A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis
Abstract
1. Introduction
2. Materials and Methods
- Search Strategy
- Literature Selection
- Inclusion and Exclusion Criteria
- ➢
- Recent scientific articles on dental fluorosis were generally accepted.
- ➢
- Research published with a high impact factor was considered.
- ➢
- Research papers published between 2010 and 2023 in peer-reviewed journals were categorized based on quality within the last 5 years.
- ➢
- Further screening of the papers between 2018 and 2023 was performed for better relevance.
- ➢
- Articles should focus on fluoride use, dental carries caused by dental fluorosis, and dental fluorosis causes, etiology, mechanisms, prevention, diagnosis, or treatment, addressing etiology, mechanisms, prevention, diagnosis, or treatment.
- ➢
- Articles should provide a comprehensive and evidence-based analysis, citing relevant literature, scientific references to support findings, and conclusions.
- ➢
- Articles must include original research studies, including experimental, observational, and clinical trials, systematic reviews, meta-analyses, or case–control studies, to provide robust and reliable evidence on dental fluorosis.
- ➢
- Studies completed prior to the year 2010 were excluded from the evaluation.
- ➢
- Data from conference papers or dissertations that reused the same samples as other articles published in peer-reviewed journals were not included.
- ➢
- Articles that did not specifically focus on fluoride use, dental fluorosis, related etiology, mechanisms, the prevention of dental fluorosis, diagnosis, or treatment were excluded.
- ➢
- Articles that were not published in peer-reviewed journals were excluded.
- Research Design
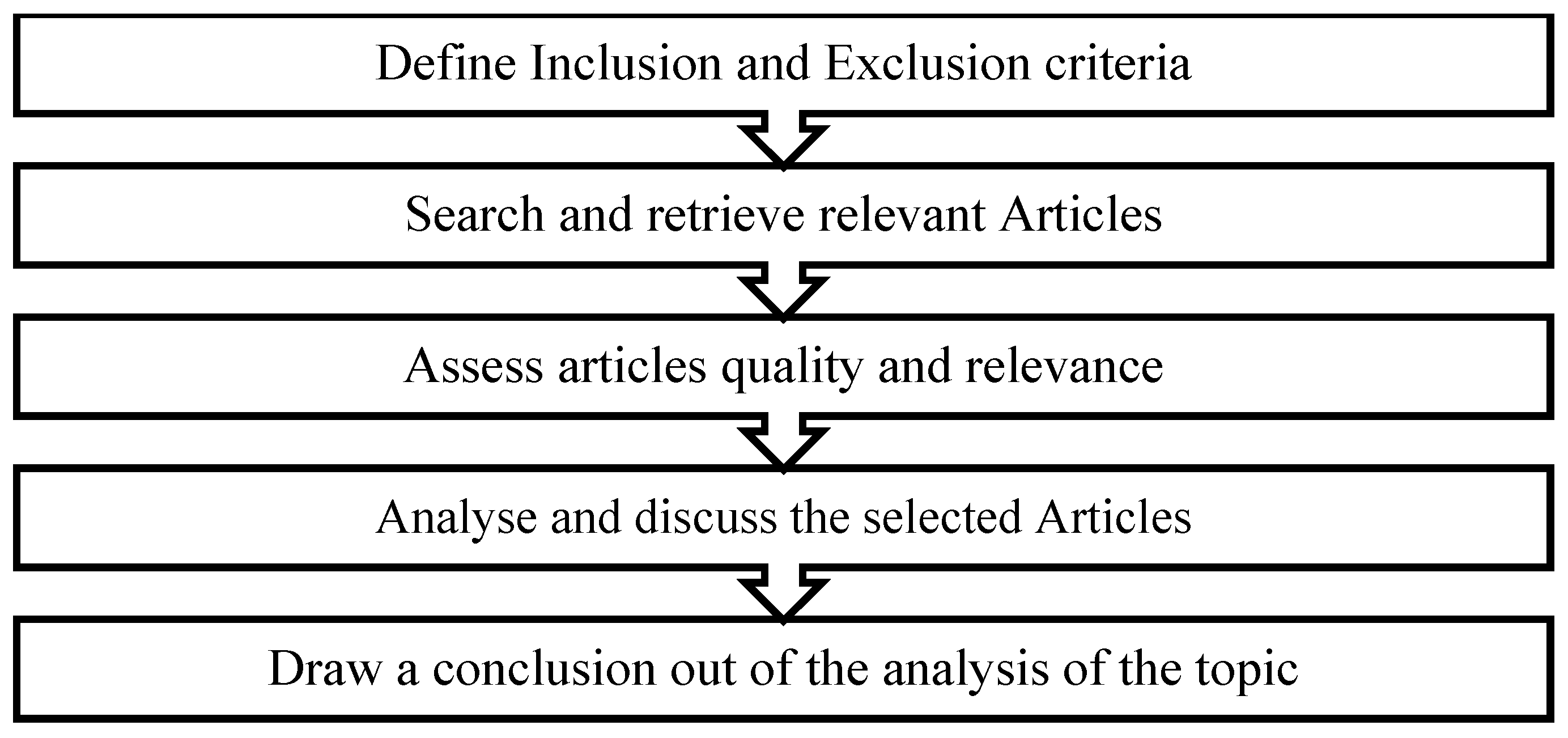
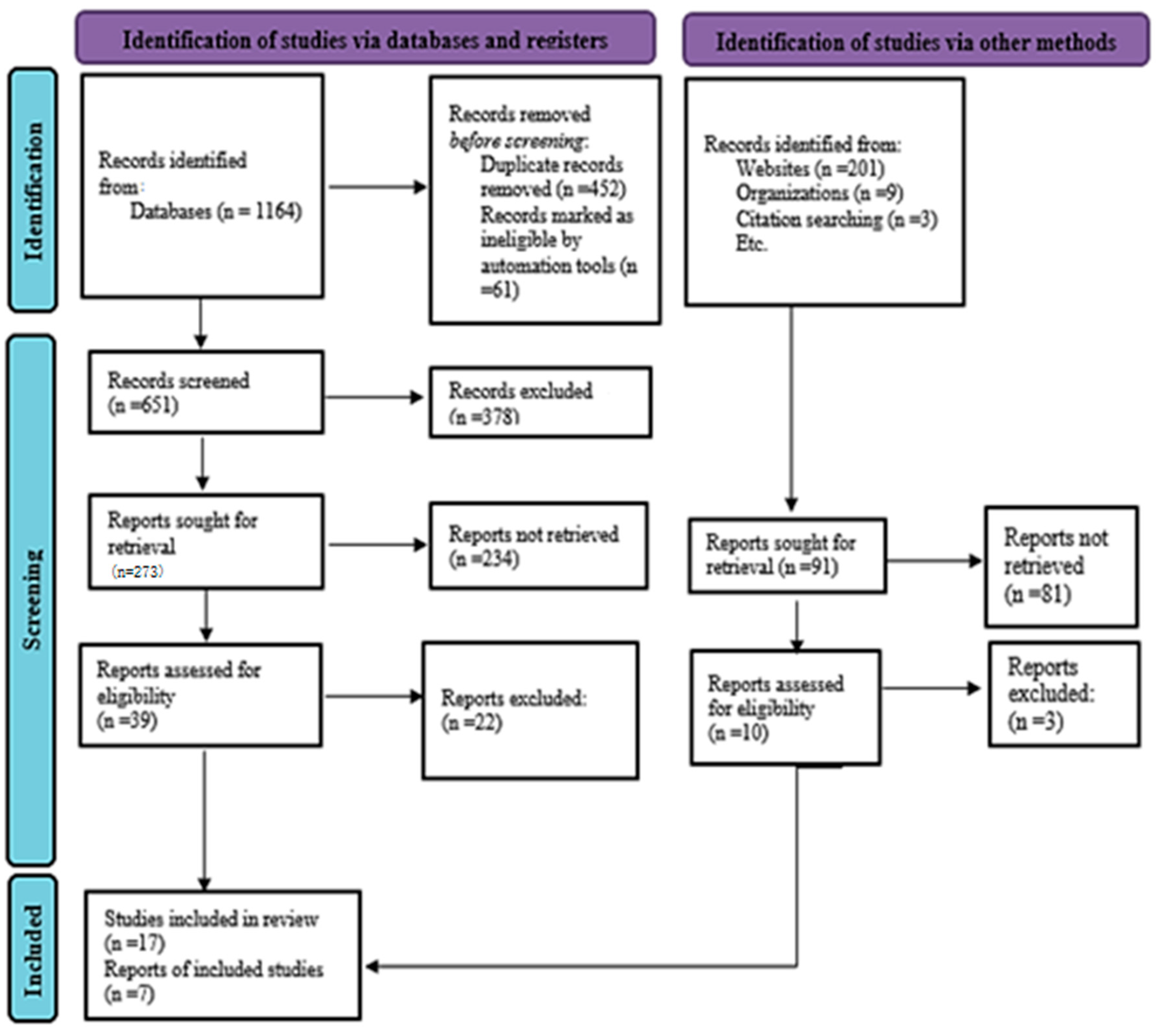
- Study Selection
- Quality Assessment
3. Results
