1. Introduction
Poor water quality is a complicated issue that affects the water management and living standards of SSF communities. One of the most important issues is water pollution, which has an impact on the social–ecological system of the coastal area. Water quality directly affects the performance and health of fish in the context of SSFs. The quality of water is a key determinant of the quality of fish reproduction, growth, survival, and the fish habitat. Water contains appropriate physical (e.g., transparency, temperature), chemical (e.g., pH, dissolved oxygen, contaminants), and biological (e.g., chlorophyll) properties that help meet the essential living requirements of aquatic life. Water pollution is caused by anthropogenic activities and natural calamities, which leads to social–ecological changes in the coastal systems. These hydrological disruptions in the coastal ecosystem have far-reaching and unanticipated repercussions, including loss of revenue, poverty, and the marginalization of SSF communities.
Many marine species rely on coastal water quality to survive, and it also supports a variety of key economic activities, such as tourism, coastal recreation, and fishing, as well as property values. Coastal waters are affected by a wide range of contaminants, both local and distant, as well as point, nonpoint, and airborne sources. Coastal waterways are polluted from all sides because of a variety of natural (i.e., cyclones, floods, and droughts) and manmade activities (i.e., aquaculture, sewage dumping, dam construction, plastic wastes). Fish is crucial for food security and nutrition, since it provides not just nutrition but also a variety of essential micronutrients that guard against hunger and diseases caused by malnutrition [
1]. SSFs employ over 90 percent of the 35 million people who are registered as fishers around the world [
2]. Water contamination is a complex issue that makes proper water management and a fair living standard challenging for SSF communities. The livelihood of SSF populations is threatened by factors such as nutrient enrichment, dangerous algal blooms, low oxygen, hypoxia, pollution, and sedimentation. Many individuals living around the shoreline rely on SSF to make a living [
3].
Water quality and small-scale fisheries are linked to several Sustainable Development Goals (SDGs) and contribute considerably to SDG 14 (Life Below Water). Several nations, financial institutions, environmental organizations, and scientific groups have vowed to assist in the achievement of the Sustainable Development Goals [
4]. SDG14b notably mentions SSFs, urging them to have secure access to resources and markets [
4,
5,
6]. The adoption of the Voluntary Guidelines for Securing Sustainable SSF in the Context of Food Security and Poverty Eradication (SSF-Guidelines) by the Food and Agriculture Organization (FAO) in 2014 marked a critical moment for SSFs due its focus on sustainable resource management in combination with the governance of tenure for sustainable fisheries [
7,
8,
9]. The SSF-Guidelines were distinct from other fisheries plans in that they were produced through a participatory, inclusive process and based on human rights [
9,
10,
11]. The objective of this paper is to examine the critical role of water quality for sustainable small-scale fisheries. Specifically, the focus is on linking the effects of water-quality deterioration from social–ecological changes to levels of vulnerability in the context of small-scale fishery systems. The aim of this paper is to give a general summary of the stressors resulting in vulnerabilities of SSF fishing communities in the Chilika Lagoon related to water quality.
Case Study and Methods
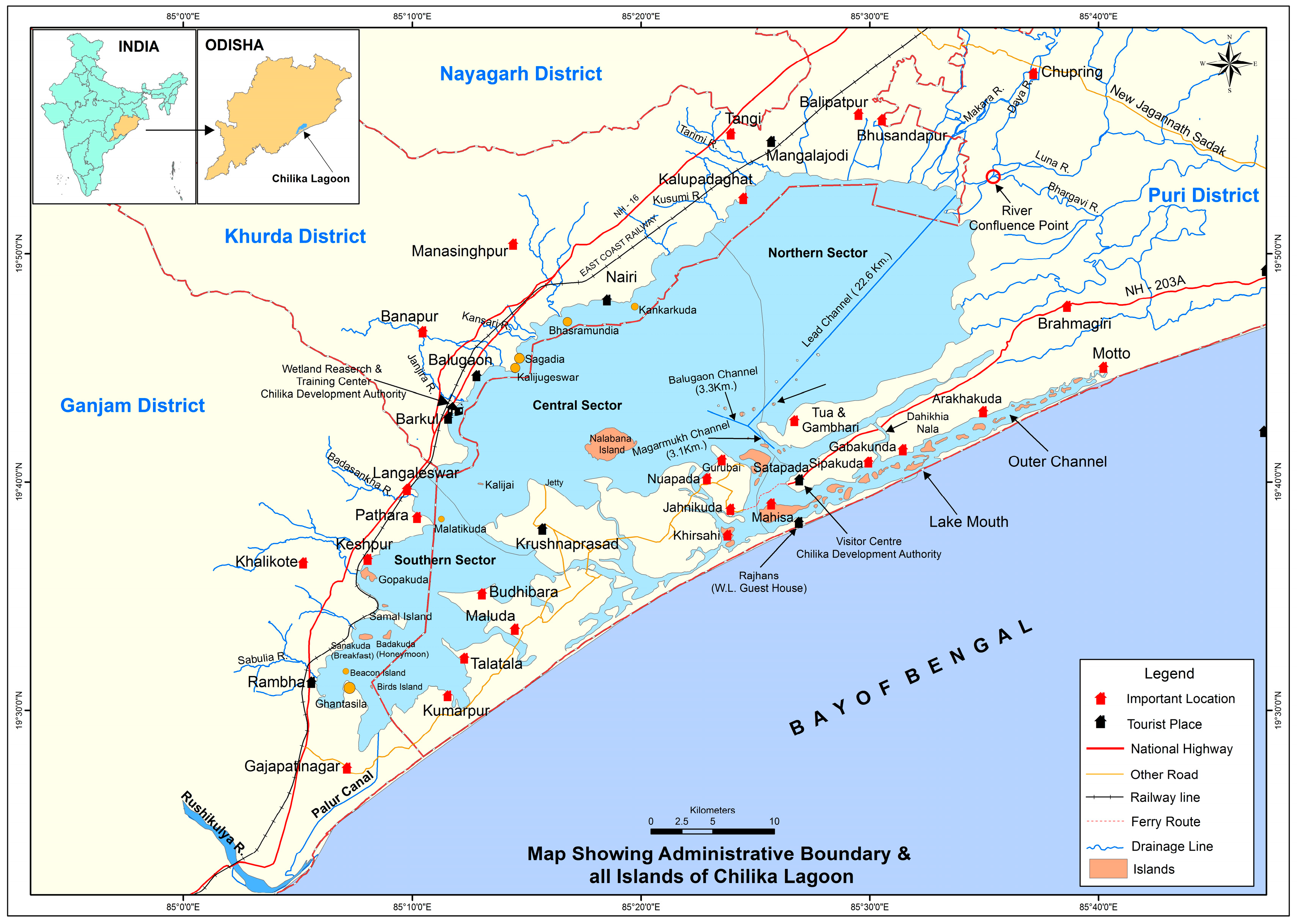
The case study location is the Chilika Lagoon on the east coast of the Bay of Bengal in India. The Chilika Lagoon is Asia’s largest brackish water lagoon. It is a major hub for biodiversity in terms of rare, fragile, endangered, and threatened species. Around 225 fish species, 710 plant species, and 800 different animal species live in the Chilika Lagoon habitat [
12]. The Chilika is known for being an estuarine-like shallow lagoon, which helps promote a highly productive ecosystem with significant livelihood opportunities [
13,
14]. Over 400,000 fishers who lived in and around the lagoon (in about 150 village units) once made their livings from the fertile fishing areas [
12]. The rich fisheries in the Chilika, which support the livelihoods of over 0.2 million fishers and produce close to 9% of the state of Odisha’s revenue from marine products, are built on a diverse and dynamic assemblage of fish, invertebrate, and crustacean species [
15,
16,
17]. The vast diversity of fish and other aquatic flora and fauna is maintained due to the hydrological connectivity of the Chilika with the Bay of Bengal, tributaries of the river Mahanadi, and a variety of streams of western catchments (
Figure 1) [
18]. About 86 percent of the fish species in the wetland are migratory and dependent on riverine and marine habitats for a portion of their life cycles [
17]. Due to rising silt loads from catchments and decreased connectivity with the sea, the Chilika had a period of rapid degradation between 1950 and 2000, such as the multiplication of invasive macrophytes, wetland shrinkage, and a significant drop in lagoon fisheries [
16].
The lagoon is very significant for the local community as a centre of cultural, religious, and spiritual activities, as well as a source of subsistence (mostly through its fisheries). The Chilika is constantly under stress from anthropogenic and natural factors, such siltation, infestation, and salinity degradation, which reduces production and biodiversity [
19]. The introduction of shrimp farming in 1980 increased pressure on the ecosystem of lagoons and ultimately had a severe negative impact on the way that lagoon fisheries were traditionally managed by local communities [
16,
20]. This caused a decline in household income and natural fish output, as well as environmental degradation and an increase in poverty [
16]. Local fishermen have been driven to obtain fish farther from their usual fishing areas due to urbanization, sea-mouth opening, tourism, and aquaculture. The livelihood and communities dependent on the fisheries are being neglected as a result of numerous social and ecological changes. The lagoon contains a variety of fresh, brackish, and salty water environments due to freshwater runoff from the drainage basin and saline water inputs from the ocean. This geographically and temporally diversified water environment supports an incredibly productive ecology [
21]. With the advent of several seasons, the lagoon’s water quality undergoes significant seasonal changes and shows various ecological traits in certain localised areas. According to its physiochemical and biological properties, the lagoon has been divided into four main sectors: the northern sector, central sector, southern sector, and outer channel [
22].
