Abstract
In this study, a novel Fe3O4/Ag3PO4/g-C3N4 magnetic composite photocatalyst was successfully synthesized, tailored specifically for the visible light-driven photocatalytic degradation of sulfonamide antibiotics, more precisely, sulfamethazine (SMZ). To analyze the fabricated samples, characterization techniques such as X-ray diffraction (XRD), scanning electron microscope (SEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), photoluminescence spectroscopy (PL), and UV-vis diffuse reflectance spectra (UV-vis) were systematically employed. The composite showcased efficient visible-light absorption and charge separation, with its peak photocatalytic performance recorded at a solution pH value of 6.0. Significantly, the Fe3O4/Ag3PO4/g-C3N4 magnetic composite photocatalyst displayed excellent stability and recyclability, consistently maintaining a high degradation efficiency of over 97% even after five consecutive cycles. Further experimentation with radical scavengers confirmed a significant decrease in photocatalytic activity, establishing that superoxide radicals (•O2−) and photo-generated holes (h+) are the primary active species during the degradation of SMZ. Overall, it provides a crucial understanding regarding the photocatalytic decomposition of sulfonamide antibiotics using magnetic composite photocatalysts. It also emphasizes the promising potential of the Fe3O4/Ag3PO4/g-C3N4 composite for tangible applications in environmental remediation.
1. Introduction
The prevalence of antibiotics in the environment, particularly sulfonamides, is an emerging issue of global concern [1,2,3]. These substances are extensively employed in both human and veterinary medicine owing to their broad-spectrum antibacterial efficacy [4,5]. Nevertheless, the ubiquity of these pollutants, driven by their persistent nature and extensive usage, is becoming a growing environmental and public health threat [6,7,8]. Conventional water treatment processes often fail to effectively remove these compounds, underscoring the urgent need for innovative and efficient strategies [9,10,11]. One such approach is photocatalytic degradation, an advanced oxidation process that promises to offer a robust solution to this challenge [12,13,14].
In recent years, the development of novel photocatalysts has been a hot research topic. Among various photocatalysts, graphitic carbon nitride (g-C3N4) has attracted considerable attention due to its unique properties, such as suitable band gap, excellent chemical stability, and non-toxic nature [15,16,17,18]. However, the photocatalytic performance of g-C3N4 is often limited by the rapid recombination of photo-generated electron-hole pairs and low light absorption capacity [19]. To overcome these limitations, the construction of heterostructures by coupling g-C3N4 with other semiconductors has been widely explored [20,21]. In this study, silver phosphate (Ag3PO4) and magnetite (Fe3O4) are promising candidates due to their strong visible light absorption and magnetic properties, respectively [22,23,24,25]. The combination of these three materials, Fe3O4/Ag3PO4/g-C3N4, is expected to exhibit enhanced photocatalytic performance under visible light irradiation due to the synergistic effects, including improved light absorption, efficient charge separation, and easy magnetic separation.
In this study, we present the synthesis and characterization of a Fe3O4/Ag3PO4/g-C3N4 composite and investigate its photocatalytic performance for the degradation of sulfamethazine (SMZ) under visible light irradiation. The results of this study provide valuable insights into the design and development of efficient and recyclable photocatalysts for the removal of sulfonamides from the environment.
2. Materials and Methods
2.1. Materials
The materials required for this experiment were obtained from various sources. Urea, silver nitrate (AgNO3), disodium hydrogen phosphate (Na2HPO4·12H2O), t-butyl alcohol (t-BuOH), and ethanol were purchased from Tianjin Xintong Fine Chemicals Company Limited, Tianjin, China. Ethylenediaminetetraacetic acid disodium (EDTA-2Na) was obtained from Beijing Chemical Works, Beijing, China. Benzoquinone (BQ) was procured from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. SMZ and Iron(III) chloride hexahydrate (FeCl3·6H2O) were sourced from Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China. All the reagents used in this research were of analytical quality and were applied directly without additional purification. All solutions were prepared using deionized water.
2.2. Preparation of Photocatalyst
2.2.1. Synthesis g-C3N4
Initially, 10 g of urea was introduced into a semi-sealed crucible, which was then heated in a muffle furnace at 550 °C for a duration of four hours. The heating rate was maintained at 2 °C per minute. Post-thermal treatment, the yielded yellow powder was left to cool down to room temperature naturally. The cooled product was subsequently rinsed thoroughly with distilled water and ethanol and then dried at 60 °C for a 12-h period. Following the drying process, the powder was subjected to an additional round of calcination in the muffle furnace, this time at 500 °C for 330 min, with a heating rate of 5 °C per minute. The final product yielded from this procedure was g-C3N4.
2.2.2. Synthesis Ag3PO4/g-C3N4
The Ag3PO4/g-C3N4 was synthesized in line with the literature, incorporating minor modifications with slight modifications. Briefly, g-C3N4 underwent sonication in 20 mL of water for 2 h, followed by the addition of 0.312 g AgNO3 and subsequent sonication for 1 h at room temperature. Further, 0.238 g of Na2HPO4·12H2O was incorporated, and the solution was stirred for an hour at 60 °C. The resulting yellow precipitate was isolated via centrifugation, rinsed thoroughly with water and ethanol, and dried at 60 °C for 24 h.
2.2.3. Synthesis Fe3O4
Magnetic Fe3O4 nanoparticles were synthesized via a solvothermal technique. Initially, a clear, yellow solution was formed by dissolving 1.35 g of FeCl3·6H2O in 40 mL of ethylene glycol. Thereafter, 3.6 g of anhydrous sodium acetate and 1.0 g of polyethylene glycol were incorporated, followed by 30 min of stirring. This solution was transferred to a 100 mL hydrothermal reaction kettle and heated to 200 °C for 8 h. The ensuing black Fe3O4 sediment was separated, cleaned three times, alternating between anhydrous ethanol and deionized water, and then dried at 60 °C for a full day to produce Fe3O4 nanoparticles.
2.2.4. Synthesis Fe3O4/Ag3PO4/g-C3N4
Fe3O4 was dispersed uniformly in a solution of 20 mL deionized water and 10 mL anhydrous ethanol via 2-h sonication. The Ag3PO4/g-C3N4 composite photocatalyst was then incorporated and mechanically stirred for 2 h, followed by another 2 h of sonication. The blend was moved into a hydrothermal reactor and subjected to a temperature of 180 °C for a duration of 8 h. After allowing natural cooling, the resultant black solution was washed thrice, alternating between anhydrous ethanol and deionized water, and subsequently dried at 60 °C for 24 h. The product derived was Fe3O4/Ag3PO4/g-C3N4.
