Characterization and Resource Potential of Li in the Clay Minerals of Mahai Salt Lake in the Qaidam Basin, China
Abstract
:1. Introduction
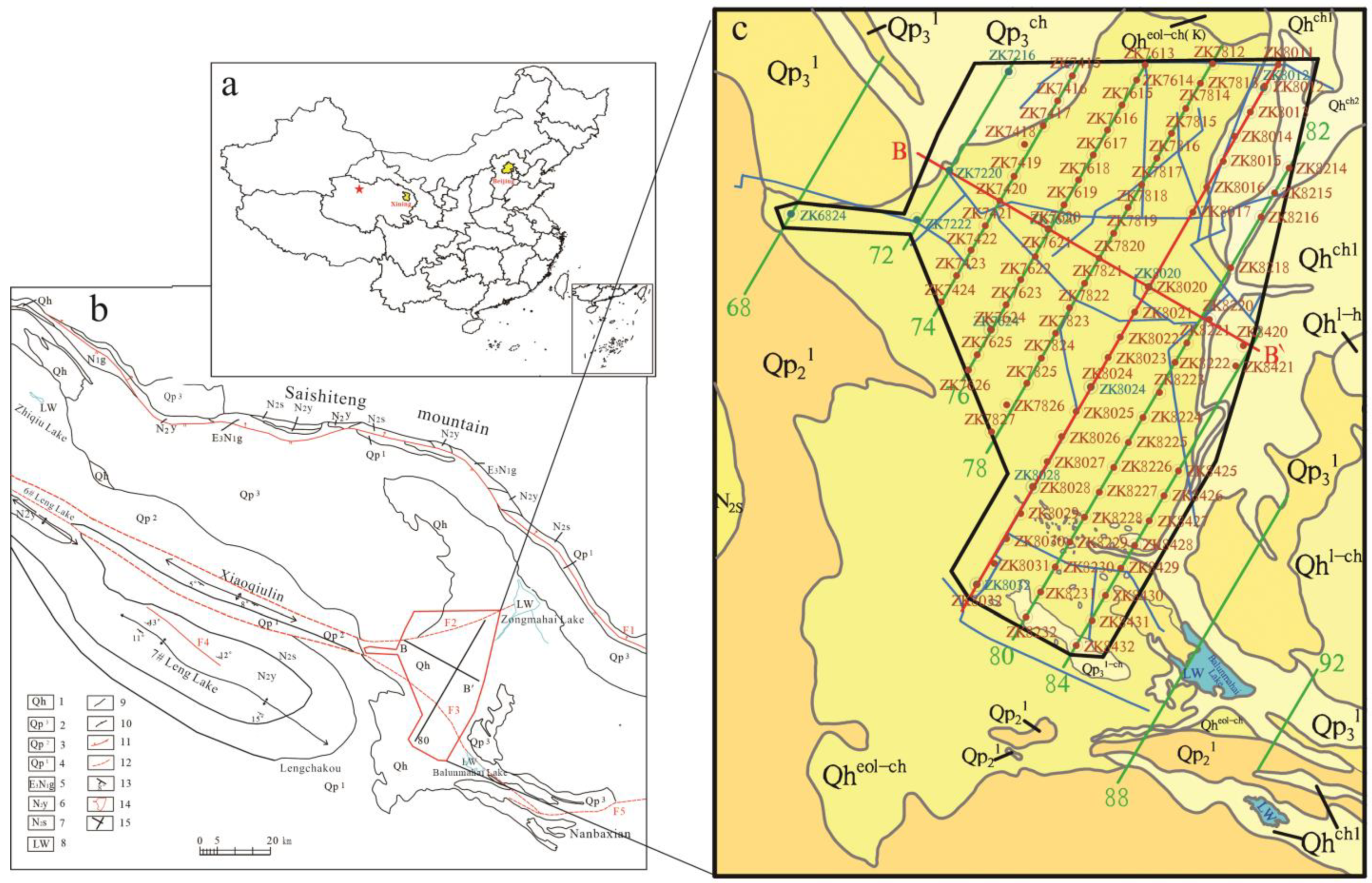
2. Geological Setting and Samples
3. Analytical Methods
3.1. Lithium Content Determination
3.2. Mineral Composition Analysis
4. Results and Discussion
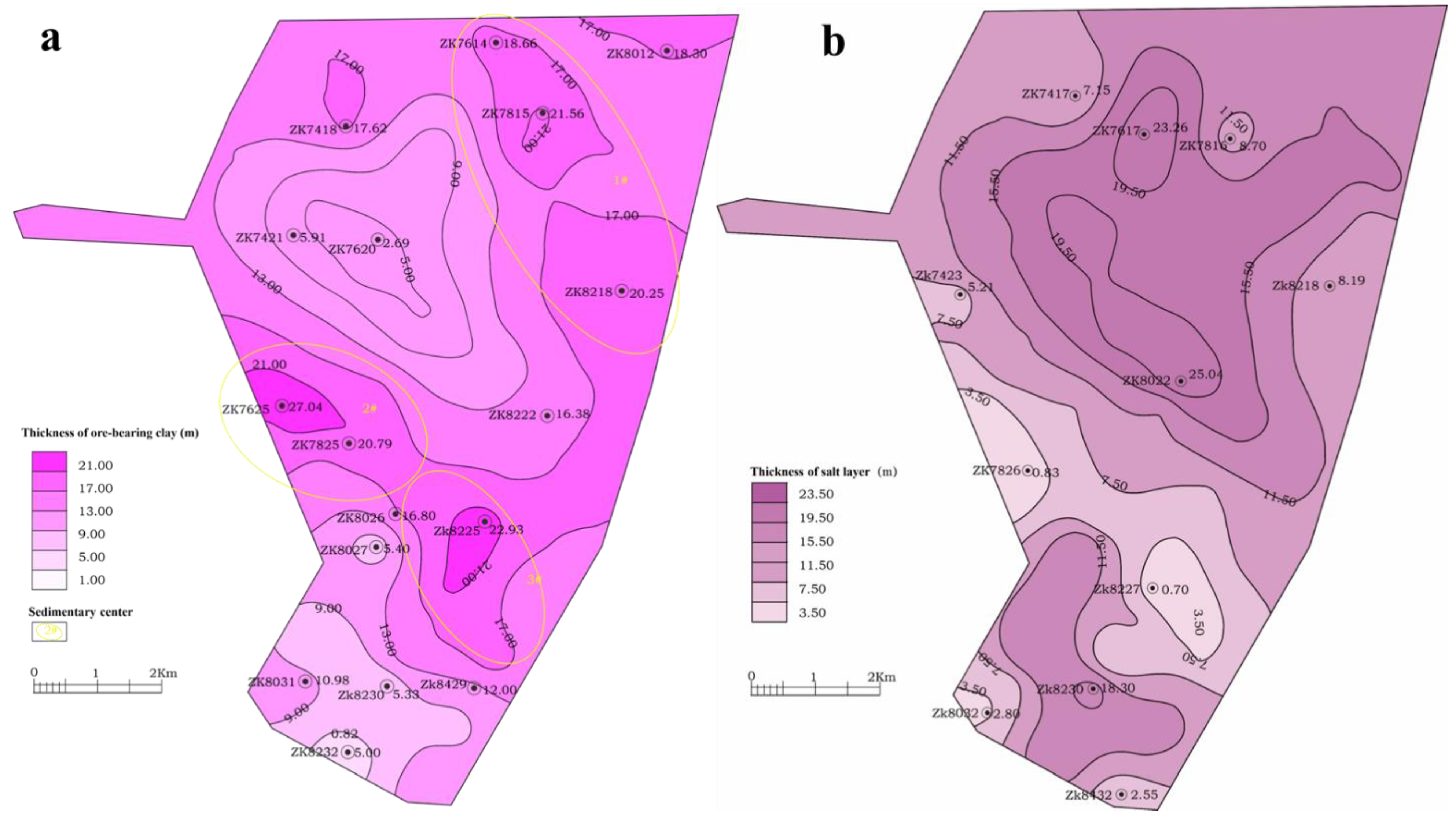
4.1. Spatial Distribution Characteristics of the Mineral Clay Layer
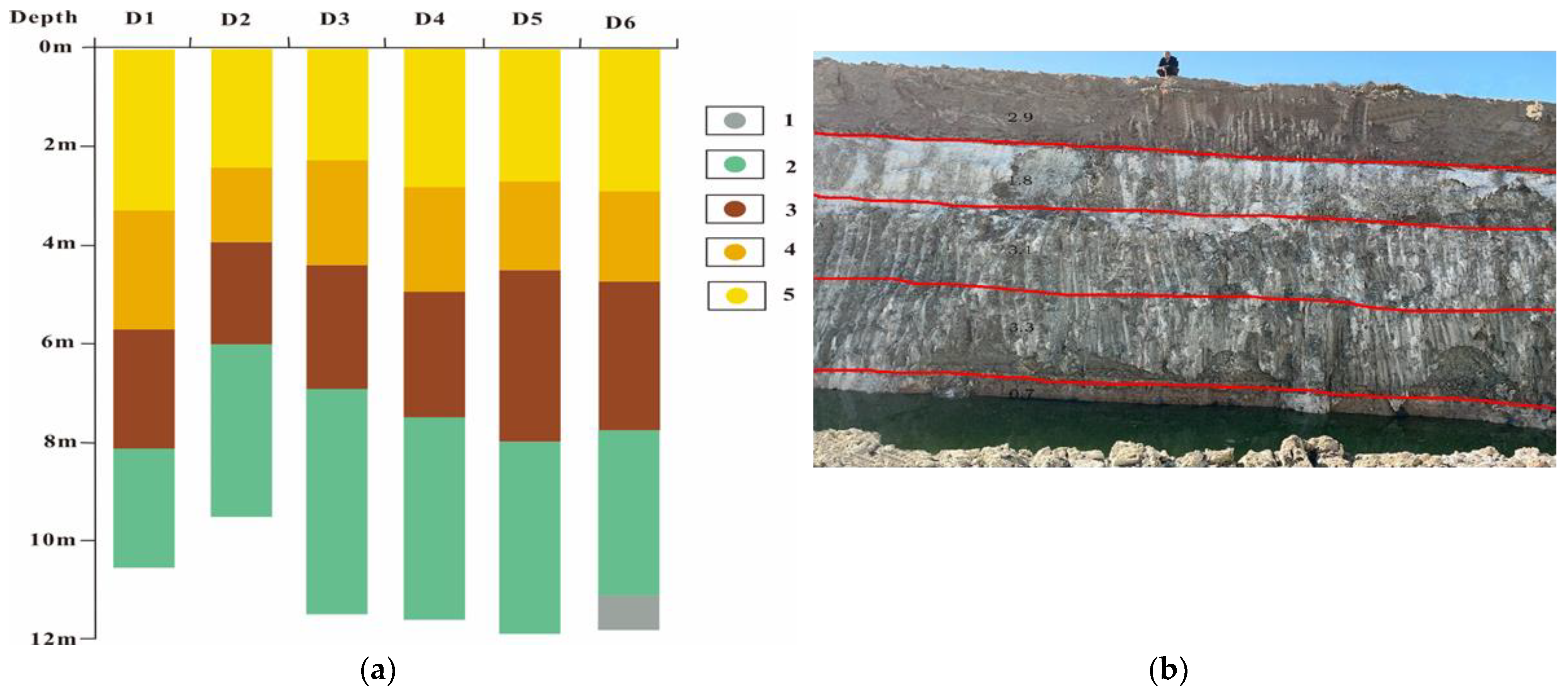
4.2. Vertical Distribution Characteristics of the Clay Layer
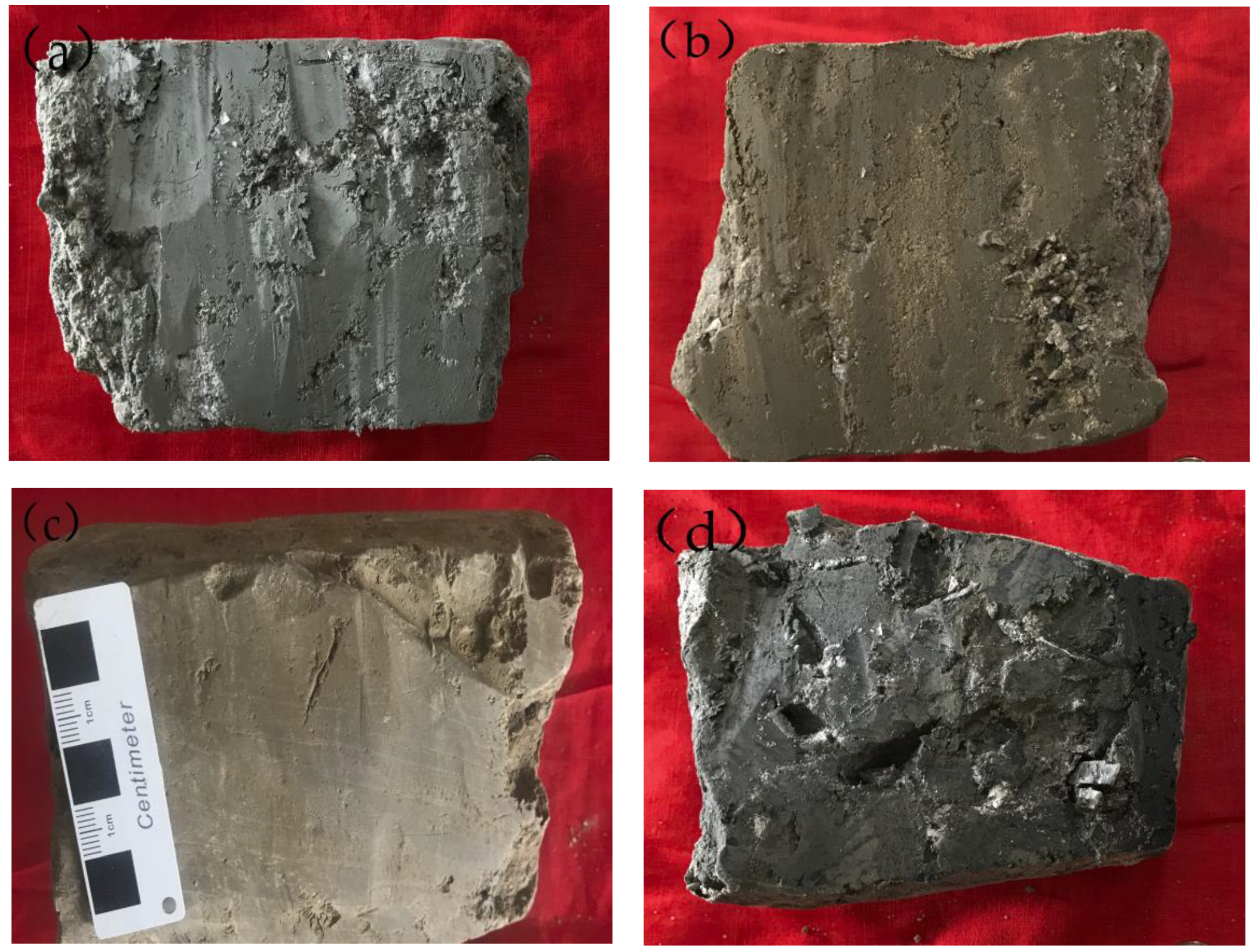
4.3. Degree of Development and Characteristics of the Clay Layer
- (1)
- Classification based on the sedimentary environment

- (2)
- Classification based on the material composition
4.4. Geochemical Characteristics of the Li Contents
- (1)
- The salt layer in the evaluation area exhibited the lowest Li contents, followed by the silty clay layers. A clear correlation was observed between the enrichment of Li and the clay content in the study area, as evidenced by the association between higher clay contents and greater Li enrichment.
- (2)
- Despite variations in the sedimentary environments, the Li contents remained stable in the gray-brown clay, gray-green clay, and black mud, with a small coefficient of variation. The average Li2O contents ranged from approximately 114.34 μg/g to 123.94 μg/g, with a coefficient of variation of 4.21%.
- (3)
- Among the different material compositions, the Li contents remained stable, exhibiting a small coefficient of variation in the clay with silt, gypsum, salt, and black mud. Among them, the average Li2O contents ranged from 117.85 μg/g to 124.59 μg/g, with a coefficient of variation of 2.97%.