A systematic literature review was employed as a research strategy to comprehend the social and environmental changes associated with water quality and their effects on the livelihood and well-being of the SSF sector, to support the data obtained, and to respond to the research questions. The process of conducting a review involves five steps, including the development of research questions, using search strategies, determining inclusion and exclusion criteria, extracting and analysing data, and reporting findings. This systematic literature review follows a structured approach to address research questions related to the impact of water quality on vulnerability in small-scale fishery (SSF) communities, the responses of small-scale fishers to these vulnerabilities, and the adaptation strategies for making small-scale fisheries more viable.
A thorough search strategy was employed, utilising databases such as Google Scholar, ScienceDirect, JSTOR, and Scopus, to gather pertinent studies regarding small-scale fisheries (SSFs), water quality, and hydrological changes in the Chilika. Boolean AND/OR operators were used to combine search phrases for improved relevance. Key terms included “water quality,” “aquaculture,” “eutrophication,” and others. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram guided the selection process, resulting in 74 suitable articles (
Figure 2). Inclusion criteria covered studies on water quality’s impact on fisheries and SSF livelihoods in lagoon settings, published in academic journals, conference proceedings, and document reports. Publications not in English, news articles, dissertations, and online sources were excluded. Quality assessment, as a critical criterion, involved using a checklist to determine research calibre, including whether chosen publications were indexed in the ISI Scopus database.
Data extraction and synthesis were conducted to summarise and group the chosen studies, leading to findings and discussions on water quality changes, vulnerability issues, and research gaps in the Chilika Lagoon. Overall, this systematic review provides insights into the relationship between water quality and SSF vulnerability, contributing to a better understanding of the dynamics in coastal ecosystems and the potential for sustainable management strategies. Although all of the aforementioned processes are described in the order they may occur, the assessment process may involve numerous activities that are initially introduced and then improved upon in later stages. As a result, qualitative research on SSFs and SES variations in the Chilika, as well as the quantitative assessment of water-quality fluctuation, are conducted. The research gap observed in the study was corroborated by finding literature related to it.
2. Drivers and Dynamics Influencing Water Quality
The Chilika Lagoon is the largest brackish water lagoon in Asia and is located between latitude 19°28′–19°54′ N and longitude 85°06′–85°35′ E [
23,
24]. In accordance with the Ramsar Convention, it was recognised as a Wetland of International Importance in 1981 [
24]. It is a shallow body of water that is roughly 65 km in length and 20 km in breadth [
25]. During the summer and monsoon seasons, the water-spread area is predicted to be 704 km
2 and 1020 km
2, respectively [
24,
26]. During the rainy season, the salinity content of the Chilika Lagoon decreases as a result of floodwaters from 52 rivers and rivulets. When the south wind starts to blow during the dry season, the supply of flood water is cut off and saline water from the Bay of Bengal takes precedence [
27,
28]. The marine, fluvial, and terrestrial habitats all interact constantly with the Chilika Lagoon. Based on coastal processes, tidal rhythm, cyclones, and storms from the Bay of Bengal, the marine environment’s influence on the lagoon regulates the interchange of water and materials between the lagoon and the sea [
29].
Anthropogenic activities and natural disasters frequently produce impacts that interfere with the health of the lagoon ecosystem [
30]. Urban, industrial, and agricultural wastes are among the various inputs received by the Chilika Lagoon, which significantly alter the water quality [
30,
31]. Freshwater that has been contaminated with silt, chemical components, and domestic trash is poured into the fluvial environment. The lagoon has undergone considerable changes in its geomorphological features as a result of changes through manmade activity in the catchment basin and the frequent occurrence of cyclones and storms [
29]. The Chilika Lagoon has historically provided livelihood opportunities to more than 300,000 fishers.
The Chilika Lagoon’s protected, shallow waters are ideal for aquaculture, particularly for the lucrative tiger prawn (Penaeus monodon) [
32]. However, the quick growth of the global shrimp market and the rise in export prices throughout the 1980s made shrimp aquaculture a significant force behind the transformation in the lagoon. Conflicts over resources resulted from local elites encroaching on traditional fisheries grounds to utilise as aquaculture farms [
33,
34].
Other drivers emerged in 2001. To address the ongoing siltation issue in the lagoon, a hydrological intervention to establish an artificial sea mouth with the Bay of Bengal was undertaken. The effects of the sea mouth backfired, since they exacerbated the ecological problem by intensifying daily water inflow and outflow, which changed the saltwater and freshwater equilibrium. Due to the negative effects of the multiple drivers, prominent being the aquaculture and sea-mouth opening, two forces functioning in concert, the social–ecological system of the lagoon was put under stress [
33]. Other factors affecting the current stage of lagoon water quality are sedimentation, which results from riverine discharge and the breakdown of macrophytes, choking of the outer channel, shifting of the inlet mouth, a decline in water area, and an increase in the vegetated area, and the opening of new inlets both on the sea and river sides [
22].
The Chilika frequently suffers cyclone landfalls, and there is significant littoral drift, which can open or close tidal inlets. Strong drift also affects the morphology, and the cyclonic events frequently result in alterations to the shoreline and the formation of new inlets [
35,
36]. These catastrophic events also have an impact on the geomorphologic processes in the Chilika Lagoon, which regulate the water exchange between the lagoon and the nearby Bay of Bengal through tidal inlets [
37]. In the Chilika, significant environmental changes resulted in biodiversity loss, the introduction of novel multispecies, and changes to the water system, including salinity variations [
34,
38]. Some significant factors that affected the water quality of the Chilika Lagoon are shown in
Table 1. For instance, one of the significant modifications to the Mahanadi River system in 1957 was the building of the Hirakud Dam. The Chilika Lagoon receives water from the Hirakud Dam. In contrast to expectations, the dam greatly increased the amount of sediment flowing into the lagoon. High rates of sedimentation entered the lagoon as a result of this. Large-scale deforestation, excessive grazing, illegal felling, and excessive silting have also occurred in the western region [
39,
40].
Importantly, Orissa state had a Super Cyclone in 1999, the deadliest cyclone to strike the state in a century. The Super Cyclone affected 15,681,072 people and 14,586 villages, 9893 people died, and 1,661,683 homes were damaged when it raced through the whole Orissa coastline region [
41]. The storm severely damaged the fishing gear and homes in and around the lagoon. In terms of damage to important infrastructure (schools, hospitals, road networks), cyclones have both short- and long-term effects on fisheries livelihoods. They also reduce access to medical support and water use. Following the 1999 Super Cyclone, those losses were noticeable in the Chilika Lagoon, which left fishing communities with traumatising perceptions [
28]. Cyclone Phailin (12 October 2013) and Hud-Hud (12 October 2014) were two severe cyclones that made landfall in Chilika. Based on its power, wind speed, amount of precipitation, and its track of making landfall, each hurricane or cyclone has unique characteristics and causes impacts that vary in intensity [
42,
43]. Despite the variations in magnitude, there is a broad agreement in the literature regarding how hurricanes have an immediate influence on suspended sediment load, water clarity, and salinity in coastal waterbodies [
43]. The distribution of salinity, ammonia, and silicate all changed significantly after the cyclonic effects [
44]. According to post-Phailin assessments, salinity has significantly decreased across all sectors while there was silicate enrichment in Chilika waters [
45]. Taxonomic analyses also revealed the presence of freshwater phytoplankton species, and the situations were concerning as hazardous cyanobacterial freshwater planktonic forms were appearing in Chilika [
43]. Seagrass meadows disappeared, freshwater weeds grew, and benthic populations changed as a result of the overall drop in salinity and turbidity [
45,
46]. On 3 May 2019, the category 4 cyclonic storm Fani, extremely dangerous and with winds of 250 km/h, made landfall close to Puri on India’s east coast. A total of 64 people lost their lives and the coastal regions of Odisha suffered significant economic losses as a result of the unexpected cyclone [
35]. Fani had a significant influence on Chilika and nearby catchment areas through gusty winds, tidal surge, heavy rain, and floods. The powerful cyclonic storm Cyclone Fani caused four new mouths to open into the sea, upsetting the delicate balance of the lagoon’s environment. This resulted in a decrease in fish output due to the incursion of additional water flow. Following Cyclone Fani, there has been an increase in pigment diversity—chlorophyll-a and fucoxanthin levels. The sudden reduction in the salinity level along with excessive turbidity will be detrimental to the fish health as well. Phytoplankton and zooplankton populations of the lagoon are impacted by the significant change in nutrients, salinity, and turbidity from cyclonic effects [
35].
