A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Source and In Vitro Establishment
2.2. In Vitro S. dominica Proliferation, Rooting, and Acclimatization
2.3. Induction and Multiplication of S. dominica Callus
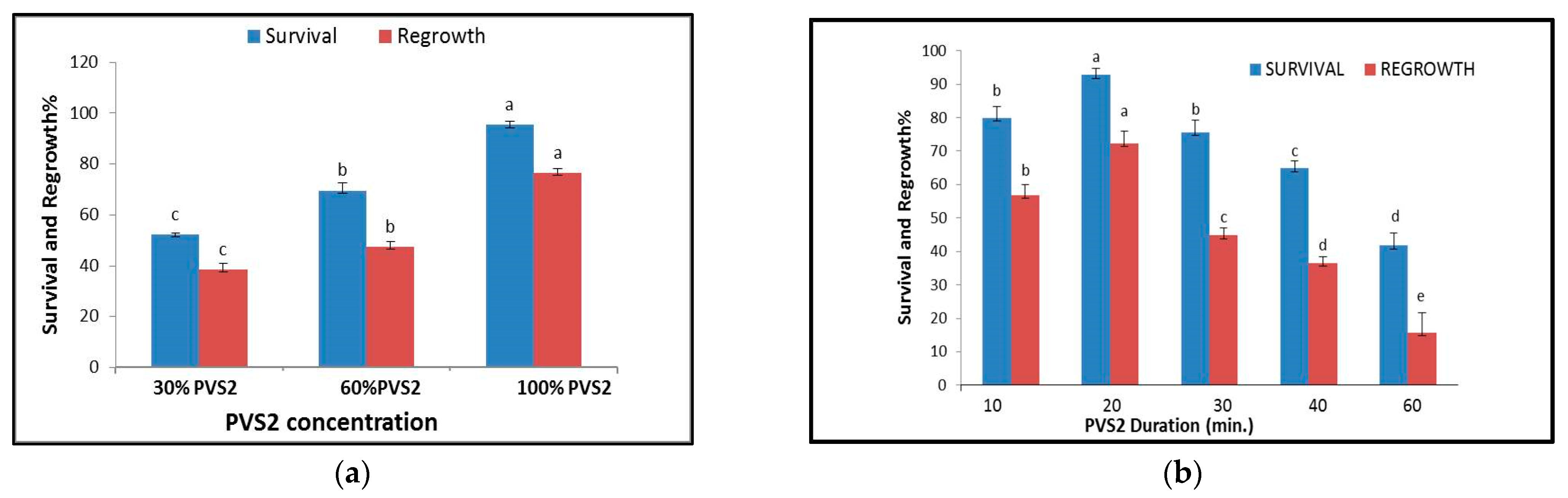
2.4. Cryopreservation of S. dominica Callus
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Sterilization and In Vitro Establishment
3.2. In Vitro S. dominica Proliferation, Rooting, and Acclimatization
3.3. Induction and Multiplication of S. dominica Callus
3.4. Cryopreservation of S. dominica Callus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niesenbaum, R.A. The integration of conservation, biodiversity, and sustainability. Sustainability 2019, 11, 4676. [Google Scholar] [CrossRef]
- Napreenko, M.G.; Antsiferova, O.A.; Aldushin, A.V.; Samerkhanova, A.K.; Aldushina, Y.K.; Baranovskiy, P.N.; Napreenko-Dorokhova, T.V.; Panov, V.V.; Konshu, E.V. New approaches to sustainable management of wetland and forest ecosystems as a response to changing socio-economic development contexts. In Innovations and Traditions for Sustainable Development; Springer: Cham, Switzerland, 2021; pp. 395–416. [Google Scholar]
- Borón, V.; Payán, E.; MacMillan, D.; Tzanopoulos, J. Achieving sustainable development in rural areas in Colombia: Future scenarios for biodiversity conservation under land use change. Land Use Policy 2016, 59, 27–37. [Google Scholar] [CrossRef]
- Khalil, A.; Hassawi, D.S.; Kharma, A. Genetic relationship among Salvia species and antimicrobial activity of their crude extract against pathogenic bacteria. Asian J. Plant Sci. 2005, 4, 544–549. [Google Scholar] [CrossRef]
- Zihlif, M.; Afifi, F.; Abu-Dahab, R.; Abdul Majid, A.M.S.; Somrain, H.; Saleh, M.M.; Naffa, R. The antiangiogenic activities of ethanolic crude extracts of four Salvia species. BMC Complement. Altern. Med. 2013, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Şenkal, B.C.; Uskutoğlu, T.; Cesur, C.; ÖZAVCI, V.; Doğan, H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk. J. Agric. For. 2019, 43, 395–404. [Google Scholar] [CrossRef]
- Goren, A.C.; Kilic, T.; Dirmenci, T.; Bilsel, G. Chemotaxonomic evaluation of Turkish species of Salvia: Fatty acid compositions of seed oils. Biochem. Syst Ecol. 2006, 34, 160–164. [Google Scholar] [CrossRef]
- Taifour, H.; El-Oqlah, A. Jordan Plant Red List; Royal Botanic Garden: Kew, UK, 2014; Volume 1. [Google Scholar]
- Taifour, H. Jordan Plant Red List; Royal Botanic Garden: Kew, UK, 2016; Volume 2. [Google Scholar]
- Abdallah, M.; Abu-Dahab, R.; Afifi, F. Composition of the essential oils from Salvia dominica L. and Salvia hormium L. grown in Jordan. Jordan J. Pharm. Sci. 2013, 6, 40–46. [Google Scholar] [CrossRef]