- (4)
- According to the average Li content in the specified region, the overall average clay value, and the presence of seven distinct clay types (grayish brown, grayish green, black carbon-bearing, silt-bearing, gypsum-bearing, and salt-bearing), the Li contents in the clay-containing minerals exhibited a consistent and minimal coefficient of change. The average Li2O contents ranged from 114.34 μg/g to 124.59 μg/g, with a coefficient of variation of 4.76%.
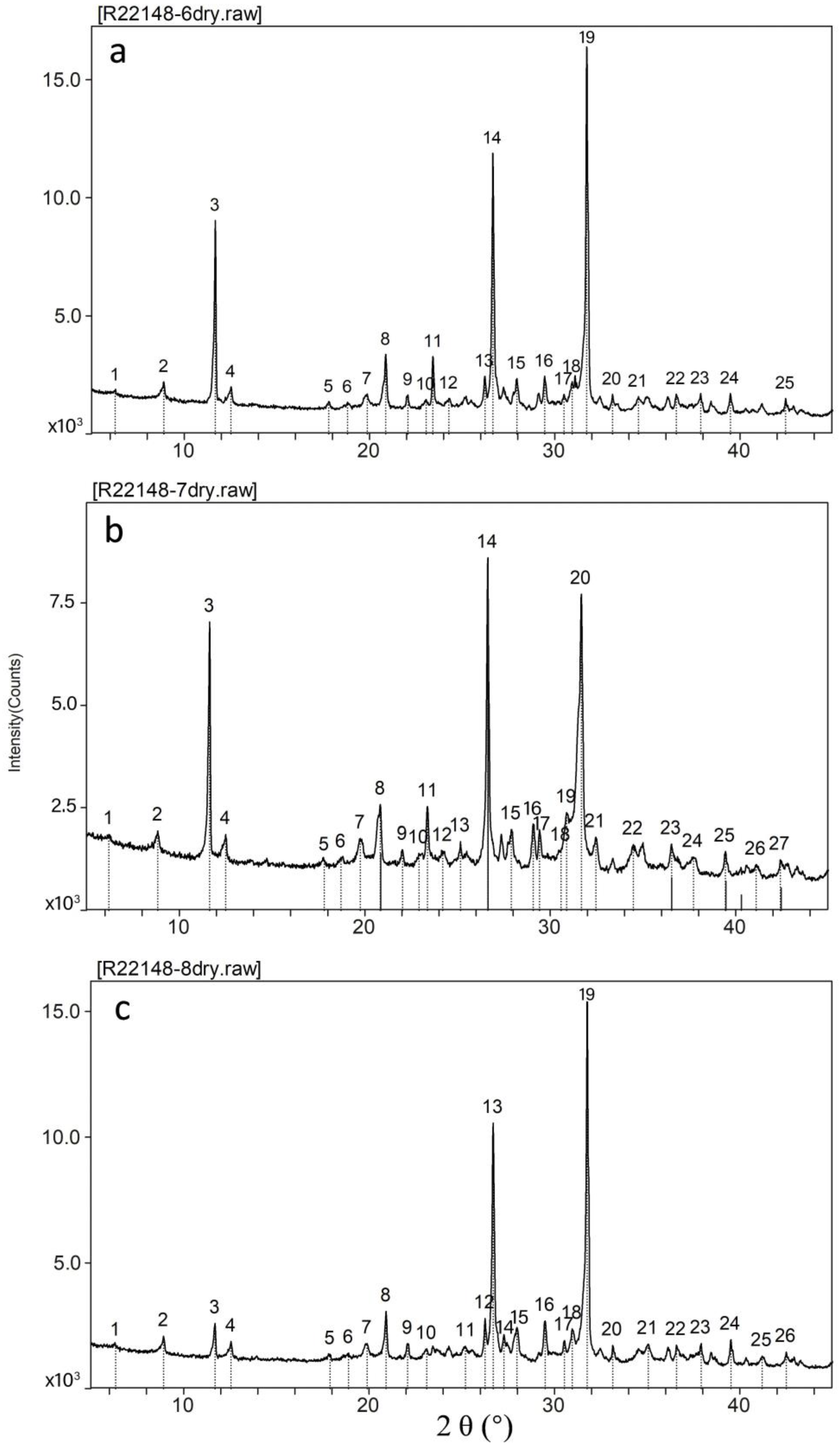
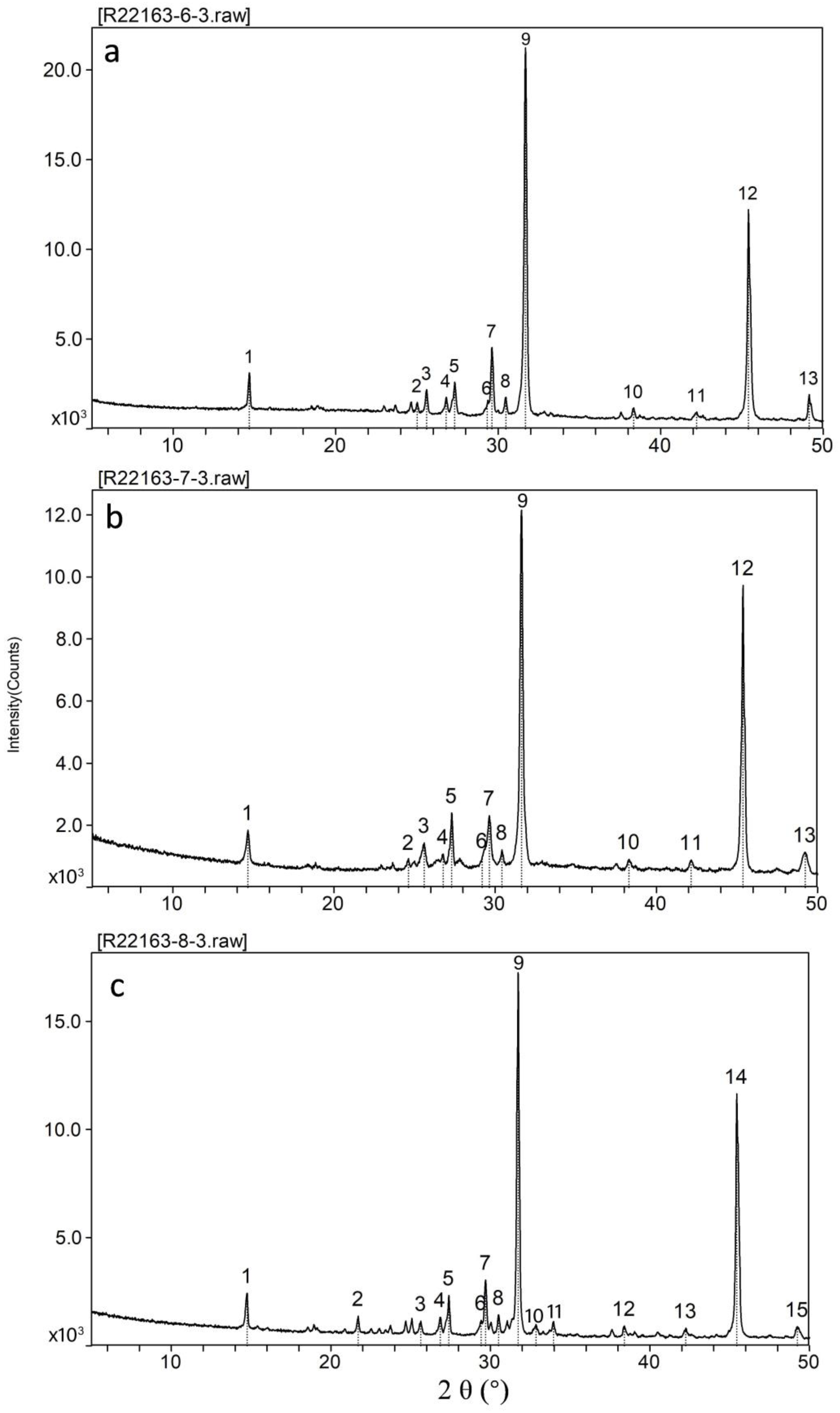
4.5. Mineral Composition
4.5.1. Mineral Compositions of Entire Samples
4.5.2. Water-Soluble Matter
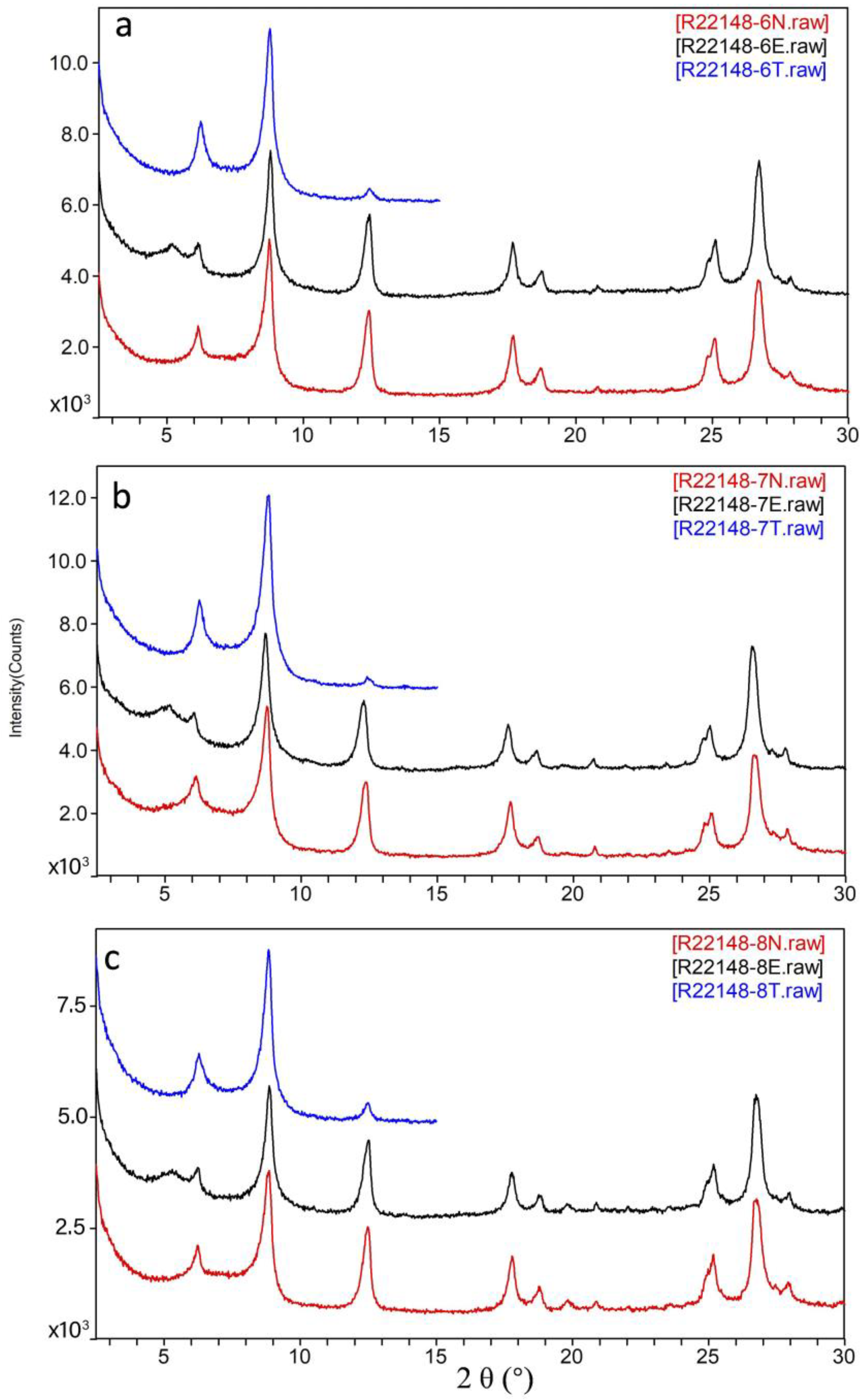
4.5.3. Clay Minerals
4.6. Occurrence Forms of Lithium in Clay Minerals
4.7. Relationship between the Lithium Contents in the Clay and Salt Lake Lithium Deposits
4.8. New Types of Lithium Deposits
4.9. Clay-Type Lithium Resource Potential in the Mahai Basin
5. Conclusions
- The spatial distribution of the mineral-bearing clay layer is influenced by the sedimentary environment of the original lake, with the salt layer and the mineral-bearing clay layer exhibiting a complementary relationship. Specifically, the clay layer is found to be thin in regions where the salt layer is present, whereas the salt layer is absent in regions where the clay layer is developed.
- In the subsidence center of the basin, the thickness variation in the mineral-bearing clay layer remains relatively constant, and the vertical stratification of the mineral-bearing clay layer is clearly discernible. From top to bottom, the units can be roughly divided into sedimentary cycles of salt-bearing silt, gray-brown clay, gray-green clay, black carbon-bearing clay, and salt.
- The evaluation area exhibits the lowest Li content in the salt layer, followed by the silt layer and the clay layer. A discernible correlation was observed between the Li enrichment and the clay content within the study area, as evidenced by the association between the elevated clay contents and the heightened Li enrichment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil-Alana, L.A.; Monge, M. Lithium: Production and estimated consumption. Evidence of persistence. Resour. Policy 2019, 60, 198–202. [Google Scholar] [CrossRef]
- Heredia, F.; Martinez, A.L.; Urtubey, V.S. The importance of lithium for achieving a low-carbon future: Overview of the lithium extraction in the ‘Lithium Triangle’. J. Energy Nat. Resour. Law 2020, 38, 213–236. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar]