Floods frequently occur in the Chilika Lagoon area. Because of the rains in 2001, 2003, 2006, 2008, 2011, and 2014, abnormal floods were observed [
47]. In Orissa state, 76 flood occurrences were reported between 1834 and 2007, despite the state government’s successful completion of flood-control measures (such as the building of dams and weirs) [
41]. According to recent studies, both the frequency and the intensity of floods have gradually increased over the decades [
28,
41]. Since 2003, Odisha has regularly kept records of flood incidents. Fishers frequently experienced losses or damage to their equipment and boats during floods [
28]. Concern over droughts also extends to the livelihoods of the Chilika population. With the exception of the monsoon season, it is likely that the average monthly rainfall near the Chilika Lagoon is substantially lower [
41]. There have been six moderate and severe droughts in various sections of Odisha between 1995 and 2004 [
28,
41]. Despite being less susceptible to droughts than agrarian communities, fishing communities struggle to acquire enough water for domestic purposes (such as drinking, bathing, and cooking) and the water that is available is frequently of poor quality [
28]. The lack of water availability, pollution, and anthropogenic pressures threaten people’s health and interfere with every element of daily living [
28,
41]. Cyclones, droughts, and floods have a common place in Chilika, and they occur every year [
47].
3. Understanding Water-Quality Variability in Relation to Social–Ecological Changes
The lagoon ecology is threatened by siltation, industrial pollution, weed growth, depletion of bioresources, and variations in salinity. Water exchange between the lagoon and the sea, which regulates salinity, siltation, macrophyte infestation, and the recruitment of marine forms, had a significant impact on the biological changes in the lagoon system and its fisheries [
30]. The primary social–ecological modifications that caused concern were restricted seawater exchange between the lagoon and the sea, siltation of the lagoon basin and the outer channel, weed infestation, eutrophication, and decrease in fish and variation in aquatic species. Environmental issues had been made worse by pollution from domestic, industrial, agricultural, and aquaculture wastes, overfishing, poor watershed management that favoured excessive sediment inputs, land reclamation for human settlement, agriculture, and aquaculture, deforestation in the catchment area, unplanned tourism, and conflicts in fisheries [
30]. The lagoon’s ecology has drastically declined, as evidenced by the loss of biodiversity and the decline in the production of fish and shellfish [
30,
48]. Water-quality degradation has a severe impact on fish production. Pollution of water changes the physical and chemical characteristics of the aquatic environment and affects the quality of the water and fish. Additionally, as crustaceans are so susceptible to water pollution, it reduces the principal food source for those species, while excessive fishing pressure hinders the growth of fish populations, as all large-sized individuals disappear from the fishery. This will result in low catch for the fishing communities, and hence they suffer economic loss and poverty. Therefore, it was advised to prevent water-quality deterioration to maintain the water quality, reduce pollution, and sustain the aquatic environment [
48]. In order to comprehend the ecological characteristics of the Chilika Lagoon, this paper looked at the variability of water-quality parameters (temperature, pH, transparency, turbidity, water depth, salinity, alkalinity, BOD, dissolved oxygen (DO), chlorophyll-a, and nutrients such as nitrites (NO
2−), nitrates (NO
3−), phosphorous, and silicates) during the years 1950 to 2015, as data from this period were available in the literature. Monitoring the physiochemical and biological features aids in determining whether the water complies with legal requirements and is secure for use by humans and the environment. It is important to note that, given the lack of comprehensive and up-to-date open data sources, this study relies on data extracted from published literature and research studies available within the specified time frame.
3.1. Variability in Physical Parameters
Physical parameters fluctuate with the quantity of heat, amount of dissolved material present in the water, and ambient pressure. Temperature and salinity variations through time and space, in addition to affecting physical attributes, are significant water-mass tracers that can be utilised to visualise lagoon water circulation. Water depth, temperature, transparency, turbidity, and salinity are the physical parameters covered in this section. The measurements of the physical parameters of lagoon waters provide a baseline data on water characteristics, particularly mixing and natural turbidity levels.
3.1.1. Water Depth, Turbidity, and Transparency
During the summer, the amount of tidal influx controls the lagoon’s depth; during the monsoon, freshwater inflow controls it. The water depth varied from 0.8 to 2.5 m during the monsoon season, whereas it was between 0.4 and 2.5 m and 0.365 and 2.5 m, respectively, during the postmonsoon and summer [
31,
49]. In coastal waters, the presence of suspended particles is a key factor in controlling light penetration. It is crucial to examine the water purity in inland and coastal water bodies to determine their water quality. Salinity, nutritional content, biological oxygen demand, pH, and chl-a are all connected with transparency in a beneficial way [
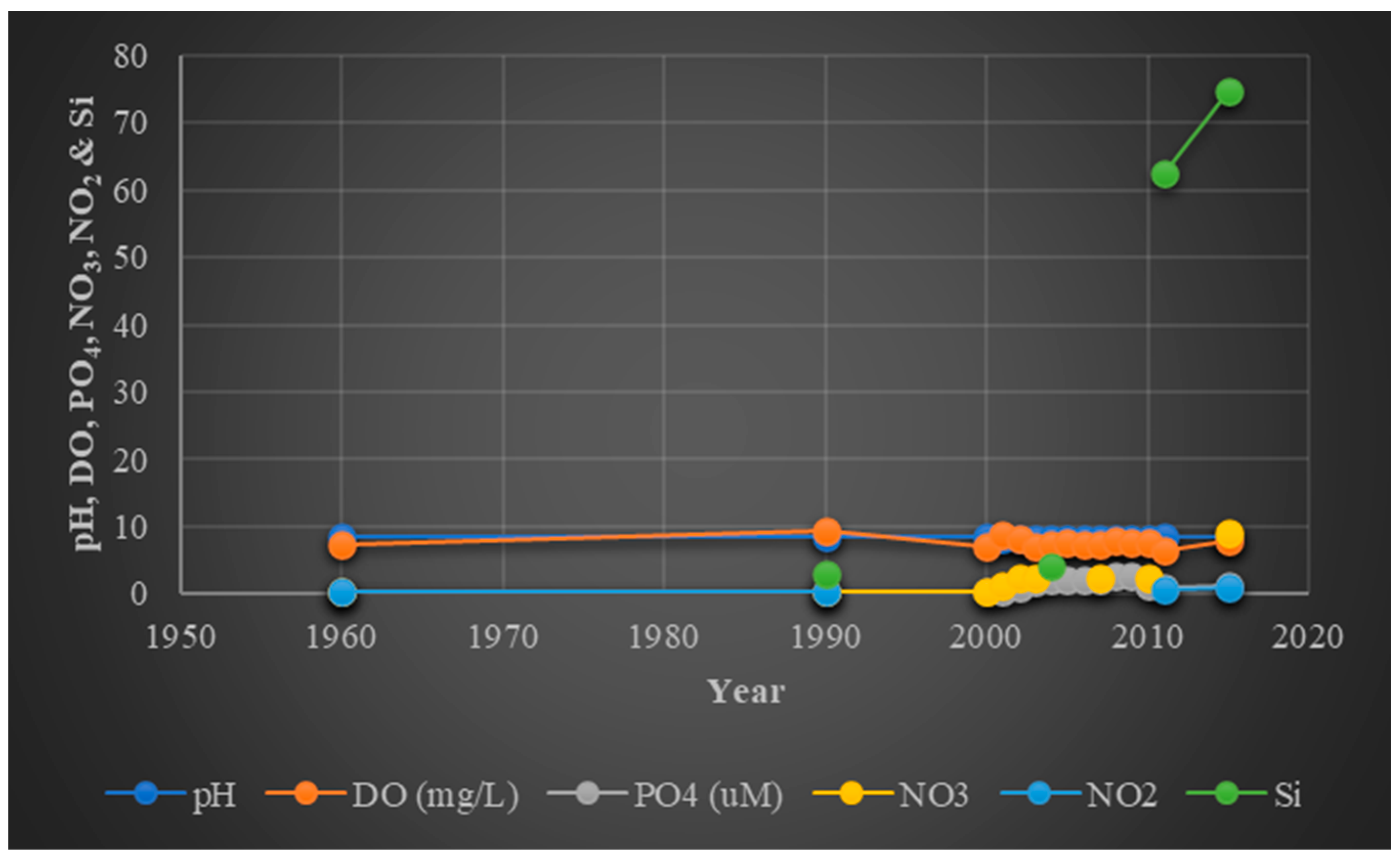
49]. These show that the lagoon’s highly turbid waters continue to contain a significant amount of these substances. The transparency decreased significantly by 25% during Cyclone Phailin in 2013. This was brought on by the high sediment load in the lagoon, which led to an increase in turbidity of 32–61 NTU (
Figure 3). Due to the integrity of the lagoon, which has been preserved to this day, transparency was then restored again within 4 months [
45].
In the lagoon, heavily suspended particulate matter (SPM) input and settling can significantly alter the water level [
52]. The lack of a substantial change in the water level between 1990 and 2015, when looking at the mean value for the entire lagoon, can be attributed to the effective outflux of suspended matter (sediment flow) from the river to the sea through the dredged channel [
52,
53]. Issues arising from aquaculture recirculation issues may be fish excrement, uneaten feed pellets, sewage-waste particles, or plankton. These particles will cause turbidity, which will prevent light from passing through, decrease photosynthesis, even affect fish production, and annihilate protective colonies of microbes and other species.
