Abstract
Anzasca Valley is part of the Monte Rosa gold district located in the Italian Central Alps. Since we do not know the effects of arsenic on the aquatic environment in Anzasca Valley, we investigated the biofilm of three streams. The three perennial streams studied are in the Anza catchment but with different lithology. Rio Rosso flows out of an ancient gold mine (Miniera dei Cani); its waters, acid mine drainages, are rich in iron and arsenic. Rio Gattera, a small stream adjacent to the Rio Rosso, flows through metamorphic rocks with mixed composition. Its waters are not acidic but contain a small amount of arsenic. Rio Roletto is upstream, with respect to the others, and drains different metamorphic rocks without arsenic. We analyzed the chemistry and the metals of the water, characterized by microscopic analysis and HPLC, the phytobenthic community living in the biofilm, and in the Rio Rosso, we measured the arsenic adsorbed in the biofilm. Sampling was performed between 2012 and 2014, and arsenic in the biofilm of Rio Rosso was measured in different seasons. In the three streams, the carotenoids of the biofilms showed the different stability of phytobenthic communities (Bacyllariophyceae vs. Cyanobacteria): in Rio Roletto and Rio Gattera, the ratio between the communities did not change; in Rio Rosso, the ratio between the communities changed completely, probably due to the peculiarity of water composition and presence of arsenic.
1. Introduction
Arsenic is a ubiquitous element on the earth and a metalloid. Several hundred arsenic minerals are known, but only a few are relatively common in crustal rocks, sediments, and soil. Pyrite is present in sediments of many rivers, lakes, oceans, and aquifers and plays a very important role in the geochemical cycles of various elements [1] often associated with arsenopyrite [2]. Minerals and other inorganic solid arsenic molecules may be classified into five groups: elemental, arsenides, arsenosulfides, arsenites, and arseniates [2]. Arsenite, As (III), exists in low-oxygen (reducing) groundwater and hydrothermal waters [3]. Arsenate, As (V), is present in oxidizing groundwater and surface waters [2]. Arsenic toxicity depends on its oxidation state: the reduced forms As (III), arsenite, are more toxic than those oxidized As (V), arsenate [4,5]. The water solubility of the arsenic molecules is very different and modifies their bioavailability, i.e., the possibility of spreading along the food web [1,2]. Arsenic is one of the most toxic metalloids in the natural environment [2], and the toxicity of the organic species is lower than that of the inorganic ones [6]. Arsenic is present worldwide [7,8,9], from natural (volcanism, erosion) and anthropogenic sources (industrial activity, pesticides) [10]. In Italy, arsenic is naturally present in significant quantities in Sardinia, Lazio, Tuscany, Lombardy, and Veneto, often associated with mining areas. Moreover, it has a wide spread in the metamorphic rocks of the Alps and in its relative alluvial deposits [5]. Arsenic in the environment can contaminate groundwater through different sources: soil, water, air, and dust. Arsenic can be uptaken from organisms with low levels of complexity that can change arsenic oxidation state: microorganisms, plankton, phytobenthos, zoobenthos, macroinvertebrates, and macrophytes. They can accumulate arsenic in different chemical structures without any problems, thanks to their metabolic defense pathways. Fishes and plants can uptake arsenic and translocate it along the food web to the final utilizer, which has a high level of complexity and low defense pathways [4,5].
The main receivers of arsenic contamination are drinking water, food, soil, and dust. It occurs through breathing, skin adsorption, and diet [11]. Recently, arsenic diet adsorption has been investigated, but drinking water remains a major source. One of the last recommendations of the World Health Organization (WHO) limits arsenic to 10 µgL−1 for drinking water [12]. This recommendation was adopted by Italy, but some municipalities have serious problems with the application. The last Italian recommendation is the D.lgs. 23 February 2023 n.18 [13], which confirmed the limit of 10 µgL−1 for drinking water, imposing more transparent procedures and recommendations to adapt to climate change, respecting the Health Risk Assessment, and that all data should be open source.
Gold mines are widespread throughout the world, and acid mine waters are a known problem. The different geological and environmental conditions, geographical location and elevation, pH, temperature, and tributaries modify the chemical–physical properties of the water and the biotic component [7,8,9].
This study was carried out in Anzasca Valley in Piedmont, Italian Central Alps, in the gold district, where, since ancient times, gold extraction has been performed. Currently, there are several abandoned mines; one of these is the “Miniera dei Cani” (located at 1475 m above sea level in the municipality of Vanzone with San Carlo), which represents a particular case of acid mine drainage. Due to the very steep conformation of the area, all the debris produced by mining was transported downward along the Anza River for processing. The present-day Rio Rosso flowing from the mine is a perennial stream impacted by the mining activity. The beginning of the mining activity is unclear; it is hypothesized to date back to Roman times, but in the Middle Ages, documents have ascertained the ownership of Captain Bonifacio Cane, a mercenary leader in the service of the Visconti Family. The mine changed owners several times over the centuries, including the Borromeo Family, until it closed around 1950 due to the gold vein exhaustion. Further information is on the website of the Municipality of Vanzone con San Carlo: http://www.acquavanzonis.it/le-miniere-dei-cani/, accessed on 30 August 2023.
