasound with other organic wastewater treatment methods.
- (2)
- Explore new work modes of ultrasound, for example, dual-frequency mode or triple-frequency mode, in order to achieve a possible reduction in energy consumption.
- (3)
- Apply numerical simulation to optimize the ultrasound reactor structure by enhancing the cavitation effect, for example, Monte Carlo simulation can assist artificial neural networks to determine the contribution of three effects of ultrasound (mechanical effect, chemical effect, and thermal effect).
- (4)
- Explore new operation methods to enhance the cavitation effect, such as adding solid particles and increasing mechanical stirring, thus improving the effectiveness of ultrasound.
6. Conclusions
- (1)
- When combined with other methods, the mechanical effect of ultrasound can clean the surface of solid material and enhance mass transfer, while the chemical effect and thermal effect of ultrasound can generate ·OH and PS to promote the reaction rate and thus accelerate the degradation of organic pollutants.
- (2)
- Ultrasound can increase the removal rate of TN and TP by promoting the growth of algae. In addition to that, ultrasound can control membrane pollution, thereby enabling the effective filtration of pollutants by the membrane.
- (3)
- In ultrasound waves, PS and PMS can generate more oxidizing free radicals to degrade organic pollutants. Additionally, ultrasound can activate nZVI to generate the FE reaction to degrade pollutants.
- (4)
- According to the different reactor types, the ultrasound reactors can be divided into two types: ultrasound cleaning tank-type reactors and ultrasound probe reactors. The ultrasound generated by the ultrasound cleaning tank reactor is more uniform, while the probe-type reactor is more convenient. In addition, there is a liquid whistle reactor, in which ultrasound is generated by a mechanical effect. According to the liquid circulation mode, the ultrasound reactors can be divided into two types as well, in which the fully mixed type is applicable to almost all reactions of the ultrasound–enhanced organic wastewater treatment, while the continuous reactor can achieve the sequential processing of ultrasound and other processing methods of organic wastewater treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Variable | Description |
| Ultraviolet | UV |
| Electrochemical | EC |
| Persulphate | PS |
| Microwave | MW |
| bonded high valent Fe | Fe (III) |
| bonded low valent Fe | Fe (II) |
| Nano zero valent iron | nZVI |
| Peroxymonosulphate | PMS |
| Bisphenol A | MPA |
| Methylene Blue | MB |
References
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The Role of Ultrasound on Advanced Oxidation Processes. Top Curr. Chem. 2016, 374, 75. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Jin, R.-C. The Application of Low-Intensity Ultrasound Irradiation in Biological Wastewater Treatment: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2728–2761. [Google Scholar] [CrossRef]
- Li, H.; Lei, H.; Yu, Q.; Li, Z.; Feng, X.; Yang, B. Effect of low frequency ultrasonic irradiation on the sonoelectro-Fenton degradation of cationic red X-GRL. Chem. Eng. J. 2010, 160, 417–422. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, J.; Li, P.; Wang, K.; Yu, X.; Zhang, M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem. Eng. J. 2016, 287, 30–37. [Google Scholar] [CrossRef]