- Hasan, M.R.; Al-Jaber, H.I.; Al-Qudah, M.A.; Zarga, M.H.A. New sesterterpenoids and other constituents from Salvia dominica growing wild in Jordan. Phytochem. Lett. 2016, 16, 12–17. [Google Scholar] [CrossRef]
- Fiore, G.; Nencini, C.; Cavallo, F.; Capasso, A.; Bader, A.; Giorgi, G.; Micheli, L. In vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 701–703. [Google Scholar]
- Al-Jaber, H.I.; Bdair, O.; Barhoumi, L.M.; Dabaibeh, R.N.; Abu-Zarga, M.H.; Abaza, I.F.; Afifi, F.U. Characterization of chemical components in organs emissions of two Salvia dominica L. populations from Jordan. J. Oleo Sci. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Radomir, A.M.; Stan, R.; Florea, A.; Ciobotea, C.M.; Bănuță, F.M.; Negru, M.; Sumedrea, D.I. Overview of the Success of In Vitro Culture for Ex Situ Conservation and Sustainable Utilization of Endemic and Subendemic Native Plants of Romania. Sustainability 2023, 15, 2581. [Google Scholar] [CrossRef]
- Tahtamouni, R.W.; Shibli, R.A.; Al-Abdallat, A.M.; Al-Qudah, T.S.; Younis, L.; Al-Baba, H.; Al-Ruwaiei, H. Cryopreservation of Thymbra spicata L. var. spicata and genetic stability assessment of the cryopreserved shoot tips after conservation. Jordan J. Biol. Sci. 2017, 10, 19–28. [Google Scholar]
- Al-Abdallat, A.M.; Shibli, R.A.; Akash, M.W.; Rabbaa, M.; Al-Qudah, T. In vitro preservation of transgenic tomato (Solanum lycopersicum L.) plants overexpressing the stress-related SlAREB1 transcription factor. Int. J. Mol. Sci. 2017, 18, 1477. [Google Scholar] [CrossRef] [PubMed]
- Gianguzzi, V.; Barone, G.; Di Gristina, E.; Sottile, F.; Domina, G. Micropropagation of Endemic Endangered Taxa of the Italian Flora: Adenostyles alpina subsp. macrocephala (Asteraceae), as a Case Study. Plants 2023, 12, 1530. [Google Scholar] [CrossRef]
- Teixeira, J.C.; Huber, C.D. The inflated significance of neutral genetic diversity in conservation genetics. Proc. Nat. Acad. Sci. USA 2021, 118, e2015096118. [Google Scholar] [CrossRef]
- Maynard, D.D.C.; Vidigal, M.D.; Farage, P.; Zandonadi, R.P.; Nakano, E.Y.; Botelho, R.B.A. Environmental, social and economic sustainability indicators applied to food services: A systematic review. Sustainability 2020, 12, 1804. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Sharma, M.; Pandotra, P. Biotechnological Interventions for Sustainable Conservation of Plant Genetic Resources in the Scenario of Climate Change. Nat. Resour. Conserv. Res. 2019, 2, 754. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the Effects of the Explant Type and Different Plant Growth Regulators on Micropropagation of Five Mediterranean Salvia spp. Native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Cuenca, S.; Amo-Marco, J.B. In vitro propagation of two spanish endemic species of salvia throught bud proliferation. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 225–229. [Google Scholar] [CrossRef]