4. Discussion
5. Conclusions
6. Limitations
7. Future Directions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsherbini, M.S.; Alsughier, Z.; Elmoazen, R.A.; Habibullah, M.A. Prevalence and severity of dental fluorosis among primary school children in AlRass, Saudi Arabia. Int. J. Med. Health Res. 2018, 4, 45–49. [Google Scholar]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Magara, Y. Fluoride in Drinking-Water; IWA Publishing: London UK, 2006. [Google Scholar]
- Wei, W.; Gao, Y.; Wang, C.; Zhao, L.; Sun, D. Excessive fluoride induces endoplasmic reticulum stress and interferes enamel proteinases secretion. Environ. Toxicol. 2013, 28, 332–341. [Google Scholar] [CrossRef]
- Waldbott, G.L. Fluoride in Food. Am. J. Clin. Nutr. 1963, 12, 455–462. [Google Scholar] [CrossRef]
- Ando, M.; Tadano, M.; Yamamoto, S.; Tamura, K.; Asanuma, S.; Watanabe, T.; Kondo, T.; Sakurai, S.; Ji, R.; Liang, C. Health effects of fluoride pollution caused by coal burning. Sci. Total Environ. 2001, 271, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Singh, R.; Damodaran, T.; Mishra, V.; Sharma, D.; Rai, D. Fluoride in groundwater: Toxicological exposure and remedies. J. Toxicol. Environ. Health Part. B 2013, 16, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Sananda Dey, B.G. Fluoride Fact on Human Health and Health Problems: A Review. Med. Clin. 2016, 2, 1360855569151406848. [Google Scholar]
- Pitzer, K.S. Fluorides of Radon and Element 118; University of California: Berkeley, CA, USA, 1975. [Google Scholar]
- Cameron, A.C.; Widmer, R.P. Handbook of Pediatric Dentistry E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2021. [Google Scholar]
- Downer, M.C.; Blinkhorn, A.S. The next stages in researching water fluoridation: Evaluation and surveillance. Health Educ. J. 2007, 66, 212–221. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1.