- Rasheed, Q.J.; Pandian, K.; Muthukumar, K. Treatment of petroleum refinery wastewater by ultrasound-dispersed nanoscale zero-valent iron particles. Ultrason. Sonochem. 2011, 18, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Abdelhay, A.; Othman, A.A.; Albsoul, A. Treatment of slaughterhouse wastewater using high-frequency ultrasound: Optimization of operating conditions by RSM. Environ. Technol. 2021, 42, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, H.; Ge, D.; Zhu, N. A novel conditioning approach for amelioration of sludge dewaterability using activated carbon strengthening electrochemical oxidation and realized mechanism. Water Res. 2022, 220, 118704. [Google Scholar] [CrossRef]
- Chang, T.; Wang, Y.; Wang, Y.Q.; Zhao, Z.; Shen, Z.; Huang, Y.; Veerapandian, S.K.P.; De Geyter, N.; Wang, C.; Chen, Q.; et al. A critical review on plasma-catalytic removal of VOCs: Catalyst development, process parameters and synergetic reaction mechanism. Sci. Total Environ. 2022, 828, 154290. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Gao, G.; Gao, S.; Xie, C.; Shi, J.-W. MnyCo3−yOx bimetallic oxide prepared by ultrasonic technology for significantly improved catalytic performance in the reduction of NOx with NH3. Fuel 2023, 352, 129159. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Li, Y.; Li, Y.; Zhou, H.; Fan, Y. A general linear free energy relationship for predicting partition coefficients of neutral organic compounds. Chemosphere 2020, 247, 125869. [Google Scholar] [CrossRef] [PubMed]
- Suna, S.; Liu, H.; Zhang, J.; Wang, W.; Xu, P.; Zhu, X.; Wang, Y.; Wan, S. Application of a novel coagulant in reservoir water treatment in Qingdao. Desalin. Water Treat. 2023, 284, 49–60. [Google Scholar] [CrossRef]
- Yaqub, A.; Ajab, H. Applications of sonoelectrochemistry in wastewater treatment system. Rev. Chem. Eng. 2013, 29, 123–130. [Google Scholar] [CrossRef]
- Scannapieco, D.; Naddeo, V.; Belgiorno, V. Control of fouling in MBRs through nanospheres addition. Desalin. Water Treat. 2014, 55, 702–711. [Google Scholar] [CrossRef]
- Qin, W.; Ma, Y.; He, T.; Hu, J.; Gao, P.; Yang, S. Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst. Nanomaterials 2022, 12, 4138. [Google Scholar] [CrossRef]
- Zhu, G.F.; Xiong, S.H.; Shi, C.; Jin, Y.; Ge, M.Q. Ceria-promoted heterogeneous Fenton-like oxidation of polyvinyl alcohol, Rhodamine-B, and Reactive Red X-3B over Fe/Cu@gamma-Al2O3 microspheres under neutral conditions. J. Alloys Compd. 2022, 924, 15. [Google Scholar] [CrossRef]
- Musielak, G.; Mierzwa, D.; Kroehnke, J. Food drying enhancement by ultrasound—A review. Trends Food Sci. Technol. 2016, 56, 126–141. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Li, J. A novel process for asparagus polyphenols utilization by ultrasound assisted adsorption and desorption using resins. Ultrason. Sonochem. 2020, 63, 104920. [Google Scholar] [CrossRef]
- Liao, T.; Xi, Y.; Zhang, L.; Li, J.; Cui, K. Removal of toxic arsenic (As()) from industrial wastewater by ultrasonic enhanced zero-valent lead combined with CuSO4. J. Hazard. Mater. 2021, 408, 124464. [Google Scholar] [CrossRef]
- Cardenas Sierra, R.S.; Zuniga-Benitez, H.; Penuela, G.A. Elimination of cephalexin and doxycycline under low frequency ultrasound. Ultrason. Sonochem. 2021, 79, 105777. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, N. A systematic review of the sonophotocatalytic process for the decolorization of dyes in aqueous solution: Synergistic mechanisms, degradation pathways, and process optimization. J. Water Process Eng. 2021, 44, 102314. [Google Scholar] [CrossRef]