3.1.2. Salinity Variation
Salinity is a measure of how many dissolved chemicals are still present after all organic matter has completely oxidised. Salinity is a very important factor that controls the Chilika Lagoon’s biogeochemical features [
46,
49,
53]. The majority of the lagoon’s biodiversity is located along a salinity gradient, and changes in species variety occur due to the salinity changes throughout the year [
52,
53]. Salinity is an important factor that affects the Chilika’s metabolism and biological productivity, which in turn supports a broad variety of species. The development, feeding habits, spawning, production, and survival of many fish and shrimp species are significantly impacted by seasonal variations in salt. Variations in the salinity regime play a significant role in the presence and absence of phytoplankton, as well as the regulation of bodily fluid levels in migratory birds, which are sensitive to changes in their environment [
54]. With the oldest data available, a high salinity trend was seen in the lagoon’s water quality in the 1960s. The salinity of the lagoon’s near-freshwater levels steadily decreased between 1995 and 1998, but it was fully restored to normal by the hydrological intervention in 2001 [
51,
52]. Comparing the postrestoration era to the artificial-sea-mouth opening, the tidal flow increased by 44% and the salinity of the lagoon by 35%. Average lagoon salinity between 2001 and 2012 ranged from 11 to 14 ppt, and it was shown to be greater during dry circumstances (
Figure 3). Due to the massive freshwater discharge and significant precipitation that occurred during Cyclone Phailin in 2013 (11.12 ppt; 2012–2013 > 8.75 ppt; 2013–14), there was a sharp drop in salinity. The rainfall in the Phailin month was 2.5 times more than in the pre-Phailin month and made up around 45% of the total precipitation in 2013; this caused frequent flooding into the lagoon [
45].
The salinity ranged from 0 to 37 ppt with an average of 11.09 ppt from 1990 to 2015 (
Figure 3), and the trend indicated that the overall salinity stayed relatively constant [
53]. Changes in salinity can impact fish behaviour in a number of ways. Intertidal biodiversity is impacted by evaporation and dilution caused by salinity, which controls the metabolism of living things. Fisheries in general and faunal diversity are being improved in large part due to salinity-level dynamics. The dynamics of salinity levels improved after reclamation. The establishment of an artificial sea mouth in 2001 had an overall beneficial effect on aquatic diversity, fish migration across the sea, and ecosystem restoration [
50,
51,
55]. The lagoon receives a sufficient amount of freshwater along with clean mixing during the monsoon season. While low salinity readings are observed in almost all lagoon locations during the majority of the winter, due to the high rate of evaporation, decreased inflow of freshwater, and influences from the tidal movement of the sea, there is a progressive increase in salinity over the summer [
45]. The hydrological intervention led to resource conflicts, overfishing, and vulnerability to fishing communities’ livelihoods. The intervention had many negative consequences, but there was an improvement in the circulation of young fish, prawns, and crabs [
30].
3.1.3. Water Temperature (WT)
The temperatures of coastal waters alter with latitude and longitude due to diurnal and seasonal fluctuations. Variations in temperature have a significant impact on shallow coastal waters. The Chilika’s freshwater ecology does not show a significant range of vertical and horizontal temperature change. Seasonally low surface temperatures prevail in the winter, with the average lagoon temperature typically remaining within a range of 28.1–29.2 °C [
54]. Temperature changes have a substantial impact on the microbial activity and mineralisation process that turns organic matter into nutrients [
56]. Despite the fact that different species of phytoplankton have similar rates of photosynthesis and growth in relation to temperature, the growth rate has been found to be lower over a range of light and temperature conditions [
52,
53]. The hydrodynamics and circulation pattern of coastal waters are directly influenced by weather variables, such as precipitation, temperature, humidity, and wind speed. In the Chilika Lagoon, a warm, subhumid, tropical monsoon climate is usual. From March to May, the temperature rises with seasonal changes before beginning to drop as the southwest monsoon arrives [
31]. In similar temporal contexts with intense solar radiation and winter cooling of surface waters, a common trend of temperature variability was discovered. There is significant seasonal variability in the trend. After Phailin, a startling drop in water temperature was seen. The drop might be caused by a combination of river water and precipitation with low temperatures. The temperature of the water and dissolved oxygen are also found to be inversely related. This might be explained by the lower solubility of oxygen in warm waters [
45]. A significant seasonal variation can be traced to the cooling of surface waters during the winter and the high solar radiation that warms the surface water during the summer [
52]. WT is connected with turbidity, which may be caused by the suspended particles’ ability to absorb significant amounts of solar radiation heat [
53].
For maintaining the wellbeing of fish and other aquatic species, the temperature of coastal waters is crucial. Along with disrupting the operations of the food web, temperature can have an impact on fish development, behaviour, and reproduction. Minor temperature changes have an impact on the metabolism of aquatic organisms and their movement patterns. Increased temperatures encourage the spread of invasive species, which can put aquatic animals at risk. Rising temperatures and decreased dissolved oxygen cause a regular stratification of the water column, which has an impact on mixing and circulation. It is sometimes assumed that diminishing seagrass is primarily caused by warming temperatures. WT has an inverse relationship with DO and a direct relationship with the salinity level. WT causes algal blooms, which in turn boosts the ability to absorb nutrients. Nutritional factors indirectly affect water clarity by encouraging phytoplankton to produce organic materials and decreasing solubility. Fisheries are impacted by a low nutrient uptake and lower DO because these two factors together stress aquatic life and have an impact on a variety of other biochemical and aesthetic water indicators (such as transparency and clarity).
3.2. Variability in Chemical Parameters
The chemical properties of lagoon waters are adversely affected by anthropogenic activities, such as the use of pesticides and fertilisers in agriculture and aquaculture. Chemical characteristics such as nutrients must therefore be measured over an extended period of time in order to create a baseline dataset for sustainable coastal conservation and management. Chemical characteristics involve parameters such as pH, alkalinity, biological oxygen demand (BOD), dissolved oxygen (DO), and nutrients such as nitrites (NO2−), nitrates (NO3−), phosphorus, and silicates (Si).
3.2.1. pH, Alkalinity, and Buffering Activity
The pH of any aquatic ecosystem regulates the biogeochemistry of carbon and nitrogen, which in turn affects biodiversity [
52,
57]. The production, respiration, and mixing of fresh and saline water with various pH conditions are the main governing factors for the pH in the Chilika [
52,
58]. Seasonal pH variations may result from phytoplankton and macrophyte carbon dioxide (CO
2) uptake, CO
2 release from respiration, and the mixing of lagoon water with various external freshwater sources [
52,
53,
57]. Overall, changes in pH values are resistant in coastal waters. The pH of pure waters is significantly impacted by the amount of CO
2 levels that alter the growth of vegetation. This is explained by the alkalinity of saltwater, which provides stronger protection against excessive CO
2 buildup. Higher alkalinity results in a greater pH-buffering capacity. Fish production depends on the carbonate-buffering process, since photosynthesis is the primary natural supply of oxygen. In coastal waters, the hydrogen-ion-concentration spectrum rises due to the influence of free CO
2 removal from the atmosphere during photosynthesis through saltwater–freshwater flow rates, water temperature, organic-matter breakdown, and salinity drop [
54].
The pH is a crucial environmental factor that affects aquatic animals’ ability to survive, as well as their physiology, metabolism, and chemical processes. In a coastal environment, pH regulates the life cycle and distribution of nutrients. Additionally, it preserves the carbonate- and bicarbonate-buffering system, which is crucial to the survival and growth of aquatic plants. The lagoon is mildly alkaline, and there are no discernible temporal variations from 1990 to 2015. When compared to the data from the 1960s, the alkalinity in 2015 appeared to be extremely low. After Phailin (October 2013 onwards), a pH drop may have occurred as a result of increased community respiration overproduction or the mixing of new water with a lower pH due to cyclone-induced precipitation and discharge [
46,
52,
57]. After Phailin, the pH declined from 8.48 (pre-Phailin period from July 2011 to September 2013) to 7.98 without being regained (post-Phailin span from October 2013 to June 2015) (
Figure 3). The ongoing decline may result from an improved respiration cycle over the predominance of freshwater intake into the lagoon in successive monsoonal cycles with a lower pH [
45]. There was a decrease in total alkalinity following the cyclone, reflecting the fact that the freshwater inflows from river discharge and the seawater from the mouth are what regulate changes in total alkalinity in the Chilika Lagoon [
53].
3.2.2. Dissolved Oxygen (DO) and Biological Oxygen Demand (BOD)
An essential indicator of an aquatic ecosystem’s health is the amount of dissolved oxygen (DO) [
53]. The DO content of typical coastal waters varies significantly both globally and seasonally. The fluctuations are a result of photosynthetic activity, bacterial nitrification, and free exchange with the environment through mixing, circulation, and wind action. Fish cannot survive below 4–5 ppm; hence, variations in DO have an impact on aquatic life. Variations in DO affect the lagoon’s capacity to absorb organic materials without negative consequences. Because of its huge area, robust photosynthetic activity, and the churning effect of winds on the coastal waters, the Chilika is often adequately oxygenated throughout the year. The near-neutral water quality and balanced productivity, as well as physical factors such as wind movement that permit mixing of oxygen with water, are what sustain the proper oxygen conditions in the Chilika [
59]. The macrophytes and seagrass may contribute more to the Chilika’s increased oxygen content than phytoplankton [
53]. The Chilika keeps its DO concentration between 6 and 8 ppm [
60]. The likelihood of an alarming reduction in the DO concentration during the night hours depends on the microbial community and their abundance in the specific region [
52]. A higher concentration of DO during the day depends on the photosynthesis [
53]. An abrupt increase in DO was caused by Cyclone Phailin and resulted in a sustained spike of 6.9–7.4 mgL
−1 in the coastal ecosystem (
Figure 4). This increase in DO may be caused by wind-induced aeration brought on by low temperatures and enhanced vertical mixing along with photosynthesis.
