Abstract
Crop yield has been a major target of plant breeding, although resistance and quality have also been important. The current climate change is calling for breeding actions to mitigate greenhouse gas (GHG) emissions. The present review focuses on opportunities from plant breeding to mitigate climate change while simultaneously securing yield and food requirements, as exemplified by winter wheat cultivation in Northern Europe. Therefore, we review the history of traditional plant breeding, the impact of climate change on crops and implications for plant breeding, opportunities to use plant breeding as a tool to mitigate climate change, and then we assess the estimated mitigation effects from plant breeding and discuss their impact on climate effects. Nitrogen uptake efficiency (NUpE) was indicated as the character with the highest potential to contribute to climate change mitigation, with positive effects also from increased straw length and stubble heights, while increased total biomass yield (root or above-ground) showed less effect. In addition to contributing to climate change mitigation, NUpE might increase profitability for growers and decrease nitrogen leakage from agricultural fields. An increase in NUpE by 15% through plant breeding has the potential to result in reduced GHG emissions corresponding to 30% of the fossil fuel use in agriculture in Sweden.
1. Introduction—Target and Aims of the Review
Plant breeding is, by definition, the art and science of changing the traits of plants through genetic improvements to produce desired characteristics for the benefit of humanity [1,2]. Thus, plant breeding strives to use diverse genetic material to change the genetic composition of desirable plants/crops and select and multiply those with the highest attributes, structure, and nutrient composition for the most suitable uses related to human requirements [3]. Plant breeding has been attributed to most crops grown today, although with substantially higher intensity in the major crops in terms of production and economic impact [4]. In all plant breeding activities, a major goal has been to target traits that contribute to a higher economic return from the cultivation of the crop [5]. Therefore, increased yield is an important trait, especially for staple crops, contributing to economic benefit for the producer and food security for the consumer [6]. A trait directly linked to yield is resistance to major diseases, which has therefore been an important breeding goal [7]. In certain crops, e.g., bread wheat and malting barley, end-use quality characteristics of importance for production have been included as breeding goals [8,9]. Breeding for environmental sustainability has been less targeted due to the lack of economic incentives, although such a target is becoming increasingly important due to ongoing climate change [10].
The present paper specifically targets the state-of-the-art knowledge on breeding for increased environmental sustainability and the opportunities that plant breeding contributes to that target. Thus, we will shortly describe the history, aims, and impact of plant breeding until the current stage. Thereafter, we summarize the current status of breeding related to climate change and estimate sustainable future opportunities, using winter wheat production in Southern Sweden as a model system.
2. Plant Breeding—History and Traditional Aims
2.1. The Historical Start of Plant Breeding and Its Current Status
Plant breeding has been a major tool for humanity since the start of the domestication of the first agricultural plants some 9000 to 11,000 years ago [11]. However, the first plant breeding activities were restricted to the selection of plants with the most desirable characteristics, such as a high yield or a good taste [12].
Modern agriculture developed rapidly from the 17th century to the mid-19th century, where the British agricultural revolution played a key role as a corner-stone for the industrial revolution [13]. Plant breeding was one, among others, of the important technologies that formed the basis for the development of modern agriculture. Plant breeding was driven by the increased growth of the human population, industrialization, and increased knowledge, development, and understanding of genetic principles [14]. The novel genetic principles were built on science by researchers such as Carl Linnaeus and Gregor Mendel. In the 1890s, the first commercial plant breeding activities started in England with the formation of Gartons Agricultural Plant Breeders, using cross-pollination of plants [15].
The next important step in plant breeding was the development of hybrid varieties, which use two inbred parental lines for crossing to result in outperforming offspring through the heterosis effect [16]. Maize was the first crop that was significantly improved using the hybrid technique in the USA, resulting in a large increase in yield at the beginning of the 20th century [17].
Currently, we see an outbreak of novel technologies in the area of plant breeding and research activities [18]. The emerging technologies are basically built on five important pillars, which include, (i) novel tools, including imaging techniques, to capture large amounts of phenotypic data, (ii) big data tools, including computer related space, power and capacity, allowing digital analyses of enormous amounts of data, (iii) novel genomic tools, revealing sequence information from the whole genome with an exactness not previously available, (iv) marker-related evaluations of traits, allowing speed breeding, where each generation only needs to produce few seeds in indoor conditions and selection is made from markers instead of from plant performance, and (v) gene editing tools, to determine specific traits, governed by certain genes to be particularly tailored. The current revolution of plant breeding tools and opportunities should thereby allow great changes in agriculture and the performance of crops, including opportunities to target more complex traits than in previous plant breeding activities. Thus, plant breeding might be a prioritized activity to solve challenging problems, such as the increasing global population and climate change, with additional threats such as pandemics and war outbreaks.
2.2. Traits of Focus in Traditional Plant Breeding
Plant breeding of crops has traditionally focused on human food security by increasing production as well as improving other traits that contribute to the economic return for the producers [19]. To increase productivity, the focus has been on increasing the yield potential (uptake, transport, and storage of metabolites such as starch and other polysaccharides and oil in the storage organs such as grains and tubers) and making the plant tolerant/resistant to biotic and abiotic stresses [20]. Among the biotic stresses, certain diseases and pests reduce the yield by up to 100%, thereby severely threatening human food security [21]. Another biotic stress that severely impacts the yield is weeds, which have the potential to compete with the crop for space, light, and soil resources [22]. Protection against biotic stress is often possible through chemical-based treatments, although breeding efforts to solve the issues are often more economically viable and ecologically friendly [23]. Most breeding efforts against biotic stresses have been carried out through the incorporation of a single dominant gene. However, the use of multiple broad-spectrum genes is known to contribute to more durable resistance/tolerance [24]. So far, breeding efforts have had a limited impact on crop architecture, a character known to be important in breeding for adaptation to environmental effects, especially abiotic stresses [25].