2.3. Characterization
Characterization of the materials was carried out using a range of techniques. X-ray diffraction (XRD) analysis was performed using a Rigaku Ultima IV diffractometer, utilizing Cu Kα radiation and scanning a 2θ range from 10° to 90°. Scanning electron microscopy (SEM) images were obtained using a FEI Quanta-PEG 450 instrument. Photoluminescence (PL) spectra were measured with an F-98 system (Shanghai, China), and Fourier-transform infrared (FT-IR) spectroscopy was conducted on a PerkinElmer Spectrum Two spectrometer. Ultraviolet-visible (UV-vis) spectroscopy was performed using a TU-1901 spectrophotometer (Beijing, China), scanning a wavelength range from 200 nm to 800 nm.
2.4. Photocatalytic Activity and Stability Measurements
The photocatalytic degradation of sulfamethazine (SMZ) by Fe3O4/Ag3PO4/g-C3N4 samples was assessed under illumination under the light of a 500 W xenon lamp equipped with a 420 nm cut-off filter. A solution of SMZ with a concentration of 3 mg/L was formulated, and 600 mg of the created photocatalysts were added, leading to a concluding volume of 50 mL after dilution. The suspension was agitated magnetically in darkness for 30 min to establish an adsorption/desorption equilibrium between SMZ and the photocatalysts prior to irradiation. Suspension aliquots of 1.5 mL were periodically drawn and passed through a 0.22 μm Millipore filter to measure the remaining SMZ concentration. Residual SMZ was quantified using high-performance liquid chromatography (HPLC, Agilent Technologies 1200-Series). The recyclability and stability of the photocatalyst were evaluated via repeated SMZ photodegradation trials. Catalyst stability was gauged over multiple reaction cycles, with the photocatalyst being magnetically retrieved and rinsed with deionized water ahead of each subsequent cycle. The efficiency of degradation (% DE) was quantified using Equation (1). By graphing the natural logarithm of the ratio between the SMZ concentration at a given time (Ct) and the initial concentration (C0) against time (t), a linear relationship should emerge. The slope of this line provides the apparent rate constant (k) for a pseudo-first-order reaction, as outlined in Equation (2).
DE = (C0 − Ct)/C0,
ln(C0/Ct) = kt,
To evaluate the role of various active free radical species in the degradation of SMZ, a free radical scavenging experiment was employed. This experiment incorporates free radical trapping agents in conjunction with the photocatalyst in the SMZ solution. Trapping agents included t-Buoh, EDTA-2Na, and BQ, each at a concentration of 1 mmolL−1. These were utilized to capture hydroxyl radicals (•OH), holes (h+), and superoxide radicals (•O2−), respectively. The predominant active species in the Fe3O4/Ag3PO4/g-C3N4 photocatalyst for SMZ degradation were deduced by comparing degradation rates before and after the introduction of these radical trapping agents. This comparison highlights the respective contribution of each active species towards the photocatalytic degradation process, elucidating the underlying reaction mechanism.
3. Results and Discussion
3.1. Charactation
The XRD analysis represented in Figure 1 provides critical insights into the crystalline structures of the g-C3N4, Ag3PO4, Fe3O4, and Fe3O4/Ag3PO4/g-C3N4 synthesized in this study. In the case of g-C3N4, two distinct diffraction peaks at 2θ values of approximately 13.0° and 27.4° are observed, corresponding to the (100) and (002) crystal planes, respectively. This alignment corresponds precisely with the standard XRD pattern of g-C3N4 (JCPDS 87-1526) [26]. The cubic phase Ag3PO4 demonstrates notable diffraction peaks at 2θ values of 20.854°, 29.660°, 33.260°, 36.541°, 42.460°, 47.760°, 52.661°, 54.981°, 57.240°, 61.621°, 69.862°, 71.859°, 73.821°, 87.241°, which can be respectively ascribed to the (110), (200), (210), (211), (220), (310), (222), (320), (321), (400), (420), (421), (332), (520) planes (JCPDS No.06-0505) [27]. For the Fe3O4, well-defined diffraction peaks are located at 2θ values of 30.206°, 35.501°, 43.190°, 53.717°, 57.221°, 62.738°, which correspond to the (220), (311), (400), (422), (511), (440) planes as referenced in the standard card (JCPDS 19-0629) [28]. In the XRD pattern of Fe3O4/Ag3PO4/g-C3N4, the presence of the g-C3N4 (002) plane and the Fe3O4 (220), (311), (400), (422), (511), (440) planes is clearly discernible. Crucially, the diffraction peaks of Fe3O4 remain unaltered, thus indicating that the crystallographic structure of Fe3O4 is preserved post-loading onto the Ag3PO4/g-C3N4 surface. The absence of additional impurity peaks signifies that no new impurities were introduced during the reaction, thereby attesting to the high purity of the resultant magnetic photocatalyst composite. Furthermore, a slight decrease in the intensity of the Fe3O4 diffraction peaks in the Fe3O4/Ag3PO4/g-C3N4 sample compared to pure Fe3O4 is suggestive of potential interactions among Fe3O4, Ag3PO4 and g-C3N4.
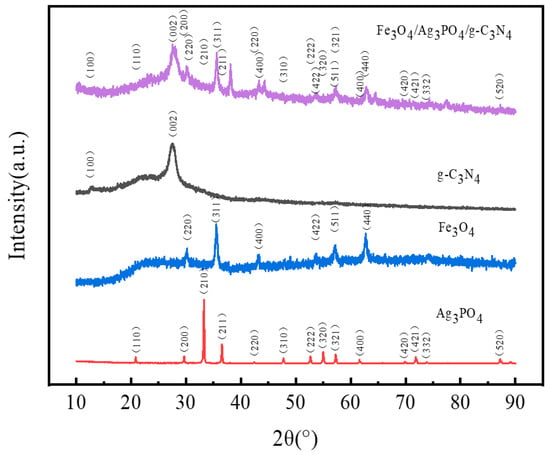
Figure 1.
XRD patterns of g-C3N4, Fe3O4, Ag3PO4 and Fe3O4/Ag3PO4/g-C3N4.