- Viswanathan, G.; Jaswanth, A.; Gopalakrishnan, S. Mapping of fluoride endemic areas and assessment of fluoride exposure. Sci. Total Environ. 2009, 407, 1579–1587. [Google Scholar] [CrossRef]
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian. J. Dent. Res. 2009, 20, 350. [Google Scholar] [CrossRef]
- Akuno, M.; Nocella, G.; Milia, E.; Gutierrez, L. Factors influencing the relationship between fluoride in drinking water and dental fluorosis: A ten-year systematic review and meta-analysis. J. Water Health 2019, 17, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Susheela, A.K. Fluorosis management programme in India. Curr. Sci. 1999, 77, 1250–1256. [Google Scholar]
- Samal, A.C.; Bhattacharya, P.; Mallick, A.; Ali, M.M.; Pyne, J.; Santra, S.C. A study to investigate fluoride contamination and fluoride exposure dose assessment in lateritic zones of West Bengal, India. Environ. Sci. Pollut. Res. 2015, 22, 6220–6229. [Google Scholar] [CrossRef]
- Kaseva, M. Contribution of trona (magadi) into excessive fluorosis—A case study in Maji ya Chai ward, northern Tanzania. Sci. Total Environ. 2006, 366, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mandinic, Z.; Curcic, M.; Antonijevic, B.; Lekic, C.P.; Carevic, M. Relationship between fluoride intake in Serbian children living in two areas with different natural levels of fluorides and occurrence of dental fluorosis. Food Chem. Toxicol. 2009, 47, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Kravchenko, J.; Atlaw, B.; McCornick, P.G.; Jeuland, M.; Merola, B.; Vengosh, A. Groundwater quality and its health impact: An assessment of dental fluorosis in rural inhabitants of the Main Ethiopian Rift. Environ. Int. 2012, 43, 37–47. [Google Scholar] [CrossRef]
- Goodley, K.L.D. Prevention of dental caries through the use of fluoride–the WHO approach Authors: Hiroshi Ogawa, Poul Erik Petersen 66–68 Download. Mouth 2016, 33, 66–68. [Google Scholar]
- Galagan, D.J.; Vermillion, J.R.; Nevitt, G.A.; Stadt, Z.M.; Dart, R.E. Climate and fluid intake. Public Health Rep. 1957, 72, 484. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, A.; Mohanty, P.K. Dental fluorosis—revisited. Biomed. J. Sci. Tech. Res. 2018, 2, 2243–2247. [Google Scholar] [CrossRef]
- Dissanayake, C. Of stones and health: Medical geology in Sri Lanka. Science 2005, 309, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.; Chandrajith, R. Medical geochemistry of tropical environments. Earth-Sci. Rev. 1999, 47, 219–258. [Google Scholar] [CrossRef]
- Satur, J.G.; Gussy, M.G.; Morgan, M.V.; Calache, H.; Wright, C. Review of the evidence for oral health promotion effectiveness. Health Educ. J. 2010, 69, 257–266. [Google Scholar] [CrossRef]
- Acheson, D. Independent Inquiry into Inequalities in Health Report; Ministry of Public Health: Brussels, Belgium, 2001.