- Lindagato, P.; Li, Y.J.; Macháček, J.; Yang, G.X.; Mungwarakarama, I.; Ndahimana, A.; Ntwali, H.P.K. Lithium Metal: The Key to Green Transportation. Appl. Sci. 2023, 13, 405. [Google Scholar] [CrossRef]
- Fan, J.J.; Tang, G.J.; Wei, G.J.; Wang, H.; Xu, Y.G.; Wang, Q.; Zhou, J.S.; Zhang, Z.Y.; Huang, T.Y.; Wang, Z.L. Lithium isotope fractionation during fluid exsolution: Implications for Li mineralization of the Bailongshan pegmatites in the West Kunlun, NW Tibet. Lithos 2020, 352–353, 105236. [Google Scholar]
- Bibienne, T.; Magnan, J.F.; Rupp, A.; Laroche, N. From mine to mind and mobiles: Society’s increasing dependence on lithium. Elements 2020, 16, 265–270. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; De Los Hoyos, C.R.; Declercq, J. Classification and Characteristics of Natural Lithium Resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability. J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- He, M.Y.; Luo, C.G.; Yang, H.J.; Kong, F.C.; Li, Y.L.; Deng, L.; Zhang, X.Y.; Yang, K.Y. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes. Ore Geol. Rev. 2020, 117, 103277. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Chen, C.; Lee, C.A.; Tang, M.; Biddle, K.; Sun, W. Lithium systematics in global arc magmas and the importance of crustal thickening for lithium enrichment. Nat. Commun. 2020, 11, 5313. [Google Scholar] [CrossRef] [PubMed]
- Lithium Salt Industry Branch of China Inorganic Salt Industry Association. 2022 Domestic Lithium Salt Industry Data Statistics; Lithium Salt Industry Branch of China Inorganic Salt Industry Association: Xining, China, 2023; pp. 1–18. [Google Scholar]
- Miao, W.L.; Zhang, X.Y.; Li, Y.L.; Li, W.X.; Yuan, X.L.; Li, C.Z. Lithium and strontium isotopic systematics in the Nalenggele River catchment of Qaidam basin, China: Quantifying contributions to lithium brines and deciphering lithium behavior in hydrological processes. J. Hydrol. 2022, 614, 128630. [Google Scholar] [CrossRef]
- Li, H.F.; Li, L.J.; Li, W. Lithium Extraction from Salt Lake Brine with High Mass Ratio of Mg/Li Using TBP-DIBK Extraction System. Separations 2023, 10, 1–15. [Google Scholar]
- Pan, T.; Li, S.P.; Wang, T.; Han, G.; Jia, J.T. Metallogenic characteristics and prospecting potential of lithium deposits in the Qinghai Province. Acta Geol. Sin. 2022, 96, 1827–1854. [Google Scholar]
- Ma, J.Y.; Hu, S.Z.; Tian, X.D. Sedimentary environment and exploitation of Maihai potash deposits in Qaidam Basin. J. Salt Lake Res. 2010, 18, 9–17. [Google Scholar]
- Hu, S.Y.; Ren, J.; Li, J.Q. Permeability and brine enrichment mechanism of brine reservoir in the Mahai Salt Lake, Qaidam Basin. Sci. Geogr. Sin. 2022, 42, 2039–2046. [Google Scholar]