Abiotic stresses include all factors that are impacting crop performance based on non-biological inputs, such as, e.g., heat, drought (low precipitation), flooding, water logging (high precipitation), high CO2 concentration, soil nutrition, etc. [26]. Breeding efforts towards abiotic stress tolerance often focus on changing the plant architecture so that it can withstand the outer environment [27]. As an example, during the 1960s and 1970s, dwarfing genes were introduced in winter wheat to counter water logging problems in the UK. The plants responded with an average additional increase in wheat grain yield (up to 3 t/ha) and improved nutrient use efficiency (NUE) because of reduced susceptibility to water logging, wind, and rain [28,29,30]. The average height of wheat plants between the 1960s and 1990s was reduced by up to 23 cm, although no further reduction in plant height was observed between 1990 and 2013 [29]. Results from the Broadbalk Wheat Experiment (1843-ongoing) conducted in Rothamsted, UK, showed that the introduction of dwarfing genes in wheat had a significant impact on harvest index, grain yield, and the concentration of minerals in the grain [30,31]. Harvest index, i.e., the ratio of harvested grain to total aboveground biomass, is a measure connected to yield, and therefore, this index has been in focus for breeding, and for winter wheat varieties, it has shown a continuous increase of approx. 0.2% per year in the last century (Figure 1). Despite the change in harvest index, the total aboveground biomass has been relatively stable (Figure 2), due to an increase in grain yield and a simultaneous decrease in residual aboveground biomass. The effects of root biomass changes over the last century have been limitedly studied, although Bektas et al. [32] have shown a general decrease over the last hundred years (Figure 3), and aquaponics studies indicate a considerable decrease in root biomass for barley [33]. A recent study indicated increased early vigor and growth in roots from old genotypes in comparison with modern varieties [34]. The increase in grain yield has had limited effect on the thousand kernel weight, grain hardness, flour yield, gluten extensibility, and relative protein content [30,35].
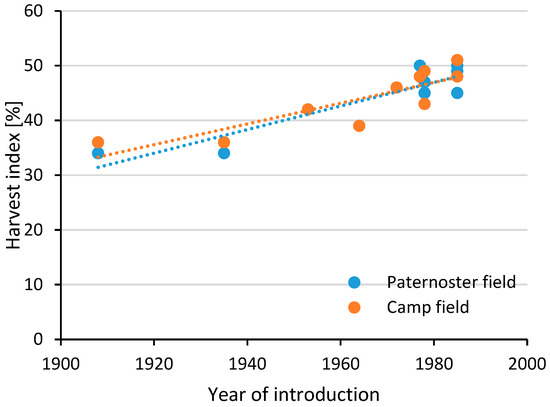
Figure 1.
Development of harvest index of winter wheat varieties. Dotted lines represent a linear regression. Based on data presented by Austin et al. [36]. Varieties not yet commercialized in 1980 were assumed to be commercialized in 1985. Paternoster refers to the field with low fertility (fertilized with 38 kg N/ha) and Camp field with high soil fertility (fertilized with 104 kg N/ha).
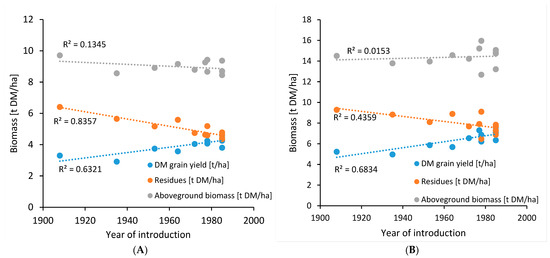
Figure 2.
Development of aboveground, grain and residual biomass in winter wheat on two different fields ((A): Paternoster, (B): Camp). Dotted lines represent a linear regression. Based on data presented by Austin et al. [36]. Varieties not yet commercialized in 1980 were assumed to be commercialized in 1985.
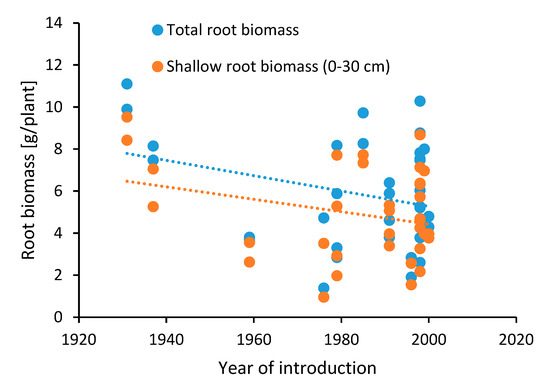
Figure 3.
Historical development of root biomass [g/plant] based on data presented by Bektas et al. [32].
3. Climate Change and Plant Breeding
3.1. Impact of Climate Change on Agricultural Crops and Implications for Plant Breeding
Climate change is one of the major global concerns, evident from observations of increasing atmospheric CO2 concentration and temperature, thereby resulting in melting glaciers and rising sea levels across the world [37]. Climate change is currently impacting a range of environmental factors that directly and indirectly influence crop adaptability and productivity. As a result, food production, food quality, and food security are severely influenced, not least in developing and vulnerable countries [38,39]. The increased frequencies and severity of abiotic and biotic stresses from climate change contribute to a change in the chemical and physical properties of the soil, plant nutrient uptake efficiency, soil microbial activity, and other biotic factors such as the activity of insects and pests [34,40].
Water deficiency in plants is known to induce changes in the physiological, morphological, biochemical, and molecular characteristics of plants. In wheat, drought stress is affecting the flag-leaf area, root and plant biomass, days to anthesis, and tillers per plant, which directly affect overall crop yield [34]. Similarly, high temperatures and drought stress during production negatively influence the yield, therefore changing the choice of areas suitable for rice production [41]. The current extremely rapid change in climate requires an upscaling of time-effective plant breeding, including the use of a wide array of genetic resources in combination with modern phenotyping and genotyping methodologies [42,43].