Arsenic release from mine drainage occurs after the oxidative dissolution of sulfide minerals (mainly arsenopyrite); arsenite and arsenate forms coexist and are controlled by oxygen availability and arsenic-oxidizing microorganisms [7]. When Rio Rosso flows out of the mine, the acid waters (pH 2.4) are extremely rich in arsenic and iron [14]. The high iron concentration confers a red color to the rocks surrounding the stream, conferring to the stream its name: Rio Rosso, in Italian, stands for “red stream”. From a scientific point of view, there is a lack of information on this site, especially concerning the biological and environmental features. For a better characterization of this area, we also investigated two other streams in the same catchment, Rio Roletto and Rio Gattera, but with a different lithology [15,16]. The streams are tributaries of the Anza River on the left side of Anzasca Valley. A preliminary investigation to understand the aquatic ecosystem life in the streams in the presence of arsenic was carried out by measuring some biotic and abiotic parameters: metals and water chemistry, arsenic amount in the biofilm, primary producers identification, and photosynthetic pigments analysis (HPLC), which can be used as a bioindicator of water quality.
2. Materials and Method
2.1. Study Area
This study was carried out in Anzasca Valley, Piedmont, Italian Central Alps, as shown in Figure 1. Anzasca Valley is a long, narrow valley closed by Monte Rosa (4634 m). The Anza River (31 km) stretches from Monte Rosa and flows to Toce River. Several small streams, on the right and on the left side of the valley, flow in the Anza River. About halfway down the valley, on the left side, near the small town Vanzone con San Carlo, there is an old abandoned gold mine (Miniera dei Cani, 1475 m above sea level) (Figure 1) (yellow triangle), and the Rio Rosso (2.2 km) flows out of the mine. We decided to investigate its stream waters in comparison with two other small streams: Rio Gattera (2.6 km), near Rio Rosso, and Rio Roletto (3.6 km), further upstream. The streams are perennial and are located in the same sub-catchment area (4.5 km2). In 2012, we sampled three sites for Rio Roletto, indicated on the map as points 1, 2, and 3 (coordinates and altitude in Table S1 (Supplementary Materials)); two for Rio Gattera, points 4 and 5; and one for Rio Rosso, point 6. After the preliminary chemical analysis (Table S1), we saw more interesting differences among the three streams at the same altitude, and the subsequent samplings were carried out at one point for each stream: sites 1, 4, and 6 for Rio Roletto, Rio Gattera, and Rio Rosso, respectively.
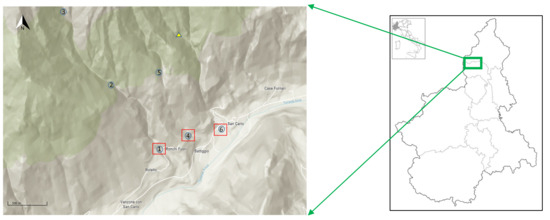
Figure 1.
Map shows the study area in Italy and sampling sites for the three streams in Anzasca Valley (Piedmont): Rio Roletto (1, 2, 3), Rio Gattera (4, 5), Rio Rosso (6). The yellow triangle indicates the gold mine. In red box successive sampling.
2.1.1. Chemistry of Stream Waters
Stream waters were collected one and a half meters from the streamside in the center of the stream. We avoided sampling during or after heavy rain events, which may affect water chemistry because of the different chemical composition between rain and stream water. In the laboratory, water was stored at 4 °C until the analysis. The samples were analyzed for pH, conductivity, alkalinity, ammonium, total organic carbon, silica, main cations (calcium, magnesium, sodium, potassium), and anions (sulfate, nitrate, chloride) using standard methods for freshwater samples [17,18] in the chemistry laboratory of the CNR IRSA in Verbania. Regular quality assurance/quality control procedures are adopted in the laboratory to assure the data quality and the comparability of the data over time, including the use of control charts and the participation of national and international intercomparisons. The limit of detection (LOD), limit of quantification (LOQ), details of the analytical methods, and the analytical quality control QA/QC procedures adopted in the laboratory can be found at http://www.idrolab.irsa.cnr.it/, accessed on 30 August 2023.
2.1.2. Metals in the Stream Waters
Metals in the stream waters were measured by ICP-OES (inductively coupled plasma–optical emission spectrometer). An ultrasonic nebulizer was used to generate fine aerosol, which is transported by argon gas. The vaporized aerosol is then passed through a thermo-electrically cooled condenser, where a large fraction of the solvent is removed. Analysis was tested against certified reference materials. Water samples were filtered at 0.45 µm (treated samples) or measured without any filtration (untreated samples). To ensure consistent results are produced from this research activity, the CNR IRSA laboratory has regularly performed analytical quality control QA/QC, with participation in national and international Working Ring Tests, such as NIVA [19] and limit of detection (LOD), and details of the method can be found on the site http://www.idrolab.irsa.cnr.it/, accessed on 30 August 2023. Water samples were filtered at 0.45 µm (treated samples) or measured without any filtration (untreated samples).
2.1.3. Diatom Identification in the Biofilm
Diatom sampling was performed following the standard procedure, EN13946 (European Standard 2014) [20], by scraping five stones (usually cobbles and small boulders) with a toothbrush. The samples for diatom identification were treated with hot hydrogen peroxide and hydrochloric acid, as described in the EN13946 procedure, and finally mounted using Naphrax (Brunel Microscopes Ltd., Chippenham, UK) on permanent slides (Zeiss Axiolab, magnification 1000×). Taxonomic identification was based on Krammer and Lange-Bertalot [21] and other authors [22,23,24,25,26,27], integrated with the papers by Potapova and Hamilton [28] and Van de Vijver [29,30] concerning the Achnanthidium minutissimum species complex, Fragilaria rumpens complex, and Fragilaria radians complex, respectively. For each sample, a minimum of 400 valves were identified using a Zeiss Axiolab microscope (Gottingen, Germania), and results were expressed as relative abundances (r.a.). Subsequently, the α-diversity and heterogeneity of the diatomic communities for each sampling site were evaluated based on the Shannon index [31] and Evenness [32], respectively. The ecological status was assessed via the calculation of the Intercalibration Common Metrics Index (ICMi) [33] and the Diatom-Based Index for Heavy Metal Pollution (ICM) [34].