As shown in Figure 2a, the g-C3N4, as prepared through the thermal oxidation exfoliation technique with urea serving as the precursor, leads to the generation of a multitude of irregularly stacked lamellar structures. These layered configurations play an instrumental role in creating a more direct route for electron transfer, thereby fostering efficient migration of photo-generated charge carriers [29]. Furthermore, the augmentation of the specific surface area and pore volume introduces an abundance of active sites for photocatalytic reactions [30]. Figure 2b represents Ag3PO4, prepared through the in-situ precipitation technique, displaying a spherically stacked morphology. A noteworthy observation from this figure is the visible agglomeration within the sample, which could potentially impact the photocatalytic efficacy due to the consequent reduction in the reactive surface area. As exhibited in Figure 2c, the solvothermal method is used to prepare Fe3O4 nanospheres. Owing to their relatively modest dimensions, these nanospheres showcase commendable dispersibility. This characteristic is beneficial as it may lead to a more homogeneous distribution of active sites and augmented accessibility for reactants, thereby potentially boosting the overall photocatalytic performance. As shown in Figure 2d, Fe3O4 nanospheres are discernibly dispersed across the Ag3PO4/g-C3N4 composite’s surface. The incorporation of Fe3O4 into the composite serves a dual purpose: It not only expedites the photocatalyst recovery due to its magnetic properties but also boosts the electron migration rates thanks to its superior electrical conductivity [30]. Furthermore, the presence of Fe3O4 may reduce the recombination of photo-induced electron-hole pairs, which, in turn, augments the photocatalytic efficiency of the composite. The combined effect of improved material properties and unique morphological features is anticipated to yield notable photocatalytic performance in the Ag3PO4/g-C3N4 composite.
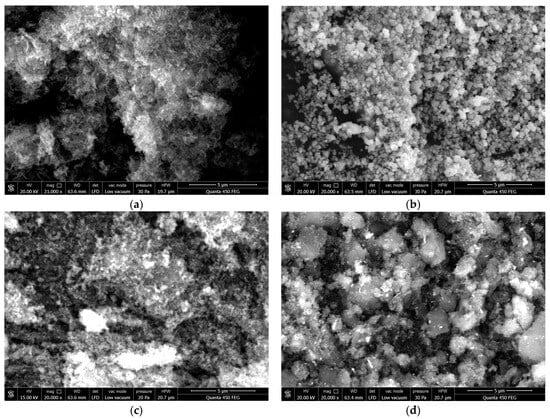
Figure 2.
SEM image of (a) g-C3N4, (b) Ag3PO4 (c) Fe3O4 and (d) Fe3O4/ Ag3PO4/g-C3N4.
Figure 3 presents the FT-IR spectra of the Fe3O4/Ag3PO4/g-C3N4 composite synthesized in this study. The composite shows pronounced absorption peaks around 810 cm−1, 1200–1700 cm−1, and 3200–3400 cm−1, closely paralleling the characteristic peaks of pure g-C3N4 [31,32]. The peak at 810 cm−1 can be attributed to the bending vibration of triazine units. The region from 1200 to 1700 cm−1 represents standard stretching vibrations of CN heterocycles. Furthermore, the absorption peak in the range of 3200–3400 cm−1 is ascribed to the O-H stretching vibration of adsorbed water molecules [33]. An additional peak, evident around 1650 cm−1, corresponds to the bending vibration of these water molecules [34]. This spectral evidence testifies to the successful creation of the g-C3N4 structure without disruption during the composite formation process, a claim further validated by XRD characterization results [35]. The integration of Ag3PO4 and g-C3N4 into the composite amplifies the surface adsorption O-H peak [36]. Further, the Fe3O4/Ag3PO4/g-C3N4 composite exhibits a distinctive Fe-O stretching vibration of Fe3O4 around 589 cm−1 [37]. This additional feature augments the O-H surface absorption peak, consequently enhancing the hydroxyl density on the Fe3O4/Ag3PO4/g-C3N4 surface and improving the adsorption performance of this magnetic composite photocatalyst [33]. These collective observations corroborate the successful preparation of the Fe3O4/Ag3PO4/g-C3N4 composite.
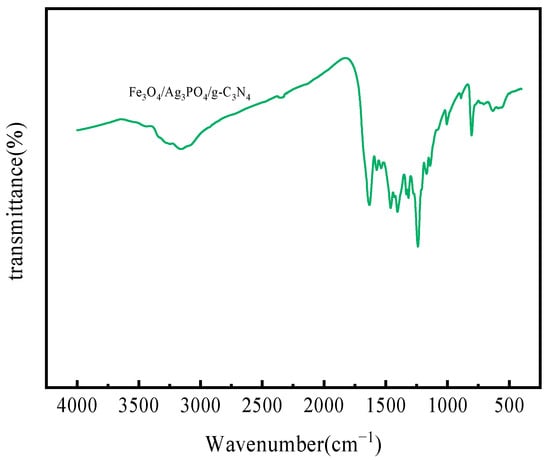
Figure 3.
FT-IR spectra of Fe3O4/Ag3PO4/g-C3N4.
XPS was employed to delve deeper into the chemical composition of the magnetic composite photocatalysts, particularly focusing on understanding the intricate interplay between g-C3N4, Ag3PO4, and Fe3O4. XPS, with its unique capability of providing information on the chemical bonds and binding states of elements within a material, offers a comprehensive analysis of the constituents and their interactions. As shown in the survey XPS spectra (Figure 4a), with the detection of carbon, nitrogen, oxygen, silver, iron elements, and phosphorous, the Fe3O4/Ag3PO4/g-C3N4 composite demonstrates the successful incorporation of all three constituents—g-C3N4, Ag3PO4, and Fe3O4. Figure 4b–e illustrates the high-resolution XPS spectra for the C 1s, N 1s, O 1s, Ag 3d, and Fe 2p regions. In these spectra, the binding energy for each sample was calibrated to the standard C 1s binding energy (284.8 eV). The C 1s spectrum for the Fe3O4/Ag3PO4/g-C3N4 composite (Figure 4b) reveals characteristic peaks at 284.8 eV, 286.4 eV, 288.3 eV, and 290.2 eV, which are attributable to the C-C, C-O, N=C-N, and O=C-O bonds, respectively [38,39,40,41]. This suggests the presence of multiple carbon-containing species within the composite, indicative of a complex interaction among the constituents. In the N 1s spectrum (Figure 4c), the peaks located at 398.8 eV (C–N–C) [42], 400.3 eV (N-(C)3) [43], and 401.4 eV (N–H) [44] corroborate with those from the g-C3N4 sample, confirming the presence of g-C3N4 in the composite. The O 1s spectrum (Figure 4d) for the Fe3O4/Ag3PO4/g-C3N4 composite displays peaks at 529.7 eV, 532.1 eV, and 533.8 eV, which attributed to lattice oxygen (OL) [45], hydroxyl oxygen (OOH) [46], and adsorbed oxygen (OA) [47], respectively. High-resolution spectra of Ag 3d for Fe3O4/Ag3PO4/g-C3N4 sample (Figure 4e) present two distinctive peaks at 368.1eV and 374.1eV, which are congruent with typical Ag+ orbitals of Ag+ 3d5/2 and Ag+ 3d3/2 [48,49]. This confirms the presence and involvement of Ag3PO4 within the composite. Lastly, the Fe 2p spectrum for the Fe3O4/Ag3PO4/g-C3N4 (Figure 4f) exhibits two prominent peaks at 709.7 eV and 722.8 eV, representing the Fe 2p3/2 orbitals of Fe2+ and Fe 2p1/2 orbitals of Fe3+ [50,51]. It indicates the successful integration and interaction of Fe3O4, Ag3PO4, and g-C3N4 in the synthesized photocatalyst.