- Kidd, E.A.; Fejerskov, O. Essentials of Dental Caries; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Nassar, Y.; Brizuela, M. The Role of Fluoride on Caries Prevention; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Zhang, S.; Wang, X.; Guo, C.; Zhang, X.; Ma, J.; Niu, P.; Chen, T. Associations of low level of fluoride exposure with dental fluorosis among US children and adolescents, NHANES 2015–2016. Ecotoxicol. Environ. Saf. 2021, 221, 112439. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; The Ottawa Hospital: Ottawa, ON, Canada, 2000. [Google Scholar]
- Mandinic, Z.; Curcic, M.; Antonijevic, B.; Carevic, M.; Mandic, J.; Djukic-Cosic, D.; Lekic, C.P. Fluoride in drinking water and dental fluorosis. Sci. Total Environ. 2010, 408, 3507–3512. [Google Scholar] [CrossRef]
- Rango, T.; Vengosh, A.; Jeuland, M.; Tekle-Haimanot, R.; Weinthal, E.; Kravchenko, J.; Paul, C.; McCornick, P. Fluoride exposure from groundwater as reflected by urinary fluoride and children’s dental fluorosis in the Main Ethiopian Rift Valley. Sci. Total Environ. 2014, 496, 188–197. [Google Scholar] [CrossRef]
- Kumar, S.; Lata, S.; Yadav, J.; Yadav, J. Relationship between water, urine and serum fluoride and fluorosis in school children of Jhajjar District, Haryana, India. Appl. Water Sci. 2017, 7, 3377–3384. [Google Scholar] [CrossRef]
- Irigoyen-Camacho, M.; Pérez, A.G.; González, A.M.; Alvarez, R.H. Nutritional status and dental fluorosis among schoolchildren in communities with different drinking water fluoride concentrations in a central region in Mexico. Sci. Total Environ. 2016, 541, 512–519. [Google Scholar] [CrossRef]
- Molina-Frechero, N.; Nevarez-Rascón, M.; Nevarez-Rascón, A.; González-González, R.; Irigoyen-Camacho, M.E.; Sánchez-Pérez, L.; López-Verdin, S.; Bologna-Molina, R. Impact of dental fluorosis, socioeconomic status and self-perception in adolescents exposed to a high level of fluoride in water. Int. J. Environ. Res. Public Health 2017, 14, 73. [Google Scholar] [CrossRef]
- Gbadebo, A. Groundwater fluoride and dental fluorosis in southwestern Nigeria. Environ. Geochem. Health 2012, 34, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, Y.; Wang, W.; Zhao, L.; Zhang, W.; Han, H.; Shi, Y.; Yu, G.; Sun, D. A national cross-sectional study on effects of fluoride-safe water supply on the prevalence of fluorosis in China. BMJ Open 2012, 2, e001564. [Google Scholar] [CrossRef]
- Juárez-López, M.L.A.; Huízar-Álvarez, R.; Molina-Frechero, N.; Murrieta-Pruneda, F.; Cortés-Aguilera, Y. Fluorine in water and dental fluorosis in a community of Queretaro state Mexico. J. Environ. Prot. 2011, 2, 744–749. [Google Scholar] [CrossRef]
- Molina-Frechero, N.; Gaona, E.; Angulo, M.; Pérez, L.S.; González, R.G.; Rascón, M.N.; Bologna-Molina, R. Fluoride exposure effects and dental fluorosis in children in Mexico City. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3664. [Google Scholar] [CrossRef]
- Firempong, C.; Nsiah, K.; Awunyo-Vitor, D.; Dongsogo, J. Soluble fluoride levels in drinking water-a major risk factor of dental fluorosis among children in Bongo community of Ghana. Ghana. Med. J. 2013, 47, 16–23. [Google Scholar] [PubMed]
- Fernandes, I.C.; Forte, F.D.S.; Sampaio, F.C. Molar-incisor hypomineralization (MIH), dental fluorosis, and caries in rural areas with different fluoride levels in the drinking water. Int. J. Paediatr. Dent. 2021, 31, 475–482. [Google Scholar] [CrossRef]
- Das, G.; Tirth, V.; Arora, S.; Algahtani, A.; Kafeel, M.; Alqarni, A.H.G.; Saluja, P.; Vij, H.; Bavabeedu, S.S.; Tirth, A. Effect of fluoride concentration in drinking water on dental fluorosis in Southwest Saudi Arabia. Int. J. Environ. Res. Public Health 2020, 17, 3914. [Google Scholar] [CrossRef]
- Rojanaworarit, C.; Claudio, L.; Howteerakul, N.; Siramahamongkol, A.; Ngernthong, P.; Kongtip, P.; Woskie, S. Hydrogeogenic fluoride in groundwater and dental fluorosis in Thai agrarian communities: A prevalence survey and case–control study. BMC Oral. Health 2021, 21, 545. [Google Scholar] [CrossRef]
- Al Warawreh, A.M.; Al Tamimi, Z.H.; Al Qatawna, M.I.; Al Momani, A.A.; Al Mhaidat, M.R.; El Naji, W.S.; AlSaraireh, S. Prevalence of dental fluorosis among Southern Jordanian population. Int. J. Dent. 2020, 2020, 8890004. [Google Scholar] [CrossRef]
- Bhowmik, A.D.; Shaw, P.; Mondal, P.; Munshi, C.; Chatterjee, S.; Bhattacharya, S.; Chattopadhyay, A. Incidence of fluorosis and urinary fluoride concentration are not always positively correlated with drinking water fluoride level. Curr. Sci. 2019, 116, 1551–1554. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Kruger, E.; Tennant, M. Spatial distribution of groundwater fluoride levels and population at risk for dental caries and dental fluorosis in Sri Lanka. Int. Dent. J. 2019, 69, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gevera, P.; Mouri, H.; Maronga, G. Occurrence of fluorosis in a population living in a high-fluoride groundwater area: Nakuru area in the Central Kenyan Rift Valley. Environ. Geochem. Health 2019, 41, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Q.; Li, G.; Wang, M.; Liu, H.; Yu, X.; Chen, J.; Li, P.; Dong, L.; Zhou, G. The cholinergic system, intelligence, and dental fluorosis in school-aged children with low-to-moderate fluoride exposure. Ecotoxicol. Environ. Saf. 2021, 228, 112959. [Google Scholar] [CrossRef] [PubMed]