- Skała, E.; Wysokińska, H. In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 596–602. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Μicropropagation and cultivation of Salvia sclarea for essential oil and sclareol production in northern Greece. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. New insights for the production of medicinal plant materials: Ex vitro and in vitro propagation of valuable Lamiaceae species from northern Africa. Curr. Plant Biol. 2021, 27, 100216. [Google Scholar] [CrossRef]
- Popova, E.; Kulichenko, I.; Kim, H.H. Critical Role of Regrowth Conditions in Post-Cryopreservation of In Vitro Plant Germplasm. Biology 2023, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Ruta, C.; De Mastro, G.; Tarraf, W.; Ancona, S.; Tagarelli, A.; Ozudogru, A.; Lambardi, M. Long-term preservation of Cicer arietinum L. germplasm by in vitro propagation and cryopreservation. Genet. Resour. Crop Evol. 2020, 67, 263–271. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Rabba’a, M.M.; Shibli, R.A.; Shatnawi, M.A. Cryopreservation of Teucrium polium L. shoot-tips by vitrification and encapsulation-dehydration. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 110, 371–382. [Google Scholar] [CrossRef]
- Al-Abdallat, A.M.; Adayileh, B.K.; Sawwan, J.S.; Shibli, R.; Al-Qudah, T.S.; Abu-Irmaileh, B.; Albdaiwi, R.N.; Almaliti, J.; Bustanji, Y. Secondary Metabolites Profiling, Antimicrobial and Cytotoxic Properties of Commiphora gileadensis L. Leaves, Seeds, Callus, and Cell Suspension Extracts. Metabolites 2023, 13, 537. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Jiang, H.; Zhang, S.; Que, Q.; Chen, X.; Zhou, W. Efficient in vitro sterilization and propagation from stem segment explants of Cnidoscolus aconitifolius (Mill.) IM Johnst, a multipurpose woody plant. Plants 2022, 11, 1937. [Google Scholar] [CrossRef]
- Hirakawa, T.; Tanno, S. In vitro propagation of Humulus lupulus through the induction of axillary bud development. Plants 2022, 11, 1066. [Google Scholar] [CrossRef]
- Kaviani, B.; Deltalab, B.; Kulus, D.; Tymoszuk, A.; Bagheri, H.; Azarinejad, T. In Vitro Propagation of Pyracantha angustifolia (Franch.) CK Schneid. Horticulturae 2022, 8, 964. [Google Scholar] [CrossRef]
- Aghilian, S.; Khajeh-Hosseini, M.; Anvarkhah, S. Evaluation of seed dormancy in forty medicinal plant species. Int. J. Agric. Crop Sci. 2014, 7, 760. [Google Scholar]
- Tursun, A.O. The effects of low temperature applications on dormancy of Salvia verticillata L. and Rumex crispus L. seeds. Pak. J. Bot. 2020, 52, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, T.S.; Shibli, R.A.; Alali, F.Q. In vitro propagation and secondary metabolites production in wild germander (Teucrium polium L.). In Vitro Cell. Dev. Biol.-Plant 2011, 47, 496–505. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Carrer, R.P.; Andrade, L.B. Micropropagation of Salvia guaranitica Benth. through axillary shoot proliferation. Braz. Arch. Biol. Technol. 2010, 53, 883–888. [Google Scholar] [CrossRef]