3.2. Opportunities of Using Plant Breeding of Agricultural Crops as a Tool to Mitigate Climate Change
Plant breeding has been identified not only as a sustainable method to address plant adaptability to a changing climate but also as a tool for its mitigation [44]. Efforts have been made to understand complex stress-adaptive mechanisms, stress uncertainty, and genotype-environment interactions in breeding for crops adaptable to the future climate [45]. Consequently, there is also a pressing need to accelerate the genetic gain of the major crops by taking into account gene x gene and genotype-environmental interactions. A comprehensive understanding of the above-mentioned factors can significantly enhance the adaptation of wheat cultivars and help in defining the goals for future wheat breeding strategies [46]. Currently, characters mitigating climate change are less studied, and breeding as a tool has been less utilized. Two crop characteristics that have an impact on climate change mitigation are (i) the ability of the crop to fix carbon dioxide and thereby contribute to the formation of soil organic carbon (SOC) [47] and (ii) the nutrient use efficiency (NUE) of the crop so that a high content of biomass is obtained with low input [48]. Modern plant breeding provides substantial opportunities for the introduction of these characters through speed breeding, bioinformatics, and big data phenomics/genomics-led approaches, together with the availability of relevant germplasm [49,50].
Both approaches above have their own systematic limitations. Modern agriculture has striven towards a lower total plant biomass production (at higher yields of harvestable product), thus the amount of plant residues potentially contributing to the SOC build-up has historically decreased, and with it, the amount of carbon potentially stabilized in the soil [29]. Currently, a return to an increase in the total plant biomass is not an aim for plant breeding, although coming requirements might lead to a shift. For NUE, a major limitation is how to make enough nutrients available in the soil with low additions of fertilizer [51].
3.3. Opportunities to Use Plant Breeding as a Tool for Carbon Sequestration by Agricultural Crops
Plant breeding efforts to increase carbon sequestration by agronomic crops have been limited until now. Carbon sequestration by crops will directly contribute to an increased SOC in the soil and thereby, a decrease in CO2 in the atmosphere [47]. Generally, SOC present in the soil is the result of two mechanisms: (i) the accumulation of organic matter through the humification of plant residues and (ii) organic compounds released from, e.g., roots during crop growth [52]. The potential to store carbon in the soil is tremendous, as most soils have the ability to store higher amounts than they do today [53]. However, differences in the degree of stabilization of carbon in the soil have been noted for above- and belowground biomass, where root biomass contributed three times more carbon to the pools than shoot [54]. Also, a higher stabilization degree was found for clay-rich soils (>40% clay), especially for the aboveground biomass and with increasing soil clay content [55].
Currently, crops such as cereals are known to contribute about 20–30% of the assimilated carbon to soil organic matter [52]. Based on the above, plant breeding activities to increase SOC are then related either to an increase in root and straw (for cereals) biomass to result in the addition of plant residues after harvesting or to exploring genetic differences in the release of organic compounds during plant growth. Until now, breeding activities for such characters have been basically lacking.
3.4. Opportunities to Breed for Nutrient Use Efficiency in Agricultural Crops
Increased NUE has not been considered a major breeding goal for agricultural crops, although some research and breeding activities have been carried out [56,57]. However, studies have also shown that the standardized selection of traits with a primary focus on yield has contributed to the loss of genes responsible for other characters such as efficient nutrient acquisition and adaptation to soil-related biotic and abiotic stresses [58]. Furthermore, old wheat cultivars, i.e., those developed before 1950, were shown to have superior mycorrhizal symbiosis, resulting in higher yields at low P availability and in acidic soils compared to modern varieties grown in similar conditions [59,60,61]. Previous studies have shown large genetic variation in phosphorus use efficiency (PUE) in wheat genotypes, which is largely related to their root architecture [62]. A recent meta-analysis has shown that mycorrhizal symbiosis can benefit the growth and yield of agriculturally significant crops by increasing phosphorus acquisition in plants [63]. However, to improve PUE in wheat, future breeding programs should include efficient screening/phenotyping for high PUE in different genotypes, identification of QTLs and genes responsible for specific root architecture for mycorrhizal symbiotic interactions, and marker-assisted selection of those specific traits [64].
The work on NUE has until now mainly focused on increasing yield and production per area [48], while reducing climate change effects has not been a major breeding target. NUE is known as a complex trait that is the result of a range of physiological characteristics [56]. Thus, the focus on breeding needs to be on a combination of effects from N regimes, genotypes, and several developmental stage traits of the plants [48]. Some of the more important characters are related to the uptake, transport, and transfer of N in the plant. The fast development of new methods allowing the determination and high-throughput screening of quantitative trait loci (QTLs) and major and minor genes involved in complex traits opens an avenue for breeding on NUE and other similar characters [56]. The fact that NUE is also related to yield would make this an important breeding objective for the future, also connecting it to climate change mitigation.
4. Assessment of Estimated Climate Mitigation Effects from Plant Breeding
4.1. Baselines for the Assessment
To understand the effects of potential breeding efforts on the GHG emission balance of wheat cultivation, an activity-based systems assessment was carried out. The applied life cycle assessment (LCA) methodology followed the standard for environmental management assessment [65]. A conventional wheat production system with a grain yield of 4 tons of dry matter (DM) per hectare was assumed as a reference system. The assessment accounted for inputs such as fertilizer, seeds, pesticide use, and other production means. Machinery operations (soil treatment, seeding, fertilizing, harvest, and transport operations) as well as the use of buildings (dryer, cereal silo) were accounted for.
Fertilization was assumed to follow a 25 kg/ha N base dose, which was complemented by additional nitrogen, potassium, and phosphorus corresponding to 2.33, 0.36, and 0.50% of grain DM yield, respectively. Greenhouse gas (GHG) emissions from N, P, and K were assumed to be 4.5, 2.3, and 0.70 kg carbon dioxide equivalents (CO2e) per kg of plant nutrient, respectively [66]. The same seeding rate of 180 kg/ha [67] was assumed for all tested alternatives, which corresponded to an energy consumption of 9 MJ/kg and a GHG emission from seed production of 50 g CO2e/MJ [68].