- Ritesh, P.; Srivastava, V.C. Understanding of ultrasound enhanced electrochemical oxidation of persistent organic pollutants. J. Water Proc. Eng. 2020, 37, 101378. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms. J. Hazard. Mater. 2021, 414, 125517. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Wang, L.; Luo, D.; Hamdaoui, O.; Vasseghian, Y.; Momotko, M.; Boczkaj, G.; Kyzas, G.Z.; Wang, C. Bibliometric analysis and literature review of ultrasound-assisted degradation of organic pollutants. Sci. Total Environ. 2023, 876, 162551. [Google Scholar] [CrossRef]
- Hassani, A.; Malhotra, M.; Karim, A.V.; Krishnan, S.; Nidheesh, P.V. Recent progress on ultrasound-assisted electrochemical processes: A review on mechanism, reactor strategies, and applications for wastewater treatment. Environ. Res. 2022, 205, 112463. [Google Scholar] [CrossRef]
- de Andrade, F.V.; Augusti, R.; de Lima, G.M. Ultrasound for the remediation of contaminated waters with persistent organic pollutants: A short review. Ultrason. Sonochem. 2021, 78, 105719. [Google Scholar] [CrossRef] [PubMed]
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpour, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochem. 2019, 58, 104633. [Google Scholar] [CrossRef]
- Prakash, L.V.; Gopinath, A.; Gandhimathi, R.; Velmathi, S.; Ramesh, S.T.; Nidheesh, P.V. Ultrasound aided heterogeneous Fenton degradation of Acid Blue 15 over green synthesized magnetite nanoparticles. Sep. Purif. Technol. 2021, 266, 118230. [Google Scholar] [CrossRef]
- Luo, X.; Gong, H.; He, Z.; Zhang, P.; He, L. Recent advances in applications of power ultrasound for petroleum industry. Ultrason. Sonochem. 2021, 70, 105337. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, J.; Tang, X.; Liu, F.; Yu, X.; Tang, X.; Jiang, H.; Gan, L. Effective ultrasound electrochemical degradation of methylene blue wastewater using a nanocoated electrode. Ultrason. Sonochem. 2014, 21, 1310–1317. [Google Scholar] [CrossRef]
- Yousefi, N.; Pourfadakari, S.; Esmaeili, S.; Babaei, A.A. Mineralization of high saline petrochemical wastewater using Sonoelectro-activated persulfate: Degradation mechanisms and reaction kinetics. Microchem. J. 2019, 147, 1075–1082. [Google Scholar] [CrossRef]
- Heredia-Rivera, U.; Ferrer, I.; Vazquez, E. Ultrasonic Molding Technology: Recent Advances and Potential Applications in the Medical Industry. Polymers 2019, 11, 667. [Google Scholar] [CrossRef]
- Oturan, M.A.; Sirés, I.; Oturan, N.; Pérocheau, S.; Laborde, J.-L.; Trévin, S. Sonoelectro-Fenton process: A novel hybrid technique for the destruction of organic pollutants in water. J. Electroanal. Chem. 2008, 624, 329–332. [Google Scholar] [CrossRef]
- Chen, X.; Bayanheshig; Jiao, Q.; Tan, X.; Wang, W. Numerical simulation of ultrasonic enhancement by acoustic streaming and thermal effect on mass transfer through a new computation model. Int. J. Heat Mass Transfer 2021, 171, 121074. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Liu, S.; Peng, Y.; Chen, J.; Yoo Ki, S. Ultrasound-assisted electrochemical treatment for phenolic wastewater. Ultrason. Sonochem. 2020, 65, 105058. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo-Aguirre, A.L.; Florez-Acosta, O.A.; Torres-Palma, R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016, 31, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Sethu, V.; Manickam, S. Kinetics and mechanism of low-frequency ultrasound driven elimination of trace level aqueous perfluorooctanesulfonic acid and perfluorooctanoic acid. Chem. Eng. Process.—Process Intensif. 2019, 142, 107542. [Google Scholar] [CrossRef]