- Zhao, Q.S.; Kong, Z.H.; Hu, S.Y.; Zhan, J.W. Geophysical exploration and application of underground brine of Mahai salt lake in Qaidam Basin. J. Jilin Univ. Earth Sci. Ed. 2023, 53, 1–11. [Google Scholar]
- Long, P.Y. Mineral Occurrence Characteristics of Shallow Solid Potassium Ore after Water Solution Mining in Mahai Salt Lake, Qaidam Basin. Master’s Thesis, Hebei GEO University, Shijiazhuang, China, 2021. [Google Scholar]
- Wang, Z.X. Holocene Sedimentary Environment and Potash Mineralization of Mahai Salt Lake, Qaidam Basin. Master’s Thesis, Hebei GEO University, Shijiazhuang, China, 2021. [Google Scholar]
- He, M.Y.; Dong, J.B.; Jin, Z.D.; Liu, C.Y.; Xiao, J.; Zhang, F.; Sun, H.; Zhao, Z.Q.; Gou, L.F.; Liu, W.G.; et al. Pedogenic processes in loess-paleosol sediments: Clues from Li isotopes of leachate in Luochuan loess. Geochim. Cosmochim. Acta 2021, 299, 151–162. [Google Scholar]
- Xu, C. Preliminary study on clay minerals in the sediments of Qinghai-Tibet Salt Lake. Chin. J. Geol. 1985, 1, 87–96. [Google Scholar]
- Wen, H.J.; Luo, C.G.; Du, S.J.; Yu, W.X.; Gu, H.N.; Ling, K.Y.; Cui, Y.; Li, Y.; Yang, J.H. Carbonate-hosted clay-type lithium deposit and its prospecting significance. Chin. Sci. Bull. 2020, 65, 53–59. [Google Scholar] [CrossRef]
- Cui, Y.; Wen, H.J.; Yu, W.X.; Luo, C.G.; Du, S.J.; Ling, K.Y.; Xu, F.; Yang, J.H. Study on the occurrence state and enrichment mechanism of lithium in lithium-rich clay rock series of the Daoshitou Formation of Lower Permian in Central Yunnan. Acta Petrol. Sin. 2022, 38, 2080–2094. [Google Scholar]
- Ling, K.Y.; Wen, H.J.; Fan, H.F.; Zhu, X.K.; Li, Z.H.; Zhang, Z.W.; Grasby, S.E. Iron loss during continental weathering in the early Carboniferous period recorded by karst bauxites. J. Geophys. Res.-Earth Surf. 2023, 128, e2022JF006906. [Google Scholar] [CrossRef]
- Ling, K.Y.; Wen, H.J.; Grasby, S.E.; Zhao, H.N.; Deng, C.Z.; Yin, R.S. The Emeishan large igneous province eruption triggered coastal perturbations and the Capitanian mass extinction: Insights from mercury in Permian bauxite beds. Chem. Geol. 2023, 617, 121243. [Google Scholar]
- Gourcerol, B.; Gloaguen, E.; Melleton, J.; Tuduri, J.; Galiegue, X. Re-assessing the European lithium resource potential—A review of hard-rock resources and metallogeny. Ore Geol. Rev. 2019, 109, 494–519. [Google Scholar]
- Williams, L.B.; Hervig, R.L. Lithium and boron isotopes in illite-smectite: The importance of crystal size. Geochim. Cosmochim. Acta 2005, 69, 5705–5716. [Google Scholar]