Working time for yield-independent operations has been estimated based on general recommendations [69]. Working time for yield-dependent operations was estimated based on the wet grain yield [t/ha] and the machinery capacity (combine harvester 33.8 t/h; capacity and the number of field trailers needed to allow a continuous combine harvest). Diesel use has been estimated from the working time [h/ha] and typical diesel consumption [L/h] [69]. Indirect energy considers energy used for material, manufacture, and maintenance [70] and is estimated based on total weight, economic lifetime, and annual use [69]. GHG emissions include emissions from diesel use (83.8 g CO2-equivalents/MJ; EC 2009 [71]) and indirect energy use (97.5 g CO2-equivalents/MJ; Börjesson and Tufvesson et al. [72]). The use of a 200 kW tractor for all field operations was assumed.
Field operations and corresponding emissions for crop cultivation were assumed to be the same for all alternatives (Table 1). Operations that depended on the grain yield, e.g., harvest, transport, and post-harvest processes, varied according to the assumed grain yield (Table 1).

Table 1.
Data on the field and other operations for all alternatives tested.
The use of 3.08 kg/ha of pesticides (active substances) was assumed according to recommendations [67]. Energy use for and carbon intensity of pesticide manufacture were assumed to be 196–288 MJ/kg active ingredient [73] and 69 g CO2e/MJ [74]. The transportation distances of grains from the field to the drying facility and from the drying facility to storage were assumed to be 10 and 50 km, respectively.
Soil organic carbon (SOC) effects were estimated as potential contributions, with stable carbon originating from crop residues. The amount of crop residues was estimated from grain DM yield and a straw:grain ratio of 0.77 [75]. The total aboveground biomass (straw + grain) and an aboveground:belowground ratio of 6.07 [76] were used to estimate the belowground DM root biomass. The amount of straw at different stubble heights was estimated using data from Nilsson and Bernesson [75]. The moisture content of grains at harvest and in storage, as well as that of straw, was assumed to be 20, 14, and 18%, respectively.
Contributions to SOC were estimated based on the amount of crop residue left to be incorporated into the soil. A carbon content of crop residues of 42.5% was assumed [77]. The SOC contribution in the form of stable carbon from root biomass was estimated based on the amount of root biomass, the carbon content, and a humification coefficient of 0.35 [54]. Furthermore, root biomass was assumed to contribute stable carbon in the form of exudates, amounting to 65% of the stable carbon from root biomass [78]. The contribution of stable carbon from aboveground crop residues was estimated using a humification coefficient of 0.15 [54].
The application of mineral fertilizer contributes to emissions of nitrous oxide (N2O). These emissions were estimated to be 1% of the amount of applied nitrogen fertilizer [79]. However, nitrogen that was immobilized in soil organic matter, estimated based on a C/N ratio of 10 in soil organic matter [80], was assumed not contribute to N2O emissions. N2O emissions were recalculated as CO2-equivalents (CO2e) using an emission factor of 298 [81].
Post-harvest processes included drying the grains from a DM content at harvest of 80% to a DM content of 86% for storage. The use of fossil oil was assumed for heat generation, using 5.62 MJ of oil for the evaporation of 1 kg of water [69]. For each MJ of oil, 0.43 MJ of electricity is used in grain drying [82]. The carbon intensities of oil and electricity were assumed to be 83.8 and 10.1 g CO2e/MJ, respectively [71,83]. Emissions from cultivation, harvest, transport, and post-harvest processes were summarized as carbon dioxide equivalents (CO2e).
4.2. Impact on Climate Change Mitigation from Plant Breeding Derived Measures
Systems assessment on increases/decreases in grain yield, straw yield, and root yield, as well as changes in UpE and agricultural practices (Figure 4), showed that increasing NUpE resulted in the most significant decrease in GHG emissions as compared to changes in biomass or agricultural practices (Figure 4, section D). Also, increasing the straw length or stubble height resulted in a decrease in GHG emissions (Figure 4, section E,F). Previous studies have shown that straw management (i.e., reducing the amount of straw removed) could increase carbon sequestration, at least on soils with high clay content [55]. Our assessment also showed that differences in the straw:grain ratio, the cultivar, and the stubble height had a high impact on SOC contribution, i.e., approx. 250–570 kg of stable carbon per hectare for a wheat crop with a grain yield of 5 tons per hectare (Figure 5). Thus, crop management, besides plant breeding, has a large impact on SOC contribution and GHG emissions. An increase in grain yield, combined with keeping the total biomass yield constant (leading to a decrease in straw and/or root biomass), generally results in increases in GHG emissions (Figure 4, section A,B). However, when the straw (except for the stubble) is removed anyway, the GHG emissions are mostly unaffected by changes in grain yield (Figure 4, section A). This effect was even more pronounced when an increased grain yield would mean both less straw and root biomass since root biomass has a considerably higher impact on SOC compared to shoot biomass (Figure 4, section B). Should an increase in grain yield also increase total biomass yield (i.e., the straw and root biomass yield are kept constant), the changes in GHG emissions would be low (Figure 4, section C).
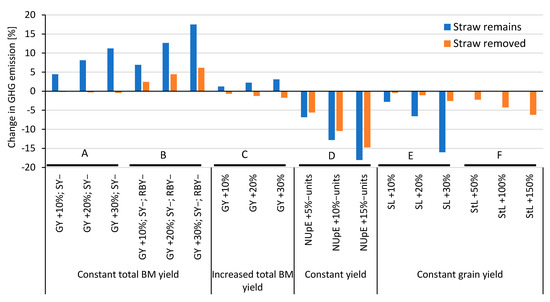
Figure 4.
Changes in greenhouse gas (GHG) emissions as caused by potential effects of breeding and management. GY = grain yield; SY = straw yield; RBY = root biomass yield; SL = Straw length; StL = stubble length; NUpE = nitrogen uptake efficiency. Sections A–F show the impact of increase and/or decrease of different factors and their combinations on relative change in GHG emissions.
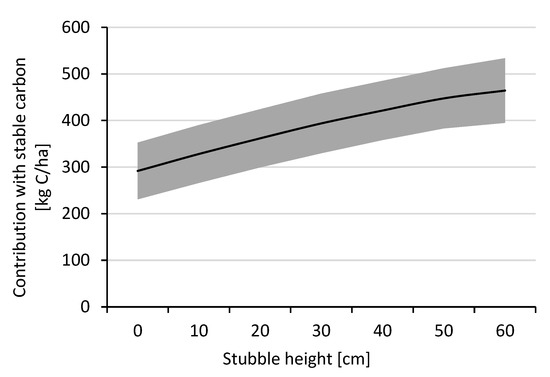
Figure 5.