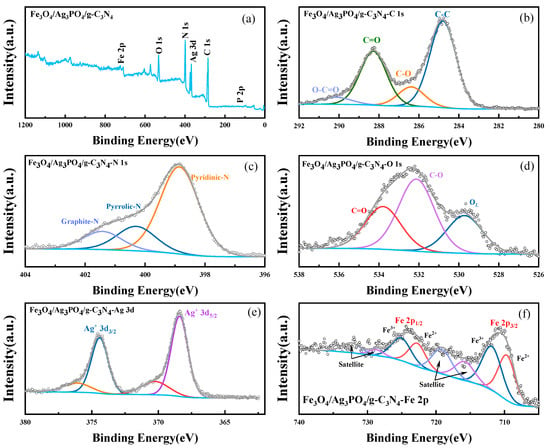
Figure 4.
(a) The high-resolution XPS spectra of Fe3O4/Ag3PO4/g-C3N4; (b) C 1s; (c) N 1s; (d) O 1s; (e) Ag 3d; (f) Fe 2p.
The efficiency of photo-generated electron-hole pair separation, a key parameter in photoreactivity, is typically assessed PL [52]. For our PL measurements, an excitation wavelength of 328 nm was used. As from Figure 5, the emission maxima of the test samples concentrate around the 463nm spectral region. The sequence of descending fluorescence intensities is as follows: g-C3N4 > Ag3PO4 > Fe3O4 > Fe3O4/Ag3PO4/g-C3N4. This hierarchy implies that the lower the fluorescence intensity, the fewer electron-hole recombination events occur. Therefore, the composite with the lowest fluorescence intensity, namely Fe3O4/Ag3PO4/g-C3N4, exhibits the least recombination rate of photo-generated electron-hole pairs. This observation substantiates the hypothesis that the composite photocatalyst configuration effectively mitigates the recombination of photo-generated electron-hole pairs, which is desirable for enhancing photocatalytic efficiency. Furthermore, corroborating our spectrographic data, Fe3O4 intrinsically exhibits commendable electronic conductivity. Such a characteristic is advantageous in facilitating the transfer of photo-generated charge carriers, a crucial step in photocatalytic reactions. This further underscores the role of compositional design in tailoring the photoreactive properties of composite photocatalysts.
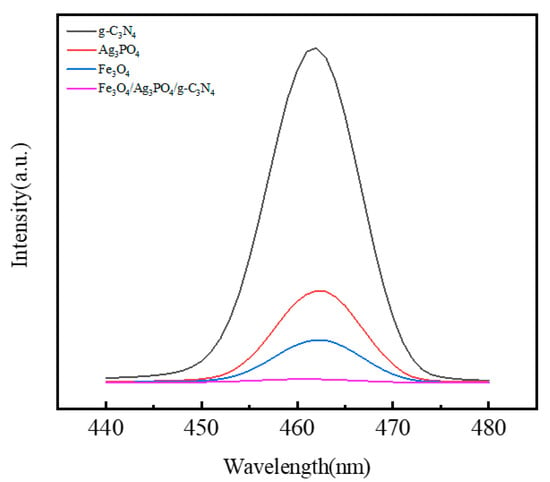
Figure 5.
PL spectra of g-C3N4, Ag3PO4, Fe3O4 and Fe3O4/Ag3PO4/g-C3N4.
The potency of a material’s photocatalytic activity is chiefly determined by its aptitude for absorbing and utilizing incident light. This work employs UV-vis diffuse reflectance spectroscopy to investigate the optical absorption properties of the magnetic composite photocatalyst Fe3O4/Ag3PO4/g-C3N4. As illustrated in Figure 6, all samples reveal capabilities of absorbing light within the ultraviolet range, with some response extending into the visible spectrum, dictated by the respective bandgap dimensions. Pristine g-C3N4 manifests an absorption cut-off around 438 nm, whereas Ag3PO4 shows an edge at about 510 nm. Based on existing literature, Fe3O4 primarily absorbs light in the ultraviolet domain. Upon integration of Fe3O4 into the composite, the absorption edge of Fe3O4/Ag3PO4/g-C3N4 is detected at around 745 nm, thereby broadening the composite’s responsiveness to visible light. According to the Tauc relation [53], The g-C3N4 possesses a bandgap energy of 2.96 eV, making it responsive primarily to ultraviolet light. Ag3PO4, with a bandgap of 2.27 eV, extends the composite’s light absorption into the visible range, thereby bolstering the efficiency under visible light irradiation. Fe3O4, having a notably smaller bandgap of 1.16 eV, aids in widening the composite’s light absorption further into the visible spectrum. When combined, the Fe3O4/Ag3PO4/g-C3N4 composite exhibits an intermediate bandgap of 2.66 eV. This collaborative interplay ensures that the composite harnesses a broader range of the light spectrum, optimizing the generation of electron-hole pairs. These bandgap energies have significant bearings on a material’s absorption properties. This intermediate bandgap leads to heightened light absorption, particularly in the visible region, as substantiated by the absorption edge observed near 745 nm. This result underscores the synergistic impact of the composite components: Fe3O4 expands the light absorption into the visible range, whereas the wider-bandgap materials (g-C3N4 and Ag3PO4) enhance the overall photocatalytic performance under UV light. As a result, under equivalent illumination, the composite photocatalyst is proficient in generating a higher yield of reactive species, thereby boosting its photocatalytic performance.
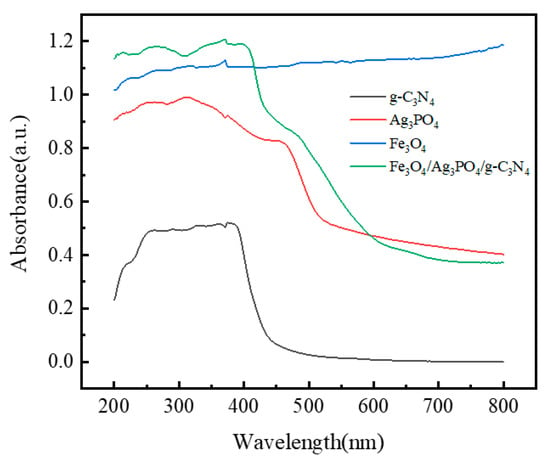
Figure 6.