- Yani, S.I.; Seweng, A.; Mallongi, A.; Nur, R.; Abdullah, M.T.; Salmah, U.; Sirajuddin, S.; Basir-Cyio, M.; Anshary, A. The influence of fluoride in drinking water on the incidence of fluorosis and intelligence of elementary school students in Palu City. Gac. Sanit. 2021, 35, S159–S163. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Chadwick, B.L.; Farnell, D.J.; Chestnutt, I.G. Factors associated with dental fluorosis among Malaysian children exposed to different fluoride concentrations in the public water supply. J. Public Health Dent. 2021, 81, 270–279. [Google Scholar] [CrossRef]
- Strużycka, I.; Olszewska, A.; Bogusławska-Kapała, A.; Hryhorowicz, S.; Kaczmarek-Ryś, M.; Grabarek, B.O.; Staszkiewicz, R.; Kuciel-Polczak, I.; Czajka-Jakubowska, A. Assessing Fluorosis Incidence in Areas with Low Fluoride Content in the Drinking Water, Fluorotic Enamel Architecture, and Composition Alterations. Int. J. Environ. Res. Public Health 2022, 19, 7153. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, L.; Zhang, T.; Xu, T.; Li, M.; Yin, W.; Jiang, Q.; Yang, Y.; Hu, T. Brick tea consumption is a risk factor for dental caries and dental fluorosis among 12-year-old Tibetan children in Ganzi. Environ. Geochem. Health 2019, 41, 1405–1417. [Google Scholar] [CrossRef]
- Silva, M.C.C.; Lima, C.C.B.; Lima, M.d.D.M.d.; Moura, L.d.F.A.d.D.; Tabchoury, C.P.M.; Moura, M.S.d. Effect of fluoridated water on dental caries and fluorosis in schoolchildren who use fluoridated dentifrice. Braz. Dent. J. 2021, 32, 75–83. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Duckworth, R.M. Fluoride: Intake and metabolism, therapeutic and toxicological consequences. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Elsevier: Amsterdam, The Netherlands, 2017; pp. 539–550. [Google Scholar]
- Whitford, G.M. Fluoride metabolism and excretion in children. J. Public Health Dent. 1999, 59, 224–228. [Google Scholar] [CrossRef]
- Agalakova, N.; Nadei, O. Inorganic fluoride and functions of brain. Crit. Rev. Toxicol. 2020, 50, 28–46. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.F.; Limeback, H. Blood is thicker than water: Flaws in a National Toxicology Program study. Med. Hypotheses 2018, 121, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.J.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride exposure and kidney and liver function among adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef]
- Clark, M.B.; Slayton, R.L.; Section on Oral Health; Segura, A.; Boulter, S.; Clark, M.B.; Gereige, R.; Krol, D.; Mouradian, W.; Quinonez, R.; et al. Fluoride Use in Caries Prevention in the Primary Care Setting. Pediatrics 2014, 134, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.N.; Shah, J. Reversal of dental fluorosis: A clinical study. J. Nat. Sci. Biol. Med. 2013, 4, 138–144. [Google Scholar] [CrossRef]
- Hamdan, M. The prevalence and severity of dental fluorosis among 12-year-old schoolchildren in Jordan. Int. J. Paediatr. Dent. 2003, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Murray, J. Gingivitis and gingival recession in adults from high-fluoride and low-fluoride areas. Arch. Oral. Biol. 1972, 17, 1269–1277. [Google Scholar] [CrossRef]
- Singh, A.; Vazirani, S.J.; Jolly, S.; Bansal, B. Endemic fluorosis. Postgrad. Med. J. 1962, 38, 150. [Google Scholar] [CrossRef][Green Version]
- Łukomska-Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokołowski, K.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Antibacterial properties of calcium fluoride-based composite materials: In Vitro study. BioMed Res. Int. 2016, 2016, 1048320. [Google Scholar]
- Brothwell, D.J.; Limeback, H. Fluorosis risk in grade 2 students residing in a rural area with widely varying natural fluoride. Community Dent. Oral. Epidemiol. 1999, 27, 130–136. [Google Scholar] [CrossRef]
- Brothwell, D.; Limeback, H. Breastfeeding is protective against dental fluorosis in a nonfluoridated rural area of Ontario, Canada. J. Hum. Lact. 2003, 19, 386–390. [Google Scholar] [CrossRef]
- Wondwossen, F.; Åstrøm, A.N.; Bjorvatn, K.; Bårdsen, A. Sociodemographic and behavioural correlates of severe dental fluorosis. Int. J. Paediatr. Dent. 2006, 16, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.K.; Bezerra de Menezes, L.M.; Dias, A.A.; Morais de Alencar, C.H.; Leitao de Almeida, M.E. Analysis of protection or risk factors for dental fluorosis in 6 to 8 year-old children in Fortaleza, Brazil. Rev. Panam. Salud Publica Pan Am. J. Public Health 2010, 28, 421–428. [Google Scholar]