Contribution of cereal crop residues depending on grain:straw ratio, cultivar, and stubble height when the straw is removed. Own calculations based on data from Nilsson and Bernesson [75]. The example corresponds to a grain yield of 4 t DM/ha. The upper and lower limits correspond to cultivars with a low and high straw:grain ratio, respectively. Calculations assumed an aboveground:belowground ratio of 5.00 and 7.14 [76] for low and high cases, respectively, humification coefficients of 0.15 and 0.35 [54] for aboveground and belowground biomass, respectively, and a carbon content of 42.5% [77].
Thus, from the assessment carried out here, plant breeding to improve NUpE is an interesting approach to mitigating climate change. According to previous estimates, excessive application of nitrogen fertilizer accounts for ca. 70% of GHG emissions associated with wheat production, which is primarily due to nitrate leaching and N2O emissions [84]. Therefore, improvement in total NUpE will not only contribute to a reduction in GHG emissions but also increase profitability for the growers and reduce nutrient leakage. These results have led to a consensus that there is an increasing need for breeding wheat cultivars with increased NUpE to reduce the input of mineral fertilizers used to maintain high yield and grain quality [85]. Strategies have also been suggested on how to increase NUpE in crops on farms based on breeding activities and variety testing schemes, including measurements of canopy N, grain N, and N harvest index on a wider material [86].
5. Long-Term Sustainability Effects of the Use of Plant Breeding Strategies in Agricultural Crops
The use of fossil fuels is by far the largest contributor to GHG emissions on a global scale. In agriculture, the use of fossil fuels and the use of mineral fertilizers are the largest contributors to GHG emissions, and agriculture contributes ca. 20% of the GHG emissions in Sweden [87]. Here, we show that an improvement of the NUpE in winter wheat grown in Sweden would allow a reduction of GHG emissions of approx. 10,000 t CO2-equivalents per year and per %-unit of NUpE change. This corresponds to a 1.2–1.6% total GHG emission decrease from winter wheat production when all straw is removed or remains, respectively. An increase of 15% in NUpE would result in GHG emission decreases corresponding to approx. 30% of the GHG emissions from fossil fuel use in agriculture in Sweden [88]. Previous studies have shown that NUE has increased in wheat over time, e.g., both under Nordic and Chinese conditions [89,90]. In Chinese wheat, the annual genetic gain in NUpE was found to be 0.71–1.03% over a time scale from 1941 [90]. Most likely, NUpE has an upper limit, and the level that is reachable is currently not known. Thus, the possibility of reaching a NUpE increase of 15% is uncertain. However, novel studies on genomic associations of agronomic traits related to NUE [91] contribute additional opportunities to breed for increased NUpE.
In addition to breeding for increased NUpE, other breeding/cultivation strategies, not mentioned above, might be available to mitigate climate change. Thus, the development of perennial crops, e.g., wheatgrass, known to contribute to a reduction in water usage, soil erosion, nutrient leaching, increased retention of soil organic carbon, and other ecosystem services, might also have the potential to mitigate climate change [92,93]. Furthermore, the use of intermediate crops for producing additional biomass, e.g., when the field would be bare otherwise, has been shown to be an effective measure to sequester carbon and to compensate for the impact on SOC from straw removal [94]. Climate change will also result in increased temperatures, leading to longer growing periods in the north. This might contribute to possibilities for double cropping (i.e., production of two full crops during the growing season in temperate climate zones), leading to a similar effect as the cultivation of intermediate crops. Assessments of the opportunities for climate change mitigation from these measures are still to be done. Basically, the limited amount of data from such cultivation systems and breeding tasks contributes to challenges in assessing their effects on the mitigation of climate change.
In order to cultivate crops with shorter growing periods, incorporating crop phenology manipulation into breeding strategies becomes crucial to enabling the plants to adapt to new growth cycles [95]. This would entail, for instance, modifying the crop’s phenotypic traits to enhance water use efficiency and NUE, aligning the crop cycle with seasonal rainfall patterns, and adjusting the duration of stem elongation while maintaining the timing of anthesis. This approach would increase the number of grains per spike and the harvest index without altering the overall water consumption of the crop [96]. Similarly, increased interest has been shown in manipulating the photosynthetic traits of plants to improve plant yield and CO2 assimilation in a continuously changing climate. Consequently, as CO2 levels are projected to rise, it becomes imperative to assess the correlation between this increase and its impact on plant yield, particularly when considering the interplay with other abiotic stresses [97].
6. Conclusions
Modern agriculture has developed since the 17th century, with plant breeding as a cornerstone. Until now, the major focus of plant breeding activities has been to increase crop yield but also to increase resistance and quality of the produce. Plant breeding to mitigate climate change has received less attention. Increased carbon sequestration and nutrient use efficiency (NUE) are two major characteristics that have an effect on climate change mitigation. Carbon sequestration is largely impacted by the accumulation of organic matter from crops as a result of biomass disposition in different plant parts. However, biomass disposition between plant parts has comparably lower effects on climate change mitigation than changes in NUE. Also, increased straw length and stubble height have a positive effect on the mitigation of climate change. Increased NUE has, besides a greenhouse gas (GHG) emission effect, other positive effects on the grower’s economic return, such as reduced fertilizer requirements, soil acidification, eutrophication, etc. A 15% increase in NUpE in Swedish winter wheat will result in a GHG emission reduction corresponding to 30% of the fossil fuel use in agriculture in Sweden.