- Nanos, C.; Tsoulpha, P.; Kostas, S.; Hatzilazarou, S.; Michail, I.; Anastasiadi, V.; Nianiou-Obeidat, I. Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use. Horticulturae 2023, 9, 847. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Khajeh-Hosseini, M.; Rashed-Mohassel, M.; Emamipoor, Y. The effect of gibberellic acid and potassium nitrate on seed dormancy of fourteen medicinal plant species (Lamiaceae). Iran. J. Seed Sci. Technol. 2018, 7, 233–242. [Google Scholar]
- Ghanbar, T.; Hosseini, B.A.H.M.A.N.; Jabbarzadeh, Z.O.H.R.E.H.; Farokhzad, A.L.I.R.E.Z.A.; Sharafi, A. High-frequency in vitro direct shoots regeneration from axillary nodal and shoot tip explants of clary sage (Salvia sclarea L.). Bulg. J. Agric. Sci. 2016, 22, 73–78. [Google Scholar]
- Yang, H.; Yuan, H.; Du, C.; Liang, L.; Chen, M.; Zou, L. Development of a Highly Efficient Shoot Organogenesis System for an Ornamental Aeschynanthus pulcher (Blume) G. Don Using Leaves as Explants. Plants 2022, 11, 2456. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of explant origin and medium plant growth regulators on in vitro shoot proliferation and rooting of Salvia tomentosa, a native sage of the Northeastern Mediterranean Basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Krzemińska, M.; Olszewska, M.A.; Owczarek, A. Cytokinin-based tissue cultures for stable medicinal plant production: Regeneration and phytochemical profiling of Salvia bulleyana shoots. Biomolecules 2021, 11, 1513. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable Exploitation of Greek Rosmarinus officinalis L. Populations for Ornamental Use through Propagation by Shoot Cuttings and In Vitro Cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Marconi, P.L.; López, M.C.; De Meester, J.; Bovjin, C.; Alvarez, M.A. In vitro establishment of Salvia hispanica L. plants and callus. Biotecnol. Veg. 2013, 13, 203–207. [Google Scholar]
- Modarres, M.; Asili, J.; Lahouti, M.; Gangali, A.; Iranshahy, M.; Sahebkar, A. Simultaneous determination of rosmarinic acid, salvianolic acid B and caffeic acid in Salvia leriifolia Benth. root, leaf and callus extracts using a high-performance liquid chromatography with diode-array detection technique. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1721–1730. [Google Scholar] [CrossRef]
- Tahtamouni, R.W.; Shibli, R.A.; Younes, L.S.; Abu-Mallouh, S.; Al-Qudah, T.S. Responses of Lantana Camara Linn. Callus Cultures to Heavy Metals Added to the Culture Media. Jordan J. Biol. Sci. 2020, 13, 551–557. [Google Scholar]