- Vigier, N.; Decarreau, A.; Millot, R.; Carignan, J.; Petit, S.; France-Lanord, C. Quantifying Li isotope fractionation during smectite formation and implications for the Li cycle. Geochim. Cosmochim. Acta 2008, 72, 780–792. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, W.P.; Yang, Y.; Cui, Y.; Xu, L.; Luo, C.G.; Wen, H.J. Experimental study on the adsorption of Li+ by clay minerals-implications for the mineralization of clay-type lithium deposit. Acta Mineral. Sin. 2022, 42, 141–153. [Google Scholar]
- Chan, L.H.; Kastner, M. Lithium isotopic compositions of pore fluids and sediments in the Costa Rica subduction zone: Implications for fluid processes and sediment contribution to the arc volcanoes. Earth Planet. Sci. Lett. 2000, 183, 275–290. [Google Scholar] [CrossRef]
- Li, Y.H.; Schoonmaker, J.E. Chemical composition and mineralogy of marine sediments. Treatise Geochem. 2003, 7, 1–35. [Google Scholar]
- Carew, T.J.; Rossi, M.E. Independent Technical Report for the Lithium Nevada Project, Nevada, USA; SRK Consulting Technical Report; SRK Consulting, Inc.: Kolkata, India, 2016. [Google Scholar]
- Benson, T.R.; Coble, M.A.; Dilles, J.H. Hydrothermal enrichment of lithium in intracaldera illite-bearing claystones. Sci. Adv. 2023, 9, eadh8183. [Google Scholar]
- Ma, S.C.; Wang, D.H.; Sun, Y. Geochronology and geochemical characteristics of Lower-Middle Triassic clay rock and their significances for prospecting clay-type lithium deposit. Earth Sci. 2019, 44, 427–440. [Google Scholar]
| Different Sedimentary Deposits | Value | Li | Li2O/(µg/g) |
|---|---|---|---|
| Silt layer | Max. | 71.80 | 154.55 |
| Min. | 12.40 | 26.69 | |
| Ave. | 31.77 | 68.38 | |
| Salt layer | Max. | 46.30 | 99.66 |
| Min. | 2.93 | 6.31 | |
| Ave. | 18.44 | 39.69 | |
| Gray-brown clay | Max. | 92.20 | 198.46 |
| Min. | 24.50 | 52.74 | |
| Ave. | 57.58 | 123.94 | |
| Gray-green clay | Max. | 86.50 | 186.19 |
| Min. | 21.30 | 45.85 | |
| Ave. | 53.12 | 114.34 | |
| Black mud | Max. | 92.50 | 199.11 |
| Min. | 19.30 | 41.54 | |
| Ave. | 54.84 | 118.04 | |
| Clay with silt | Max. | 84.90 | 182.75 |
| Min. | 26.80 | 57.69 | |
| Ave. | 54.75 | 117.85 | |
| Clay with gypsum | Max. | 93.80 | 201.90 |
| Min. | 21.30 | 45.85 | |
| Ave. | 57.88 | 124.59 | |
| Clay with salt | Max. | 91.80 | 197.60 |
| Min. | 23.90 | 51.44 | |
| Ave. | 56.26 | 121.10 |
| Mineral Category | Mineral Name | Content (%) | ||
|---|---|---|---|---|
| Grayish Brown Clay | Black Mud | Grayish Green Clay | ||
| Siliceous mineral | Quartz | 25.3 | 26.3 | 24.9 |
| Plagioclase | 9.6 | 7.6 | 9.9 | |
| Clay mineral | Illite | 15.2 | 15.5 | 15 |