The biochemical oxygen demand (BOD) measures the amount of organic matter in a given ecosystem [
52]. The oxygen shortage caused by the biodegradation of organic compounds in the water body provides information on the level of pollution [
53]. A high BOD level may be caused by the decomposition of macrophytes and weeds due to increased salinity, the mixing of decomposed organic waste, increased wind movement, and the churning of sediments. After Phailin, there was a decline in the BOD that has persisted ever since. The decrease may be brought on by strong freshwater drainage discharging organic materials [
45]. The average BOD was around 2.66 mgL
−1 [
53]. A low BOD presumably exists when the lagoon receives less organic effluent. Low levels of DO are caused by the rapid breakdown of plant debris during the summer. Increased water temperature, shallow water depth, and high BOD levels are all factors in rapid decomposition. Lagoon transparency was reduced and the oxygen intake for organic-matter decomposition increased due to the turbid water inflow from the river and land runoff. The accessible plankton population and submerged macrophytes may have contributed to the high oxygen levels and increased rate of photosynthesis [
45]. Fish deaths are caused by low amounts of DO because aquatic species need oxygen to survive, similar to humans. The amount of oxygen readily available affects the rate of feeding, degree of movement, and water temperature.
The amount of oxygen that a body of water can absorb increases with temperature, salinity, and height. Monitoring oxygen demand guarantees that the marine environment’s water is protected, which can be used as a tool to assess the health of the ecosystem. The rate of photosynthesis, surface-water diffusion, water turbulence, and tidal action all significantly affect the amount of DO. Reduced DO has a negative effect on aerobic biota, particularly on benthic organisms. In lagoons with high flushing rates, saltwater intrusion causes the stratification to break down, allowing the water column to converge. The increased temperature increases the likelihood and severity of hypoxic situations in constrained lagoons with poor flushing rates and large fertiliser inputs. This causes a steady change in the distribution of biodiversity and a loss of species. The Chilika Lagoon is well saturated in terms of DO; hence, the oxygen needed for nitrification is also seen to be insignificant when compared to the oxygen needed for bacterial respiration [
52,
57]. DO and chlorophyll-a maintained a positive correlation during the summer, which may indicate that the lagoon’s primary productivity plays a significant role in regulating oxygen distribution, as it does in other tropical estuary ecosystems [
53].
3.2.3. Nutrient Disparity and Trace Elements
In the marine ecosystem, nutrients are one of the most important factors that influence how living things grow, reproduce, and carry out their metabolic processes. The distribution of nutrients is primarily influenced by coastal patterns, seasonal changes, and freshwater input from rivers and surface streams [
45]. It is generally known that some nutrients, such as nitrate–nitrogen and phosphate–phosphorus, have a limiting role in the development of algal cells in marine environments. Due to interactions between freshwater movement and the sea, the Chilika Lagoon is a rich source of nutrients and is significantly impacted by water features. In the Chilika Lagoon, the concentrations of nitrate and phosphate typically range from 0.036 to 1.96 ppm and 0.2 to 4.66 ppm, respectively (
Figure 4) [
52]. Point-source inputs to the catchment areas of estuarine systems in urban areas with high nutrient loads also contribute a sizeable portion of the nutrient burden. In shallow habitats such as the Chilika Lagoon, bottom sediments can also act as a secondary source of nutrients for the water column. Surface runoff and river discharge into the lagoon during the monsoon season, inorganic nitrogen fertiliser use, NOx emissions from fossil-fuel combustion, and nitrogen fixation in agricultural systems may also contribute as sources of nitrogen [
52,
53]. The amount of nitrate present in the lagoon indicates that the coastal mechanism is advantageously active and shows high values during the postmonsoon season.
For the growth of autotrophic phytoplankton, algae, and macrophytes, phosphate (PO
4) is a crucial inorganic nutrient. A lack of PO
4 can hinder the development of the plankton population, while an abundance of it can cause the water body to become eutrophic. Along with riverine input, other factors that may have a substantial impact on the Chilika’s concentration level include the release of phosphate from sediments as a result of wind-induced water churning and the release of PO
4 from SPM in more saline conditions. The amount of phosphorus (P) that enters the coastal lagoon depends on the type of rocks present and how severely they are weathered. The amount of P available to the algae in the aquatic ecosystem is, however, governed by the availability of other elements [
51,
53]. Monsoon season is when phosphorus concentrations are highest. Heavy precipitation and terrestrial runoff may be to blame for the high P concentration. Phosphates play a vital function as an inorganic nutrient for macrophyte and phytoplankton growth when they are liberated from sediments by wind-churning water.
The health of aquatic ecosystems depends heavily on elements found in minute concentrations in seawater, sometimes referred to as trace elements, such as silica (Si). For the generation of silica frustules, the diatom growth rate is dependent on the silica concentration. There are few details about the Si concentration in the Chilika. The Chilika Lagoon has a silicate level that ranges from 0.5 to 10.2 ppm (
Figure 4) [
45]. Premonsoon silicate concentrations were lowest in the southern sector and peaked in the northern sector during the postmonsoon season [
45,
54,
61]. The silicate concentration varied greatly in space between 0.1 and 258 μM, which may have been caused by sedimentary-particle adsorption, the coprecipitation of soluble silicon and iron, chemical reactions with clay minerals, or phytoplankton silicate absorption for metabolism. Rivers’ weathered silicate content is carried to the lagoon, where it results in a high silicate content. During Phailin, a 69% spike in silicate was seen. Silicate concentration was balanced with saline water after two months, returning to normal levels [
45]. The biogeochemical cycle and seasonal variations regulate the nutrient stoichiometry in coastal lagoons. Plankton population and variety vary as a result of this interaction. High nutrient concentration is caused by runoff from large rivers and agricultural drainage canals that are connected to the lagoon. Data indicate a positive association with salinity during summer, which is probably caused by microbial-organic-matter breakdown [
31,
45]. The influx of freshwater containing silicate from northeast rivers may be the cause of the high silicate in the northern sector. The consumption of silicates by phytoplankton, particularly diatoms and silicoflagellates, for their biological activity may be the cause of the low silicate concentration [
52,
53].
The dispersion of contaminants from runoff produced by river networks may be the cause of higher concentrations of nutrients. From agricultural land, soil, farm fertilisers, and pesticides used for agriculture were washed into lagoon waters [
62]. The large-scale spatial–temporal variability of nitrate and phosphate was produced in the coastal environment as a result of the rapid phytoplankton assimilation and the augmentation of surface runoff. The mineralisation cycle results in a larger nutrient buildup in riverine discharge zones because it releases nutrients to the environment because of higher residence times. Salinity and nutrient buildup show a negative link. The minimal nitrogen removal by fixation or denitrification may be caused by the reported productivity drop following Phailin. Additionally, the oxygen level has been kept steady. Following the Phailin, there was an abrupt drop in the PO
4 concentration. Dilution effects from mixing river water or consumption of low-saline suspended-particle matter can be the reason for this drop. A month after Phailin, the concentration drop was reversed. Overall fluctuations in the nutrient concentration could be brought on by sediment absorption, seawater exchange, and water-mass balance [
45]. Extreme algal blooms that deplete oxygen pose a serious hazard to the aquatic ecosystem, including hypoxia, habitat loss, and the depletion of natural resources. Along with natural nutrient spikes from coastal ocean upwelling, land- and ocean-based sources, urban wastewater discharge, and agricultural runoff, impacts from aquaculture on the coastal ecosystem through uneaten feed and fish wastes are significant. The total effects of the fish and water-quality decline have a severe impact on SSF livelihoods and raise poverty rates.