UV-vis spectra of g-C3N4, Ag3PO4, Fe3O4, and Fe3O4/Ag3PO4/g-C3N4.
3.2. Photocatalyst Performance
The inherent stability of SMZ poses a significant challenge to its degradation under visible light in the absence of a photocatalyst. After 60 min of visible light irradiation, the photocatalysts in dark conditions exhibited a negligible removal efficiency for SMZ, less than 5%. This observation suggests that adsorption by the photocatalysts plays a limited part in the entire process. To delve deeper into how solution pH affects the breakdown of SMZ under visible light with Fe3O4/Ag3PO4/g-C3N4 serving as a photocatalyst, we conducted photocatalytic experiments with initial pH values adjusted to 5.0, 6.0, 7.0, 8.0, and 9.0. The results showed that the degradation efficiencies of SMZ by Fe3O4/Ag3PO4/g-C3N4 within 60 min were 93.41%, 99.21%, 93.28%, 96.77%, and 98.01% at pH values of 5.0, 6.0, 7.0, 8.0, and 9.0, respectively (as shown in Figure 7). The corresponding degradation kinetic constants were 0.04602, 0.07673, 0.04369, 0.05426, and 0.06140 min−1. These results suggest that the degradation efficiency and kinetics of SMZ are significantly influenced by the pH of the solution. The highest degradation efficiency was observed at pH 6.0, indicating that the photocatalytic activity of Fe3O4/Ag3PO4/g-C3N4 is optimal in slightly acidic conditions. The degradation kinetic constants also showed a similar trend, with the highest value at pH 6.0. At pH 6.0, the photocatalyst likely assumes an optimal surface charge, preventing aggregation and fostering the generation of reactive oxygen species (ROS). This, in turn, elevates the photocatalytic degradation of SMZ. Furthermore, it is imperative to understand the dual acidic-basic nature of SMZ. Given the molecule’s intrinsic acidic and basic functional groups, its speciation can vary with pH, influencing its interaction dynamics with the photocatalyst and consequently affecting its degradation efficiency.
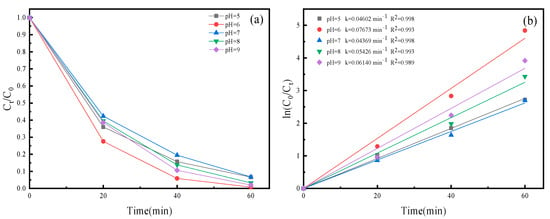
Figure 7.
(a) Photocatalytic performance of Fe3O4/Ag3PO4/g-C3N4 with different pH for SMZ under visible light. (b) Plots of ln(C0/Ct) versus irradiation time for SMZ.
Assessing the photochemical stability of a photocatalyst is crucial to ascertaining its viability for real-world applications. We conducted an extensive stability evaluation for the Fe3O4/Ag3PO4/g-C3N4 magnetic composite photocatalyst via five repeated cycles of photocatalytic degradation of SMZ under identical experimental parameters. For these tests, the pH of the solution was maintained at 6, and the visible light degradation of SMZ was observed over a period of 60 min. As shown in Figure 8, it was noted that degradation rates of 99.21%, 99.01%, 98.87%, 97.92%, and 97.37% in these sequential degradation cycles, respectively. Remarkably, even after five cycles, the degradation rate sustained above 97%, signifying a negligible decrease in performance. This robust performance across multiple cycles illustrates the outstanding endurance and photochemical stability of the Fe3O4/Ag3PO4/g-C3N4 composite. The negligible depreciation in its photocatalytic efficiency suggests a commendable recyclability potential for the composite. This demonstration of consistent high-performance degradation across multiple cycles, with minimal loss in efficiency, is a highly promising indicator of the composite’s real-world applicability.
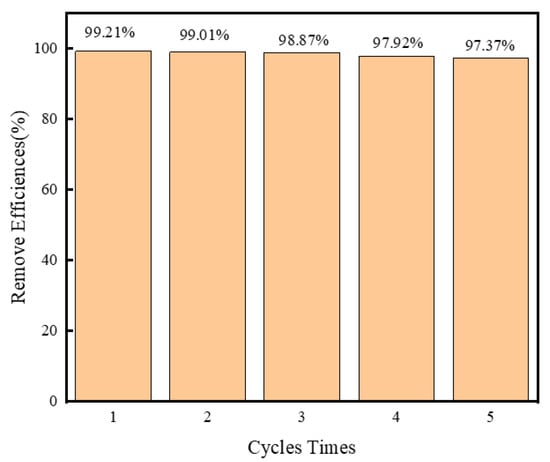
Figure 8.
Stability and reusability test of Fe3O4/Ag3PO4/g-C3N4 for SMZ.
3.3. Photocatalytic Mechanism
Photocatalytic oxidation processes involve key reactive species such as photo-generated (h+), superoxide radical(•O2−), and hydroxyl radical(•OH) [54,55,56]. To unveil the nuances of the reaction mechanism, especially when applying the Fe3O4/Ag3PO4/g-C3N4 photocatalyst to the degradation of SMZ, we investigated the impact of several scavengers. The selection included BQ, an •O2− scavenger [57], t-BuOH, a scavenger of •OH [58], and EDTA-2Na, a quencher for h+ [59]. This series of tests was devised to elucidate the respective roles of these reactive species in the photocatalytic process. The experiment was conducted with an initial solution pH set at 6 and under visible light irradiation, creating a highly consistent environment to evaluate the effects of these scavengers on the photocatalytic degradation of SMZ. Figure 9 presents these effects, revealing how the efficiency of SMZ degradation varies with the introduction of different scavengers. The results demonstrated that the degradation rate of SMZ remained largely unaffected upon the addition of t-BuOH. This strongly suggests that •OH has a relatively minor role in the photocatalytic degradation process [60]. Conversely, the introduction of BQ and EDTA-2Na led to a significant decrease in the degradation rate. This data implies that •O2− and h+ are the primary active species responsible for the degradation of SMZ in the presence of the Fe3O4/Ag3PO4/g-C3N4 photocatalyst and under visible light conditions [61]. Drawing from these observations, we propose a detailed mechanism for the photocatalytic degradation of SMZ by the Fe3O4/Ag3PO4/g-C3N4 photocatalyst under visible light. Upon irradiation, the photocatalyst triggers the excitation of electrons to the conduction band, leaving high h+ in the valence band. This electron-hole pair generation is a critical initial step in photocatalytic reactions. A salient feature of our Fe3O4/Ag3PO4/g-C3N4 photocatalyst is its heterojunction structure, which serves to inhibit the recombination of photo-generated electron-hole pairs. This is crucial, as such recombination often leads to a decrease in photocatalytic efficiency. The suppressed recombination, in our case, likely contributes to the enhanced photocatalytic activity observed. In our reaction system, dissolved oxygen adsorbed on the photocatalyst surface can react with photo-generated electrons (e−) to produce •O2−, another reactive species. The photocatalyst’s large specific surface area provides abundant active sites, further facilitating the migration of photo-generated carriers and thus increasing the possibility of reaction with the adsorbed oxygen. In summary, our findings strongly suggest that the two primary active species, •O2− and h+, play crucial roles in the degradation of SMZ. They interact to efficiently break down SMZ into smaller organic molecules or even fully mineralize it into CO2 and H2O [62]. Meantime, the photocatalytic degradation mechanism diagram of Fe3O4/Ag3PO4/g-C3N4 under visible irradiation is shown in Figure S1.