| Chlorite | 5.0 | 4.5 | 5.1 | |
| Yimeng mixed layer | 4.3 | 5.1 | 4.1 | |
| Kaolinite | 2.2 | 2 | 2.3 | |
| Carbonate mineral | Aragonite | 5.7 | 0 | 6 |
| Dolomite | 4.1 | 4.8 | 3.9 | |
| Calcite | 6.2 | 4.2 | 6.7 | |
| Sulfate mineral | Gypsum | 6.9 | 18.5 | 5.6 |
| Salt minerals | Halite | 9.8 | 8.3 | 9.7 |
| Carnallite | 5.3 | 2.9 | 6.4 | |
| Metal sulfide | Pyrite | 0.4 | 0.2 | 0.5 |
| Classification Features | Mineralization | Ore-Forming Geological Body | Ore Scale | Ore Structure (Hydrochemical Type) | Mineral | Occurrence Form of Minerals |
|---|---|---|---|---|---|---|
| Quaternary modern salt lake deposits | Evaporative sedimentation | Salt lake brine, salt | Large, extra large | Solid-liquid phase coexists | KCl, NaCl, MgCl2, MgSO4, Potassium halite, carnallite, halite, mirabilite, etc. | Intergranular brine and pore brine; salt minerals |
| Sand–gravel pore brine deposit | Chemical deposition | Pressurized brine | Small, medium | Liquid phase | KCl, NaCl, MgCl2, MgSO4, etc. | Intergranular brine, pore brine, and fissure water |
| Paleogene–Neogene salt deposits | Chemical deposition | Salt | Small, medium, large | Solid phase | Celestite, strontianite, gypsum, halite, etc. | Salt minerals |
| Clay-type deposit | Sedimentary adsorption | Clay | Large, extra large | Solid phase | Li bearing illite, chlorite, kaolinite, and Yimeng mixed bed | Adsorption in clay minerals or occurrence in the mineral lattice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.; Chen, J.; He, M.-Y.; Ding, C.; Ma, Y.; Liang, H.; Zhang, T.; Du, X. Characterization and Resource Potential of Li in the Clay Minerals of Mahai Salt Lake in the Qaidam Basin, China. Sustainability 2023, 15, 14067. https://doi.org/10.3390/su151914067
Pan T, Chen J, He M-Y, Ding C, Ma Y, Liang H, Zhang T, Du X. Characterization and Resource Potential of Li in the Clay Minerals of Mahai Salt Lake in the Qaidam Basin, China. Sustainability. 2023; 15(19):14067. https://doi.org/10.3390/su151914067
Chicago/Turabian StylePan, Tong, Jianzhou Chen, Mao-Yong He, Chengwang Ding, Yuliang Ma, Hui Liang, Tao Zhang, and Xiaochun Du. 2023. "Characterization and Resource Potential of Li in the Clay Minerals of Mahai Salt Lake in the Qaidam Basin, China" Sustainability 15, no. 19: 14067. https://doi.org/10.3390/su151914067
APA StylePan, T., Chen, J., He, M.-Y., Ding, C., Ma, Y., Liang, H., Zhang, T., & Du, X. (2023). Characterization and Resource Potential of Li in the Clay Minerals of Mahai Salt Lake in the Qaidam Basin, China. Sustainability, 15(19), 14067. https://doi.org/10.3390/su151914067