3.3. Variability in Biological Parameters
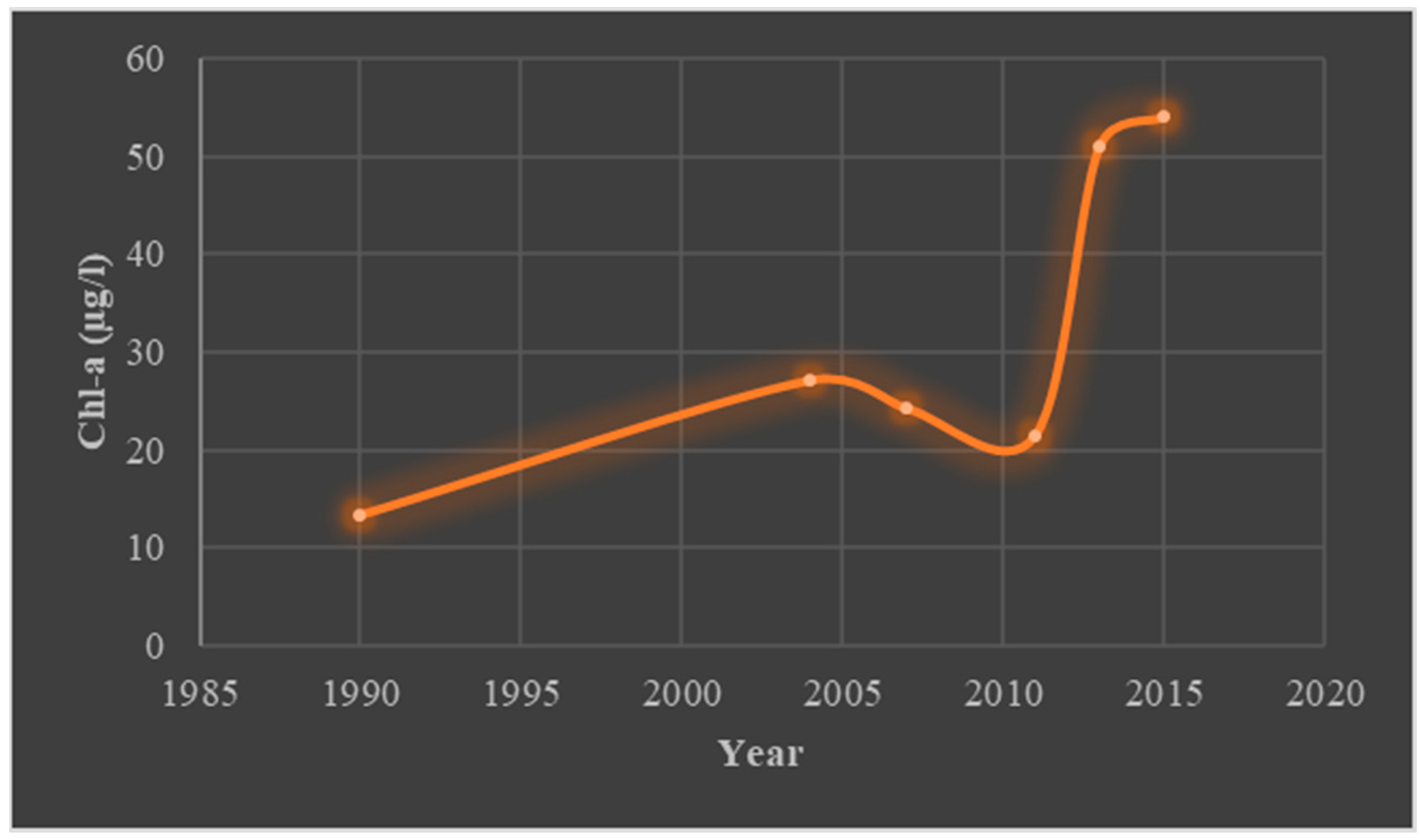
Green algae, blue–green algae, diatoms, and dinoflagellates make up the four major groups of algae in the Chilika Lagoon. The northern–central sections were overrun with green and blue–green algae, while the outer channel was dominated by diatoms. The most important component of the coastal lagoon is chl-a. Chl-a encourages the growth of phytoplankton and is a reliable indicator of the presence of algae in the marine ecosystem. Chl-a typically fluctuated in the Chilika between 0.13 and 51.10 gL
−1 (
Figure 5). In 2001, the artificial-sea-mouth opening resulted in a high concentration of 54.04 gL
−1 [
25,
62]. The availability of macronutrients, variability of salinity, dissolved oxygen (DO), water-column transparency, suspended matter, river discharge, and seawater influx all have a significant impact on the concentration of chlorophyll-a in the lagoon [
63]. Following Phailin, through wind-mediated churning, a sharp increase in chlorophyll content was noticed due to the contribution of benthic chlorophyll [
45]. An increase in nutrients carried by the runoff encouraged phytoplankton development. In general, nitrogenous nutrients regulated phytoplankton development. Tropical cyclones increase the amount of nutrients in the water column, which is likely to encourage the growth of phytoplankton. However, simultaneous high-suspended-sediment intake through mixing and runoff results in less light reaching the lagoon, which slows the growth of phytoplankton [
63]. Higher suspended particles block light from entering, changing the photosynthetic process. Algal blooms cause DO levels to drop. The concentration of chl-a will increase as the bloom gets larger. Lagoon waters become murky due to the bloom, which reduces clarity and light penetration. Another important factor that controls the growth and distribution of phytoplankton is salinity. Some lagoon areas showed a direct correlation between chl-a and DO while displaying an antagonistic correlation with salinity, turbidity, and depth. This shows that the shallow lagoon sections encouraged photosynthesis at an appropriate light intensity, producing oxygen [
64,
65]. The seasonal distribution of chl-a in the lagoon is significantly influenced by the interaction between the sea and the river, which has an impact on the physicochemical cycles [
63].
Chlorophyll concentration can be used as a gauge of the phytoplankton biomass that has an impact on plant growth due to differences in water quality. The changes in chl-a concentrations are connected to seasonal swings. Chl-a content aids in investigating the pace of algal blooms and their effects on fish populations. Fish habitats are preserved and fisheries are protected from vulnerability by identifying safe fish populations. The functioning of the shoreline is hampered by the addition of nitrogen and phosphorus to coastal waters. The nutrient introduction came from sewage intrusion, waste dumping from the industrial and domestic sectors, macrophyte litter, water exchanges between the lagoon and the sea, and various inputs from anthropogenic sources. The introduction was caused by agricultural runoff containing fertilisers and pesticides containing ammonia and urea. The water quality was affected by changes in the nutrient content, stoichiometric fluctuations, and benthic chlorophyll mixing from bottom-sediment churning, which put a strain on fisheries and the organisms that depended on them [
63,
64].
The impacts of various drivers associated with water quality on fisheries are listed in
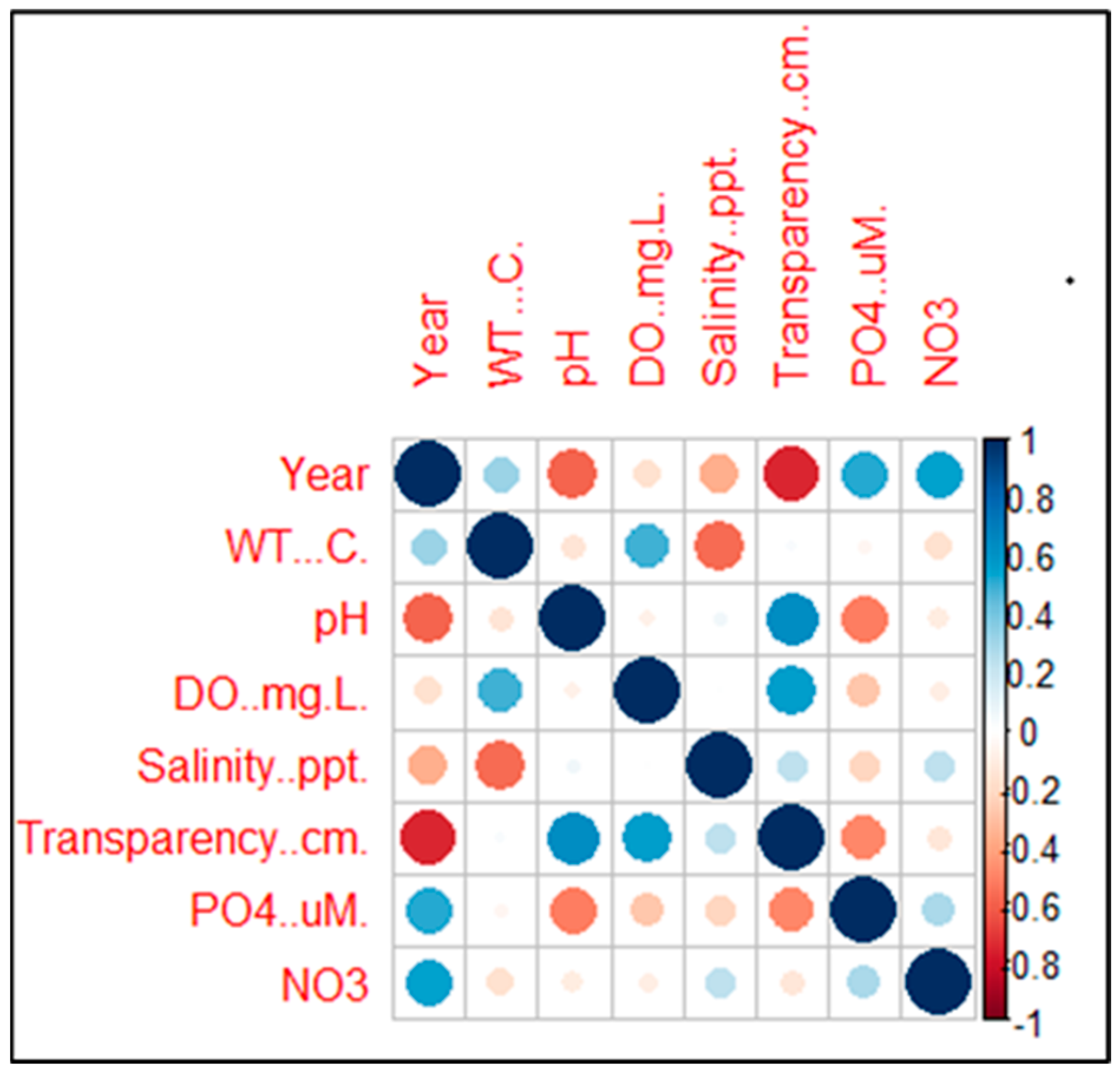
Table 2, which explores why water-quality deterioration is a clear threat to the livelihoods of SSFs. Varying species of fish have evolved to different circumstances of water quality, making them crucial components of aquatic ecosystems and indicators of water quality. Fish are extremely sensitive to a wide range of water-quality factors, such as pH, turbidity, temperature, and dissolved-oxygen concentrations. They are also a member of the food chain. The correlation of water-quality parameters in the Chilika Lagoon from 1950 to 2015 is indicated in
Figure 6. The deterioration of the lagoon water environment and the services it offers is a major contributor to the fall in fish productivity. Addressing the root causes of the depletion emphasises the necessity of comprehensive SSF management measures. Integrating multiple environmental effects and repercussions of vulnerabilities in SSF communities is a key goal of the study.