- Lemraski, M.G.; Eftekhari, M.; Faraji, M.; Zarrini, S.S. Study of callus induction in common sage (Salvia officinalis L.). Int. J. Agric. Crop Sci. (IJACS) 2014, 7, 386–389. [Google Scholar]
- Mederos-Molina, S. In vitro callus induction and plants from stem and petiole explants of Salvia canariensis L. Plant Tissue Cult. 2004, 14, 167–172. [Google Scholar]
- Hemmati, N.; Cheniany, M.; Ganjeali, A. Effect of plant growth regulators and explants on callus induction and study of antioxidant potentials and phenolic metabolites in Salvia tebesana Bunge. Bot. Serbica 2020, 44, 163–173. [Google Scholar] [CrossRef]
- Martinez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of the carbon source and plant growth regulators (PGRs) in the induction and maintenance of an in vitro callus culture of Taraxacum officinale (L) weber Ex FH Wigg. Agronomy 2021, 11, 1181. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: Modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Delgado-Aceves, L.; González-Arnao, M.T.; Santacruz-Ruvalcaba, F.; Folgado, R.; Portillo, L. Indirect somatic embryogenesis and cryopreservation of Agave tequilana Weber cultivar ‘Chato’. Plants 2021, 10, 249. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Q.; Feng, C.-H.; Wang, M.-R.; Hu, L.-Y.; Volk, G.; Wang, Q.-C. Recovery patterns, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J. Biotechnol. 2015, 214, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Juan-Vicedo, J.; Ramírez-Luna, J.E.; Piqueras, A.; Casas, J.L. Micropropagation and cryopreservation by vitrification of the Spanish endemic medicinal plant Sideritis leucantha Cav. subsp. leucantha (Lamiaceae). In Vitro Cell. Dev. Biol.-Plant 2021, 57, 1057–1065. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Kulus, D.; Vacaro de Souza, A.; Kaviani, B.; Vicente, E.F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Chenshu, A.; Wang, X.; Yuan, X.; Zhao, B.; Wang, Y. Optimization of cryopreservation of Artemisia annua L. callus. Biotechnol. Lett. 2003, 25, 35–38. [Google Scholar] [CrossRef]
- Popova, E.V.; Lee, E.J.; Wu, C.H.; Hahn, E.J.; Paek, K.Y. A simple method for cryopreservation of Ginkgo biloba callus. Plant Cell Tissue Organ Cult. (PCTOC) 2009, 97, 337–343. [Google Scholar] [CrossRef]
- Bulbarela-Marini, J.E.; Gómez-Merino, F.C.; Galindo-Tovar, M.E.; Pastelín-Solano, M.C.; Murguía-González, J.; Núñez-Pastrana, R.; Castañeda-Castro, O. Ratio of Somaclonal Variation and the Phytohormonal Content of Citrus× latifolia in Three In Vitro Culture Systems. J. Plant Growth Regul. 2023, 42, 3356–3364. [Google Scholar] [CrossRef]