- Matouq, M.; Al-Ayed, O.; Al-Anber, Z.; Al-Shannag, M.; Kloub, N.; Tagawa, T.; Aljbour, S. Wastewater Treatment Resulting from an Oil Shale Retorting at High Frequency Ultrasound Waves with a Chemical Elemental Analysis. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1878–1884. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Zhu, J.-N.; Kong, F.; Xing, D.; Zhao, L.; Ma, J.; Ren, N.-Q.; Liu, B.-F. Ultrasonic enhanced simultaneous algal lipid production and nutrients removal from non-sterile domestic wastewater. Energy Convers. Manag. 2019, 180, 680–688. [Google Scholar] [CrossRef]
- Chang, J.-H.; Ellis, A.V.; Yan, C.-T.; Tung, C.-H. The electrochemical phenomena and kinetics of EDTA–copper wastewater reclamation by electrodeposition and ultrasound. Sep. Purif. Technol. 2009, 68, 216–221. [Google Scholar] [CrossRef]
- Radi, M.A.; Nasirizadeh, N.; Mirjalili, M.; Rohani Moghadam, M. Ultrasound-assisted electrochemical decolorization of anthraquinone dye C.I Reactive Blue 49, its optimization and synergic effect: A comparative study. Int. J. Environ. Sci. Technol. 2018, 16, 2455–2464. [Google Scholar] [CrossRef]
- Souza, F.L.; Saéz, C.; Lanza, M.R.V.; Cañizares, P.; Rodrigo, M.A. Removal of herbicide 2,4-D using conductive-diamond sono-electrochemical oxidation. Sep. Purif. Technol. 2015, 149, 24–30. [Google Scholar] [CrossRef]
- Dionisio, D.; Motheo, A.J.; Sáez, C.; Canizares, P.; Rodrigo, M.A. Coupling Ultrasound to the Electro-Oxidation of Methyl Paraben Synthetic Wastewater: Effect of Frequency and Supporting Electrolyte. ChemElectroChem 2018, 6, 1199–1205. [Google Scholar] [CrossRef]
- Johin, J.; Nidheesh, P.V.; Sivasankar, T. Sono-electro-chemical Treatment of Reactive Black 5 Dye and Real Textile Effluent Using MnSO4/Na2S2O8 Electrolytes. Arabian J. Sci. Eng. 2019, 44, 9987–9996. [Google Scholar] [CrossRef]
- Darvishi Cheshmeh Soltani, R.; Jorfi, S.; Alavi, S.; Astereki, P.; Momeni, F. Electrocoagulation of textile wastewater in the presence of electro-synthesized magnetite nanoparticles: Simultaneous peroxi- and ultrasonic-electrocoagulation. Sep. Sci. Technol. 2019, 55, 945–954. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Qu, G.; Ren, N.; Zou, H.; Hu, Y.; Qiu, W. Research on dynamics and mechanism of treatment on phenol simulated wastewater by the ultrasound cooperated electro-assisted micro-electrolysis. Water Environ. Res. 2021, 93, 1243–1253. [Google Scholar] [CrossRef]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serrà, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 2022, 427, 132006. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Wen, D.; Zheng, J.; Zhang, B. Pilot-scale treatment of atrazine production wastewater by UV/O3/ultrasound: Factor effects and system optimization. J. Environ. Manag. 2017, 203, 182–190. [Google Scholar] [CrossRef]
- Giannakis, S.; Papoutsakis, S.; Darakas, E.; Escalas-Canellas, A.; Petrier, C.; Pulgarin, C. Ultrasound enhancement of near-neutral photo-Fenton for effective E. coli inactivation in wastewater. Ultrason. Sonochem. 2015, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef] [PubMed]
- Panahian, Y.; Arsalani, N.; Nasiri, R. Enhanced photo and sono-photo degradation of crystal violet dye in aqueous solution by 3D flower like F-TiO2(B)/fullerene under visible light. J. Photochem. Photobiol. A 2018, 365, 45–51. [Google Scholar] [CrossRef]