The variability of water-quality parameters resulting from social–ecological changes have significant effects on the overall ecosystem and fisheries. Sedimentation, caused by factors such as shrinking lagoon volume and sediment coring, leads to decreased water clarity and reduced biological productivity. Artificial-sea-mouth construction disrupts salinity and pH levels, affecting fishers and leading to a decline in fish production. Aquaculture contributes to nutrient and waste pollution, eutrophication, and habitat destruction, negatively impacting natural fish production. Dam construction, tourism activities, cyclones, and floods/droughts also cause changes in water parameters, habitat damage, and conflicts/displacement of fishers. These cumulative impacts have far-reaching consequences, including the displacement of traditional-water users, depletion of groundwater, and compromised food safety and security. It is crucial to address these issues comprehensively to protect coastal ecosystems and sustain livelihoods.
4. Key Vulnerabilities from Water-Quality Issues
Understanding fishing communities’ existing circumstances and pinpointing their needs is essential to determining what makes SSF communities vulnerable, crafting workable legislative solutions to address that vulnerability, and discovering chances to increase viability [
66]. It is believed that vulnerability is a multifaceted, complicated, extremely dynamic, and relative term whose research is highly inter- and transdisciplinary [
67]. Someone or something (e.g., small-scale fisheries are more vulnerable than large-scale fisheries) may be considered vulnerable [
6,
67]. SSFs are at risk because of a wide range of complex environmental, economic, and political stressors and changes [
68]. SSF communities are particularly sensitive to local and global change processes because of their heavy reliance on natural resources and close linkages to the coastal environment [
2]. The vulnerabilities that fishing communities in the Chilika Lagoon confront are outlined in
Figure 7, which links the causes and effects on the water quality.
Inherent issues in the socioeconomic conditions and political environments in fisheries, as well as natural and anthropogenic impacts, increase SSF vulnerability and restrict their ability to sustain viable livelihoods [
69].
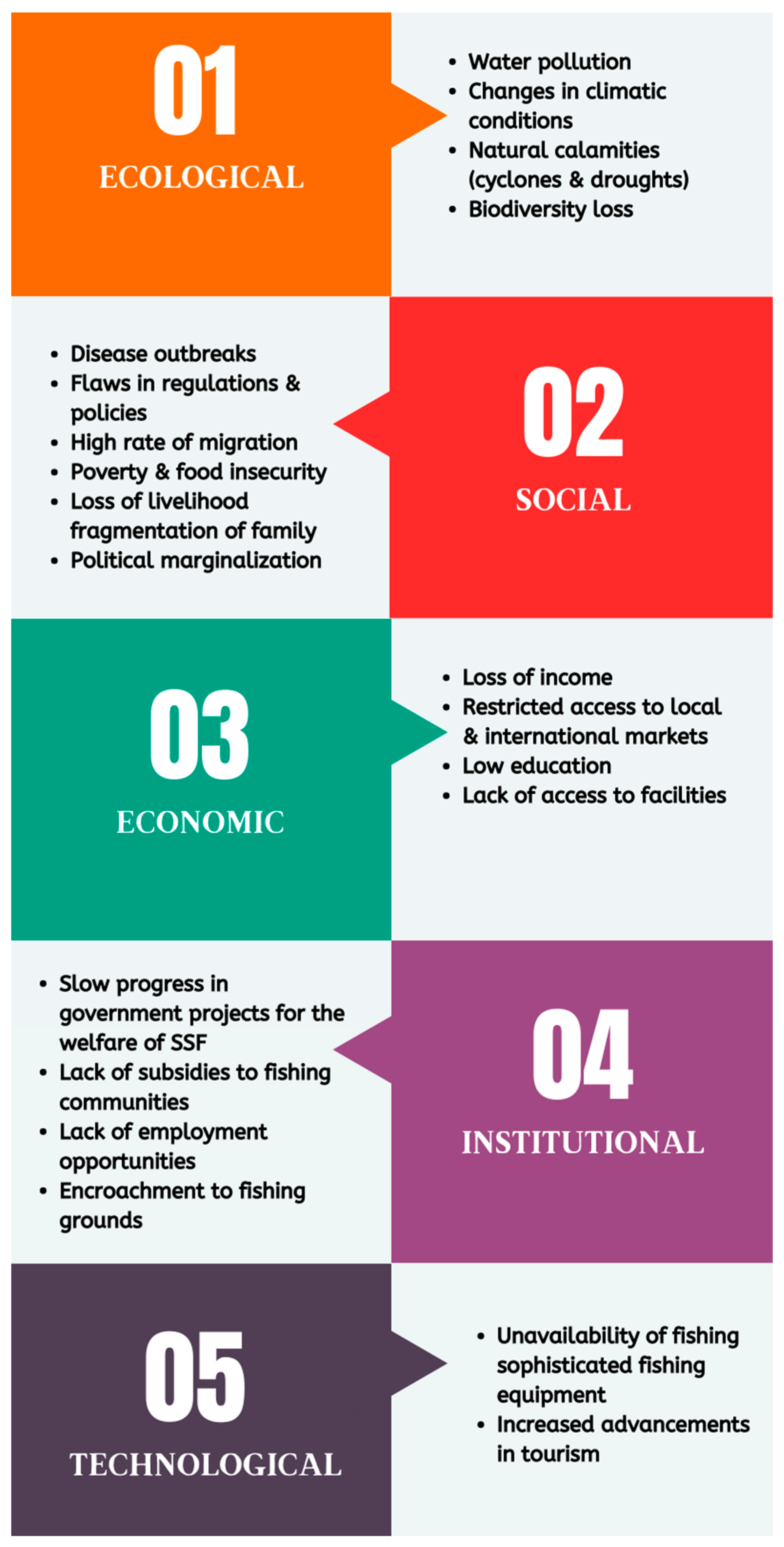
Figure 7 shows the major domain categories in the Chilika’s vulnerability into five groups: ecological, social, economic, institutional, and technical.
Ecological Vulnerability: This pertains to the ecological domain and natural resources within the lagoon, such as water, the status of biodiversity, natural drivers, and various climate-change factors. The changes in water-quality parameters in the Chilika Lagoon are the result of hydrological interventions, biodiversity loss due to anthropogenic activities and natural calamities, and erratic rainfall patterns and sedimentation.
Social Vulnerability: Different fishery-dependent factors are used to analyse social vulnerability: the unemployment rate, poverty, job opportunities for women, food, and nutritional security [
40,
70]. Communities that rely heavily on fishing are more likely to be socially vulnerable than other coastal communities because of their close dependency on fish stock. These findings highlight the importance of continuing to investigate climate change and social vulnerability to develop adaptation strategies [
71].
Economic Vulnerability: Savings, income, credits, and loans are all part of the economic domain. Natural disasters and anthropogenic activities have caused a significant increase in the amount of damage to SSF communities [
40,
66]. Families in communities near to the water bodies are at high risk of losing their homes and lives due to the unexpected natural drivers of change, such as cyclones. Among the economic vulnerabilities of fishing communities identified were low revenue due to fewer fish, restricted access to local and international markets, personal safety concerns due to unemployment or more frequent hazardous natural calamities, and poverty leading to less education and nutritional insecurity.
Institutional Vulnerability: The institutional domain of vulnerability refers to the role of community-based laws and governmental regulations influencing access to natural or financial resources. Rich businesspeople from outside the lagoon-built shrimp farming in the Chilika led to the displacement of local fishing villages from their resource base [
40,
72]. Issues of access and entitlements have arisen because of developments concerning fishing-area encroachment and lease [
12,
72]. Improper public policies, disputes in access rights to fishery resources, ineffective stakeholder engagement, and lack of management and planning created institutional vulnerabilities. These put the livelihoods of traditional fishing communities in jeopardy, creating instability in the sector and harming the fragile Chilika ecosystem [
12].