2.1.4. Benthic Algal Community of the Biofilm
Benthic algal determinations were carried out on subsamples from each site. They were also preserved with acetic Lugol’s solution; the reagents were all of analytical grade by VWR (Milan, Italy). The benthic algal taxonomic composition was analyzed under a Leica 4000B inverted microscope (Leica Srl, Milan, Italy) according to the norm CEN 15204 modified [35]. Hence, sedimentation chambers were filled with diluted sediment samples and evaluated at 400×–1000× magnification following the Üthermol method [36] and Lund [37], classifying the taxa to the species level whenever possible. Further, an aliquot from each sample was used for the identification of algae in temporary slides at 1000× magnification under lightfield, darkfield, and phase contrast microscopy techniques to characterize them morphologically. Image analyses were also used to perform identification.
2.1.5. Quantification of Carotenoid and Chlorophyll a in the Biofilm
The carotenoids were extracted overnight at 4 °C, in the dark, from the freeze biofilm using 90% acetone. Spectrophotometric evaluation of chlorophylls a and pheophytin was performed following the method reported by Steinman and Lamberti [38]. The reagents were all of analytical grade by VWR (Milan, Italy). The data from each experiment represent the mean (±standard deviation, SD) of three replicates.
HPLC analysis on the same extracts was performed with a Thermo Fisher Scientific (Waltham, MA, USA) “UltiMate LC System” composed of a UV/VIS detector and a Photodiode Array BioLC detector [39]; reagents were HPLC Lichrosolv by VWR (Milan, Italy). The identification of carotenoids was based on the retention time and on the UV spectrum of each peak in the database of spectra of pigments most widespread in aquatic ecosystems.
2.1.6. Arsenic in the Biofilm of Rio Rosso
Dried biofilms (50 °C) of Rio Rosso from 2012 to 2014 were analyzed to measure arsenic by ICP-MS in the University laboratory of Milan. Aliquots of 150 mg of dried biofilm samples were digested by a microwave digestor system (Anton Paar MULTIWAVE-ECO) in Teflon tubes filled with 10 mL of 65% nitric acid by employing a one-step temperature ramp (the temperature is increased to 180 °C in 20 min and maintained for 10 min). After 20′ of cooling time, the mineralized samples were transferred to polypropylene test tubes, and the final volume was registered. Then, samples were properly diluted (1:100) with 1.3 M of nitric acid in MILLI-Q water, and the concentration of elements was measured via inductively coupled plasma mass spectrometry (BRUKER Aurora-M90 ICP-MS). To check the nebulization performance, an aliquot of 2 mgL−1 of an internal standard solution (72Ge, 89Y, 159Tb) was added to the samples and to the calibration curve to give a final concentration of 20 µg L−1.
Typical polyatomic analysis interferences were removed using CRI (collision–reaction interface) with an H2 flow of 70 mL min−1 flown through a skimmer cone [40]. All reagents were of analytical grade by VWR (Milan, Italy). The data from each experiment represent the mean (±standard deviation, SD) of three replicates.
3. Results
3.1. Water Characterization
3.1.1. Water Chemistry of the Three Streams
Fieldwork was carried out in September 2012. Along the altitudinal gradient, we sampled three sites in Rio Roletto at different altitudes: two sites for Rio Gattera and one site for Rio Rosso (Figure 1). Samples location, altitude, and water chemical data measured are reported in Table S1 (Supplementary Materials). Some differences were present along the same stream at different altitude points, but the more interesting differences were at circa some altitude in the three streams, particularly site 1 for Rio Roletto (710 m above sea level), site 4 (680 m above sea level) for Rio Gattera, and site 6 for Rio Rosso (570 m above sea level). The stream did not make it possible to sample at the same altitude. The pH of Rio Roletto (7.80, 7.85, 7.93) and Gattera (8.05, 8.00) was slightly higher than Rio Rosso (pH 7.40); the difference was small, but it reflects their different ion composition. Conductivity values increased from Rio Roletto (74.4–64.3, µS cm−1) to Rio Rosso (230.8 µS cm−1) while the total alkalinity, on the contrary, decreased from Rio Roletto (0.591, 0.607, 539 meq L−1) to Rio Rosso (0.172 meq L−1). The Rio Rosso showed the lowest buffer capacity among the three streams, probably associated with a gradient depending on the catchment lithology composition. High concentrations of sulfate (100 mg L−1), calcium (32.60 mg L−1), and magnesium (6.70 mg L−1) in the Rio Rosso derived from the arsenopyrite and shale present in the gold mine. The acid drainage in the mine solubilized many elements, including silica, present five times higher than the other two streams. The total organic carbon in all streams was very low. From these preliminary data, we decided to increase the sampling effort by limiting to one site for each stream but performing the sampling with higher time resolution to follow the pattern of the water chemistry in different seasons. In the histogram, Figure 2, data for the three streams are shown as an average of eight different samplings collected from 2012 to 2014 in all seasons: site 1 for Rio Roletto, 4 for Rio Gattera, and 6 for Rio Rosso. The different chemical composition of the Rio Rosso stream was evident and associated with the water drainage of the gold mine.