- Şener, Y.; Tosun, G.; Kahvecioğlu, F.; Gökalp, A.; Koc, H. Fluoride levels of human plasma and breast milk. Eur. J. Dent. 2007, 1, 21–24. [Google Scholar] [CrossRef] [PubMed]
| Sample | Adults, Children, Global |
|---|---|
| Phenomenon of Interest | Effect of fluoride intake |
| Design | Review of already-published articles using search engines |
| Evaluation | Dental fluorosis |
| Research Type | Case report; controlled study; cohort, retrospective, longitudinal, and qualitative analyses |
| No. | Search Strategy (PICO—Population Intervention Comparison Outcomes) |
|---|---|
| 1 | Water (tw) OR dihydrogen oxide (tw) water based (tw) liquid (tw) OR liquid (tw) OR liquid with fluoride* (tw) OR fluoride containing liquid* (tw) OR well water* (tw) tap water* (tw) OR drinking water* (tw) OR fluoridated water* (tw) OR fluoridation* (tw) OR teeth malformation* (tw) OR dental fluorosis (tw) OR fluoride level (tw) OR fluoridated meals* (tw) OR tooth decay. |
| 2 | Water (Abstract and Keywords) OR liquid (Abstract and Keywords) OR fluoride containing liquid*(Abstract and Keywords) OR well water*(Abstract and Keywords) tap water*(Abstract and Keywords) OR drinking water*(Abstract and Keywords) OR fluoridated water*(Abstract and Keywords) OR fluoridation*(Abstract and Keywords) OR teeth malformation*(Abstract and Keywords) OR dental fluorosis (Abstract and Keywords) OR fluoride level (Abstract and Keywords) |
| 3 | Water (tw) OR liquid (tw) OR liquid with fluoride* (tw) OR fluoride containing liquid*(tw) OR well water* (tw) tap water* (tw) OR drinking water* (tw) OR fluoridated water* (tw) OR fluoridation* (tw) OR teeth malformation* (tw) OR dental fluorosis (tw) OR Fluoride level (tw) |
| PICO | Search Strategy |
|---|---|
| Population | Adults, teenagers, malnourished, infants |
| Intervention | Acceptable levels of fluoride ions (0.6 mg/L) in drinking water, breastfeeding for newborns and young children, and use of fluoridated water |
| Comparison | Fluoride water, water supply, tap water, drinking water fluoridation, well water, ground water |
| Outcome | Teeth malformation, dental fluorosis, dental caries, fluoride level |
| Sr. # | Title | Author | Year | Reference | Country | Sample Age | Description | Score |
|---|---|---|---|---|---|---|---|---|
| 1 | Fluoride in drinking water and dental fluorosis | Mandinic, Z.; Curcic, M.; Antonijevic, B.; Carevic, M.; Mandic, J.; Djukic-Cosic, D.; Lekic, C.P | 2010 | [34] | Serbia | 12 years | Fluoride levels in drinking water were strongly connected to prevalence of dental fluorosis and hair fluoride levels. | 9 |
| 2 | Fluoride exposure from groundwater as reflected by urinary fluoride and children’s dental fluorosis in the Main Ethiopian Rift Valley | Rango, T.; Vengosh, A.; Jeuland, M.; Tekle-Haimanot, R.; Weinthal, E.; Kravchenko, J.; Paul, C.; McCornick, P | 2014 | [35] | Ethiopia | 10–15 years | This study’s findings suggest that many children drink water from sources with high fluoride levels, have high fluoride levels in their urine, and suffer significant adverse. consequences to their dental health due to dental fluorosis. | 9 |
| 3 | Relationship between water, urine and serum fluoride and fluorosis in school children of Jhajjar District, Haryana, India | Kumar, S.; Lata, S.; Yadav, J.; Yadav, J | 2017 | [36] | India | Children | Fluorosis was shown to be correlated with fluoride levels in water, urine, and serum. | 9 |
| 4 | Nutritional status and dental fluorosis among schoolchildren in communities with different drinking water fluoride concentrations in a central region in Mexico | Irigoyen-Camacho, M.; Pérez, A.G.; González, A.M.; Alvarez, R.H | 2016 | [37] | Mexico | 8–12 years | Dental fluorosis in the TFI categories that impact the whole tooth surface was more prevalent in children with low height-for-age. | 8 |
| 5 | Impact of Dental Fluorosis, Socioeconomic Status and Self-Perception in Adolescents Exposed to a High Level of Fluoride in Water | Molina-Frechero, N.; Nevarez-Rascón, M.; Nevarez-Rascón, A.; González-González, R.; Irigoyen-Camacho, M.E.; Sánchez-Pérez, L.; López-Verdin, S.; Bologna-Molina, R | 2017 | [38] | Mexico | 15 years | Self-perceptions of dental fluorosis affected adolescents. | 7 |
| 6 | Groundwater Fluoride and dental fluorosis in Southwestern Nigeria | Gbadebo, A. | 2012 | [39] | Nigeria | Not specified | Indicated a prevalence of dental fluorosis due to the average person’s exposure to fluoride in their drinking water. | 9 |