Author Contributions
E.J. conceived the idea, received the funding, and wrote the manuscript with co-authors. T.P. performed the analysis and wrote various parts of the manuscript. F.M. wrote and edited various parts of the manuscript. All authors provided input for the literature review, manuscript preparation, proofreading, and critical analysis of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Swedish Government Research Funding from The Swedish Foundation for Strategic Environmental Research (Mistra) (Grant No. DIA2018-24#8) and SLU Grogrund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bernardo, R. Essentials of Plant Breeding; Stemma Press: Woodbury, MN, USA, 2014. [Google Scholar]
- Cowling, W.A. Sustainable plant breeding. Plant Breed. 2013, 132, 1–9. [Google Scholar] [CrossRef]
- Acquuah, G. Principles of Plant Genetics and Breeding, 2nd ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Heisey, P.W.; Srinivasan, C.S.; Thirtle, C.G. Public sector plant breeding in a privatizing world. In Agriculture Information: Resource Economics Division USDA-ERS; Vol. Bulletin No. 772; Economic Research Service, United States Department of Agriculture: Washington, DC, USA, 2001. [Google Scholar]
- Luby, J.J.; Shaw, D.V. Plant breeders’ perspectives on improving yield and quality traits in horticultural food crops. HortScience 2009, 44, 20–22. [Google Scholar]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar]
- Gaunt, R. The relationship between plant disease severity and yield. Annu. Rev. Phytopathol. 1995, 33, 119–144. [Google Scholar] [CrossRef]
- Johansson, E.; Prieto-Linde, M.L.; Jönsson, J.Ö. Effects of Wheat Cultivar and Nitrogen Application on Storage Protein Composition and Breadmaking Quality. Cereal Chem. 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Holm, L.; Malik, A.H.; Johansson, E. Optimizing yield and quality in malting barley by the governance of field cultivation conditions. J. Cereal Sci. 2018, 82, 230–242. [Google Scholar]
- Lammerts van Bueren, E.T.; Struik, P.C.; van Eekeren, N.; Nuijten, E. Towards resilience through systems-based plant breeding. A review. Agron. Sustain. Dev. 2018, 38, 42. [Google Scholar]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef]
- Breseghello, F.; Coelho, A.S.G. Traditional and Modern Plant Breeding Methods with Examples in Rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef]
- Kerridge, E. The Agricultural Revolution; Routledge, Taylor and Francis: London, UK, 2006. [Google Scholar]
- Overton, M. Agricultural Revolution in England: The Transformation of the Agrarian Economy 1500–1850; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Vega, L. Fundamentals of Genetics; Scientific e-Resources; ED-Tech Press: London, UK, 2019. [Google Scholar]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and hybrid crop breeding: A multidisciplinary review. Front. Genet. 2021, 12, 643761. [Google Scholar]
- Lee, E.; Tracy, W. Modern Maize Breeding. In Handbook of Maize; Genetics and Genomics; Bennetzen, J., Hake, S., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Varshney, R.K.; Dubey, A. Novel Genomic Tools and Modern Genetic and Breeding Approaches for Crop Improvement. J. Plant Biochem. Biotechnol. 2009, 18, 127–138. [Google Scholar]
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Review: Improving global food security through accelerated plant breeding. Plant Sci. 2019, 287, 110207. [Google Scholar] [PubMed]
- Zhang, H.; Li, Y.; Zhu, J.-K. Developing naturally stress-resistant crops for a sustainable agriculture. Nat. Plants 2018, 4, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Rahmatov, M.; Otambekova, M.; Muminjanov, H.; Rouse, M.N.; Hovmøller, M.S.; Nazari, K.; Steffenson, B.J.; Johansson, E. Characterization of stem, stripe and leaf rust resistance in Tajik bread wheat accessions. Euphytica 2019, 215, 55. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, R.; Chauhan, B.S. Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop Protect. 2018, 103, 65–72. [Google Scholar] [CrossRef]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Yazdani, M. Inducing Novel Resistance Gene in Wheat towards Stem Rust to Improve Food Security; Series 2022:1; Swedish University of Agricultural Sciences (SLU): Alnarp, Sweden, 2022. [Google Scholar]
- Ando, K.; Grumet, R.; Terpstra, K.; Kelly, J.D. Manipulation of plant architecture to enhance crop disease control. CABI Rev. 2007, 2007. [Google Scholar] [CrossRef]
- Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy 2022, 12, 2795. [Google Scholar]
- Snowdon, R.J.; Wittkop, B.; Chen, T.-W.; Stahl, A. Crop adaptation to climate change as a consequence of long-term breeding. Theor. Appl. Genet. 2021, 134, 1613–1623. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Berry, P.M.; Kendall, S.; Rutterford, Z.; Orford, S.; Griffiths, S. Historical analysis of the effects of breeding on the height of winter wheat (Triticum aestivum) and consequences for lodging. Euphytica 2015, 203, 375–383. [Google Scholar] [CrossRef]
- Guzmán, C.; Autrique, E.; Mondal, S.; Huerta-Espino, J.; Singh, R.P.; Vargas, M.; Crossa, J.; Amaya, A.; Peña, R.J. Genetic improvement of grain quality traits for CIMMYT semi-dwarf spring bread wheat varieties developed during 1965–2015: 50 years of breeding. Field Crops Res. 2017, 210, 192–196. [Google Scholar]
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Bektas, H.; Hohn, C.E.; Waines, J.G. Root and shoot traits of bread wheat (Triticum aestivum L.) landraces and cultivars. Euphytica 2016, 212, 297–311. [Google Scholar] [CrossRef]
- Bertholdsson, N.O.; Kolodinska Brantestam, A. A century of Nordic barley breeding—Effects on early vigour root and shoot growth, straw length, harvest index and grain weight. Eur. J. Agron. 2009, 30, 266–274. [Google Scholar]