- Van de Moortel, W.; Kamali, M.; Sniegowski, K.; Braeken, L.; Degrève, J.; Luyten, J.; Dewil, R. How Photocatalyst Dosage and Ultrasound Application Influence the Photocatalytic Degradation Rate of Phenol in Water: Elucidating the Mechanisms Behind. Water 2020, 12, 1672. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-assisted photocatalytic degradation of sulfadiazine using MgO@CNT heterojunction composite: Effective factors, pathway and biodegradability studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Bahrami, H.; Eslami, A.; Nabizadeh, R.; Mohseni-Bandpi, A.; Asadi, A.; Sillanpää, M. Degradation of trichloroethylene by sonophotolytic-activated persulfate processes: Optimization using response surface methodology. J. Clean. Prod. 2018, 198, 1210–1218. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F. Combination of UVC-LEDs and ultrasound for peroxymonosulfate activation to degrade synthetic dye: Influence of promotional and inhibitory agents and application for real wastewater. Environ. Sci. Pollut. Res. Int. 2018, 25, 6003–6014. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Kakavandi, B.; Azizi, M.; Golshan, M. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A. Chem. Eng. J. 2018, 331, 729–743. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Synthesis of poly (vinylpyrrolidone)/Fe3O4@SiO2 nanoporous catalyst by γ-rays and evaluation their sono-photo-Fenton degradation of toluidine blue under magnetic field. Appl. Organomet. Chem. 2021, 35, e6388. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martinez-Pachon, D.; Moncayo-Lasso, A.; Ibanez, M.; Hernandez, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Ondruschka, B.; Cravotto, G. Degradation of Phenol under Combined Irradiation of Microwaves and Ultrasound. Environ. Sci. Technol. 2008, 42, 8083–8087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Thang Nguyen, T.; Guo, M.; Gao, X. Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Appl. Surf. Sci. 2021, 566, 150654. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Li, C.; Liu, W.; Lichtfouse, E. High increase in biodegradability of coking wastewater enhanced by Mn ore tailings in Fenton/O3 combined processes. Int. J. Environ. Sci. Technol. 2020, 18, 173–184. [Google Scholar] [CrossRef]
- Li, J.; Pham, A.N.; Dai, R.; Wang, Z.; Waite, T.D. Recent advances in Cu-Fenton systems for the treatment of industrial wastewaters: Role of Cu complexes and Cu composites. J. Hazard. Mater. 2020, 392, 122261. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Cai, Y.-F.; Zhang, Q.-J.; Wang, H.; Liu, Y.-L. Fe/Acid-montmorillonite as effective Fenton-like catalyst for the removal of methylene blue. J. Chem. Technol. Biotechnol. 2022, 97, 3163–3171. [Google Scholar] [CrossRef]
- Liu, T.; He, F.W.; Zhang, Y.Q. Synergistic Degradation of Acid Scarlet Dyeing Wastewater by the Ultrasound/Fenton Method. Appl. Mech. Mater. 2013, 448–453, 34–37. [Google Scholar] [CrossRef]
- Bremner, D.H.; Molina, R.; Martínez, F.; Melero, J.A.; Segura, Y. Degradation of phenolic aqueous solutions by high frequency sono-Fenton systems (US–Fe2O3/SBA-15–H2O2). Appl. Catal. B 2009, 90, 380–388. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Han, J.; Gong, H.; Meng, L.; Mei, X.; Sun, Y.; Qi, L.; Gan, L. Degradation of emerging contaminants by sono-Fenton process with in situ generated H2O2 and the improvement by P25-mediated visible light irradiation. J. Hazard. Mater. 2020, 391, 122229. [Google Scholar] [CrossRef]