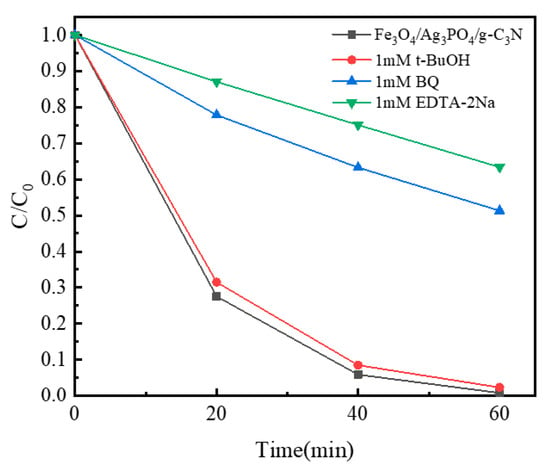
Figure 9.
Effects of a series of scavengers on the degradation efficiency of SMZ Fe3O4/Ag3PO4/g-C3N4 under visible light irradiation.
4. Conclusions
In summary, this research provides a thorough investigation into the creation, characterization, and utilization of a novel Fe3O4/Ag3PO4/g-C3N4 magnetic composite photocatalyst designed specifically for the visible-light-driven photocatalytic degradation of sulfonamide antibiotics, focusing especially on SMZ. An integral revelation of the study underlines the significance of solution pH in the degradation process, demonstrated through methodically designed experiments. These findings indicate that an optimal photocatalytic performance was achieved at a solution pH value of 6.0. Crucially, the Fe3O4/Ag3PO4/g-C3N4 magnetic composite photocatalyst displayed exceptional stability and recyclability, with the ability to sustain a degradation efficiency exceeding 97% through five successive cycles. The insights gleaned from this comprehensive investigation not only contribute to our understanding of the photocatalytic degradation of sulfonamide antibiotics using magnetic composite photocatalysts but also underscore the promising potential of the Fe3O4/Ag3PO4/g-C3N4 magnetic composite for practical applications in environmental remediation. To elucidate the role of various active radicals in the photocatalytic reactions, additional scavenger tests with different chemicals were performed. The results showed a considerable decrease in the percent degradation efficiency in the presence of radical scavengers such as BQ and EDTA-2Na, thus proving that the generation of •O2− and h+ are the primary active species possessing high redox ability during the photocatalytic process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151713279/s1. Figure S1: The photocatalytic degradation mechanism diagram of Fe3O4/Ag3P2O4/g-C3N4.
Author Contributions
Conceptualization, K.L., M.C. and L.C.; methodology, W.X., S.Z. and Y.H.; software M.C. and W.X.; investigation, M.C., W.X. and S.Z.; resources, K.L. and L.C.; data curation, K.L.; writing—original draft preparation, K.L., M.C. and W.X.; writing—review and editing, K.L. and L.C.; visualization, Y.H.; supervision, K.L. and L.C.; funding acquisition, K.L. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (51878316) and the Science and Technology Research Planning Project of Jilin Provincial Department of Education (JJKH20220297KJ, JJKH20220279KJ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All authors thank the editor and anonymous reviewers for their constructive comments and suggestions to improve the quality of this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Ding, S.; Li, M. Research Progress on Photo Degradation of Sulfonamide Antibiotics in Water Environment. Agro Food Ind. Hi-Tech 2017, 28, 3479–3482. [Google Scholar]
- Zhou, J.; Yun, X.; Wang, J.; Li, Q.; Wang, Y. A review on the ecotoxicological effect of sulphonamides on aquatic organisms. Toxicol. Rep. 2022, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Mushtaq, S.; Naz, S.; Farooq, U.; Zaidi, A.; Bukhari, S.M.; Rauf, A.; Mubarak, M.S. Sulfonamides as Potential Bioactive Scaffolds. Curr. Org. Chem. 2018, 22, 818–830. [Google Scholar] [CrossRef]
- Rakesh, K.P.; Wang, S.-M.; Leng, J.; Ravindar, L.; Asiri, A.M.; Marwani, H.M.; Qin, H.-L. Recent Development of Sulfonyl or Sulfonamide Hybrids as Potential Anticancer Agents: A Key Review. Anti-Cancer Agents Med. Chem. 2018, 18, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Liu, X.; Song, L.; Hamid, N.; Zhang, R. Sulfonamide and tetracycline in landfill leachates from seven municipal solid waste (MSW) landfills: Seasonal variation and risk assessment. Sci. Total Environ. 2022, 825, 153936. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qian, Y.; Su, C.; Cheng, W.; Li, J.; Wahlqvist, M.L.; Chen, H. Prevalence of bacterial resistance within an eco-agricultural system in Hangzhou, China. Environ. Sci. Pollut. Res. 2016, 23, 21369–21376. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Zhang, T.; Jin, L.; Han, Q.; Zhang, D.; Lin, K.; Cui, C. Occurrence and removal of sulfonamide antibiotics and antibiotic resistance genes in conventional and advanced drinking water treatment processes. J. Hazard. Mater. 2018, 360, 364–372. [Google Scholar] [CrossRef]
- Lucas, D.; Badia-Fabregat, M.; Vicent, T.; Caminal, G.; Rodriguez-Mozaz, S.; Balcazar, J.L.; Barcelo, D. Fungal treatment for the removal of antibiotics and antibiotic resistance genes in veterinary hospital wastewater. Chemosphere 2016, 152, 301–308. [Google Scholar] [CrossRef]
- Qi, K.; Chen, M.; Dai, R.; Li, Q.; Lai, M.; Wang, Z. Development of an Electrochemical Ceramic Membrane Bioreactor for the Removal of PPCPs from Wastewater. Water 2020, 12, 1838. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.-S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar]