- Lan, Y.; Chawade, A.; Kuktaite, R.; Johansson, E. Climate Change Impact on Wheat Performance-Effects on Vigour, Plant Traits and Yield from Early and Late Drought Stress in Diverse Lines. Int. J. Mol. Sci. 2022, 23, 3333. [Google Scholar]
- Fufa, H.; Baenziger, P.S.; Beecher, B.S.; Graybosch, R.A.; Eskridge, K.M.; Nelson, L.A. Genetic improvement trends in agronomic performances and end-use quality characteristics among hard red winter wheat cultivars in Nebraska. Euphytica 2005, 144, 187–198. [Google Scholar] [CrossRef]
- Austin, R.B.; Bingham, J.; Blackwell, R.D.; Evans, L.T.; Ford, M.A.; Morgan, C.L.; Taylor, M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. J. Agric. Sci. 1980, 94, 675–689. [Google Scholar]
- Chapman, S.C.; Chakraborty, S.; Dreccer, M.F.; Howden, S.M. Plant adaptation to climate change—Opportunities and priorities in breeding. Crop Pasture Sci. 2012, 63, 251–268. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Kettlewell, P.S.; Poulton, P.R.; Hollins, P.D. Grain quality in the Broadbalk Wheat Experiment and the winter North Atlantic Oscillation. J. Agric. Sci. 2008, 146, 541–549. [Google Scholar] [CrossRef]
- Mendelsohn, R. The Impact of Climate Change on Agriculture in Asia. J. Integr. Agric. 2014, 13, 660–665. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A. Climate Change and Agriculture Paper. Plant Breed. Clim. Chang. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar]
- Mukamuhirwa, A.; Persson Hovmalm, H.; Ortiz, R.; Nyamangyoku, O.; Prieto–Linde, M.L.; Ekholm, A.; Johansson, E. Effect of intermittent drought on grain yield and quality of rice (Oryza sativa L.) grown in Rwanda. J. Agron. Crop Sci. 2020, 206, 252–262. [Google Scholar] [CrossRef]
- Cooper, M.; Messina, C.D.; Podlich, D.; Totir, L.R.; Baumgarten, A.; Hausmann, N.J.; Wright, D.; Graham, G. Predicting the future of plant breeding: Complementing empirical evaluation with genetic prediction. Crop Pasture Sci. 2014, 65, 311–336. [Google Scholar]
- Pilbeam, D.J. Breeding crops for improved mineral nutrition under climate change conditions. J. Exp. Bot. 2015, 66, 3511–3521. [Google Scholar]
- Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability 2011, 3, 1944–1971. [Google Scholar]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [PubMed]
- Raffo, M.A.; Jensen, J. Gene × gene and genotype × environment interactions in wheat. Crop Sci. 2023, 63, 1779–1793. [Google Scholar]
- Crews, T.E.; Rumsey, B.E. What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review. Sustainability 2017, 9, 578. [Google Scholar]
- Cormier, F.; Foulkes, J.; Hirel, B.; Gouache, D.; Moënne-Loccoz, Y.; Le Gouis, J. Breeding for increased nitrogen-use efficiency: A review for wheat (T. aestivum L.). Plant Breed. 2016, 135, 255–278. [Google Scholar]
- Boote, K.J.; Ibrahim, A.M.H.; Lafitte, R.; McCulley, R.; Messina, C.; Murray, S.C.; Specht, J.E.; Taylor, S.; Westgate, M.E.; Glasener, K.; et al. Position Statement on Crop Adaptation to Climate Change. Crop Sci. 2011, 51, 2337–2343. [Google Scholar]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Sec. 2017, 12, 31–37. [Google Scholar] [PubMed]
- Ferrante, A.; Nocito, F.F.; Morgutti, S.; Sacchi, G.A. Plant Breeding for Improving Nutrient Uptake and Utilization Efficiency. In Advances in Research on Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 221–246. [Google Scholar]
- Kumar, R.; Pandey, S.; Pandey, A. Plant roots and carbon sequestration. Curr. Sci. 2006, 91, 885–890. [Google Scholar]
- Lal, R. Global Potential of Soil Carbon Sequestration to Mitigate the Greenhouse Effect. Crit. Rev. Plant Sci. 2003, 22, 151–184. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef]
- Poeplau, C.; Kätterer, T.; Bolinder, M.A.; Börjesson, G.; Berti, A.; Lugato, E. Low stabilization of aboveground crop residue carbon in sandy soils of Swedish long-term experiments. Geoderma 2015, 237–238, 246–255. [Google Scholar] [CrossRef]
- Cormier, F.; Le Gouis, J.; Dubreuil, P.; Lafarge, S.; Praud, S. A genome-wide identification of chromosomal regions determining nitrogen use efficiency components in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 2679–2693. [Google Scholar]
- Chawade, A.; Armoniené, R.; Berg, G.; Brazauskas, G.; Frostgård, G.; Geleta, M.; Gorash, A.; Henriksson, T.; Himanen, K.; Ingver, A.; et al. A transnational and holistic breeding approach is needed for sustainable wheat production in the Baltic Sea region. Physiol. Plant. 2018, 164, 442–451. [Google Scholar]
- Wissuwa, M.; Mazzola, M.; Picard, C. Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 2008, 321, 409. [Google Scholar]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat cultivars and ancestors: A synthesis. Can. J. Bot. 1993, 71, 512–518. [Google Scholar]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can. J. Bot. 1992, 70, 2032–2040. [Google Scholar] [CrossRef]
- Egle, K.; Manske, G.; Römer, W.; Vlek, P.L.G. Improved phosphorus efficiency of three new wheat genotypes from CIMMYT in comparison with an older Mexican variety. J. Plant Nutr. Soil Sci. 1999, 162, 353–358. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Phosphorus-use efficiency in wheat genotypes. J. Plant Nutr. 1999, 22, 331–340. [Google Scholar] [CrossRef]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus Acquisition Efficiency Related to Root Traits: Is Mycorrhizal Symbiosis a Key Factor to Wheat and Barley Cropping? Front. Plant Sci. 2018, 9, 1–21. [Google Scholar]
- van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar]
- SS-EN ISO 14044; Environmental Management-Lifecycle Assessment-Requirements and Guidelines. International Standard Organization: Geneva, Switzerland, 2006.