Technological Vulnerability: The technological domain of vulnerability refers to the major equipment and practices required to expand fishing activity, such as boats, gears, and infrastructure. For example, sophisticated equipment against the invasion of barnacles is fundamental to fishers in the Chilika. Lack of such equipment led to excessive loan-taking by the community, which intensified poverty [
40,
73]. At the same time, while tourism proves lucrative for some groups, the improper technology that handles waste further harms fishing communities. Local fishing communities are aware of the danger that tourism operations pose to dolphins, as well as ecological disturbance and mortality [
40]. Poor sanitation technology is harming the aquatic life, which harms fishers’ livelihoods.
SSF communities in the Chilika are at risk from polluted water in terms of hygiene, since they are compelled to live substandard lives and eat subpar food because of their poor incomes. Fishermen had to discontinue their livelihood activities for weeks at a time due to resource disputes, variations in water quality, and climate variation. We used water quality as a driver result in the three-dimensional phase. First, vulnerability in terms of lack of wellbeing: pollution and biodiversity loss are caused by poor water quality. These have an impact on SSFs’ lives and cause poverty. Second, lack of access to resources or capital causes vulnerability. Hydrological interventions, tourism, and global markets cause resource disputes and low income. Third, vulnerability based on loss of resilience: ongoing disturbance or pressure on Chilika waters reduces the lagoon’s ability to purify itself, which has the unintended consequence of causing eutrophication and pollution. This analysis offers a solid foundation for investigating different SSF groups’ coping mechanisms and adaptation strategies in the Chilika Lagoon. It is crucial to design policies that aim to protect the SSF communities’ livelihoods by comprehending the water-related vulnerabilities of fishing communities and their coping and adaptation mechanisms [
74]. In general, efforts to lessen susceptibility to water-quality degradation should concentrate on lowering sensitivity and exposure while simultaneously boosting local adaptation capability. Creating precise management goals that balance conflicting objectives and that take into account multiple goals, such as conservation-based, social, cultural, political, economic, and biological objectives of marine social–ecological systems, will be another crucial step in addressing the effects of water quality [
74]. Fishing-industry perceptions of water-related pollution and unregulated activities, as well as rivalry for space, were not adequately investigated in the context of the Chilika Lagoon. In the anticipated transition to the Blue Growth and Blue Economy agendas, the suffering of the fishermen may become much more complicated. State government and public service institutions, such as the Chilika Development Authority, as entities should support fisheries management and governance, as well as address injustice to small-scale fishers.
Water-quality degradation is projected to fundamentally raise the vulnerability of fishery-based livelihoods in the future. The intensity and severity of stressors contributing to water pollution could have significant effects on the people and livelihoods of SSF communities in the coastal regions and jeopardise the sustainability and resilience of coastal ecosystems if effective adaptation planning and lagoon management measures are not implemented. To improve the environment for SSF communities, proper coping and adaptation strategies, as well as policies including water-management measures, should be developed. This study suggests that, if the fishing communities have access to fishing, fishermen do not view themselves as being poor. They rely on fishing for their food security, but it puts them at risk due to fish decline, falling catches, and other uncontrollable variables, including the increase in the cyclone frequency and water-quality degradation.
Figure 8 illustrates the overall relationship between SSF communities’ vulnerability and viability. The Chilika Lagoon’s fish and other aquatic resources are harmed by the opening of the sea mouth, shrimp aquaculture, frequent industrial and commercial encroachments (dam construction and tourism), and recurring natural disasters (cyclones, floods, and droughts). As a result, the Chilika’s social–ecological system is disturbed. These elements result in multifaceted vulnerabilities, such water pollution, resource overuse, biodiversity loss, disease outbreaks, poor living conditions, and migration. There is a lack of knowledge regarding the interaction and link between water quality and SSFs, which is addressed in this research, on a vulnerability and viability level. Though SSF communities are expected to be vulnerable due to the disastrous social–ecological changes, the question of whether all the drivers are now currently proposed in the literature as the factors related to water-quality parameters has to be addressed. In this paper, we report the impacts of various water-quality drivers on the livelihoods of SSF communities. We also report the various broad categories of vulnerabilities, such as ecological, social, economic, institutional, and technical, that cover all the major effects on the coastal fisheries of the Chilika Lagoon.
The study also offers directions to increase the viability of the SSF community through a variety of coping and adaptation measures by exploring the opportunities and constraints already in place. In order to attain higher economic, social, and environmental sustainability and resilience, policymakers need to incorporate the importance of water quality into policy formulations and implementations. We have already highlighted that there is a lack of analytical information on water quality readily available to policymakers. Our study bridges this gap by providing the key parameters of water quality that are necessary for the sustainable management of fisheries resources. The graphic representation aids in comprehending the complex relationships between SSF communities’ vulnerability and viability. Understanding these connections will aid in the implementation of effective strategies for SSF sustainability. SSFs are critical to the ecological and social wellbeing of communities around the world. They aid millions of people by establishing food security, promoting excellent health and nutrition, reducing poverty, and ensuring economic security.
5. Conclusions
Water-quality deterioration poses significant threats to SSF communities’ climate and water justice in the Chilika Lagoon. This paper has identified the unique challenges that water-quality issues can cause to fishing communities and the compounded injustice of combating social–ecological changes alongside a climate crisis. SSF communities must establish stronger and culturally sensitive mitigation and adaptation frameworks that are representative of the communities undertaking the coping strategies due to the combination of fisheries and water injustices they face. There needs to be a strong emphasis on protecting water quality to improve the livelihoods of SSF communities and guarantee the continuity of the Chilika’s history, culture, and ceremonial customs of fishing communities for future generations. An approach to social and water justice that is more inclusive could help the government address the effects of social–ecological problems and its repercussions for vulnerable fishing communities. Considering the synergistic impacts of ecological, social, economic, institutional, and technical vulnerabilities can enhance adaptive legislative mechanisms to address the effects of water-quality deterioration and boost the participation of traditional fishing folks in the decision-making process. We recommend that fishery policies need to be improved in order to accommodate social–ecological changes and include water justice. Through either improved public engagement or new types of adaptation assessment processes, other existing legislation should be improved to explicitly reflect the social and environmental justice implications of poor water quality consequences on the livelihoods of SSFs.
Enhancing the communities’ ability to take more proactive, preventative measures based on current water-quality conditions, the status of biodiversity, and the state of fisher livelihoods are a way to lessen vulnerability. One of the biggest dangers to fisheries and fish-population recovery is poor water quality. This study uses social–ecological indicators related to water-quality degradation to examine aspects of vulnerability to describe the vulnerability of fishery-based livelihoods. A stronger cooperation between fisheries and water-quality professionals is required to guarantee that protective water quality is maintained while fisheries are properly managed. The findings show that a range of factors affected SSF livelihoods’ ability to adjust. The availability of fishing techniques, education, diversification of sources of income, water-quality-monitoring systems, fishing gear, and knowledge of water quality had a significant impact on how vulnerable the fishing households will be. Other coastal communities throughout the world can benefit from this information as well.
Reaching the public, stakeholders, decision-makers, and others is crucial and can be accomplished by communicating water quality and fisheries research, evaluation, and outcomes through the media and visual materials, such as infographics, figures, and illustrations. The importance of these studies and issues can be increased by media coverage, which can also greatly enhance the credibility of the field of water-quality science, fisheries policies, and the organizations that are engaged in it. The difficulty for laypeople to obtain and comprehend research outcomes is sometimes attributed to academic jargon and information that is overly text heavy. The graphics with pictures and a minimum of text can explain the ideas, procedures, and outcomes of this social–ecological study to nontechnical audience, such as fisherfolks. One of our recommendations is to start slowly and develop expertise and confidence, regardless of whether they are drafting a fact sheet, filming a video, or planning a press conference to effectively communicate the significance and outcomes of various drivers that result in the vulnerability of small-scale fisheries. Using scientific visualisations for science communication can be used as a practical tool for bridging the knowledge gap between scientists and the general public. A holistic perspective of water quality, including the key parameters being properly understood, along with the many connections water quality has with SSF social–ecological systems, and actions to include the key parameters into fishery policy management, can have significant implications for sustainable and viable small-scale fisheries systems.
We would also like to address the limitations of our study that emerged due to various challenges encountered during the research process. Despite our best efforts, there were several inherent limitations that need to be acknowledged: (i) Data-collection challenges amidst COVID-19: The COVID-19 pandemic presented substantial challenges to our data-collection efforts. The restrictions on movement and access to physical resources, including field-data collection and research institutions, limited our ability to gather a wider range of data sources; (ii) Lack of open data availability: We encountered difficulties in accessing open data relevant to our study. Many governmental and nongovernmental agencies were preoccupied with the pandemic response, resulting in delayed or unavailable responses to our data requests; (iii) Unavailability of primary data sources: As a secondary literature review, our study relied heavily on published data from various sources. The unavailability of primary data sources limited our ability to conduct in-depth analysis and validation of our findings; (iv) Scope of literature reviewed: Our study’s scope was constrained by the available literature, which could impact the comprehensiveness of our findings. Some nuances and emerging trends in the field might not have been fully captured due to limitations in the literature itself. Despite these challenges, we believe that our study contributes valuable insights into the existing body of knowledge on the topic. Our research emphasises the importance of addressing such limitations and calls for collaborative efforts among researchers, institutions, and agencies to ensure the availability of accurate and comprehensive data for future studies.