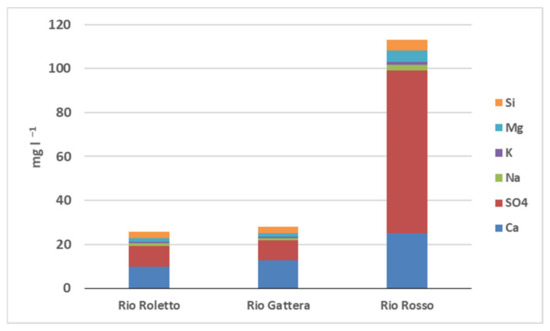
Figure 2.
Some chemical parameters of the waters of three streams. Site: 1, Rio Roletto; 4, Rio Gattera; 6, Rio Rosso. Data are the average value of eight different samplings.
3.1.2. Metals in the Stream Waters
Metals in the stream waters were measured with ICP-OES, waters were sampled in July and October 2013, and data were reported in the cumulative histogram in Figure 3. In Figure 3A, Rio Roletto and Rio Gattera, and in Figure 3B, Rio Rosso, scales were completely different; a large amount of metals were present in Rio Rosso, which explains the high conductivity of the stream. The standard method provided a step with filtration; in the case of Rio Roletto (A treated), there was a loss of aluminum and iron, and arsenic was not present (i.e., it was under the detection limit). In the Rio Gattera (A untreated), arsenic was present in a small amount, 9 µg L−1, and was completely lost by filtration together with iron (A treated). Regarding the pH of the stream water, iron was present as hydroxides and could form complexes with arsenic ions. In the Rio Rosso stream (B), after filtration (treated), iron and aluminum, present as hydroxides, complexed with arsenic and copper, were removed. In the sample, 12 µg L−1 of arsenic was present; the colloidal dispersion remained on the filter, and the filtrated water showed the loss of elements. In the untreated sample, the amount of arsenic was 109 µg L−1. The water of Rio Rosso presented a micro-turbidity, which was not detected with the standard methods; the Rio Rosso samples showed a pale opaque pink color.
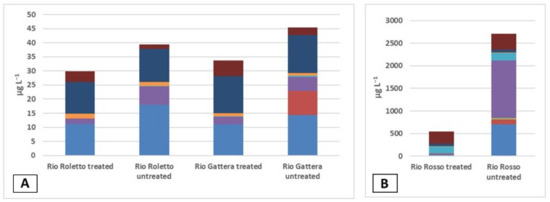
Figure 3.
Metals in stream waters: (A) Rio Roletto, treated and untreated; Rio Gattera, treated and untreated. (B) Rio Rosso, treated and untreated. Al  ; As
; As  ; Cu
; Cu  ; Fe
; Fe  ; Mn
; Mn  ; Ni
; Ni  ; Sr
; Sr  ; Zn
; Zn  .
.
 ; As
; As  ; Cu
; Cu  ; Fe
; Fe  ; Mn
; Mn  ; Ni
; Ni  ; Sr
; Sr  ; Zn
; Zn  .
.
3.2. Biofilm Characterization
3.2.1. Diatom Identification
A total of 43 species belonging to 22 genera have been detected to the highest taxonomic resolution possible (Supplementary Material Table S2). The number of dominant species (r.a. greater than 10%) varied between two and four in each stream, and that of subdominant species (r.a. greater than 5% but less than or equal to 10%) varied between one and three (Table 1). The genera most rich in taxa was Gomphonema (eight), followed by Achnanthidium (five), Fragilaria (four), Nitzschia and Encyonema (three each), and Cocconeis and Cymbella (two each), while all other genera were represented by only one taxon.

Table 1.
Diatom biodiversity and ecological status of three streams in Anzasca Valley. The contribution in number and percentage of the species present in the ICM dataset was also reported.
Approximately 33% of the total identified taxa are included in the red list [41] (Table S2 Supplementary Material). The 16% are species whose abundance and frequency are estimated to be decreasing and are present in low abundance in all sites. Taxa defined as “highly endangered” are 9% of the total number and account for approximately 1/3 of the population in all three streams. Species classified in “danger of extinction” are also included (about 7%) and are present only in Rio Roletto (Figure 4).
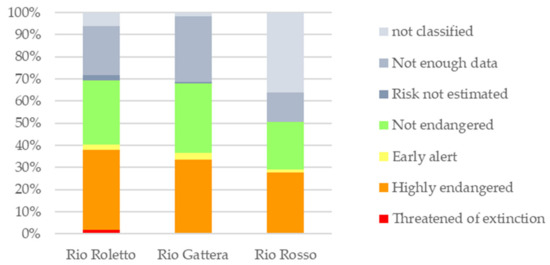
Figure 4.
Diatom community composition of the three streams in Anzasca Valley according to the red list [41].
The three streams showed low and comparable Shannon (H’) and Pielou (E) index values; the most common and abundant diatoms were achnanthoid taxa described within the Achnanthidium minutissimum complex (A. minutissimum, A. linearis, A macrocephalum and Achnanthes linearis f. curta). Such assemblages that account for 60–70% of abundance in the study sites correspond to the first degree of pollution in other metal-contaminated sites [34]. Other abundant taxa were Achnanthidium pyrenaicum (max abundance 23%) and Diatoma mesodon (12%). The latter is quite typical of mountain streams, is sensitive to organic pollution, and is found in fairly clean waters.