- Ciggin, A.S.; Sarica, E.S.; Doğruel, S.; Orhon, D. Impact of ultrasonic pretreatment on Fenton-based oxidation of olive mill wastewater—Towards a sustainable treatment scheme. J. Clean. Prod. 2021, 313, 127948. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Heterogeneous Sono-Fenton treatment of decabromodiphenyl ether (BDE-209): Debromination mechanism and transformation pathways. Sep. Purif. Technol. 2019, 209, 914–920. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; Zhang, D. Degradation of C.I. Acid Orange 7 by ultrasound enhanced heterogeneous Fenton-like process. J. Hazard. Mater. 2009, 172, 654–660. [Google Scholar] [CrossRef]
- Siddique, M.; Farooq, R.; Price, G.J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. Sonochem. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Sung, C.F.; Lin, J.G. Degradation of carbofuran in aqueous solution by ultrasound and Fenton processes: Effect of system parameters and kinetic study. J. Hazard. Mater. 2010, 178, 320–325. [Google Scholar] [CrossRef]
- Asghar, A.; Ramzan, N.; Jamal, B.U.; Maqsood, M.; Sajjadi, B.; Chen, W.Y. Low frequency ultrasonic-assisted Fenton oxidation of textile wastewater: Process optimization and electrical energy evaluation. Water Environ. J. 2019, 34, 523–535. [Google Scholar] [CrossRef]
- Gonzalez Labrada, K.; Alcorta Cuello, D.R.; Saborit Sanchez, I.; Garcia Batle, M.; Manero, M.H.; Barthe, L.; Jauregui-Haza, U.J. Optimization of ciprofloxacin degradation in wastewater by homogeneous sono-Fenton process at high frequency. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2018, 53, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, S.G.; Morcali, M.H.; Akarsu, S.; Ziba, C.A.; Dolaz, M. Comparison of classic Fenton with ultrasound Fenton processes on industrial textile wastewater. Sustain. Environ. Res. 2018, 28, 165–170. [Google Scholar] [CrossRef]
- Basturk, E.; Alver, A. Modeling azo dye removal by sono-fenton processes using response surface methodology and artificial neural network approaches. J. Environ. Manag. 2019, 248, 109300. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, Z.; Wang, S.; Kong, F. Synergistic Degradation of Chloramphenicol by an Ultrasound-Enhanced Fenton-like Sponge Iron System. Water 2021, 13, 3561. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, P.; Qi, H.; Ma, J.; Wang, J. Removal of residual functionalized ionic liquids from water by ultrasound-assisted zero-valent iron/activated carbon. Environ. Technol. 2019, 40, 2504–2512. [Google Scholar] [CrossRef]
- Jin, X.; Zhuang, Z.; Yu, B.; Chen, Z.; Chen, Z. Functional chitosan-stabilized nanoscale zero-valent iron used to remove acid fuchsine with the assistance of ultrasound. Carbohydr. Polym. 2016, 136, 1085–1090. [Google Scholar] [CrossRef]
- Barzegar, G.; Jorfi, S.; Zarezade, V.; Khatebasreh, M.; Mehdipour, F.; Ghanbari, F. 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: Reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 2018, 201, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Bisphenol A degradation using waste antivirus copper film with enhanced sono-Fenton-like catalytic oxidation. Chemosphere 2021, 276, 130218. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Wang, H. Ultrasound assisted Fenton-like degradation of dyes using copper doped graphitic carbon nitride. Water Sci. Technol. 2021, 84, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Movahedian Attar, H.; Darvishmotevalli, M.; Moradnia, M. Degradation of 4-chlorophenol from aqueous solution using ultrasound/persulphate: Prediction by RSM. Int. J. Environ. Anal. Chem. 2020, 102, 6030–6040. [Google Scholar] [CrossRef]