- Lantz, M.; Prade, T.; Ahlgren, S.; Björnsson, L. Biogas and ethanol from wheat grain or straw: Is there a trade-off between climate impact, avoidance of iLUC and production cost? Energies 2018, 11, 2633. [Google Scholar]
- Hansson, P.; Saltzman, I.-L.; Bååth Jacobsson, S.; Petersson, P. Produktionsgrenskalkyler för Växtodling-Efterkalkyler för år 2014-Södra Sverige; Hushållningssällskapen Kalmar-Kronoberg-Blekinge, Krisitanstad, Malmöhus och Halland: Kristianstad, Sweden, 2014. [Google Scholar]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.-E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing energy crops for biogas production–Yields, energy input and costs in cultivation using digestate and mineral fertilisation. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Makinkalkylgruppen. Maskinkostnader 2022; Swedish Rural Economy and Agricultural Societies Malmöhus: Bjärred, Sweden, 2022. [Google Scholar]
- Börjesson, P. Energianalyser av Biobränsleproduktion i Svenskt Jord-och Skogsbruk-Idag och Kring 2015; Department of Technology and Society, Lund University: Lund, Sweden, 1994. [Google Scholar]
- European Commission. Directive on the Promotion of the Use of Energy from Renewable Sources (2009/28/EC); European Parliament and Council of the European Union: Brussels, Belgium, 2009.
- Börjesson, P.; Tufvesson, L.; Lantz, M. Life Cycle Assessment of Biofuels in Sweden; 9188360962; Lund University: Lund, Sweden, 2010; p. 88. [Google Scholar]
- Green, M. Energy in pesticide manufacture, distribution and use. Energy World Agric. 1987, 2, 166–177. [Google Scholar]
- Audsley, E.; Stacey, K.; Parsons, D.J.; Williams, A.G. Estimation of the Greenhouse Gas Emissions from Agricultural Pesticide Manufacture and Use; Cranfield University: Bedford, UK, 2009. [Google Scholar]
- Nilsson, D.; Bernesson, S. Halm som Bränsle-Del 1: Tillgångar och Skördetidpunkter; Report/Department of Energy and Tecnology, SLU: Uppsala, Sweden, 2009. [Google Scholar]
- Hakala, K.; Heikkinen, J.; Sinkko, T.; Pahkala, K. Field trial results of straw yield with different harvesting methods, and modelled effects on soil organic carbon. A case study from Southern Finland. Biomass Bioenergy 2016, 95, 8–18. [Google Scholar]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar]
- Bolinder, M.; Kätterer, T.; Andrén, O.; Parent, L. Estimating carbon inputs to soil in forage-based crop rotations and modeling the effects on soil carbon dynamics in a Swedish long-term field experiment. Can. J. Soil Sci. 2012, 92, 821–833. [Google Scholar] [CrossRef]
- IPCC. Chapter 11: N2O Emissions from Managed Soils and CO2 Emissions from Lime and Urea Application; IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Jonasson, S.; Neuman, L. Alternativ till Spannmålstorkning Med Fossil Energi; LRF Media: Stockholm, Sweden, 2015. [Google Scholar]
- Gode, J.; Martinsson, F.; Hagberg, L.; Öman, A.; Höglund, J.; Palm, D. Miljöfaktaboken 2011-Uppskattade Emissionsfaktorer för Bränslen, el, Värme och Transporter; Värmeforsk: Stockholm, Sweden, 2011. [Google Scholar]
- Mortimer, N.; Elsayed, M.; Horne, R. Energy and Greenhouse Gas Emissions for Bioethanol Production from Wheat Grain and Sugar Beet; Resources Research Unit School of Environment and Development, Sheffield Hallam University: Sheffield, UK, 2004. [Google Scholar]
- Gaju, O.; Allard, V.; Martre, P.; Snape, J.W.; Heumez, E.; LeGouis, J.; Moreau, D.; Bogard, M.; Griffiths, S.; Orford, S.; et al. Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res. 2011, 123, 139–152. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Sweden: CO2 Country Profile. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 31 January 2023).
- Sepa Arbetsmaskiner, Utsläpp av Växthusgaser. Available online: https://www.naturvardsverket.se/data-och-statistik/klimat/vaxthusgaser-utslapp-fran-arbetsmaskiner/ (accessed on 24 January 2023).
- Muurinen, S.; Slafer, G.A.; Peltonen-Sainio, P. Breeding Effects on Nitrogen Use Efficiency of Spring Cereals under Northern Conditions. Crop Sci. 2006, 46, 561–568. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, Z.; Li, Y.; Zhang, Y.; Dong, H.; Fang, Y.; Han, L.; Xu, W.; Hu, L. Genetic improvement analysis of nitrogen uptake, utilization, translocation, and distribution in Chinese wheat in Henan Province. Field Crops Res. 2022, 277, 108406. [Google Scholar]
- Shi, H.; Chen, M.; Gao, L.; Wang, Y.; Bai, Y.; Yan, H.; Xu, C.; Zhou, Y.; Xu, Z.; Chen, J.; et al. Genome-wide association study of agronomic traits related to nitrogen use efficiency in wheat. Theor. Appl. Genet. 2022, 135, 4289–4302. [Google Scholar]
- Bell, L.W.; Wade, L.J.; Ewing, M.A. Perennial wheat: A review of environmental and agronomic prospects for development in Australia. Crop Pasture Sci. 2010, 61, 679–690. [Google Scholar]
- Cui, L.; Ren, Y.; Murray, T.D.; Yan, W.; Guo, Q.; Niu, Y.; Sun, Y.; Li, H. Development of Perennial Wheat Through Hybridization Between Wheat and Wheatgrasses: A Review. Engineering 2018, 4, 507–513. [Google Scholar]
- Björnsson, L.; Prade, T. Sustainable Cereal Straw Management: Use as Feedstock for Emerging Biobased Industries or Cropland Soil Incorporation? Waste Biomass Valorization 2021, 12, 5649–5663. [Google Scholar]
- Sheehan, H.; Bentley, A. Changing times: Opportunities for altering winter wheat phenology. Plants People Planet 2021, 3, 113–123. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant Breeding and Drought in C3 Cereals: What Should We Breed For? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a Changing Global Climate: Scaling Up and Scaling Down in Crops. Front. Plant Sci. 2020, 11, 1–29. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).