Rio Rosso also presented an abundance of teratologic forms higher than 7%, concerning mainly A. minutissimum complex species. These pioneer species, typical at the beginning of the colonization process due to their short generation time, high resistance to physical disturbances, and high efficiency in nutrient uptake [42], are suitable for changing environments that are sometimes stressful and unpredictable. They can develop in a wide range of environments, but their detoxification mechanisms are likely not efficient and may not be protected by teratological damage [34]. The three streams are not impacted by organic pollution. In fact, the assessment of the quality status based on the application of the Intercalibration Common Metrics index (ICMi) attributed high ecological class to each one (reference value belonging to the “Alpine siliceous macrotype”).
We also applied the new ICM (Índice de Contaminación por Metales) developed for rivers affected by AMD (acid mine drainage). Among the 49 taxa identified in our study, only 6 in Rio Roletto, 6 in Rio Gattera, and 11 in Rio Rosso were included in the dataset used for the construction of the ICM index, but they accounted for 64%, 69%, and 77% of abundance, respectively (Table 1). According to ICM, the water-quality classes attributed to the rivulet in Anzasca Valley were “moderately polluted” for Rio Roletto and “polluted” for Rio Gattera and mainly Rio Rosso.
3.2.2. Biofilm Taxonomic Identification of Benthic Algal Community
The number of taxa observed in each sample site for Cyanobacteria and Bacyllariophyta is represented in Figure 5; the most abundant division found was Bacillariophyceae at Rio Roletto. Taxa among Cyanobacteria were filamentous oscillatoriales with the genera Phormidium, Leptolyngbya, and Lyngbya; the coccoid species Geitleribactron; and filamentous oscillatoriales Hyella. Cyanobacteria genera Leptolyngbya and Lyngbya were found in all sampling sites, while the chroococcalean Geitleribactron was found only at Rio Roletto. Rio Rosso was the richest in Cyanobacteria taxa among the three sites with seven taxa, followed by Rio Gattera and Rio Roletto with five taxa (Figure 6). Cyanobacteria can accumulate metals internally, and studies have shown that they can survive and reproduce in metal-contaminated habitats [43]. Cyanobacteria can uptake and concentrate arsenic from the surrounding environment [44,45]; furthermore, Synechocystis and Phormidium are known as arsenic-resistant genera [46,47].
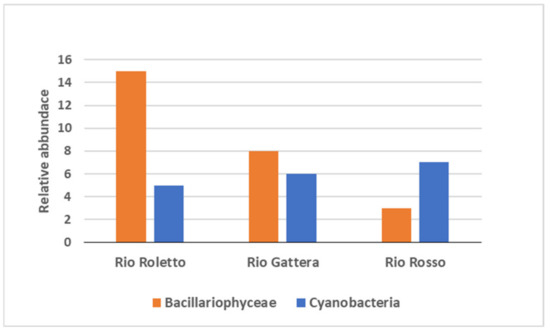
Figure 5.
Relative abundance of benthic algal community in the three streams.
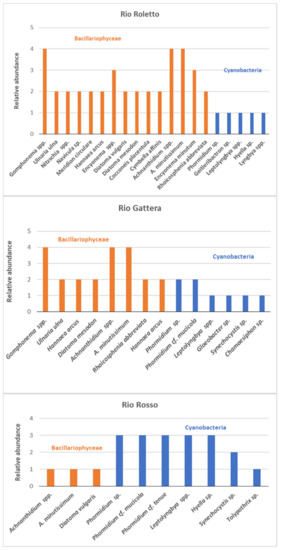
Figure 6.
Relative abundance of benthic algal community for each stream.
3.3. Quantification of Carotenoid and Chlorophylls in the Biofilm of the Three Streams
Using spectrophotometer analysis, we quantified the carotenoids and chlorophylls of the biofilm in the three streams in 2013, in March and July, which are two typical seasons to test mountain streams. The results are reported in Table 2. In the Rio Rosso stream, the values of the total chlorophyll a measured varied between 0.475 and 3.878 μg/cm2. The highest values were detected in July 2013. In March, at the end of winter, the temperature and the sun radiation were low, and the concentration of photosynthetic pigments was scarce, indicating a low development of biofilm. Rio Gattera showed a lower value of chlorophylls from 0.120 to 0.222 μg/cm2, while a higher value (0.201 to 0.466 μg/cm2) was detected in Rio Roletto. Total carotenoids were similar in Rio Roletto and Rio Gattera in March but showed higher value in Rio Rosso. This difference was also observed in July. Total carotenoids and chlorophylls are higher in the Rio Rosso stream, which might be due to greater exposition to the sun than in Rio Gattera and Rio Roletto. Carotenoids can be utilized by photosynthetic organisms to protect against solar radiation [48] and could also be utilized to protect against metal oxidative stress, but additional investigations are necessary to discriminate the role of these two factors.

Table 2.
Total carotenoids and chlorophyll a in the biofilm in the three streams.
Carotenoid and chlorophyll a composition were identified by HPLC [49]. Percentage of the major specific carotenoid as pick area are reported in Table 3. Fucoxanthin, myxoxanthophill, myxol-like (a myxol glycosilated carotenoid identified from the HPLC spectra library [50]), and beta carotene were the most abundant carotenoids. Fucoxanthin can be utilized as biomarkers for diatom algae, while the myxol-like molecules can be utilized as markers for Cyanobacteria [51]. Beta carotene was indicative of carotenoid expression but not selective for different species. In the chromatogram, only the identified peaks were considered carotenoid molecules; other peaks were present but not considered since they were considered a degradation product.