- Moradnia, M.; Noorisepehr, M.; Salari, M.; Darvishmotevalli, M. Optimization of 2-Chlorophenol Removal Using Ultrasound/Persulfate: Prediction by RSM Method, Biodegradability Improvement of Petrochemical Refinery Wastewater. Arabian J. Sci. Eng. 2021, 47, 6931–6939. [Google Scholar] [CrossRef]
- Weng, C.H.; Tsai, K.L. Ultrasound and heat enhanced persulfate oxidation activated with Fe(0) aggregate for the decolorization of C.I. Direct Red 23. Ultrason. Sonochem. 2016, 29, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Malakotian, M.; Asadzadeh, S.N.; Khatami, M.; Ahmadian, M.; Heidari, M.R.; Karimi, P.; Firouzeh, N.; Varma, R.S. Protocol encompassing ultrasound/Fe3O4 nanoparticles/persulfate for the removal of tetracycline antibiotics from aqueous environments. Clean Technol. Environ. Policy 2019, 21, 1665–1674. [Google Scholar] [CrossRef]
- Li, D.J.; Li, M.; Wang, Y.; Gong, C.P. Optimization of the operating parameters for online ultrasonic on controlling membrane fouling in SMBR. Desalin. Water Treat. 2013, 51, 3832–3839. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci. Total Environ. 2020, 708, 135068. [Google Scholar] [CrossRef]
- Lin, X.; Lu, K.; Hardison, A.K.; Liu, Z.; Xu, X.; Gao, D.; Gong, J.; Gardner, W.S. Membrane inlet mass spectrometry method (REOX/MIMS) to measure 15N-nitrate in isotope-enrichment experiments. Ecol. Indic. 2021, 126, 107639. [Google Scholar] [CrossRef]
- Wen, X.; Sui, P.; Huang, X. Exerting ultrasound to control the membrane fouling in filtration of anaerobic activated sludge--mechanism and membrane damage. Water Sci. Technol. 2008, 57, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Secondes, M.F.; Naddeo, V.; Belgiorno, V.; Ballesteros, F., Jr. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, C.P. Decomposition of nitrotoluenes in wastewater by sonoelectrochemical and sonoelectro-Fenton oxidation. Ultrason. Sonochem. 2014, 21, 840–845. [Google Scholar] [CrossRef]
- Meroni, D.; Djellabi, R.; Ashokkumar, M.; Bianchi, C.L.; Boffito, D.C. Sonoprocessing: From Concepts to Large-Scale Reactors. Chem. Rev. 2022, 122, 3219–3258. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, L.; Hu, X.; Wu, P.; Wang, X.; Huang, C.; Wang, X.; Deng, D. Ultrasound assisted, thermally activated persulfate oxidation of coal tar DNAPLs. J. Hazard. Mater. 2016, 318, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Lin, K.-Y.A.; Kakavandi, B.; Hassani, A. Enhanced electro-peroxone using ultrasound irradiation for the degradation of organic compounds: A comparative study. J. Environ. Chem. Eng. 2020, 8, 104167. [Google Scholar] [CrossRef]
- Demir, N.; Gunduz, G.; Dukkanci, M. Degradation of a textile dye, Rhodamine 6G (Rh6G), by heterogeneous sonophotoFenton process in the presence of Fe-containing TiO2 catalysts. Environ. Sci. Pollut. Res. Int. 2015, 22, 3193–3201. [Google Scholar] [CrossRef]



| No. | Study System | Pollutants | Ultrasound | Experimental Conditions | Removal Efficiency | Ref. | |
|---|---|---|---|---|---|---|---|
| Power (W) | Frequency (kHz) | ||||||
| 1 | Ultrasound–EC | MB | 300 | - | reaction time = 60 min, V = 20 V, Na2SO4 = 15 g L−1 | 94.9% | [31] |
| 2 | Blue 49 | 150 | 35 | pH = 8.3, reaction time = 80.6 min, V = 0.7 V, dye = 10 mg L−1 | COD = 90.1% | [42] | |