| 7 | A national cross-sectional Study on the Effects of fluoride-safe Water Supply on the Prevalence of Fluorosis in China | Wang, C.; Gao, Y.; Wang, W.; Zhao, L.; Zhang, W.; Han, H.; Shi, Y.; Yu, G.; Sun, D | 2012 | [40] | China | Children and adults | This study showed that the incidence of dental and skeletal fluorosis has decreased dramatically due to fluoride-free water supply programs. | 9 |
| 8 | Fluorine in Water and Dental Fluorosis in a Community of Queretaro State Mexico | Juárez-López, M.L.A.; Huízar-Álvarez, R.; Molina-Frechero, N | 2011 | [41] | Mexico | Children | high incidence of dental fluorosis | 9 |
| 9 | Fluoride Exposure Effects and Dental Fluorosis in Children in Mexico City | Molina-Frechero, N.; Gaona, E.; Angulo, M.; Pérez, L.S.; González, R.G.; Rascón, M.N.; Bologna-Molina, R | 2015 | [42] | Mexico | 10–12 years | Because of the correlation between how often children brushed their teeth and the absence of parental monitoring, dental fluorosis was more common and severe. | 9 |
| 10 | Soluble fluoride levels in drinking water-a major risk factor for dental fluorosis among children in the Bongo community of Ghana | Firempong, C.; Nsiah, K.; Awunyo-Vitor, D.; Dongsogo, J. | 2013 | [43] | Ghana | Children | High fluoride levels in the water supply were closely linked to dental fluorosis. | 9 |
| Sr.# | Title | Author | Year | Reference | Country | Sample | Description | Score |
|---|---|---|---|---|---|---|---|---|
| 1 | Molar-incisor hypomineralization (MIH), dental fluorosis and caries in rural areas with different fluoride levels in the drinking water | Fernandes, I. C., Forte, F. D. S., & Sampaio, F. C | 2021 | [44] | Brazil | Children | School MIH prevalence and drinking water F-levels were unrelated. Dental fluorosis and MIH severity were linked in areas with moderate-to-high drinking water fluoride levels. | 9 |
| 2 | Associations of low level of fluoride exposure with dental fluorosis among U.S. Children and Adolescents, NHANES 2015–2016 | Dong, H., Yang, X., Zhang, S., Wang, X., Guo, C., Zhang, X., Ma, J., Niu, P. and Chen, T | 2021 | [32] | China | Children and adolescents | Dental fluorosis was linked to exposure to fluoride, whether in the water or the blood. | 9 |
| 3 | Effect of Fluoride Concentration in Drinking Water on Dental Fluorosis in Southwest Saudi Arabia | Das, G., Tirth, V., Arora, S., Algahtani, A., Kafeel, M., Alqarni, A.H.G., Saluja, P., Vij, H., Bavabeedu, S.S. and Tirth, A. | 2020 | [45] | KSA | 9–50 years | The fluoride levels in the bodies of those who drank well water were higher. | 9 |
| 4 | Hydrogeogenic fluoride in groundwater and dental fluorosis in Thai agrarian communities: a prevalence survey and case– control study | Rojanaworarit, C., Claudio, L., Howteerakul, N., Siramahamongkol, A., Ngernthong, P., Kongtip, P., & Woskie, S. | 2021 | [46] | Thailand | Children | High rates of dental fluorosis were seen in fluoride-endemic regions, where residents drink groundwater laced with naturally occurring fluoride. | 9 |
| 5 | Prevalence of Dental Fluorosis among Southern Jordanian Population | Al Warawreh, A. M., Al Tamimi, Z. H., Al Qatawna, M. I., Al Momani, A. A., Al Mhaidat, M. R., El Naji, W. S., & AlSaraireh, S. | 2020 | [47] | Jordan | 12–52 years | Fluorosis was more prevalent in extremely mild and localized forms among tap-water-drinking individuals. | 9 |
| 6 | Incidence of fluorosis and urinary fluoride concentration is not always positively correlated with drinking water fluoride level | Bhowmik, A. D., Shaw, P., Mondal, P., Munshi, C., Chatterjee, S., Bhattacharya, S., & Chattopadhyay, A. | 2019 | [48] | India | N/M | As previously reported, high fluoride levels in crops and vegetables cultivated in fluoride-affected regions contributed to fluorosis, but fluoride from other sources also had a significant impact. | 9 |
| 7 | Spatial distribution of groundwater fluoride levels and the population at risk for dental caries and dental fluorosis in Sri Lanka | Ranasinghe, N., Kruger, E., & Tennant, M. | 2019 | [49] | Sri Lanka | N/M | 12% of 12-year-olds had dental fluorosis, whereas 81.4% of those in low-fluoride areas had tooth decay. A total of 82.4% of the population lived in low-fluoride zones, whereas 11.2% were at risk of serious health issues from fluoride absorption. | 9 |
| 8 | Occurrence of fluorosis in a population living in a high-fluoride groundwater area: Nakuru area in the Central Kenyan Rift Valley | Gevera, P., Mouri, H., & Maronga, G. | 2019 | [50] | Kenya | N/M | According to the findings, dental fluorosis was substantially more common in the younger population. | 9 |
| 9 | The cholinergic system, intelligence, and dental fluorosis in school-aged children with low-to-moderate fluoride exposure | Wang, S., Zhao, Q., Li, G., Wang, M., Liu, H., Yu, X., … & Wang, A. | 2021 | [51] | China | Children | Children’s cholinergic system impairment was linked to mild-to-moderate fluoride exposure. | 9 |