- Liu, Z.; Liu, Y.; Sun, X.; Ji, H.; Liu, W.; Cai, Z. Construction of Z-scheme Ag/AgVO3/carbon-rich g-C3N4 heterojunction for enhanced photocatalytic degradation of sulfamethiadiazole: DFT calculation and mechanism study. Chem. Eng. J. 2022, 433, 133604. [Google Scholar]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Chen, P.; Chen, L.; Ge, S.; Zhang, W.; Wu, M.; Xing, P.; Rotamond, T.B.; Lin, H.; Wu, Y.; He, Y. Microwave heating preparation of phosphorus doped g-C3N4 and its enhanced performance for photocatalytic H-2 evolution in the help of Ag3PO4 nanoparticles. Int. J. Hydrogen Energy 2020, 45, 14354–14367. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B-Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B-Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Zhao, Q.; Niu, T.; Pan, L.; Xu, P.; Zhang, F.; Wu, W.; Ni, T. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar]
- Ismael, M. Environmental remediation and sustainable energy generation via photocatalytic technology using rare earth metals modified g-C3N4: A review. J. Alloys Compd. 2023, 931, 167469. [Google Scholar]
- Liu, X.; Wang, S.; Yang, F.; Zhang, Y.; Yan, L.; Li, K.; Guo, H.; Yan, J.; Lin, J. Construction of Au/g-C3N4/ZnIn2S4 plasma photocatalyst heterojunction composite with 3D hierarchical microarchitecture for visible-light-driven hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2900–2913. [Google Scholar] [CrossRef]
- Ni, T.J.; Zhang, H.; Yang, Z.B.; Zhou, L.P.; Pan, L.K.; Li, C.L.; Yang, Z.J.; Liu, D. Enhanced adsorption and catalytic degradation of antibiotics by porous 0D/3D Co3O4/g-C3N4 activated peroxymonosulfate: An experimental and mechanistic study. J. Colloid Interface Sci. 2022, 625, 466–478. [Google Scholar] [PubMed]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [PubMed]
- Afkari, M.; Masoudpanah, S.M.; Hasheminiasari, M.; Alamolhoda, S. Effects of iron oxide contents on photocatalytic performance of nanocomposites based on g-C3N4. Sci. Rep. 2023, 13, 6203. [Google Scholar]
- Wang, J.; Shao, X.; Liu, J.; Zhang, Q.; Ji, X.; Tian, G. Mesoporous magnetic g-C3N4 nanocomposites for photocatalytic environmental remediation under visible light. Ecotoxicol. Environ. Saf. 2020, 205, 111147. [Google Scholar] [PubMed]
- Zuo, S.; Xia, D.; Guan, Z.; Yang, F.; Zan, J.; Xu, H.; Huang, M.; Li, D. Magnetite/graphite carbon nitride composite for peroxymonosulfate non-radical activation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125895. [Google Scholar]
- Cao, Y.; Zhang, Z.; Long, J.; Liang, J.; Lin, H.; Lin, H.; Wang, X. Vacuum heat-treatment of carbon nitride for enhancing photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 17797–17807. [Google Scholar]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate. CrystEngComm 2012, 14, 2966–2973. [Google Scholar] [CrossRef]
- Chen, J.; Huang, K.; Liu, S. Hydrothermal preparation of octadecahedron Fe3O4 thin film for use in an electrochemical supercapacitor. Electrochim. Acta 2009, 55, 1–5. [Google Scholar]
- Ai, F.J.; Sun, T.Y.; Xu, Z.F.; Wang, Z.G.; Kong, W.; To, M.W.; Wang, F.; Zhu, G.Y. An upconversion nanoplatform for simultaneous photodynamic therapy and Pt chemotherapy to combat cisplatin resistance. Dalton Trans. 2016, 45, 13052–13060. [Google Scholar]
- Zhang, G.; Lan, Z.-A.; Lin, L.; Lin, S.; Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar]
- Li, M.; Wang, S.; Gao, H.; Yin, Z.; Chen, C.; Yang, H.; Fang, L.; Veerabhadrappa, J.A.; Yi, Z.; Li, D. Selective removal of antibiotics over MgAl2O4/C3N4/YMnO3 photocatalysts: Performance prediction and mechanism insight. J. Am. Ceram. Soc. 2023, 106, 2420–2442. [Google Scholar]
- Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, characterization of g-C3N4/SrTiO3 heterojunctions and photocatalytic activity for organic pollutants degradation. Catalysts 2018, 8, 554. [Google Scholar]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and characterization of efficient ZnO/g-C3N4 nanocomposites photocatalyst for photocatalytic degradation of methylene blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Lu, P.; Hu, X.; Li, Y.; Zhang, M.; Liu, X.; He, Y.; Dong, F.; Fu, M.; Zhang, Z. Construction of CeO2/YMnO3 and CeO2/MgAl2O4/YMnO3 photocatalysts and adsorption of dyes and photocatalytic oxidation of antibiotics: Performance prediction, degradation pathway and mechanism insight. Appl. Surf. Sci. 2023, 608, 154977. [Google Scholar]
- Lu, P.; Hu, X.; Li, Y.; Zhang, M.; Liu, X.; He, Y.; Dong, F.; Fu, M.; Zhang, Z. One-step preparation of a novel SrCO3/gC3N4 nano-composite and its application in selective adsorption of crystal violet. RSC Adv. 2018, 8, 6315–6325. [Google Scholar]
- Zhao, Z.; Ma, H.; Feng, M.; Li, Z.; Cao, D.; Guo, Z. In situ preparation of WO3/g-C3N4 composite and its enhanced photocatalytic ability, a comparative study on the preparation methods. Eng. Sci. 2019, 7, 52–58. [Google Scholar]
- Varotsis, C.; Woodruff, W.H.; Babcock, G.T. Time-resolved Raman detection of. nu.(Fe-O) in an early intermediate in the reduction of oxygen by cytochrome oxidase. J. Am. Chem. Soc. 1989, 111, 6439–6440. [Google Scholar]
- Yang, N.; Li, G.; Wang, W.; Yang, X.; Zhang, W. Photophysical and enhanced daylight photocatalytic properties of N-doped TiO2/g-C3N4 composites. J. Phys. Chem. Solids 2011, 72, 1319–1324. [Google Scholar]
- Xiao, C.; Zhang, L.; Wang, K.; Wang, H.; Zhou, Y.; Wang, W. A new approach to enhance photocatalytic nitrogen fixation performance via phosphate-bridge: A case study of SiW12/K-C3N4. Appl. Catal. B Environ. 2018, 239, 260–267. [Google Scholar]