| 3 | Cephalosporin pharmaceutical | - | 45 | reaction time = 30 min, current of density = 8 mA cm−2 | COD = 94.0% | [4] | |
| 4 | 2,4-dichlorophenoxyacetic acid | 200 | 24 | pH = 4.0, power consumption = 302.3 kWh m−3, flow rate = 1 × 10−3 dm3 min−1, NaCl = 3 g L−1 | - | [43] | |
| 5 | Ultrasound–EC–chloride media | Methyl paraben | - | 20 | pH = 3.0, V = 2.8 V, methyl paraben = 100 mg dm−3, Na2SO4 = 3 g cm−3 | TOC = 100% | [44] |
| 6 | Ultrasound–EC–MnSO4/Na2S2O8 | Black 5 | 44 | - | pH = 8.05, V = 8 V, Na2SO4 = 100 mg L−1, MnSO4 = 75 mg L−1 | TOC = 90.0% | [45] |
| 7 | Ultrasound–EC–PS | High saline petrochemical wastewater | 300 | 130 | pH = 3.0, reaction time = 120 min, PS = 20 mmol L−1 | COD = 91.2% | [32] |
| 8 | Ultrasound–EC–FE | 2,4-dinitrotoluene, 2,4,6-trinitrotoluene | 20 | 28 | pH = 3.0, Fe3+ = 0.1 mmol L−1, 2,4-D = 1 mmol L−1, DNOC = 0.5 mmol L−1, AB = 0.025 mmol L−1, Na2SO4 = 0.05 mol L−1 | 100% | [34] |
| 9 | Red X-GRL | 160 | 20 | pH = 3,0, Fe2+ = 5 mmol L−1, Na2SO4 = 0.05 mol L−1, H2SO4 = 1 mol L−1, NaOH = 1 mmol L−1, T = 30 °C | 56.2% | [3] | |
| 10 | Textile wastewater | 350 | 40 | pH = 5.0, reaction time = 90 min, V = 0.5 V, COD = 1.25 × 103 mg L−1, electrolyte = 6 g L−1, PS = 0.5 mmol L−1 | COD = 96.0% | [46] | |
| 11 | Ultrasound–micro electrolysis | Phenol | 300 | 28 | pH = 4.0, V = 3 V, iron dosage = 50 g L−1, iron: carbon = 1:1, phenol = 100 mg L−1 | 88.6% | [47] |
| 12 | Ultrasound–electrodeposition | EDTA-Cu | 157–300 | 20 | pH = 7.0, reaction time = 220 min, voltage gradient = 1.0 V cm−1 | 84.0% | [41] |
| Advantages | Disadvantages |
|---|---|
| No secondary pollution; Accelerate the reaction rate; Reducing dosage of chemicals; Reducing wastewater treatment cost by combining with other wastewater treatment methods. | Consuming electrical energy; Making noise. |
| Reactors | Advantages | Disadvantages |
| Ultrasound cleaning tank-type reactor | Ultrasound wave is evenly distributed; Can easily control temperature. | Limiting the reactor size. |
| Ultrasound probe reactor | Uneven distribution of ultrasound wave; The probe is prone to corrosion. | Easy operation. |
| Liquid whistle reactor | Application in homogenization of oily wastewater. | Ancient, with certain limitations. |
| Types | Advantages | Disadvantages |
|---|---|---|
| Fully mixed reactor | Mix the solution evenly. Convenient combination with other processing methods. | Unable to process continuously. |
| Continuous reactor | Continuous processing. | Complex design and high cost. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Li, L.; Wang, K.; Huang, X.; Han, Y.; Ma, X.; Wang, M.; Lv, X.; Bai, X. Study and Application Status of Ultrasound in Organic Wastewater Treatment. Sustainability 2023, 15, 15524. https://doi.org/10.3390/su152115524
Wang N, Li L, Wang K, Huang X, Han Y, Ma X, Wang M, Lv X, Bai X. Study and Application Status of Ultrasound in Organic Wastewater Treatment. Sustainability. 2023; 15(21):15524. https://doi.org/10.3390/su152115524
Chicago/Turabian StyleWang, Nannan, Liangwei Li, Kai Wang, Xitong Huang, Yanhe Han, Xuejiao Ma, Menghan Wang, Xiao Lv, and Xinming Bai. 2023. "Study and Application Status of Ultrasound in Organic Wastewater Treatment" Sustainability 15, no. 21: 15524. https://doi.org/10.3390/su152115524
APA StyleWang, N., Li, L., Wang, K., Huang, X., Han, Y., Ma, X., Wang, M., Lv, X., & Bai, X. (2023). Study and Application Status of Ultrasound in Organic Wastewater Treatment. Sustainability, 15(21), 15524. https://doi.org/10.3390/su152115524