- Biswas, P.; Kumar, N. Application of Molecular Markers for the Assessment of Genetic Fidelity of In Vitro Raised Plants: Current Status and Future Prospects. In Molecular Marker Techniques; Springer: Singapore, 2023; pp. 233–256. [Google Scholar]
- Duta-Cornescu, G.; Constantin, N.; Pojoga, D.M.; Nicuta, D.; Simon-Gruita, A. Somaclonal variation—Advantage or disadvantage in micropropagation of the medicinal plants. Int. J. Mol. Sci. 2023, 24, 838. [Google Scholar] [CrossRef]
- Taji, A.; Prakash, N.; Lakshmanan, P. In Vitro Plant Breeding; Food Products Press: New York, NY, USA, 2002. [Google Scholar]
- Castillo, N.R.F.; Bassil, N.V.; Wada, S.; Reed, B.M. Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell. Dev. Biol.-Plant 2010, 46, 246–256. [Google Scholar] [CrossRef]
- Matsumoto, T.; Akihiro, T.; Maki, S.; Mochida, K.; Kitagawa, M.; Tanaka, D.; Niino, T. Genetic stability assessment of Wasabi plants regenerated from long-term cryopreserved shoot tips using morphological, biochemical and molecular analysis. CryoLetters 2013, 34, 128–136. [Google Scholar] [PubMed]


| Cytokinin Type | Concentration (mg L−1) | Number of Microshoots/Explant | Shoot Height (cm) | Callus Diameter (cm) |
|---|---|---|---|---|
| BAP y | 0.0 (control) | 1.20 ± 0.12 d * | 1.36 ± 0.11 b | 0.13 ± 0.02 c |
| 0.4 | 2.50 ± 0.15 c | 2.38 ± 0.090 a | 0.13 ± 0.01 c | |
| 0.8 | 2.20 ± 0.12 c | 2.18 ± 0.080 a | 0.61 ± 0.07 ab | |
| 1.2 | 4.80 ± 0.12 a | 2.46 ± 0.14 a | 0.84 ± 0.10 a | |
| 2.0 | 3.0 ± 0.16 b | 1.44 ± 0.07 b | 0.52 ± 0.01 b | |
| Kinetin | 0.0 (control) | 1.40 ± 0.19 b | 1.36 ± 0.09 b | 0.30 ± 0.07 c |
| 0.4 | 1.40 ± 0.19 b | 2.60 ± 0.22 a | 0.52 ± 0.11 bc | |
| 0.8 | 2.60 ± 0.18 a | 1.94 ± 0.35 ab | 0.72 ± 0.08 b | |
| 1.2 | 1.30 ± 0.12 b | 1.98 ± 0.26 ab | 0.83 ± 0.02 b | |
| 2.0 | 1.80 ± 0.25 a | 2.46 ± 0.25 a | 1.2 ± 0.10 a | |
| TDZ | 0.0 (control) | 1.20 ± 0.19 d | 1.40 ± 0.070 c | 0.18 ± 0.04 d |
| 0.4 | 2.20 ± 0.20 c | 1.46 ± 0.12 c | 0.58 ± 0.11 cd | |
| 0.8 | 2.80 ± 0.12 c | 2.14 ± 0.20 b | 0.68 ± 0.10 c | |
| 1.2 | 6.30 ± 0.30 a | 2.76 ± 0.19 b | 1.40 ± 0.05 b | |
| 2.0 | 4.40 ± 0.18 b | 3.70 ± 0.11 a | 2.18 ± 0.18 a |
| Auxin Type | Concentration (mg L−1) | Number of Roots | Root Length (cm) | Shoot Height (cm) |
|---|---|---|---|---|
| NAA y | 0.0 (control) | 0.00 ± 0.00 d * | 0.00 ± 0.00 e | 1.42 ± 0.04 d |
| 0.5 | 1.3 ± 0.21 c | 0.5 ± 0.08 d | 1.65 ± 0.06 d | |
| 1.0 | 2.5 ± 0.17 b | 0.89 ± 0.09 c | 2.22 ± 0.85 c | |
| 1.5 | 6.6 ± 0.34 a | 2.76 ± 0.08 a | 4.99 ± 0.15 a | |
| 2.0 | 2.6 ± 0.16 b | 1.31 ± 0.16 b | 2.78 ± 0.19 b | |
| IAA | 0.0 (control) | 0.0 ± 0.0 c | 0.00 ± 0.0 c | 1.27 ± 0.04 d |
| 0.5 | 0.7 ± 0.26 c | 0.15 ± 0.06 c | 1.48 ± 0.04 cd | |
| 1.0 | 1.8 ± 0.32 b | 0.44 ± 0.05 b | 1.78 ± 0.09 c | |
| 1.5 | 3.5 ± 0.22 a | 1.04 ± 0.06 a | 2.52 ± 0.11 a | |
| 2.0 | 0.4 ± 0.16 c | 0.20 ± 0.09 bc | 2.19 ± 0.07 b | |
| IBA | 0.0 (control) | 0.0 ± 0.00 c | 0.00 ± 0.0 c | 1.24 ± 0.03 c |