- Zhang, J.; Ma, Z. Porous g-C3N4 with enhanced adsorption and visible-light photocatalytic performance for removing aqueous dyes and tetracycline hydrochloride. Chin. J. Chem. Eng. 2018, 26, 753–760. [Google Scholar]
- Qi, K.; Lv, W.; Khan, I.; Liu, S.-Y. Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Dementjev, A.; De Graaf, A.; Van de Sanden, M.; Maslakov, K.; Naumkin, A.; Serov, A. X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Xing, W.; Li, C.; Wang, Y.; Han, Z.; Hu, Y.; Chen, D.; Meng, Q.; Chen, G. A novel 2D/2D carbonized poly-(furfural alcohol)/g-C3N4 nanocomposites with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2017, 115, 486–492. [Google Scholar] [CrossRef]
- Butt, F.K.; Hauenstein, P.; Kosiahn, M.; Garlyyev, B.; Dao, M.; Lang, A.; Scieszka, D.; Liang, Y.; Kreuzpaintner, W. An innovative microwave-assisted method for the synthesis of mesoporous two dimensional g-C3N4: A revisited insight into a potential electrode material for supercapacitors. Microporous Mesoporous Mater. 2020, 294, 109853. [Google Scholar] [CrossRef]
- Huang, J.-G.; Guo, X.-T.; Wang, B.; Li, L.-Y.; Zhao, M.-X.; Dong, L.-L.; Liu, X.-J.; Huang, Y.-T. Synthesis and photocatalytic activity of Mo-doped TiO2 nanoparticles. J. Spectrosc. 2015, 2015, 681850. [Google Scholar] [CrossRef]
- Tang, C.; Liu, E.; Fan, J.; Hu, X.; Ma, Y.; Wan, J. A graphitic-C3N4-hybridized Ag3PO4 tetrahedron with reactive {111} facets to enhance the visible-light photocatalytic activity. RSC Adv. 2015, 5, 91979–91987. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, H. One-pot preparation of NaBiO3/PNMA composite: Surface properties and photocatalytic performance. Appl. Surf. Sci. 2021, 544, 148910. [Google Scholar] [CrossRef]
- Tan, J.; Peng, J.; Li, Z.; Liu, D.; Li, W. Ag@ AgBr/Ag3PO4 Nanocomposites as Photocatalyst for Degradation of Rhodamine B. Int. J. Electrochem. Sci. 2021, 16, 210744. [Google Scholar] [CrossRef]
- Ren, J.; Chai, Y.; Liu, Q.; Zhang, L.; Dai, W.-L. Intercorrelated Ag3PO4 nanoparticles decorated with graphic carbon nitride: Enhanced stability and photocatalytic activities for water treatment. Appl. Surf. Sci. 2017, 403, 177–186. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Perero, S.; Wang, Q.; Chen, Q. Structural, optical and magnetic properties of Fe3O4 sputtered TeO2–PbO–B2O3 and PbO–Bi2O3–B2O3 glasses for sensing applications. J. Non-Cryst. Solids 2015, 408, 43–50. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 2021, 281, 130718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, S.P.; Yang, P. Bright and tunable photoluminescence from the assembly of red g-C3N4 nanosheets. J. Lumin. 2021, 235, 118055. [Google Scholar] [CrossRef]
- Khan, J.A.; Qasim, M.; Singh, B.R.; Singh, S.; Shoeb, M.; Khan, W.; Das, D.; Naqvi, A.H. Synthesis and characterization of structural, optical, thermal and dielectric properties of polyaniline/CoFe2O4 nanocomposites with special reference to photocatalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, H.; Park, J.; Moon, G.-H.; Vequizo, J.J.M.; Yamakata, A.; Lee, J.; Choi, W. How g-C3N4 works and is different from TiO2 as an environmental photocatalyst: Mechanistic view. Environ. Sci. Technol. 2019, 54, 497–506. [Google Scholar] [CrossRef]
- Ma, X.; Wang, G.; Qin, L.; Liu, J.; Li, B.; Hu, Y. Cheng, H. Z-scheme g-C3N4-AQ-MoO3 photocatalyst with unique electron transfer channel and large reduction area for enhanced sunlight photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 288, 120025. [Google Scholar] [CrossRef]
- Gan, H.; Yi, F.; Zhang, H.; Qian, Y.; Jin, H.; Zhang, K. Facile ultrasonic-assisted synthesis of micro–nanosheet structure Bi4Ti3O12/g-C3N4 composites with enhanced photocatalytic activity on organic pollutants. Chin. J. Chem. Eng. 2018, 26, 2628–2635. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Li, K.; Zhang, W.-D. On the heterostructured photocatalysts Ag3VO4/g-C3N4 with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2015, 324, 324–331. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Xue, Y.; Yao, G.; Zheng, S. A facile synthesis of g-C3N4/TiO2 hybrid photocatalysts by sol–gel method and its enhanced photodegradation towards methylene blue under visible light. Adv. Powder Technol. 2016, 27, 330–337. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.; Kadam, A.N.; Bathula, C.; Ghfar, A.A.; Kim, H.; Lee, S.-W. Synergistic photocatalysis of Z-scheme type Fe2O3/g-C3N4 heterojunction coupled with reduced graphene oxide. Surf. Interfaces 2022, 30, 101910. [Google Scholar] [CrossRef]
- Shi, W.; Fang, W.-X.; Wang, J.-C.; Qiao, X.; Wang, B.; Guo, X. pH-controlled mechanism of photocatalytic RhB degradation over gC3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef]
- Aissani, T.; Yahiaoui, I.; Boudrahem, F.; Yahia Cherif, L.; Fourcad, F.; Amrane, A.; Aissani-Benissad, F. Sulfamethazine degradation by heterogeneous photocatalysis with ZnO immobilized on a glass plate using the heat attachment method and its impact on the biodegradability. React. Kinet. Mech. Catal. 2020, 131, 471–487. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Guo, W.; Ren, Q.; Ding, Y.; Chen, S.; Chen, J.; Jia, X. Ag3VO4/gC3N4/diatomite ternary compound reduces Cr (vi) ion in aqueous solution effectively under visible light. RSC Adv. 2022, 12, 7671–7679. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).