Table 3.
Principal carotenoids identified by HPLC in July 2013.
To understand the biofilm behavior better, we expressed the results as percentages on extract in four different samplings, two in 2013 and two in 2014, in two different seasons. Data were reported in Figure 7. In the biofilm of Rio Roletto and Rio Gattera sampling in July 2013, Bacyllariophyceae (80%) were mainly present, while Cyanobacteria were only 20%. In Rio Rosso, instead, the Cyanobacteria represented 75%, while the Bacyllariophyceae represented 25%. The Rio Roletto and Rio Gattera were mountain streams, very low in nutrient content, and during the seasons, presented small changes. Biofilm was mainly constituted by Bacyllariopyiceae, as confirmed by diatoms and the benthic algal community. Rio Rosso waters presented low buffer capacity; many environmental variables (temperature, rains, and flow rate) could change the water chemistry and the biofilm community, as presented in Figure 6. The number of taxa was small (Figure 5), and the water, reached in metals, was of scarce quality. In these conditions, Cyanobacteria could often develop in large amounts with peculiar biofilm, very high, and rich in extruded polymeric substance to protect the phytobentonic organisms.
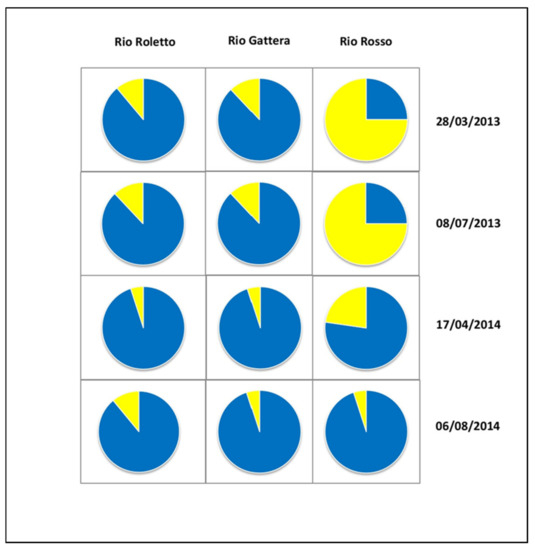
Figure 7.
Carotenoid quantification by HPLC: four different samples in 2013 and 2014 for the three streams: yellow is Cyanobacteria, blue is Bacyllariophyceae.
3.4. Arsenic Quantification of the Biofilm of Rio Rosso
In the water of Rio Rosso, the water chemistry identified large amounts of Arsenic. In the untreated water samples of Rio Rosso, the total content of heavy metals was 50 times higher compared to the untreated waters of Rio Roletto and Rio Gattera. We decided to investigate the presence of arsenic in the biofilm of Rio Rosso to understand the fate of arsenic in the water. Data are shown in Figure 8. The first investigation of Arsenic assimilated or polydisperse on the biofilm in different seasons from 2012 to 2014 showed the constant presence of arsenic with a medium value of 8 µg mg−1 d.w., but with some high values in winter, probably due to low temperature and scarce solubility of iron hydroxide and low flow rate. The amount of arsenic was very high and could select the organisms living in the Rio Rosso stream; only the more resistant and with high metal tolerance could survive. The presence of arsenic (V) as a prevalence form can be determined by the value of pH between 6 and 7.5 and in highly oxygenated waters.
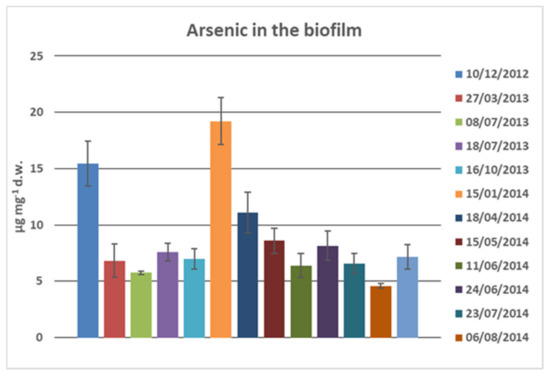
Figure 8.
Arsenic in the biofilm of Rio Rosso: µg mg−1 for dry weight (d.w.). Data collected in different samplings are reported with different colors. Errors bars of three replicates.
4. Discussion and Conclusions
The waters of the three streams were different, even though they were in the same catchment area. Rio Roletto was completely different, and without arsenic, its biofilm presented the higher biodiversity for a mountain stream among the three streams in the total algal community. Rio Gattera contains a small amount of arsenic, and a reduced relative taxa abundance was present in the biofilm. Rio Rosso flows out of the mine, and its water is rich in acid drainage and many metals; the taxa present in the biofilm were few, and there were organisms living in polluted environments. Diatom analysis confirms this hypothesis; in Rio Rosso, teratologic diatoms were also present due to the water chemistry. Surprising the pH of Rio Rosso streams was not as acidic as expected; a small tributary flowed in the stream after the mine, and before the sampling site, a spectacular red waterfall was present, which oxygenates the water and promotes the precipitation of iron and aluminum hydroxides complexed with arsenic. When Rio Rosso flows in Anza streams, the red trace of the streambed disappears, and the water is diluted in the Anza stream coming from the Monte Rosa glacier. These results are preliminary data and the first investigation of the aquatic ecosystem of the three streams. Further investigations are necessary to clarify the role of arsenic in the aquatic environment of Rio Rosso and the arsenic spread in Anzasca Valley. The biofilm of Rio Rosso changes its communities and stores a large amount of arsenic; this peculiar behavior, probably due to external polymeric substances (EPS), will be investigated in future work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151914217/s1, Table S1, Chemical characterization of the stream water: Rio Roletto, Rio Gattera, Rio Rosso sampled 18/09/2012; Table S2, List of diatomic species; Table S3, List of taxa collected from three rivers: Rio Roletto, Rio Gattera, and Rio Rosso.