| 0.5 | 2.2 ± 0.20 b | 0.27 ± 0.05 c | 1.25 ± 0.02 bc | |
| 1.0 | 4.0 ± 0.21 a | 0.42 ± 0.02 b | 1.44 ± 0.03 b | |
| 1.5 | 2.8 ± 0.29 b | 1.07 ± 0.04 a | 1.87 ± 0.04 a | |
| 2.0 | 0.5 ± 0.16 c | 0.24 ± 0.10 b | 1.22 ± 0.03 a |
| Cytokinin Type | Concentration (mg L−1) | Fresh Weight (g) | Texture | Color |
|---|---|---|---|---|
| y Kinetin | z C | 1.11 ± 0.02 d * | Friable | White yellow |
| 0.5 | 1.24 ± 0.01 d | Semi-friable | White yellow | |
| 1.0 | 1.65 ± 0.04 c | Semi-friable | White yellow | |
| 1.5 | 2.75 ± 0.06 b | Semi-friable | White yellow | |
| 2.0 | 3.61 ± 0.05 a | Semi-friable | White yellow | |
| BAP | z C | 1.14 ± 0.03 e * | Friable | White yellow |
| 0.5 | 1.74 ± 0.08 d | Friable | White yellow | |
| 1.0 | 2.51 ± 0.05 c | friable | White yellow | |
| 1.5 | 3.73 ± 0.07 b | Friable | White yellow | |
| 2.0 | 5.81 ± 0.10 a | Friable | White yellow | |
| TDZ | z C | 1.07 ± 0.01 d | Friable | White yellow |
| 0.5 | 1.46 ± 0.08 c | Compact | Brown | |
| 1.0 | 2.79 ± 0.03 a | Compact | Brown | |
| 1.5 | 1.79 ± 0.04 b | Compact | Brown | |
| 2.0 | 1.74 ± 0.09 b | Compact | Brown |
| Vitrification Solution Type x | Survival % | Recovery % |
| PVS2 z | 95.4 ± 4.2 a * | 71.8 ± 5.5 a |
| HF-MS + 15%DMSO + 1 M sucrose | 36.8 ± 5.6 b | 23.2 ± 3.1 b |
| HF-MS + 30%DMSO + 1 M sucrose | 0.0 ± 0.0 d | 0.0 ± 0.0 d |
| PVS3 | 22.8 ± 2.2 c | 13.8 ± 2.7 c |
| Loading Solution Type y | Survival % | Recovery % |
| 1.0 M sucrose + HF-MS + 2 M glycerol | 5.4 ± 5.5 d | 0 ±0.00 c |
| 0.5 M sucrose + 5% DMSO + HF media | 33.2 ± 3.9 b | 8.4 ± 3.2 b |
| 0.75 M sucrose + 5% DMSO + HF media | 22.2 ± 3.03 | 7.4 ± 2.8 b |
| 0.5 M sucrose + 10% DMSO + HF media | 16.8 ± 7.3 c | 0 ± 0.00 c |
| 0.75 M sucrose + 10% DMSO + HF media | 13.4 ± 2.4 cd | 0 ± 0.00 c |
| 0.4 M sucrose + 2M glycerol + HF media f | 84.2 ± 2.7 a | 71.8 ± 5.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qudah, T.S.; Shibli, R.A.; Zatimeh, A.; Tahtamouni, R.W.; Al-Zyoud, F. A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan. Sustainability 2023, 15, 14218. https://doi.org/10.3390/su151914218
Al-Qudah TS, Shibli RA, Zatimeh A, Tahtamouni RW, Al-Zyoud F. A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan. Sustainability. 2023; 15(19):14218. https://doi.org/10.3390/su151914218
Chicago/Turabian StyleAl-Qudah, Tamara S., Rida A. Shibli, Ahmad Zatimeh, Reham W. Tahtamouni, and Firas Al-Zyoud. 2023. "A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan" Sustainability 15, no. 19: 14218. https://doi.org/10.3390/su151914218
APA StyleAl-Qudah, T. S., Shibli, R. A., Zatimeh, A., Tahtamouni, R. W., & Al-Zyoud, F. (2023). A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan. Sustainability, 15(19), 14218. https://doi.org/10.3390/su151914218