| 10 | The influence of fluoride in drinking water on the incidence of fluorosis and intelligence of elementary school students in Palu City | Yani, S.I., Seweng, A., Mallongi, A., Nur, R., Abdullah, M.T., Salmah, U., Sirajuddin, S., Basir-Cyio, M. and Anshary, A., | 2021 | [52] | Indonesia | 6–12 years | Fluorosis was more prevalent in high-F areas, where pupils also had lower IQs than those in low-F areas. | 8 |
| 11 | Factors associated with dental fluorosis among Malaysian children exposed to different fluoride concentrations in the public water supply | Mohd Nor, N.A., Chadwick, B.L., Farnell, D.J. and Chestnutt, I.G., | 2021 | [53] | Malaysia | 9–12 years | Children born after the fluoride content in the water was altered had a reduced prevalence of fluorosis. Even when the fluoride content in the water was lowered, fluorosis was still a significant concern. | 9 |
| 12 | Assessing Fluorosis Incidence in Areas with Low Fluoride Content in the Drinking Water, Fluorotic Enamel Architecture, and Composition Alterations | Strużycka, I., Olszewska, A., Bogusławska-Kapała, A., Hryhorowicz, S., Kaczmarek-Ryś, M., Grabarek, B. O., … & Czajka-Jakubowska, A | 2022 | [54] | Poland | 15–25 years | Among the clinical sample, 89 patients were found to have dental fluorosis of varied degrees (12.8%). Compared to teeth without fluorosis, individuals with mild and severe fluorosis had considerably greater protein (p 0.001) and fluoride levels (p 0.001) in their enamel. | 9 |
| 13 | Brick tea consumption is a risk factor for dental caries and dental fluorosis among 12-year-old Tibetan children in Ganzi | Zhang, R., Cheng, L., Zhang, T., Xu, T., Li, M., Yin, W., Jiang, Q., Yang, Y. and Hu, T., | 2019 | [55] | China | 12 years | Dental fluorosis and dental caries were more common in children whose mothers drank brick tea regularly. The dental health of children born to mothers who drink less brick tea during pregnancy and breastfeeding may improve. | 9 |
| 14 | Effect of fluoridated water on dental caries and fluorosis in schoolchildren who use fluoridated dentifrice | Silva, M. C. C., Lima, C. C. B., Lima, M. D. D. M. D., Moura, L. D. F. A. D. D., Tabchoury, C. P. M., & Moura, M. S. D. | 2021 | [56] | Brazil | 5–12 years | Both the frequency and severity of dental caries were reduced in children and adolescents who drank fluoridated water as opposed to those who used fluoridated toothpaste. Fluorosis, ranging in severity from extremely low to substantial, has been linked to water fluoridation in teenagers. | 9 |
| First Author, Year | Representativeness of the Exposed Variable | Selection of the Unexposed Variable | Ascertainment of Exposure | The Outcome of Interest Not Present at the Start of the Study | Control for Important Factors or Additional Factors | Outcome Assessment | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Variables | No Response Rate | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| [34] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [35] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [36] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [37] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | 8 |
| [38] | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✕ | ✕ | 7 |
| [39] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [40] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [41] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [42] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [43] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [44] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [45] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [46] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [47] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [48] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [49] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [50] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [51] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [52] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | 8 |
| [53] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [54] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [55] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
| [56] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer, M.F. A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis. Sustainability 2023, 15, 12227. https://doi.org/10.3390/su151612227
Umer MF. A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis. Sustainability. 2023; 15(16):12227. https://doi.org/10.3390/su151612227
Chicago/Turabian StyleUmer, Muhammad Farooq. 2023. "A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis" Sustainability 15, no. 16: 12227. https://doi.org/10.3390/su151612227
APA StyleUmer, M. F. (2023). A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis. Sustainability, 15(16), 12227. https://doi.org/10.3390/su151612227