Author Contributions
Conceptualization, N.G., A.M., L.S. and G.B.; methodology, N.G., A.L., S.M., M.A., L.S. and G.B.; formal analysis, A.O., P.G., G.T. and G.L.; writing—original draft preparation, N.G., G.B., S.M. and M.A.; writing—review and editing, N.G., G.B., S.M., M.A. and A.L.; supervision, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge Rosario Mosello for his support in Rio Rosso chemistry, Marzia Ciampitiello for her support on hydrology, and Andrea Piscedda for his considerable support in the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowell, R.J.; Alpers, C.N.; Jamieson, H.E.; Nordstrom, D.K.; Majzlan, J. The environmental geochemistry of arsenic an overview. Rev. Mineral. Geochem. 2014, 79, 1–16. [Google Scholar] [CrossRef]
- Henke, K.R. Arsenic: Environmental Chemistry, Health Treats and Waste Treatment; Ed Wiley: Chichester, UK, 2009. [Google Scholar]
- Ahmad Abbasnejad, A.; Mirzaie, A.; Derakhshani, R.; Esmaeilzadeh, E. Arsenic in groundwaters of the alluvial aquifer of Bardsir plain, SE Iran. Environ. Earth Sci. 2013, 69, 2549–2557. [Google Scholar] [CrossRef]
- Bini, C.; Bech, J. PHEs, Environment and Human Health; Springer: London, UK, 2014; pp. 57–59. [Google Scholar]
- Zuzolo, D.; Cicchella, D.; Demetriades, A.; Birke, M.; Albanese, S.; Dinelli, E.; Lima, A.; Valera, P.; De Vivo, B. Arsenic: Geochemical distribution and age-related health risk in Italy. Environ. Res. 2020, 182, 109076. [Google Scholar] [CrossRef] [PubMed]
- WHO. Arsenic and Arsenic Compounds. Environmental Health Criteria 224; World Health Organization: Geneva, Switzerland, 2001; p. 512. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf?ua=1 (accessed on 30 August 2023).
- Paikaray, S. Arsenic Geochemistry of Acid Mine Drainage. Mine Water Environ. 2015, 34, 181–196. [Google Scholar] [CrossRef]
- Bondu, R.; Casiot, C.; Pistre, S.; Batiot-Guilhe, C. Impact of past mining activities on water quality in a karst area in the Cévennes region, Southern France. Sci. Total Environ. 2023, 873, 162274. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Remigio, A.; Rubinos, D.A.; ·Van der Ent, A.; Edraki, M. Geochemical cycles of arsenic in historic tin tailings from multiple ore sources: An example from Australia. Water Air Soil Pollut. 2021, 232, 497. [Google Scholar] [CrossRef]
- Gombert, S.; Galsomiès, L.; Rauch de Trauberberg, C.; Leblond, S.; Losno, S.; Colin, J.L.; Charré, B. Atmosphérique par les Métaux: Biosurveillance des Retombées; EDP Science: Paris, France, 2005; p. 108. [Google Scholar]
- EFSA Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3897. [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; p. 564. Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf?sequence=1 (accessed on 30 August 2023).
- Dlgs. 23 Febbraio 2023 n. 18 Qualità delle Acque Destinate al Consumo Umano. Available online: https://www.gazzettaufficiale.it/eli/gu/2023/03/06/55/sg/pdf (accessed on 30 August 2023).
- Quaranta, E.; Mosello, R. Le acque arsenicali-ferruginose di Vanzone (Valle Anzasca, Novara) Studi recenti finalizzati all’utilizzo terapeutico. Oscellana 1995, 4, 230–237. [Google Scholar]
- Carta Geologica d’Italia, Istituto Geografico Militare 1913, Foglio 15 Domodossola. Available online: http://sgi.isprambiente.it/geologia100k/mostra_foglio.aspx?numero_foglio=15 (accessed on 30 August 2023).
- Carta Geologica d’Italia, Istituto Geografico Militare 1927, Foglio 30 Varallo. Available online: http://sgi.isprambiente.it/geologia100k/mostra_foglio.aspx?numero_foglio=30 (accessed on 30 August 2023).
- APHA, AWWA, WEF Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012.
- APAT, IRSA-CNR Metodi Analitici per le Acque; APAT Manuali e Linee Guida 29; 2003. Available online: https://www.irsa.cnr.it/wp/?page_id=5435 (accessed on 30 August 2023). (In Italian).
- Hovind, H.; Skjelkvåle, B.L.; Faafeng, B. Intercomparison 0923: pH, Cond, HCO3, NO3, +NO2, Cl, SO4, Ca, Mg, Na, K, Fe, Mn, Cd, Pb, Cu, Ni and Zn, NIVA-Report SNO 5845 2009, ICP-Waters Report 986/2009; Norsk Institutt for Vannforskning: Oslo, Norway, 2006. [Google Scholar]
- EN 13946:2014; Water Quality—Guidance for the Routine Sampling and Preparation of Benthic Diatoms from Rivers and Lakes. European Committee for Standardization: Brussels, Belgium, 2014; p. 17.
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa. In Bacillariophyceae; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 2018; Volume 2/1: Naviculaceae, Volume 2/2: Bacillariaceae, Epithemiaceae, Surirellaceae, Volume 2/3: Centrales, Fragilariaceae, Eunotiaceae, Volume 2/4: Achnanthaceae, 1986–2004, p. 876–596–576–468. [Google Scholar]
- Lange-Bertalot, H. Volume 2: Navicula Sensu Stricto, 10 Genera Separated from Navicula Sensu Stricto, Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Germany, 2001; pp. 1–526. [Google Scholar]
- Krammer, K. Volume 1: The Genus Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Germany, 2000; pp. 1–703. [Google Scholar]
- Krammer, K. Volume 3: Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Germany, 2002; pp. 1–584. [Google Scholar]
- Krammer, K. Volume 4: Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella Supplements to cymbelloid taxa. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Germany, 2003; pp. 1–530. [Google Scholar]
- Lange-Bertalot, H.; Witkowski, A.; Bąk, M. Volume 6: Eunotia and some related genera. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Germany, 2011; pp. 1–747. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Book: Oberreifenberg, Germany, 2017; pp. 1–942. [Google Scholar]
- Potapova, M.G.; Hamilton, P.B. Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. J. Phycol. 2007, 43, 561–575. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Williams, D.M.; Schuster, T.M.; Kusber, W.; Cantonati, M.; Wetzel, C.E.; Ector, L. Analysis of the Fragilaria rumpens complex (Fragilariaceae, Bacillariophyta) with the description of two new species. Fottea 2022, 22, 93–121. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Williams, D.M.; Kusber, W.; Cantonati, M.; Hamilton, P.B.; Wetzel, C.E.; Ector, L. Fragilaria radians (Kützing) D.M. Williams et Round, the correct name for F. gracilis (Fragilariaceae, Bacillariophyta): A critical analysis of this species complex in Europe. Fottea 2022, 22, 256–291. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–125. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975; pp. 1–165. [Google Scholar]
- Mancini, L.; Sollazzo, C. Metodo per la valutazione dello stato ecologico delle acque correnti: Comunità diatomiche. Rapp. Istisan 2009, 9, 1–32. [Google Scholar]
- Fernández, M.R.; Martín, G.; Corzo, J.; de la Linde, A.; García, E.; López, M.; Sousa, M. Design and Testing of a New Diatom-Based Index for Heavy Metal Pollution. Arch. Environ. Contam. Toxicol. 2018, 74, 170–192. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 15204; Water Quality—Guidance Standard for the Routine Analysis of Phytoplankton Abundance and Composition Using Inverted Microscopy (Utermohl Technique). CEN Management Centre: Brussels, Belgium, 2006; pp. 1–40.
- Utermöhl, H. Zur Vervollkommung der quantitative Phytoplankton Methodik. Mitt.—Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Steinman, A.D.; Lamberti, G.A.; Leavitt, P.R. Biomass and Pigments of Benthic Algae. In Methods in Stream Ecology, 2nd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Accademic Press: San Diego, CA, USA, 2007; pp. 357–379. [Google Scholar] [CrossRef]
- Lami, A.; Guilizzoni, P.; Marchetto, A. High resolution analysis of fossil pigments, carbon, nitrogen and sulphur in the sediment of eight European Alpine Lakes: The Molar project. J. Limnol. 2000, 59, 25–28. [Google Scholar] [CrossRef]
- Bertazzini, A.; Sacchi, G.A.; Forlani, G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. J. Plant Physiol. 2018, 226, 145–153. [Google Scholar] [CrossRef]
- Hofmann, G.; Lange-Bertalot, H.; Werum, M.; Klee, R. Rote Liste und Gesamtartenliste der limnischen Kieselalgen (Bacil-lariophyta) Deutschlands. In Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 7: Pflanzen; Metzing, D., Hofbauer, N., Ludwig, G., Matzke-Hajek, G., Eds.; Naturschutz und Biologische Vielfalt; Landwirtschaftsverlag: Münster, Germany, 2018; Volume 70, pp. 601–708. [Google Scholar]
- B-Béres, V.; Lukács, Á.; Török, P.; Kókai Zs Novák, Z.; T-Krasznai, E.; Tóthmérész, B.; Bácsi, I. Combined eco-morphological functional groups are reliable indicatorsof colonisation processes of benthic diatom assemblages in a lowland stream. Ecol. Indic. 2016, 64, 31–38. [Google Scholar] [CrossRef]
- Fiore, M.F.; Trevors, J.T. Cell composition and metal tolerance in cyanobacteria. Biometals 1994, 7, 83–103. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Pal, R. Response of cyanobacteria to arsenic toxicity. J. Appl. Phycol. 2010, 23, 293–299. [Google Scholar] [CrossRef]
- Banerjee, M. Arsenic. A threatening environmental issue and role of cyanobacteria in toxicity mitigation. J. Ecophysiol. Occup. Health 2008, 8, 153–159. [Google Scholar]
- Ferrari, S.G.; Silva, P.G.; González, M.D.; Navoni, J.A.; Silva, H.J. Arsenic tolerance of cyanobacterial strains with potential use in biotechnology. Rev. Argent Microbiol. 2013, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, L.H.; Bai, R.; Huang, H.; Sun, G. Accumulation and transformation of arsenic in the blue-green alga Synechocysis sp. PCC6803. Water Air Soil Pollut. 2012, 223, 1183–1190. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosynthesis and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).